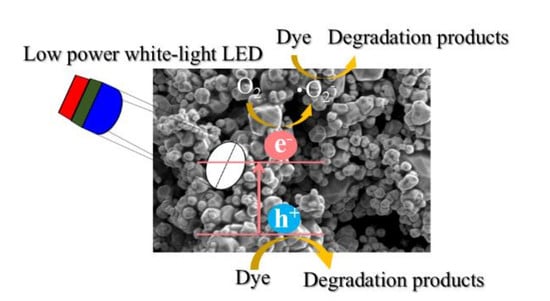
Facile Synthesis and Characterization of Ag3PO4 Microparticles for Degradation of Organic Dyestuffs under White-Light Light-Emitting-Diode Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation
2.3. Characterization
2.4. Photocatalytic Degradation Activity
2.5. Study of Recombination Rate of Electron–Hole Pairs and Charge Separation Efficiency
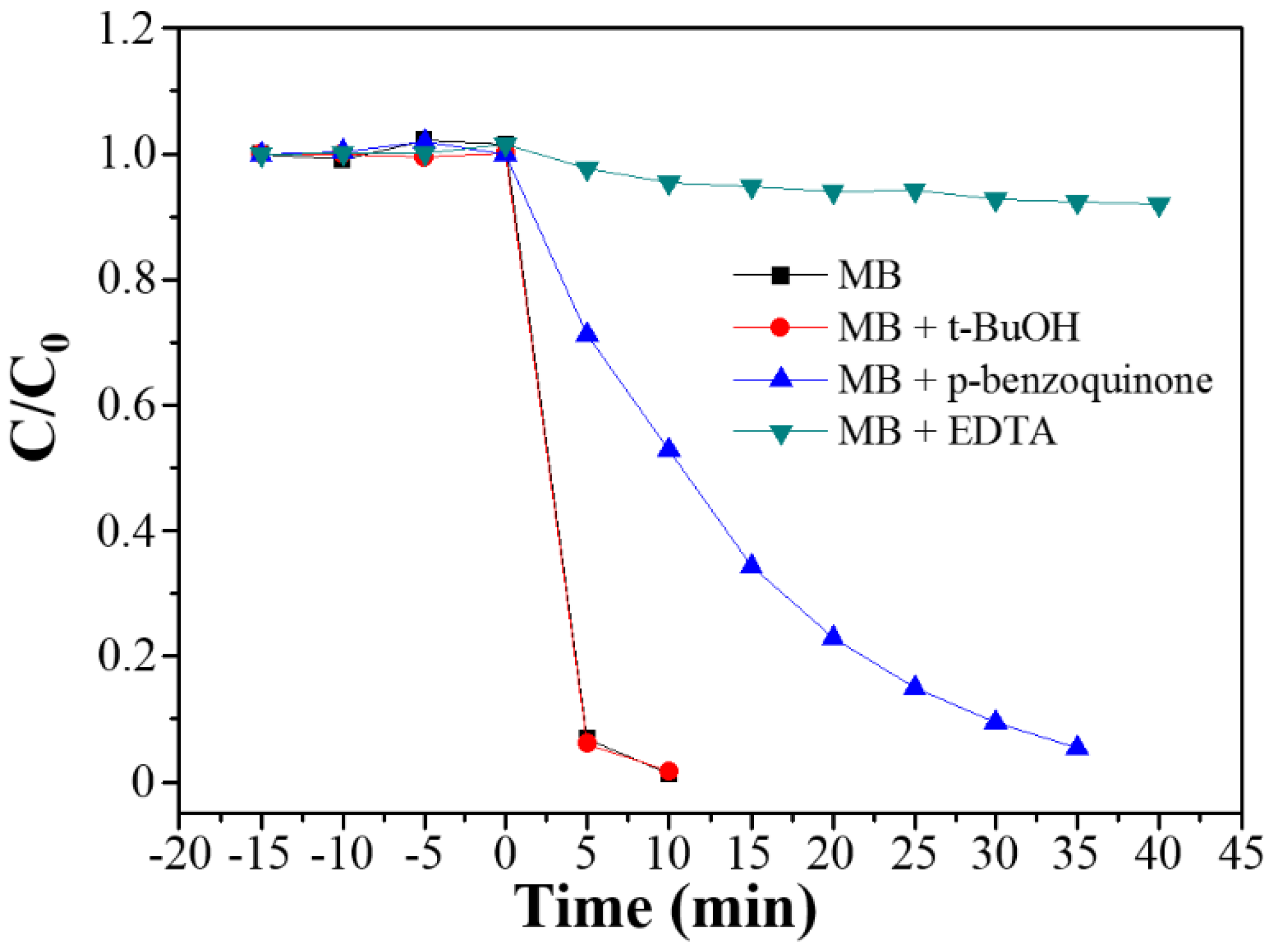
2.6. Scavenger Test
3. Results and Discussion
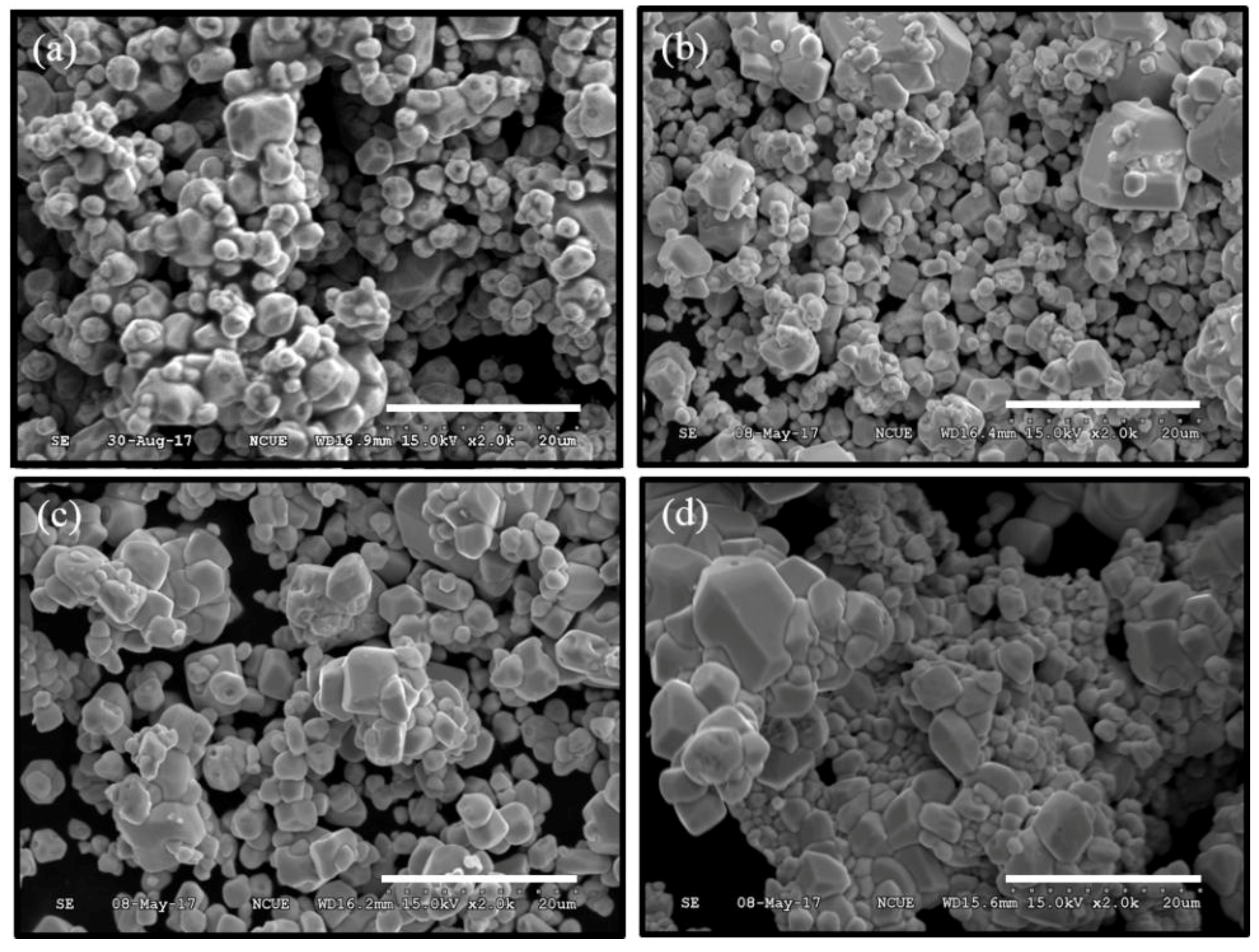
3.1. Crystalline Phase and Morphology
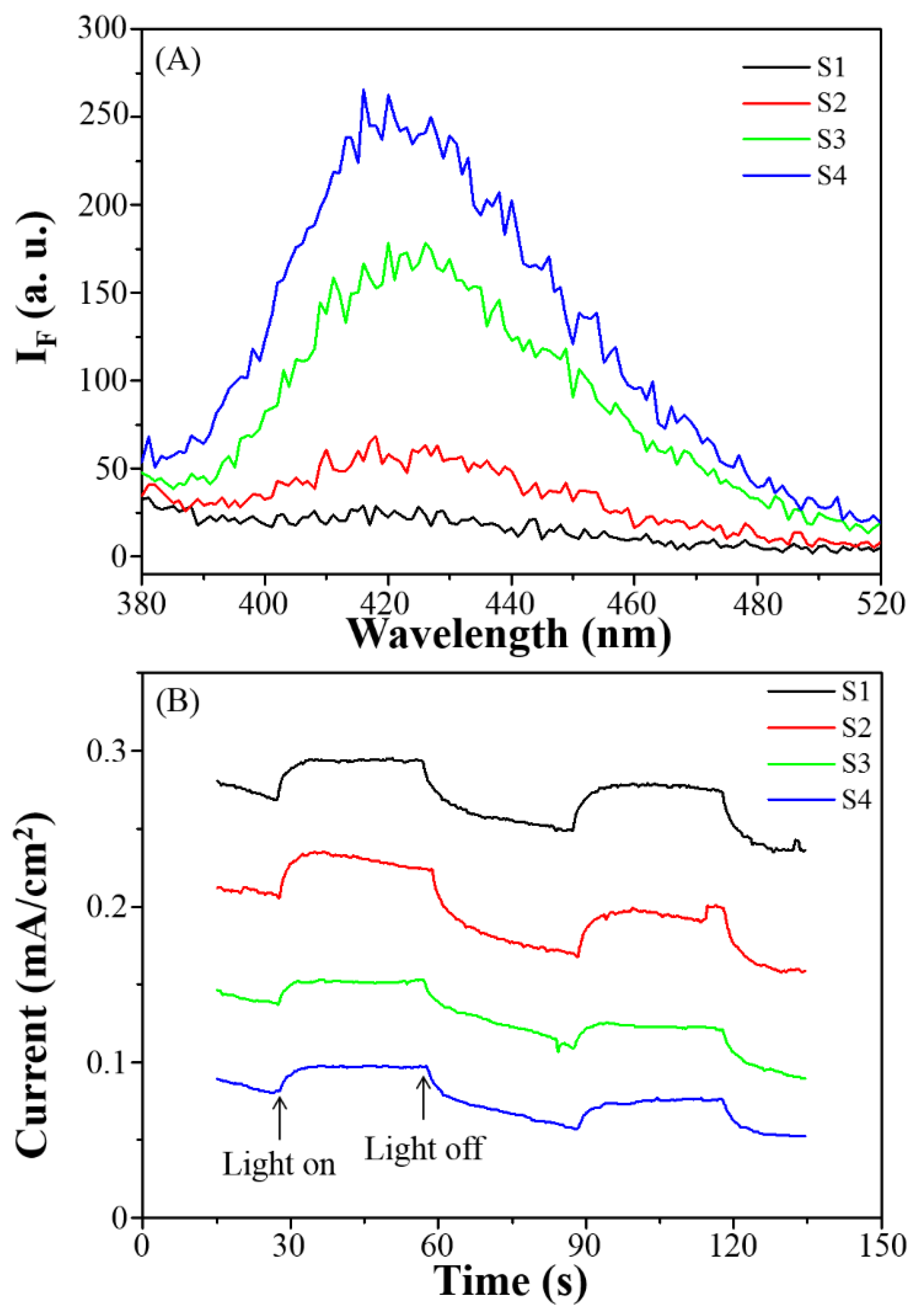
3.2. Photophysical Properties and Specific Surface Area
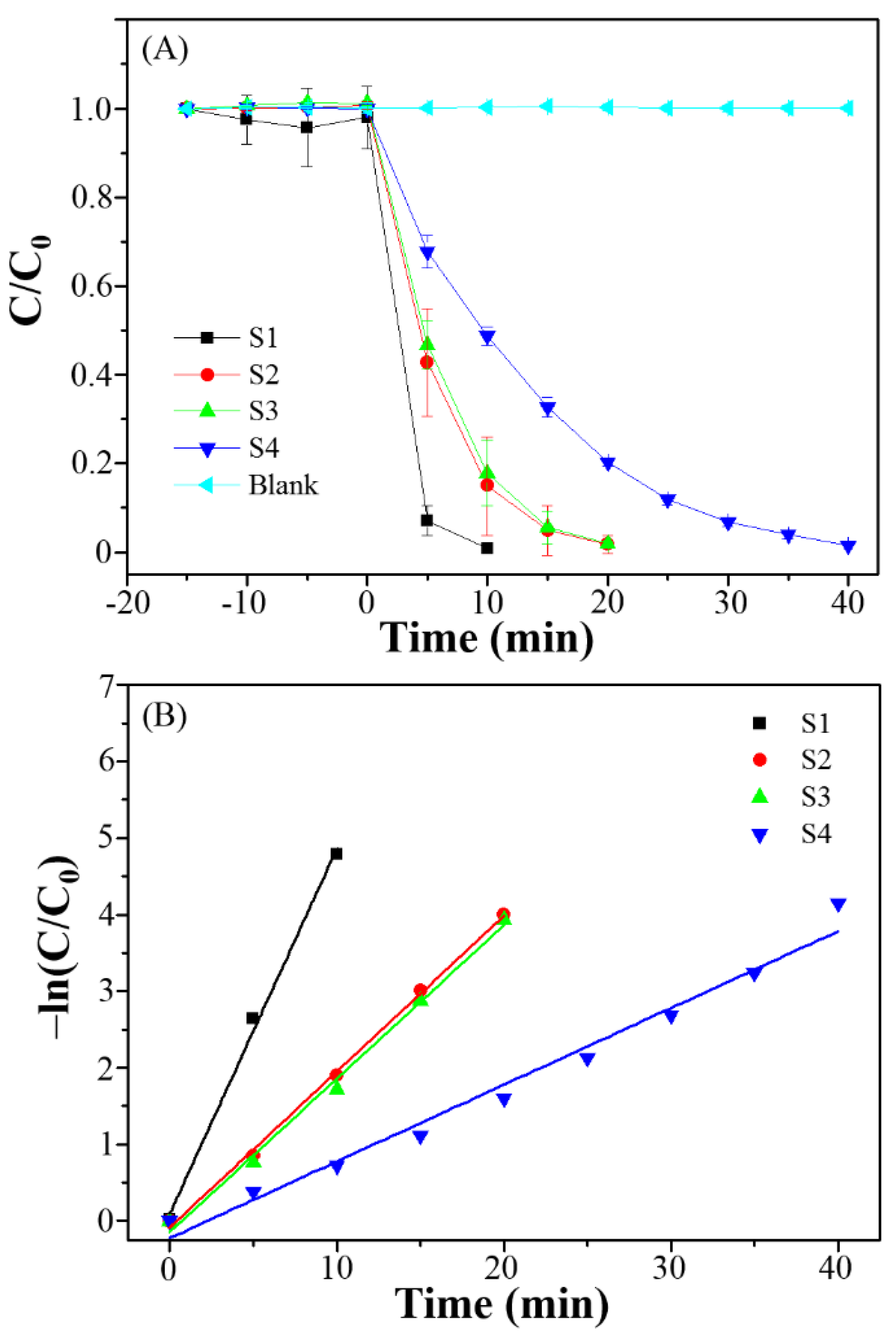
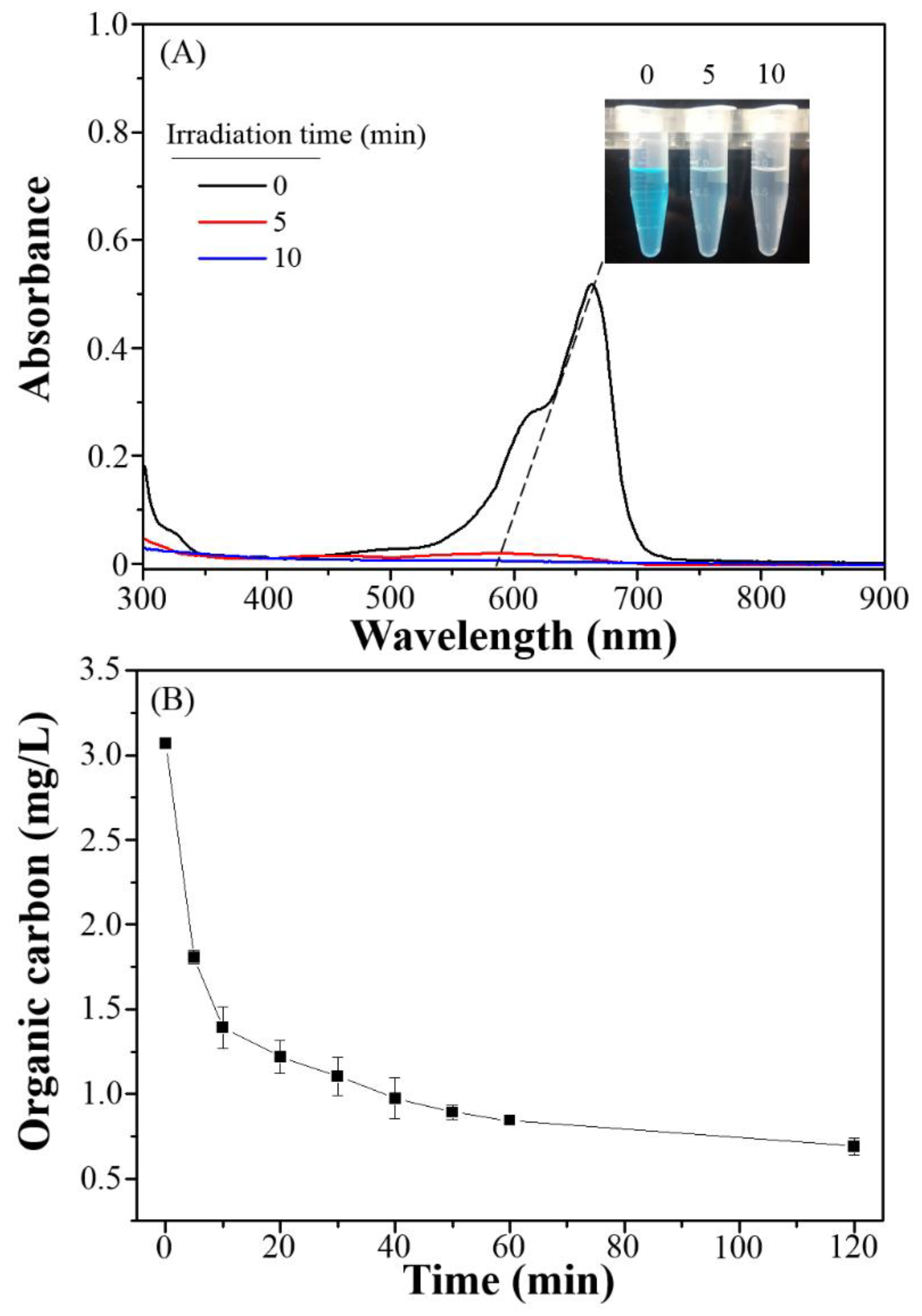
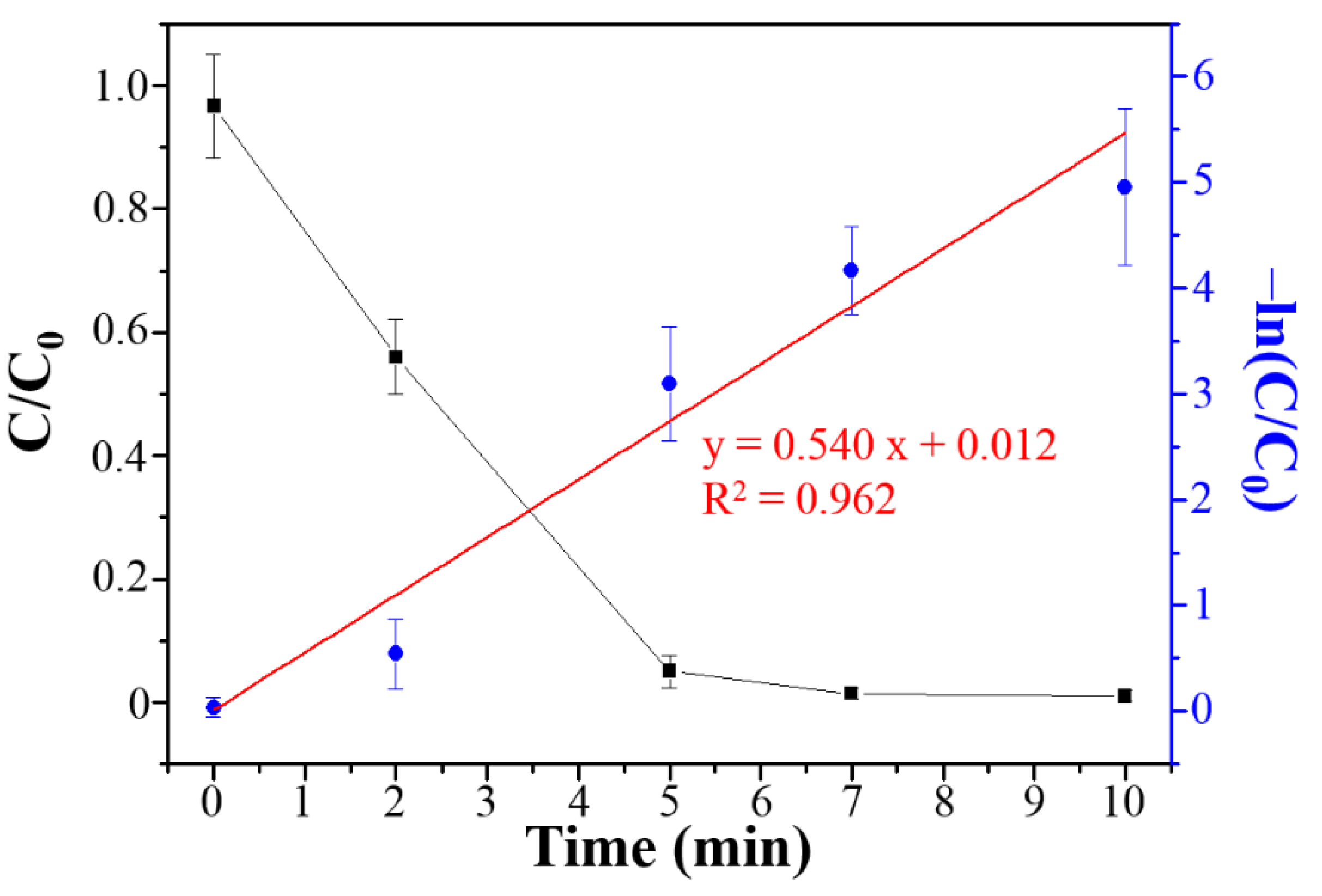
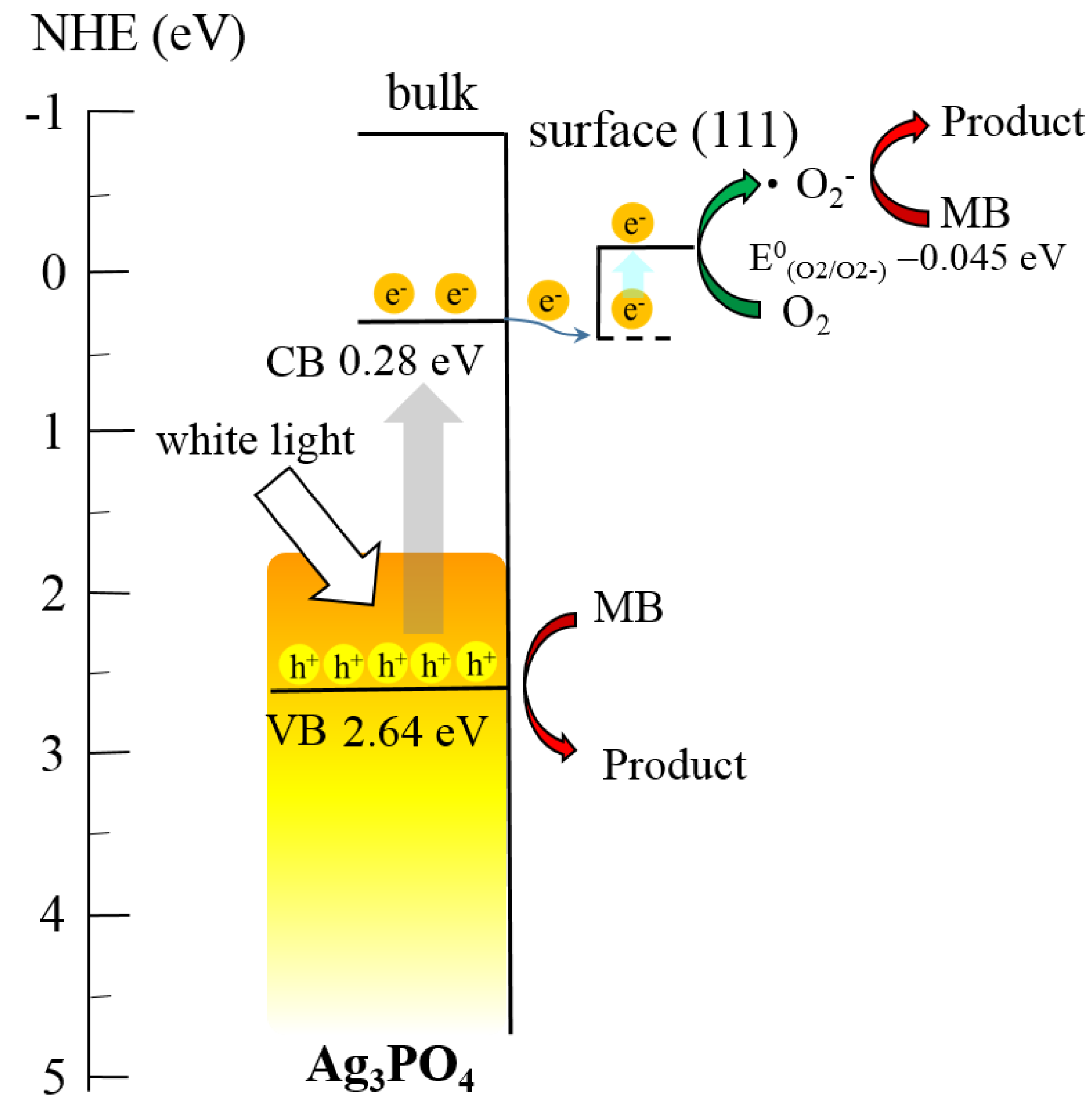
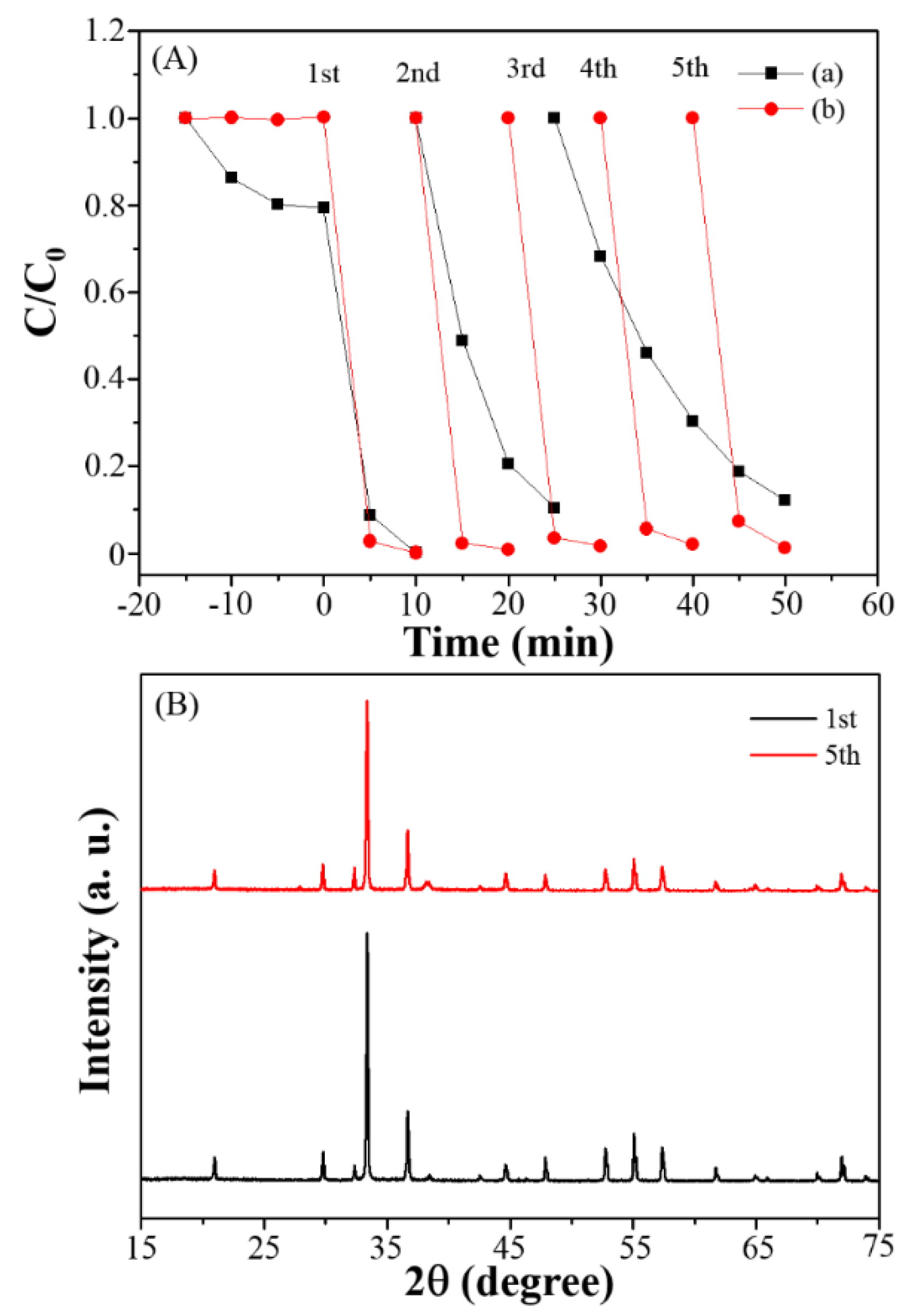
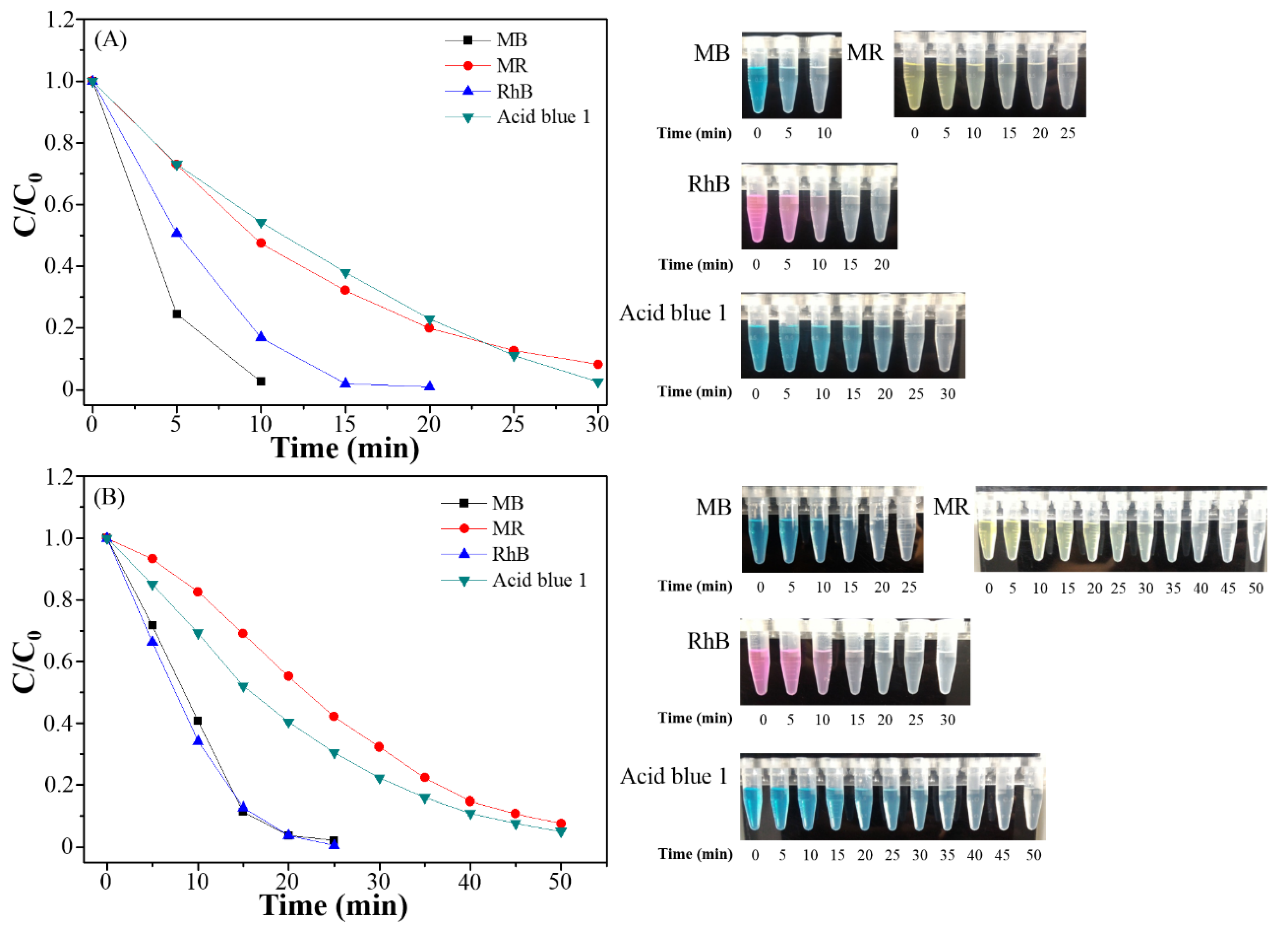
3.3. Photocatalytic Degradation, Mechanism and Applications
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ince, N.H. Ultrasound-Assisted Advanced Oxidation Processes for Water Decontamination. Ultrason. Sonochem. 2018, 40, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Ayed, L.; Asses, N.; Chammem, N.; Ben Othman, N.; Hamdi, M. Advanced Oxidation Process and Biological Treatments for Table Olive Processing Wastewaters: Constraints and a Novel Approach to Integrated Recycling Process: A Review. Biodegradation 2017, 28, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Rtimi, S.; Pulgarin, C. Light-Assisted Advanced Oxidation Processes for the Elimination of Chemical and Microbiological Pollution of Wastewaters in Developed and Developing Countries. Molecules 2017, 22, 1070. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Capodaglio, A.G. Can Radiation Chemistry Supply a Highly Efficient Ao(R)P Process for Organics Removal from Drinking and Waste Water? A Review. Environ. Sci. Pollut. Res. 2017, 24, 20187–20208. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Corena, J.R.; Bergendahl, J.A.; Hart, F.L. Advanced Oxidation of Five Contaminants in Water by UV/TiO2: Reaction Kinetics and Byproducts Identification. J. Environ. Manag. 2016, 181, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Bethi, B.; Sonawane, S.H.; Rohit, G.S.; Holkar, C.R.; Pinjari, D.V.; Bhanvase, B.A.; Pandit, A.B. Investigation of TiO2 Photocatalyst Performance for Decolorization in the Presence of Hydrodynamic Cavitation as Hybrid Aop. Ultrason. Sonochem. 2016, 28, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Havlikova, L.; Satinsky, D.; Solich, P. Aspects of Decontamination of Ivermectin and Praziquantel from Environmental Waters Using Advanced Oxidation Technology. Chemosphere 2016, 144, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Tokumura, M.; Sugawara, A.; Raknuzzaman, M.; Habibullah-Al-Mamun, M.; Masunaga, S. Comprehensive Study on Effects of Water Matrices on Removal of Pharmaceuticals by Three Different Kinds of Advanced Oxidation Processes. Chemosphere 2016, 159, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Guzman, P.; Silva-Agredo, J.; Florez, O.; Giraldo-Aguirre, A.L.; Pulgarin, C.; Torres-Palma, R.A. Selecting the Best Aop for Isoxazolyl Penicillins Degradation as a Function of Water Characteristics: Effects of Ph, Chemical Nature of Additives and Pollutant Concentration. J. Environ. Manag. 2017, 190, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Morales, M.; Tsapekos, P.; Awais, M.; Gulfraz, M.; Angelidaki, I. TiO2/UV Based Photocatalytic Pretreatment of Wheat Straw for Biogas Production. Anaerobe 2017, 46, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Bohdziewicz, J.; Kudlek, E.; Dudziak, M. Influence of the Catalyst Type (TiO2 and Zno) on the Photocatalytic Oxidation of Pharmaceuticals in the Aquatic Environment. Desalin. Water Treat. 2016, 57, 1552–1563. [Google Scholar] [CrossRef]
- Dong, H.R.; Zeng, G.M.; Tang, L.; Fan, C.Z.; Zhang, C.; He, X.X.; He, Y. An Overview on Limitations of TiO2-Based Particles for Photocatalytic Degradation of Organic Pollutants and the Corresponding Countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, S.; Zhang, Z.; Dong, X.; Liu, T. Hydrothermal Etching Fabrication of TiO2@Graphene Hollow Structures: Mutually Independent Exposed {001} and {101} Facets Nanocrystals and Its Synergistic Photocaltalytic Effects. Sci. Rep. 2016, 6, 33839. [Google Scholar] [CrossRef] [PubMed]
- Veisi, F.; Zazouli, M.A.; Ebrahimzadeh, M.A.; Charati, J.Y.; Dezfoli, A.S. Photocatalytic Degradation of Furfural in Aqueous Solution by N-Doped Titanium Dioxide Nanoparticles. Environ. Sci. Pollut. Res. 2016, 23, 21846–21860. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Hassan, M.; Gomes, V.G. Hybrid Nanostructures Based on Titanium Dioxide for Enhanced Photocatalysis. Appl. Catal. A Gen. 2015, 489, 1–16. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z.; et al. An Orthophosphate Semiconductor with Photooxidation Properties under Visible-Light Irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gao, Q.; Qin, J.; Yang, X.; Li, Y.; Tang, H. Morphology-Controlled Synthesis of Ag3PO4 Microcubes with Enhanced Visible-Light-Driven Photocatalytic Activity. Ceram. Int. 2013, 39, 9715–9720. [Google Scholar] [CrossRef]
- Yang, Z.-M.; Liu, Y.-Y.; Xu, L.; Huang, G.-F.; Huang, W.-Q. Facile Shape-Controllable Synthesis of Ag3PO4 Photocatalysts. Mater. Lett. 2014, 133, 139–142. [Google Scholar] [CrossRef]
- Dong, L.; Wang, P.; Wang, S.; Lei, P.; Wang, Y. A Simple Way for Ag3PO4 Tetrahedron and Tetrapod Microcrystals with High Visible-Light-Responsive Activity. Mater. Lett. 2014, 134, 158–161. [Google Scholar] [CrossRef]
- Hao, Z.; Ai, L.; Zhang, C.; Niu, Z.; Jiang, J. Self-Sacrificial Synthesis of Porous Ag3PO4 Architectures with Enhanced Photocatalytic Activity. Mater. Lett. 2015, 143, 51–54. [Google Scholar] [CrossRef]
- Dong, P.; Yin, Y.; Xu, N.; Guan, R.; Hou, G.; Wang, Y. Facile Synthesis of Tetrahedral Ag3PO4 Mesocrystals and Its Enhanced Photocatalytic Activity. Mater. Res. Bull. 2014, 60, 682–689. [Google Scholar] [CrossRef]
- Wan, J.; Sun, L.; Fan, J.; Liu, E.; Hu, X.; Tang, C.; Yin, Y. Facile Synthesis of Porous Ag3PO4 Nanotubes for Enhanced Photocatalytic Activity under Visible Light. Appl. Surf. Sci. 2015, 355, 615–622. [Google Scholar] [CrossRef]
- Guo, X.; Chen, C.; Yin, S.; Huang, L.; Qin, W. Controlled Synthesis and Photocatalytic Properties of Ag3PO4 Microcrystals. J. Alloys Compd. 2015, 619, 293–297. [Google Scholar] [CrossRef]
- Li, J.; Ji, X.; Li, X.; Hu, X.; Sun, Y.; Ma, J.; Qiao, G. Preparation and Photocatalytic Degradation Performance of Ag3PO4 with a Two-Step Approach. Appl. Surf. Sci. 2016, 372, 30–35. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Z.; Zhang, Z.; Zhang, X.; Wen, R.; Liu, Y.; Fang, M.; Wu, X. Controlled Synthesis and Photocatalytic Properties of Rhombic Dodecahedral Ag3PO4 with High Surface Energy. Appl. Surf. Sci. 2016, 389, 56–66. [Google Scholar] [CrossRef]
- Frontistis, Z.; Antonopoulou, M.; Petala, A.; Venieri, D.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Photodegradation of Ethyl Paraben Using Simulated Solar Radiation and Ag3PO4 Photocatalyst. J. Hazard. Mater. 2017, 323, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-S.; Su, H.-J.; Hsieh, P.-L.; Chiang, Y.-W.; Huang, M.-H. Synthesis of Ag3PO4 Crystals with Tunable Shapes for Facet-Dependent Optical Property, Photocatalytic Activity, and Electrical Conductivity Examinations. ACS Appl. Mater. Interfaces 2017, 9, 39086–39093. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zheng, Y.F.; Zhou, H.; Yin, H.Y.; Song, X.C. The Effect of Synthesis Temperature on the Morphologies and Visible Light Photocatalytic Performance of Ag3PO4. J. Taiwan Inst. Chem. Eng. 2016, 60, 328–334. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, X.; Zhao, Y.; Qiang, Y. Effect of Annealing Temperature and Time on Structure, Morphology and Visible-Light Photocatalytic Activities Ag3PO4 Microparticles. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2015, 201, 57–65. [Google Scholar] [CrossRef]
- Huang, G.-F.; Ma, Z.-L.; Huang, W.-Q.; Tian, Y.; Jiao, C.; Yang, Z.-M.; Wan, Z.; Pan, A. Ag3PO4 semiconductor Photocatalyst: Possibilities and Challenges. J. Nanomater. 2013, 2013, 1–8. [Google Scholar]
- Liu, J.H.; Li, X.; Liu, F.; Lu, L.H.; Xu, L.; Liu, L.W.; Chen, W.; Duan, L.M.; Liu, Z.R. The Stabilization Effect of Surface Capping on Photocatalytic Activity and Recyclable Stability of Ag3PO4. Catal. Commun. 2014, 46, 138–141. [Google Scholar] [CrossRef]
- Luo, L.; Li, Y.Z.; Hou, J.T.; Yang, Y. Visible Photocatalysis and Photostability of Ag3PO4 Photocatalyst. Appl. Surf. Sci. 2014, 319, 332–338. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Tseng, C.-S.; Tseng, P.-J.; Huang, C.-W.; Wu, T.; Lin, Y.-W. Synthesis and Characterization of Ag/Ag3PO4 Nanomaterial Modified BiPO4 Photocatalyst by Sonochemical Method and Its Photocatalytic Application. J. Mater. Sci. Mater. Electron. 2017, 28, 11886–11899. [Google Scholar] [CrossRef]
- Cheng, L.-W.; Tsai, J.-C.; Huang, T.-Y.; Huang, C.-W.; Unnikrishnan, B.; Lin, Y.-W. Controlled Synthesis, Characterization and Photocatalytic Activity of BiPO4 Nanostructures with Different Morphologies. Mater. Res. Express 2014, 1. [Google Scholar] [CrossRef]
- Huang, C.-K.; Wu, T.; Huang, C.-W.; Lai, C.-Y.; Wu, M.-Y.; Lin, Y.-W. Enhanced Photocatalytic Performance of BiVO4 in Aqueous AgNO3 Solution under Visible Light Irradiation. Appl. Surf. Sci. 2017, 399, 10–19. [Google Scholar] [CrossRef]
- Huang, C.-W.; Wu, M.-Y.; Lin, Y.-W. Solvothermal Synthesis of Ag Hybrid BiPO4 Heterostructures with Enhanced Photodegradation Activity and Stability. J. Colloid Interf. Sci. 2017, 490, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-Y.; Chen, Y.-J.; Lai, C.-Y.; Lin, Y.-W. Synthesis, Characterization, Enhanced Sunlight Photocatalytic Properties, and Stability of Ag/Ag3PO4 Nanostructure-Sensitized BiPO4. RSC Adv. 2015, 5, 43854–43862. [Google Scholar] [CrossRef]
- Lin, H.L.; Ye, H.F.; Xu, B.Y.; Cao, J.; Chen, S.F. Ag3PO4 Quantum Dot Sensitized BiPO4: A Novel P-N Junction Ag3PO4/BiPO4 with Enhanced Visible-Light Photocatalytic Activity. Catal. Commun. 2013, 37, 55–59. [Google Scholar] [CrossRef]
- Ma, X.L.; Li, H.H.; Wang, Y.H.; Li, H.; Liu, B.; Yin, S.; Sato, T. Substantial Change in Phenomenon of “Self-Corrosion”on Ag3PO4/TiO2 Compound Photocatalyst. Appl. Catal. B Environ. 2014, 158, 314–320. [Google Scholar] [CrossRef]
- Wang, H.; Bai, Y.S.; Yang, J.T.; Lang, X.F.; Li, J.H.; Guo, L. A Facile Way to Rejuvenate Ag3PO4 as a Recyclable Highly Efficient Photocatalyst. Chem. Eur. J. 2012, 18, 5524–5529. [Google Scholar] [CrossRef] [PubMed]

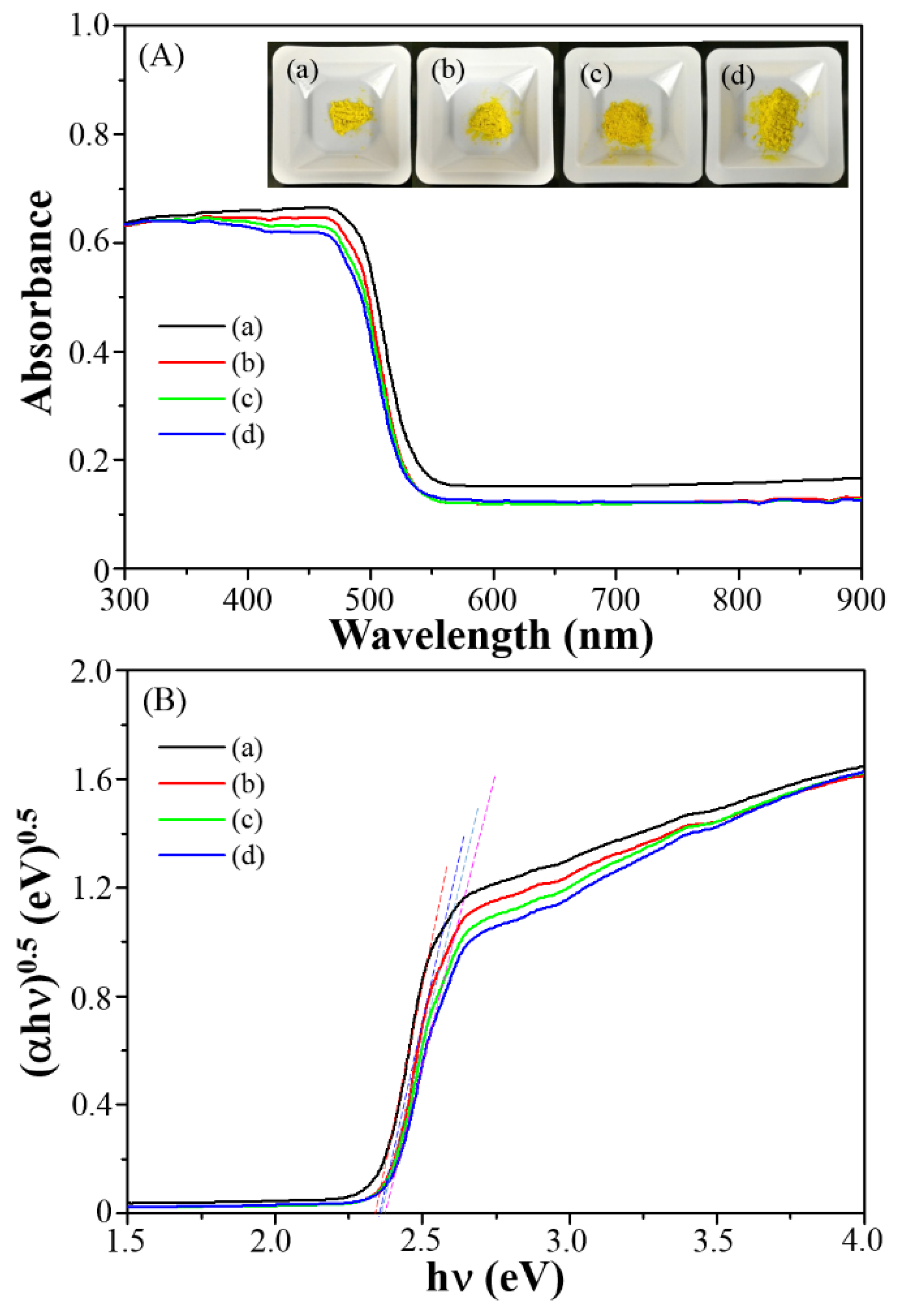
| Series | Eg (eV) | ECB (eV) | EVB (eV) | SBET (m2·g−1) |
|---|---|---|---|---|
| S1 | 2.36 | 0.28 | 2.64 | 0.65 |
| S2 | 2.38 | 0.27 | 2.65 | 0.55 |
| S3 | 2.39 | 0.27 | 2.66 | 0.48 |
| S4 | 2.41 | 0.26 | 2.67 | 0.36 |
| Series | First-Order Kinetic Equation | k (min−1) | R2 | Half-Life (min) |
|---|---|---|---|---|
| S1 | y = 0.478x + 0.100 | 0.478 | 0.9932 | 1.45 |
| S2 | y = 0.203x − 0.087 | 0.203 | 0.9978 | 3.41 |
| S3 | y = 0.200x − 0.147 | 0.200 | 0.9931 | 3.47 |
| S4 | y = 0.100x − 0.224 | 0.100 | 0.9783 | 6.93 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, C.-S.; Wu, T.; Lin, Y.-W. Facile Synthesis and Characterization of Ag3PO4 Microparticles for Degradation of Organic Dyestuffs under White-Light Light-Emitting-Diode Irradiation. Materials 2018, 11, 708. https://doi.org/10.3390/ma11050708
Tseng C-S, Wu T, Lin Y-W. Facile Synthesis and Characterization of Ag3PO4 Microparticles for Degradation of Organic Dyestuffs under White-Light Light-Emitting-Diode Irradiation. Materials. 2018; 11(5):708. https://doi.org/10.3390/ma11050708
Chicago/Turabian StyleTseng, Chi-Shun, Tsunghsueh Wu, and Yang-Wei Lin. 2018. "Facile Synthesis and Characterization of Ag3PO4 Microparticles for Degradation of Organic Dyestuffs under White-Light Light-Emitting-Diode Irradiation" Materials 11, no. 5: 708. https://doi.org/10.3390/ma11050708