Long-Term Effectiveness, under a Mountain Environment, of a Novel Conservation Nanomaterial Applied on Limestone from a Roman Archaeological Site
Abstract
:1. Introduction
2. Materials and Methods
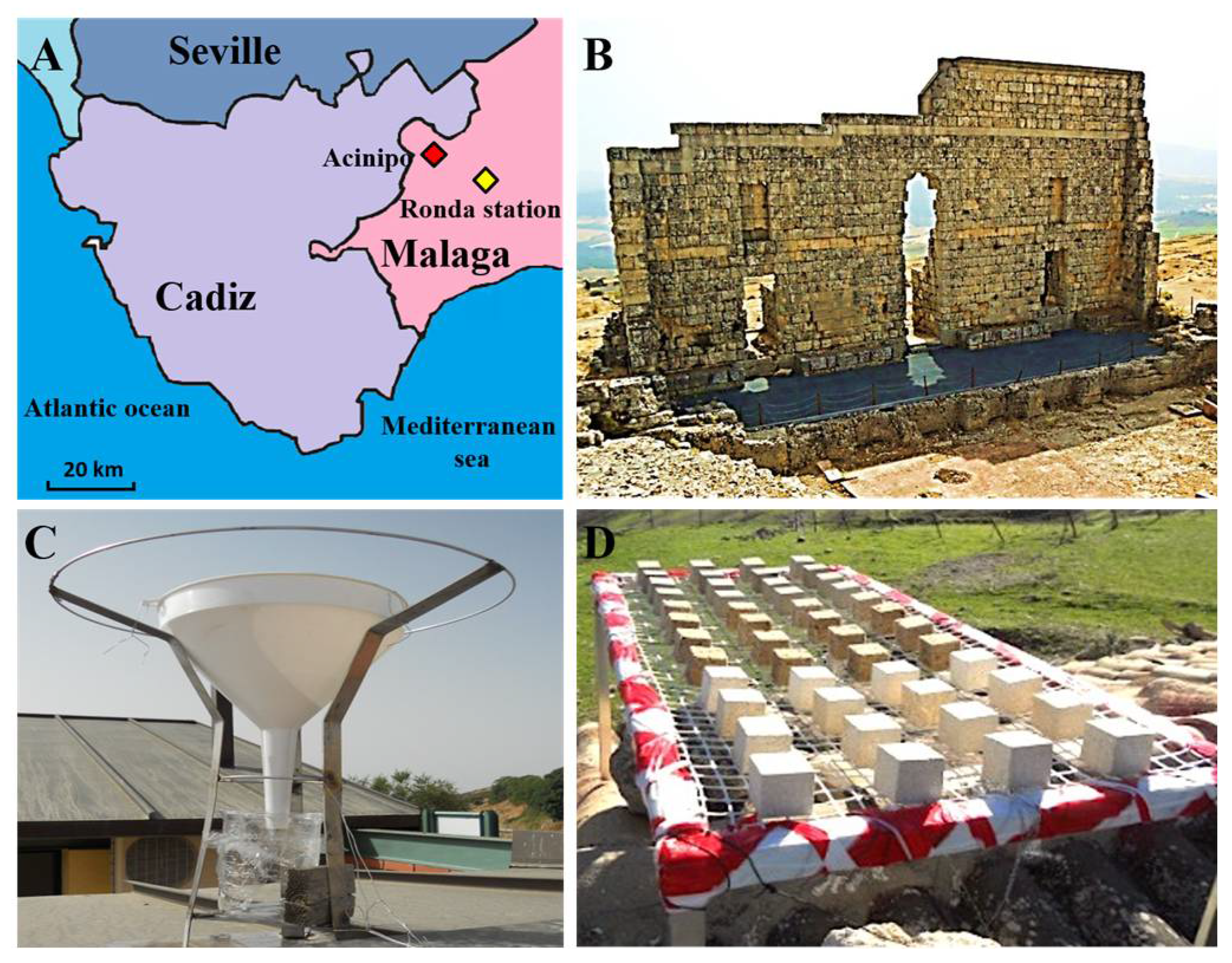
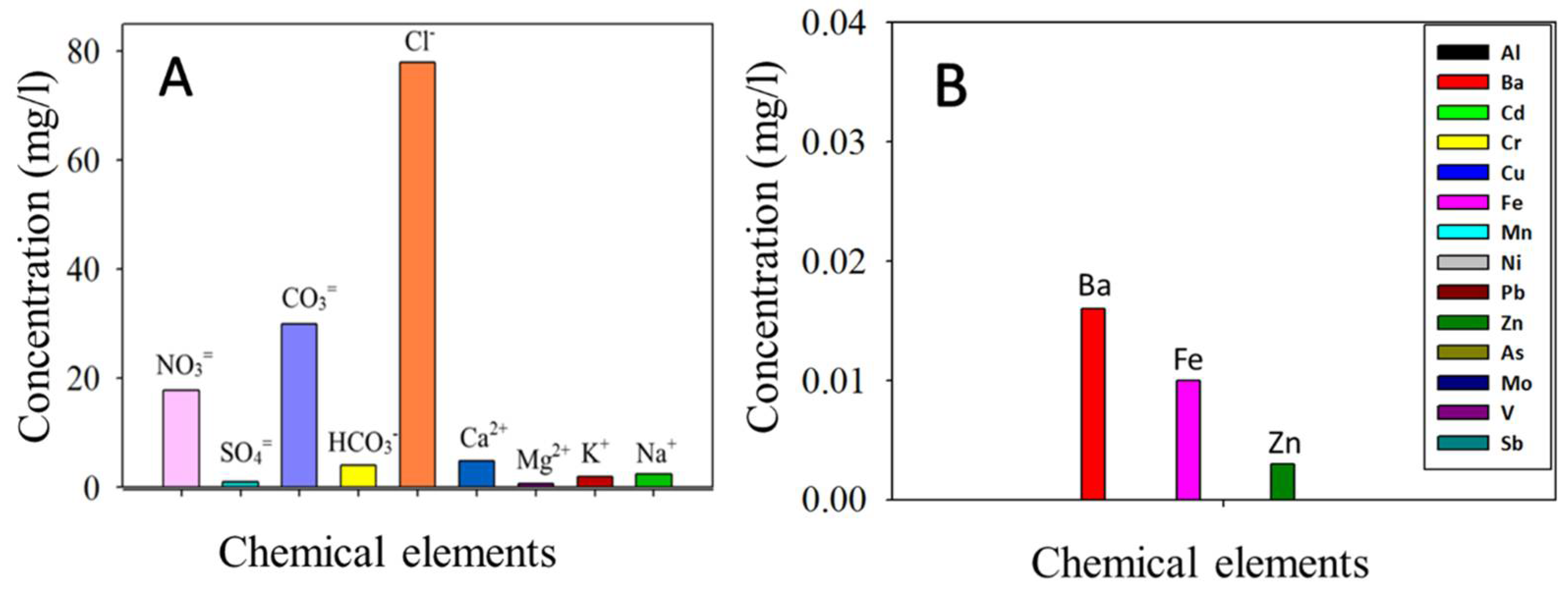
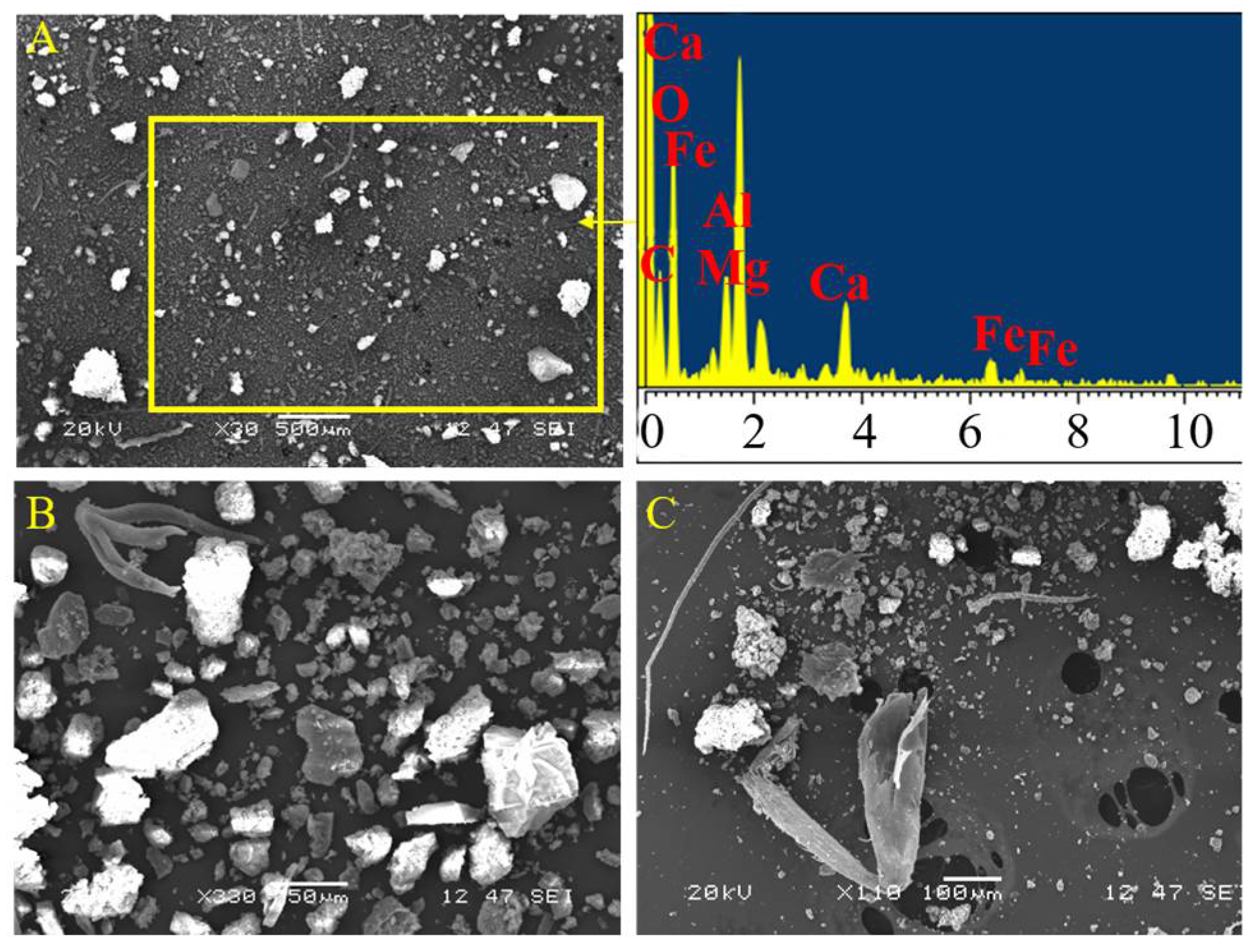
2.1. Evaluation of the Archaeological Site Environment and the Acinipo Limestone
2.2. Product Synthesis and Application on Acinipo Limestone Samples
2.3. Assessment of Effectiveness and Durability
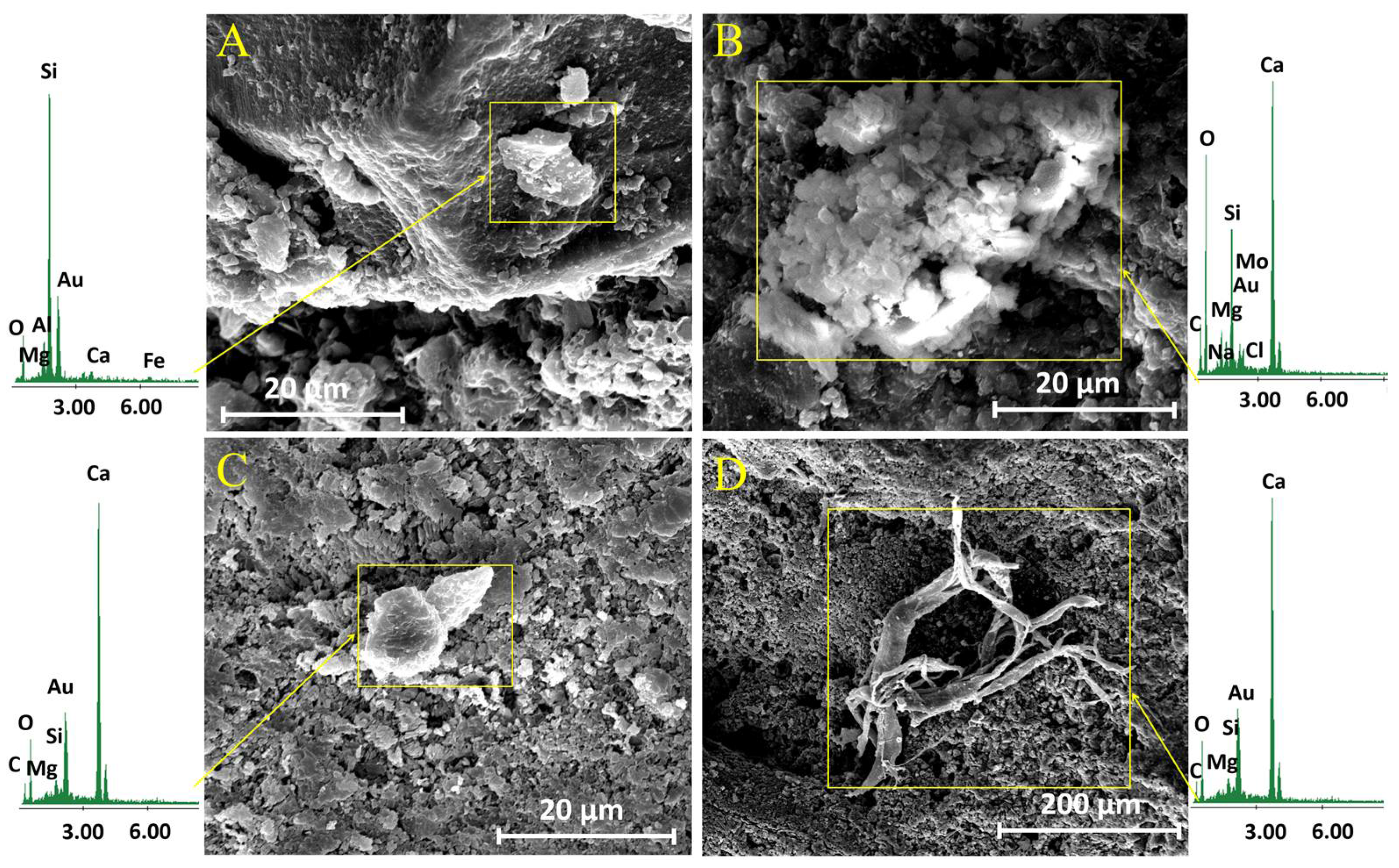
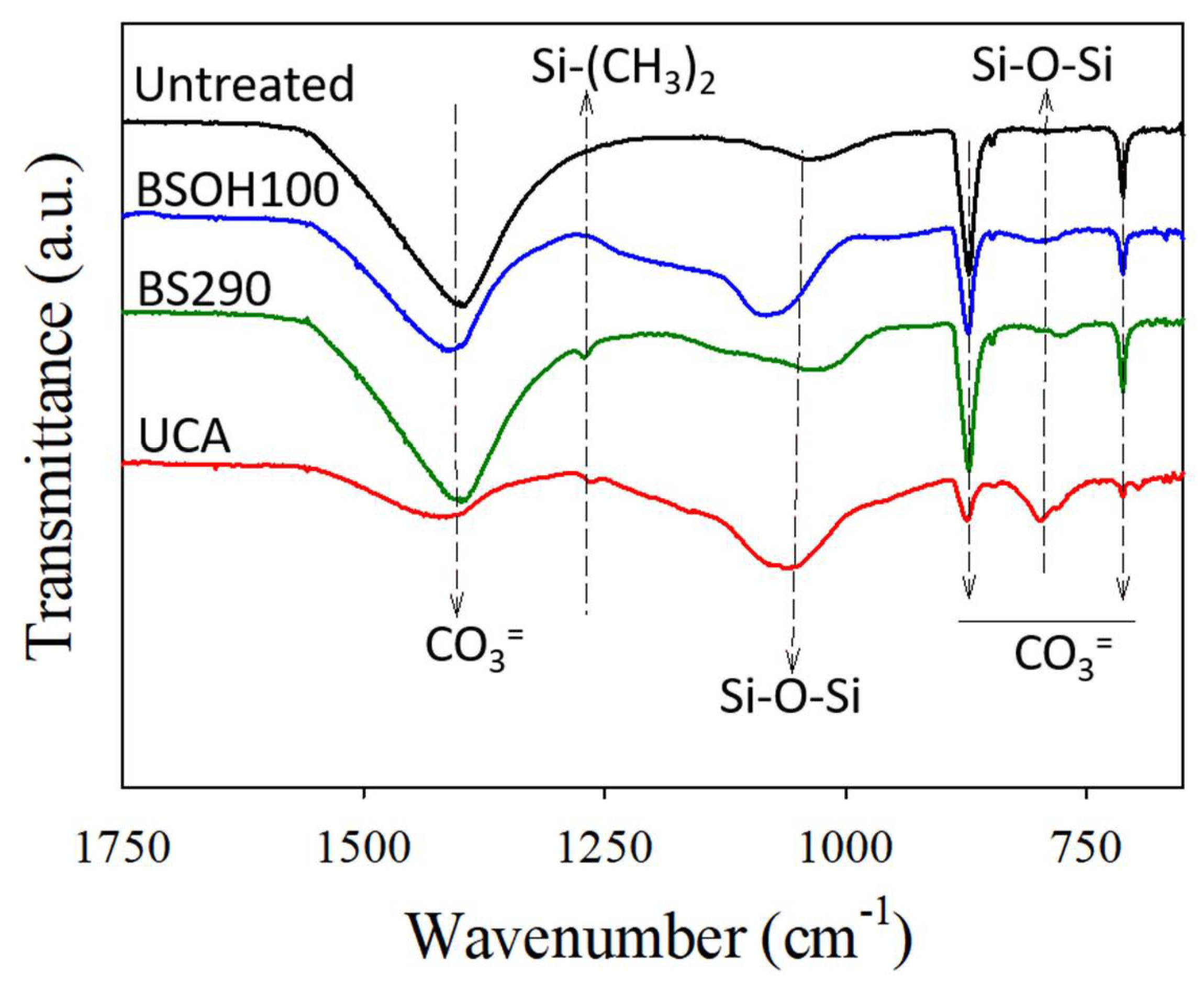
2.3.1. Limestone-Product Interaction
2.3.2. Effectiveness of the Products on the Limestone
2.3.3. Negative Effects Induced by the Products on the Limestone
2.3.4. Evaluation of the Depth of Product Penetration
3. Results and Discussion
3.1. Evaluation of the Archaeological Site Environment and the Acinipo Limestone
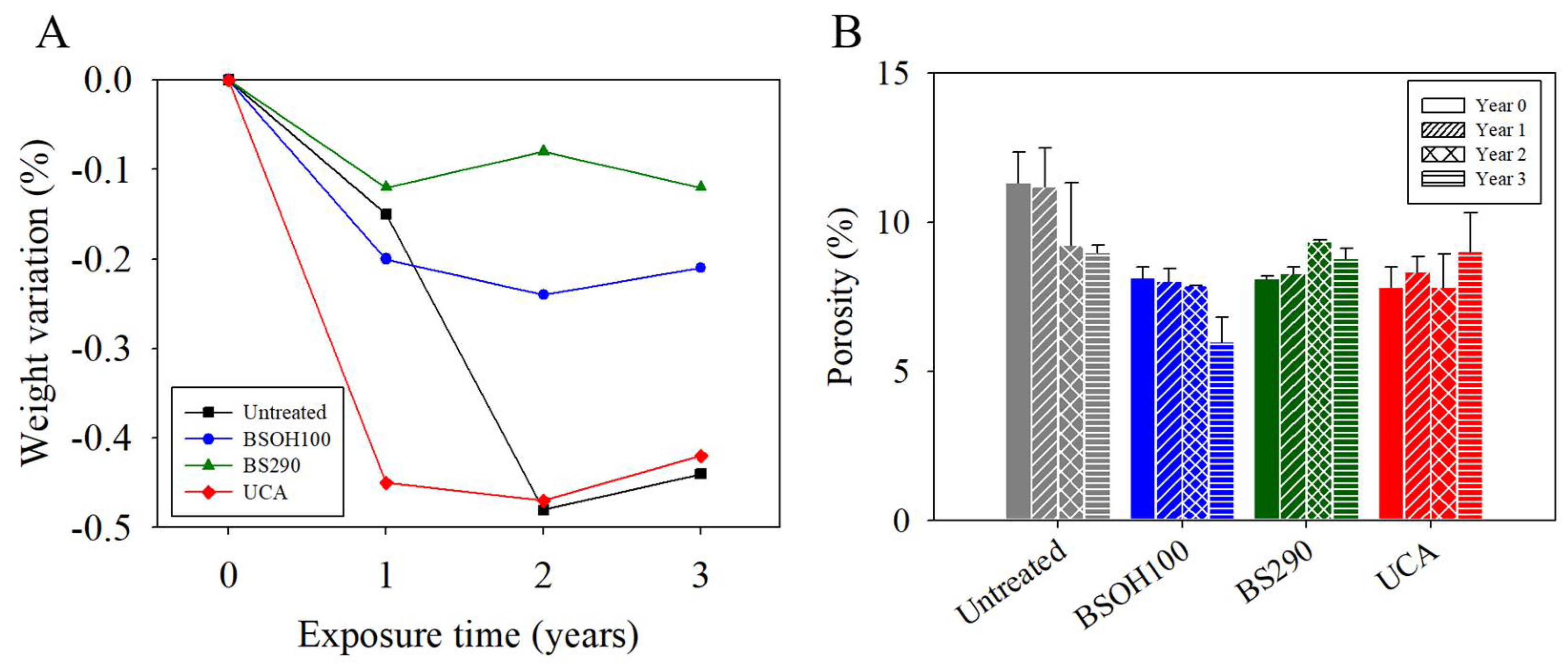
3.2. Assessment of Effectiveness and Durability
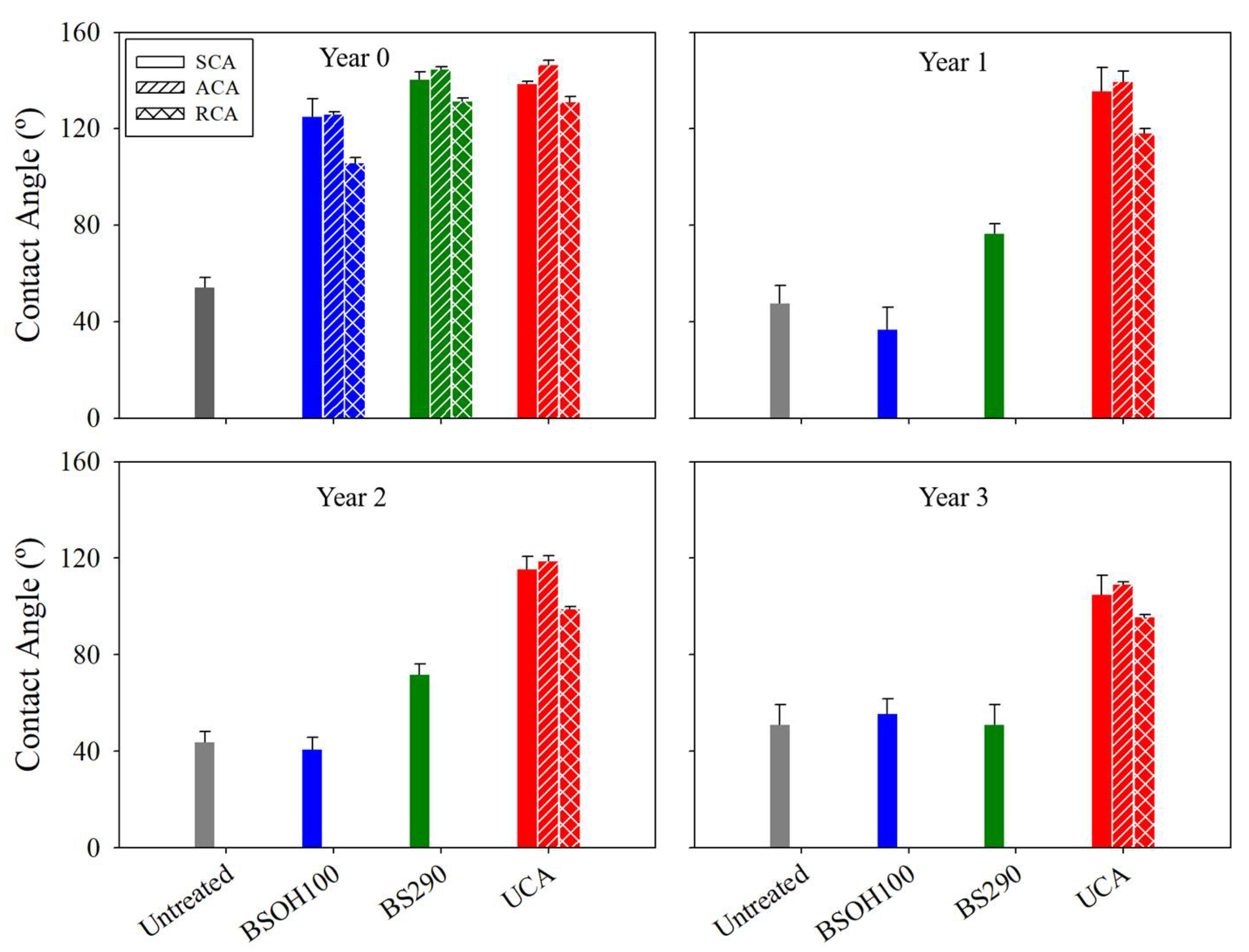
3.2.1. Limestone-Product Interaction
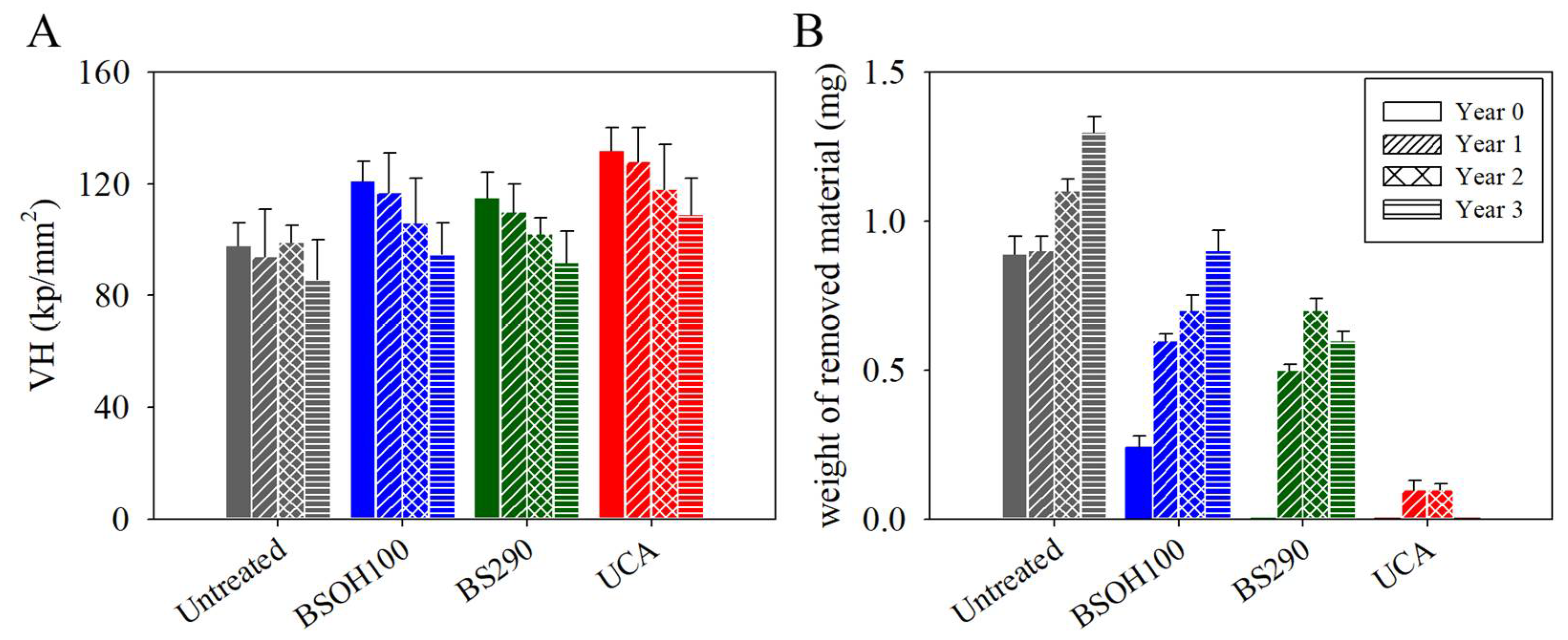
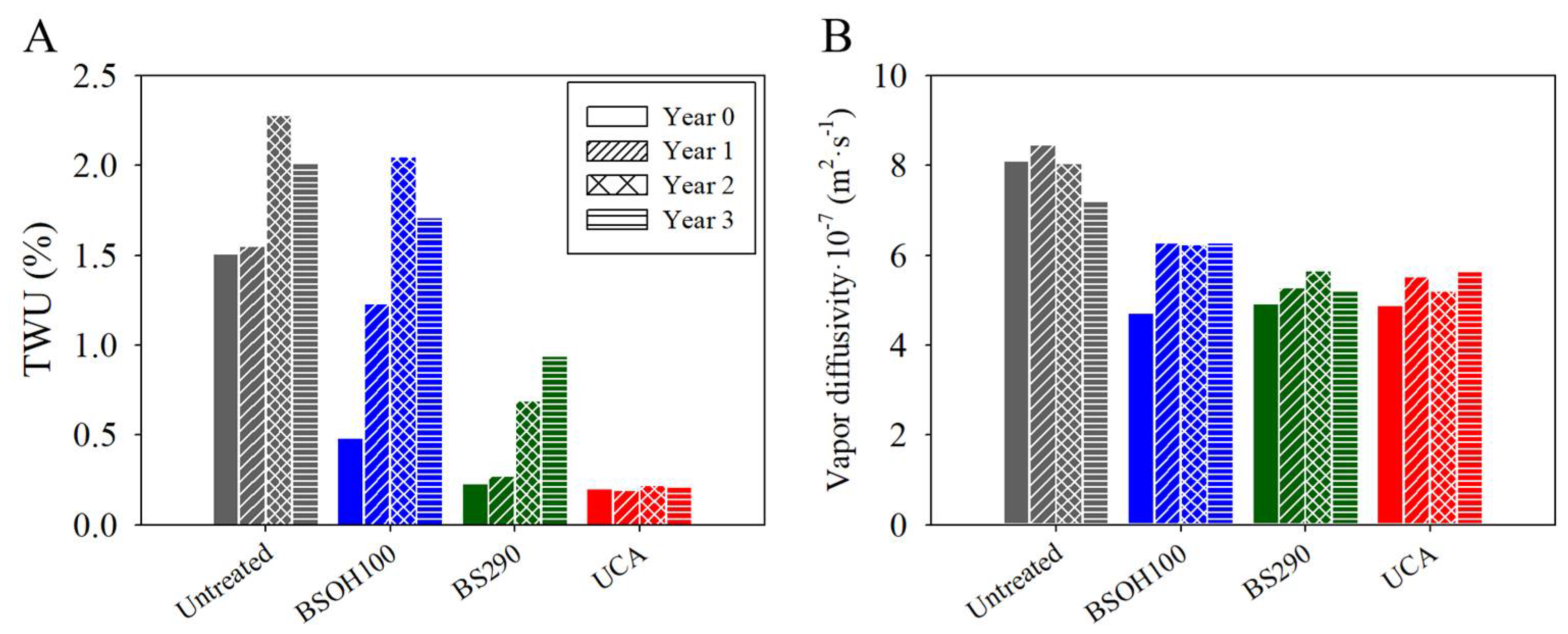
3.2.2. Effectiveness of the Products on the Limestone
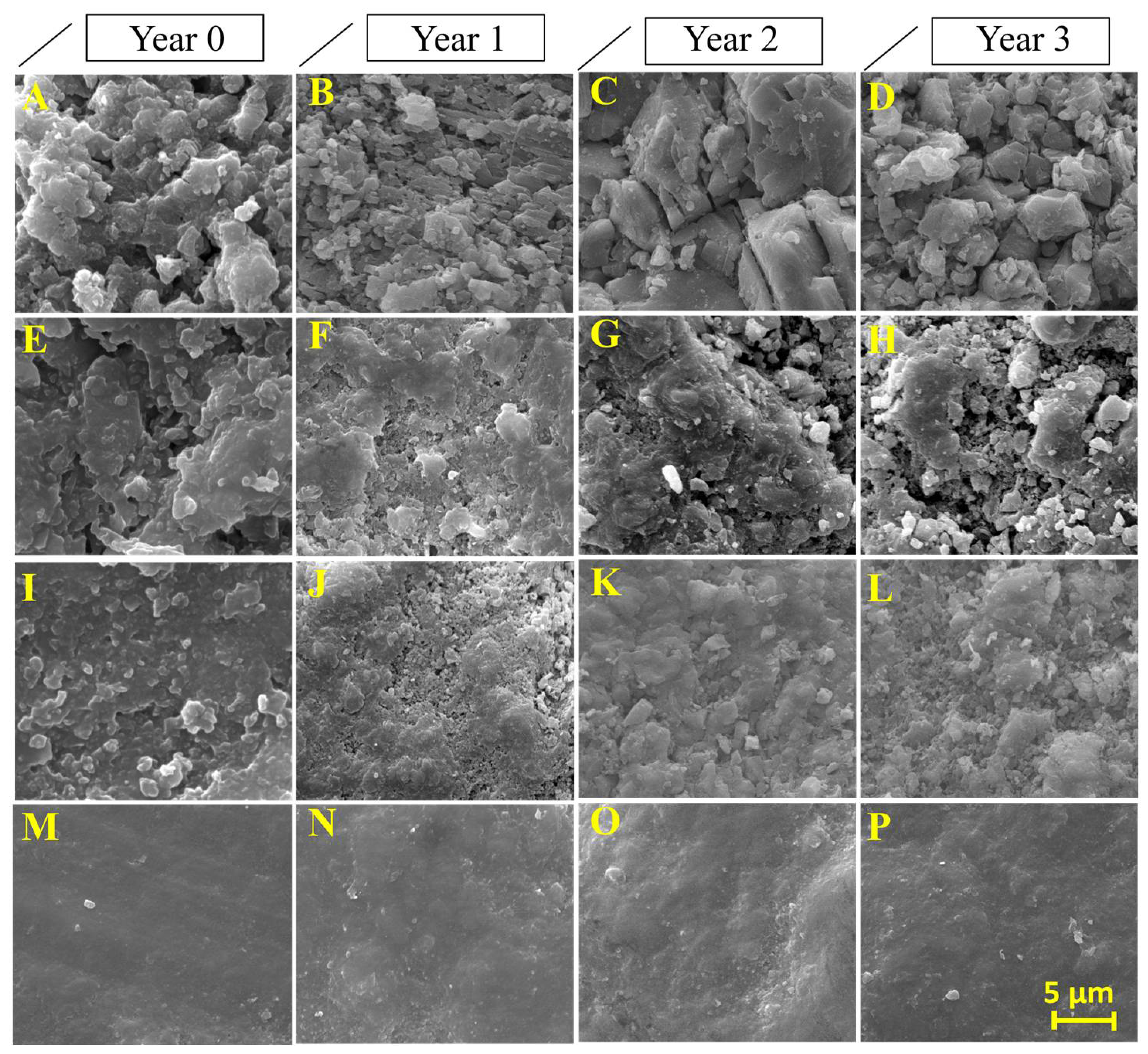
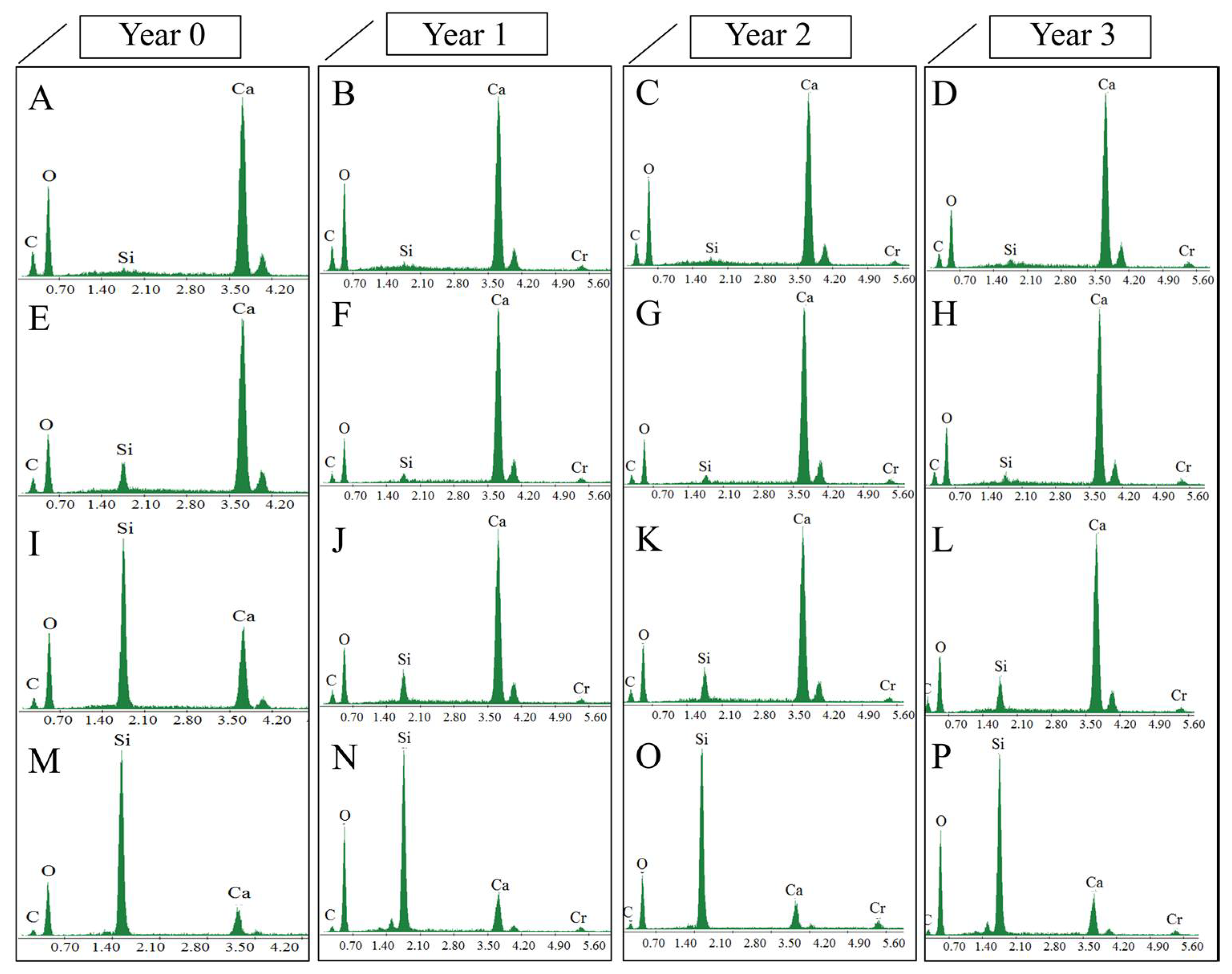
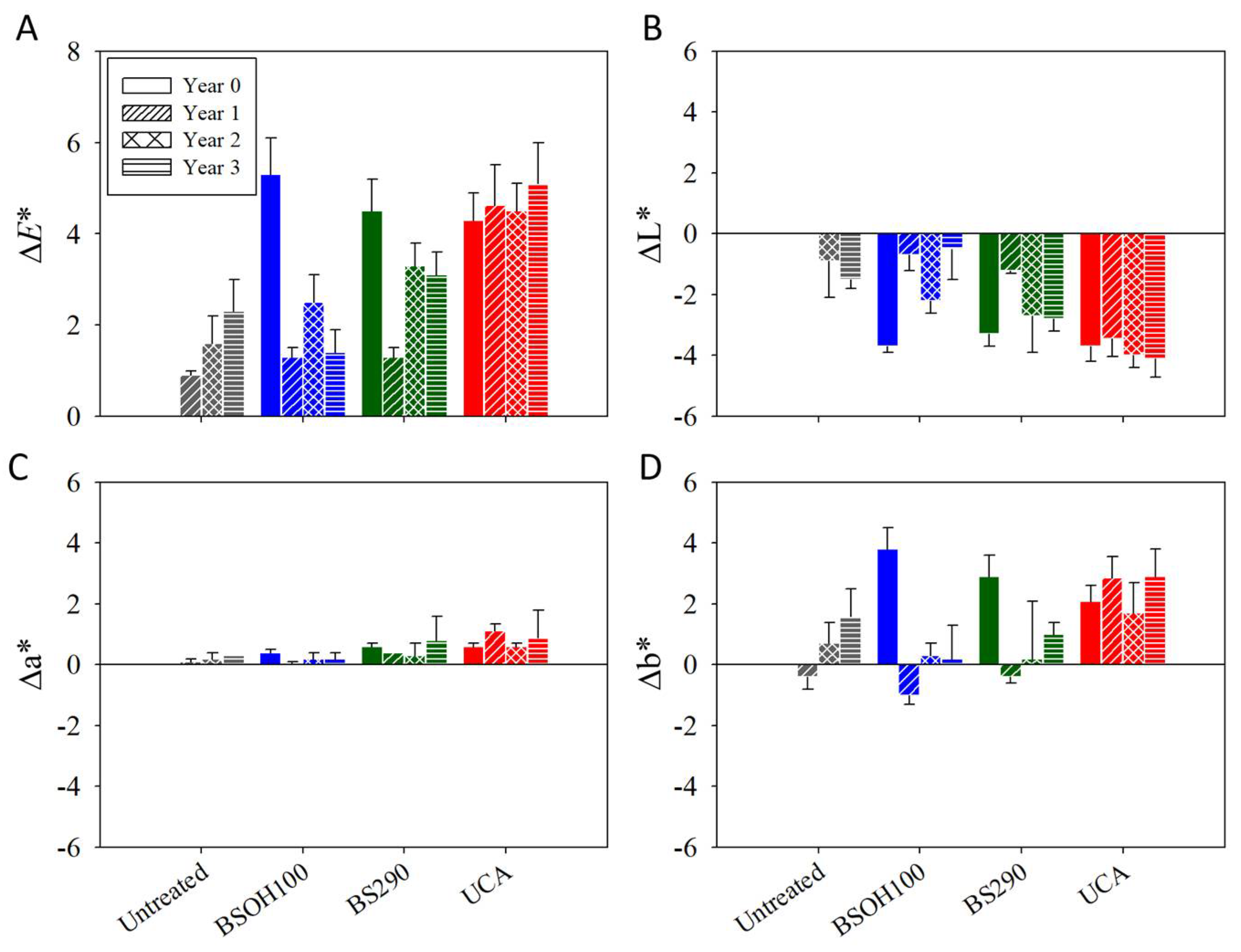
3.2.3. Negative Effects Induced by the Products on the Limestone
3.2.4. Evaluation of the Depth of the Products Penetration
4. Conclusions
- The environmental study carried out in the Acinipo Archaeological site highlights that the limestone decay was caused by salts, deposition of other pollutants, and the growth of microorganisms, with water being the main vehicle for the decay agents. Thus, effectiveness of an innovative alkoxysilane-based product with hydrophobic and consolidant properties, preventing water ingress, was tested on limestone samples that were extracted from the Acinipo Roman Theatre.
- The evaluated commercial products showed a poor compatibility with the carbonate stone under study, producing discontinuous aggregates on its surface. In addition, a low penetration and an absence of long-lasting performance were observed for these commercial products.
- In the case of the innovative nanomaterial under study, an adequate compatibility and adherence to the limestone, producing a long-term effective, homogeneous, and continuous coating with a depth of penetration of up to 10 mm, were observed. This compatibility was explained in terms of the presence of effective interactions between the n-octylamine integrated in the product and the carbonate substrate.
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wheeler, G. Alkoxysilanes and the Consolidation of Stone, 1st ed.; The Getty Conservation Institute: Los Angeles, CA, USA, 2005; ISBN 13 978-0-89236-815-0. [Google Scholar]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2–SiO2–PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Scherer, G.W.; Wheeler, G.E. Proceedings of the 4th International Symposium on the Conservation of Monuments; Zezza, M., Papachristodoulou, K., Eds.; Technical Chamber of Greece: Rhodes, Greece, 1997; Volume 3, p. 355. [Google Scholar]
- Mosquera, M.J.; Pozo, J.; Esquivias, L.; Rivas, T.; Silva, B. Application of mercury porosimetry to the study of xerogels used as stone consolidants. J. Non-Cryst. Solids 2002, 311, 185–194. [Google Scholar] [CrossRef]
- Doehne, E.; Price, C.A. Stone Conservation, 2nd ed.; The Getty conservation institute: Los Angeles, CA, USA, 2010; ISBN 978-1-60606-046-9. [Google Scholar]
- Mosquera, M.J.; de los Santos, D.M.; Montes, A.; Valdez-Castro, L. New nanomaterials for consolidating Stone. Langmuir 2008, 24, 2772–2778. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, M.J.; de los Santos, D.M.; Rivas, T. Surfactant-synthesized ormosils with application to stone restoration. Langmuir 2010, 26, 6737–6745. [Google Scholar] [CrossRef] [PubMed]
- Illescas, J.F.; Mosquera, M.J. Producing surfactant-synthesized nanomaterials in situ on a Building Substrate, without Volatile Organic Compounds. ACS Appl. Mater. Interfaces 2012, 4, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Illescas, J.F.; Mosquera, M.J. Surfactant-synthesized PDMS/silica sanomaterials improve robustness and stain resistance of carbonate stone. J. Phys. Chem. C 2011, 115, 14624–14634. [Google Scholar] [CrossRef]
- Remzova, M.; Sasek, P.; Frankeova, D.; Slizkova, Z.; Rathousky, J. Effect of modified ethylsilicate consolidants on the mechanical properties of sandstone. Constr. Build. Mater. 2016, 112, 674–681. [Google Scholar] [CrossRef]
- Xu, F.; Li, D.; Zhang, Q.; Zhang, H.; Xu, J. Effects of addition of colloidal silica particles on TEOS-based stone protection using n-octylamine as a catalyst. Prog. Org. Coat. 2012, 75, 429–434. [Google Scholar] [CrossRef]
- Salazar-Hernandez, C.; Alquiza, M.J.P.; Salgado, P.; Cervantes, J. TEOS-colloidal silica-PDMS-OH hybrid formulation used for stone consolidation. Appl. Organomet. Chem. 2010, 24, 481–488. [Google Scholar] [CrossRef]
- Luo, Y.; Xiao, L.; Zhang, X. Characterization of TEOS/PDMS/HA nanocomposites for appliation as consolidant/hydrophobic products on sandstones. J. Cult. Herit. 2015, 16, 470–478. [Google Scholar] [CrossRef]
- Carrascosa, L.A.M.; Facio, D.S.; Mosquera, M.J. Producing superhydrophobic roof tiles. Nanotechnology 2016, 27, 095604. [Google Scholar] [CrossRef] [PubMed]
- Favaro, M.; Mendichi, R.; Ossola, F.; Simon, S.; Tomasin, P.; Vigato, P.A. Evaluation of polymers for conservation treatments of outdoor exposed stone monuments. Part I: Photo-oxidative weathering. Polym. Degrad. Stab. 2006, 91, 3083–3096. [Google Scholar] [CrossRef]
- Favaro, M.; Mendichi, R.; Ossola, F.; Simon, S.; Tomasin, P.; Vigato, P.A. Evaluation of polymers for conservation treatments of outdoor exposed stone monuments. Part II: Photo-oxidative and salt-induced weathering of acrylic-silicone. Polym. Degrad. Stab. 2007, 92, 335–351. [Google Scholar] [CrossRef]
- Mosquera, M.J.; Carrascosa, L.A.M.; Badreldin, N. Producing superhydrophobic/oleophobic coatings on Cultural Heritage building materials. Pure Appl. Chem. 2017, 90. [Google Scholar] [CrossRef]
- De Rosario, I.; Elhaddad, F.; Pan, A.; Benavides, R.; Rivas, T.; Mosquera, M.J. Effectiveness of a novel consolidant on granite: Laboratory and in situ results. Constr. Build. Mater. 2015, 76, 140–149. [Google Scholar] [CrossRef]
- De Rosario, I.; Rivas, T.; Buceta, G.; Feijoo, J.; Mosquera, M.J. Surfactant-Synthesized consolidants applied to a granitic medieval Necropolis in NW Spain. Laboratory and in situ effectiveness evaluation. Int. J. Archit. Herit. 2017, 11, 1166–1176. [Google Scholar] [CrossRef]
- Nwaubani, S.O.; Dumbelton, J. A practical approach to in-situ evaluation of surface-treated structures. Constr. Build. Mater. 2001, 15, 199–212. [Google Scholar] [CrossRef]
- Bonazza, A.; Vidorni, G.; Natali, I.; Ciantelli, C.; Giosuè, C.; Tittarelli, F. Durability assessment to environmental impact of nano-structured consolidants on Carrara marble by field exposure tests. Sci. Total Environ. 2017, 575, 23–32. [Google Scholar] [CrossRef] [PubMed]
- De Ferri, L.; Lottici, P.P.; Lorenzi, A.; Montenero, A.; Salvioli-Mariani, E. Study of silica nanoparticles—Polysiloxane hydrophobic treatments for stone-based monument protection. J. Cult. Herit. 2011, 12, 356–363. [Google Scholar] [CrossRef]
- Cappelletti, G.; Fermo, P.; Camiloni, M. Smart hybrid coatings for natural stones conservation. Prog. Org. Coat. 2015, 78, 511–516. [Google Scholar] [CrossRef]
- Corcione, C.E.; De Simone, N.; Santarelli, M.L.; Frigione, M. Protective properties and durability characteristics of experimental and commercial organic coatings for the preservation of porous stone. Prog. Org. Coat. 2017, 103, 193–203. [Google Scholar] [CrossRef]
- Gherardi, F.; Gulotta, D.; Goidanich, S.; Colombo, A.; Toniolo, L. On-site monitoring of the performance of innovative treatments for marble conservation in architectural heritage. Herit. Sci. 2017, 5. [Google Scholar] [CrossRef]
- Lhaddad, F.; Carrascosa, L.A.M.; Mosquera, M.J. Long-term effectiveness, under a coastal environment, of a novel conservation nanomaterial applied on sandstone from a Roman archaeological site. J. Cult. Herit. 2018, in press. [Google Scholar]
- Mosquera, M.J.; Santos, D.; Rivas, T.; Sanmartín, P.; Silva, B. New nanomaterials for protecting and consolidating stone. J. Nano Res. 2009, 8. [Google Scholar] [CrossRef]
- Bernabe, J.M.; Carretero, M.I.; Galan, E. Mineralogy and origin of atmospheric particles in the industrial area of Huelva. Atmos. Environ. 2005, 39, 6777–6789. [Google Scholar] [CrossRef]
- Drdàcký, M.; Lesàk, J.; Rescic, S.; Slìžkovà, Z.; Tiano, P.; Valach, J. Standardization of peeling tests for assessing the cohesion and consolidation characteristics of historic stone surfaces. Mater. Struct. 2012, 45, 505–552. [Google Scholar] [CrossRef] [PubMed]
- Drdàcký, M.; Lesàk, J.; Niedoba, K.; Valach, J. Peeling tests for assessing the cohesion and consolidation characteristics of mortar and render surfaces. Mater. Struct. 2015, 48, 1947–1963. [Google Scholar] [CrossRef]
- UNE-EN 1925, Natural Stone Test Methods, Determination of Water Absorption Coefficient by Capillarity; AENOR: Madrid, Spain, 1999.
- Berns, R.S. Billmeyer and Saltzman’s Principles of Color Technology, 3rd ed.; Wiley-Interscience: New York, NY, USA, 2000; ISBN 978-0-471-19459-0. [Google Scholar]
- Mosquera, M.J.; Benı́tez, D.; Perry, S.H. Pore structure in mortars applied on restoration: Effect on properties relevant to decay of granite buildings. Cem. Concr. Res. 2002, 32, 1883–1888. [Google Scholar] [CrossRef]
- Prieto-Taboada, N.; Ibarrondo, I.; Gomez-Laserna, O.; Martinez-Arkarazo, I.; Olazabal, M.A.; Madariaga, J.M. Buildings as repositories of hazardous pollutants of anthropogenic origin. J. Hazard. Mater. 2013, 248–249, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Cong, Z.; Kang, S.; Liu, X.; Wang, G. Elemental composition of aerosol in the Nam Co region, Tibetan Plateau, during summer monsoon season. Atmos. Environ. 2007, 41, 1180–1187. [Google Scholar] [CrossRef]
- Turkington, A.V.; Martin, E.; Viles, H.A.; Smith, B.J. Surface change of sandstone samples exposed to a polluted urban atmosphere over a six-year period: Belfast, Northern Ireland. Build. Environ. 2003, 38, 1205–1216. [Google Scholar] [CrossRef]
- Facio, D.S.; Mosquera, M.J. Simple Strategy for Producing Superhydrophobic Nanocomposite Coatings in Situ on a Building Substrate. ACS Appl. Mater. Interfaces 2013, 5, 7517–7526. [Google Scholar] [CrossRef] [PubMed]
- Maravelaki-kalaitzaki, P.; Bertoncello, R.; Biscontin, G. Evaluation of the initial weathering rate of Istria stone exposed to rain action, in Venice, with X-ray photoelectron spectroscopy. J. Cult. Herit. 2002, 3, 273–282. [Google Scholar] [CrossRef]
- Maravelaki-kalaitzaki, P.; kallithrakas-kontos, N.; Korakaki, D.; Agioutantis, Z.; Maurigiannakis, S. Evaluation of silicon-based strengthening agents on porous limestones. Prog. Org. Coat. 2006, 57, 140–148. [Google Scholar] [CrossRef]
- Moropoulou, A.; Kouloumbi, N.; Haralampopoulos, G.; Konstanti, A.; Michailidis, P. Criteria and methodology for the evaluation of conservation interventions on treated porous stone susceptible to salt decay. Prog. Org. Coat. 2003, 48, 259–270. [Google Scholar] [CrossRef]
- Cardell, C.; Delalieux, F.; Roumpopoulos, K.; Moropoulou, A.; Auger, A.; Van Grieken, R. Salt-induced decay in calcareous stone monuments and buildings in a marine environment in SW France. Constr. Build. Mater. 2003, 17, 165–179. [Google Scholar] [CrossRef]
- Papida, S.; Murphy, W.; May, E. Enhancement of physical weathering of building stones by microbial populations. Int. Biodeterior. Biodegrad. 2000, 46, 305–317. [Google Scholar] [CrossRef]
- Striegela, M.F.; Guin, E.B.; Hallett, K.H.; Sandoval, D.; Swingle, R.; Knox, K.; Best, F.; Fornea, S. Air pollution, coatings, and cultural resources. Prog. Org. Coat. 2003, 48, 281–288. [Google Scholar] [CrossRef]
- Zendri, E.; Biscontin, G.; Nardini, I.; Riato, S. Characterization and reactivity of silicatic consolidants. Constr. Build. Mater. 2007, 21, 1098–1106. [Google Scholar] [CrossRef] [Green Version]
- Sdiri, A.; Higashi, T.; Hatta, T.; Jamoussi, F.; Tase, N. Mineralogical and spectroscopic characterization, and potential environmental use of limestone from the Abiod formation, Tunisia. Environ. Earth Sci. 2010, 61, 1275–1287. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G. Spectroscopic study of phase transitions in natural calcite mineral. Spectrochim. Acta Mol. Biomol. Spectrosc. 2008, 69, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Cardell, C.; Guerra, I.; Romero-Pastor, J.; Cultrone, G.; Rodriguez-Navarro, A. Innovative Analytical Methodology Combining Micro-X-ray Diffraction, Scanning Electron Microscopy-Based Mineral Maps, and Diffuse Reflectance Infrared Fourier Transform Spectroscopy to Characterize Archeological Artifacts. Anal. Chem. 2009, 81, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Tellez, L.; Rubio, J.; Rubio, F.; Morales, E.; Oteo, J.L. Synthesis of inorganic-organic hybrid materials from TEOS, TBT and PDMS. J. Mater. Sci. 2003, 38, 1773–1780. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, H.; Xiao, B.; Yan, L.; Lv, H.B. Jiang. Sol−Gel Preparation of PDMS/Silica Hybrid Antireflective Coatings with Controlled Thickness and Durable Antireflective Performance. J. Phys. Chem. C 2010, 114, 19979–19983. [Google Scholar] [CrossRef]
- Wheeler, G.; Méndez-Vivar, J.; Fleming, S. The Use of Modified Zr-nPropoxide in the Consolidation of Calcite: A Preliminary Study Focused into the Conservation of Cultural Heritage. J. Sol-Gel Sci. Technol. 2003, 26, 1233–1237. [Google Scholar] [CrossRef]
- Xu, F.; Xiang, N.; Li, D.; Yu, J.; Wu, D.; Zhang, Q. Use of coupling agents for increasing passivants and cohesion ability of consolidant on limestone. Prog. Org. Coat. 2004, 77, 1613–1618. [Google Scholar] [CrossRef]
- Demjén, Z.; Pukánszky, B.; Nagy, J. Possible coupling reactions of functional silanes and polypropylene. Polymer 1999, 40, 1763–1773. [Google Scholar] [CrossRef]
- Demjén, Z.; Pukánszky, B.; Földes, E.; Nagy, J. Interaction of Silane Coupling Agents with CaCO3. J. Colloid Interface Sci. 1997, 190, 427–436. [Google Scholar] [CrossRef]
- Mrowetz, M.; Balcerski, W.; Colussi, A.J.; Hoffmann, M.R. Oxidative power of nitrogen-doped TiO2 photocatalysts under visible illumination. J. Phys. Chem. B 2004, 108, 17269–17272. [Google Scholar] [CrossRef]
- Han, A.J.; Guo, J.; Yu, H.; Zeng, Y.; Huang, Y.F.; He, H.Y.; Long, Y.C. The Leading Role of Association in Framework Modification of Highly Siliceous Zeolites with Adsorbed Methylamine. ChemPhysChem 2006, 7, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.G.S.; Airoldi, C.J. Different neutral surfactant template extraction routes for synthetic hexagonal mesoporous silicas. J. Mater. Chem. 2002, 12, 3823–3826. [Google Scholar] [CrossRef]
- Della Volpe, C.; Penati, A.; Peruzzzi, R.; Siboni, S.; Toniolo, L.; Colombo, C. The combined effect of roughness and heterogeneity on contact angles: The case of polymer coating for stone protection. J. Adhes. Sci. Technol. 2000, 14, 273–299. [Google Scholar] [CrossRef]
- Delgado-Rodrigues, J.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Miliani, C.; Velo Simpson, M.L.; Scherer, G.W. Particle-modified consolidants: A study on the effect of particles on sol–gel properties and consolidation effectiveness. J. Cult. Herit. 2007, 8, 1–6. [Google Scholar] [CrossRef]
- Camaiti, M.; Bugani, S.; Bernard, E.; Morselli, L.; Matteini, M. Effects of atmospheric NOx on biocalcarenite coated with different conservation products. Appl. Geochem. 2007, 22, 1248–1254. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhaddad, F.; Carrascosa, L.A.M.; Mosquera, M.J. Long-Term Effectiveness, under a Mountain Environment, of a Novel Conservation Nanomaterial Applied on Limestone from a Roman Archaeological Site. Materials 2018, 11, 694. https://doi.org/10.3390/ma11050694
Elhaddad F, Carrascosa LAM, Mosquera MJ. Long-Term Effectiveness, under a Mountain Environment, of a Novel Conservation Nanomaterial Applied on Limestone from a Roman Archaeological Site. Materials. 2018; 11(5):694. https://doi.org/10.3390/ma11050694
Chicago/Turabian StyleElhaddad, Farid, Luis A. M. Carrascosa, and Maria J. Mosquera. 2018. "Long-Term Effectiveness, under a Mountain Environment, of a Novel Conservation Nanomaterial Applied on Limestone from a Roman Archaeological Site" Materials 11, no. 5: 694. https://doi.org/10.3390/ma11050694





