Polymeric Micelles of Biodegradable Diblock Copolymers: Enhanced Encapsulation of Hydrophobic Drugs
Abstract
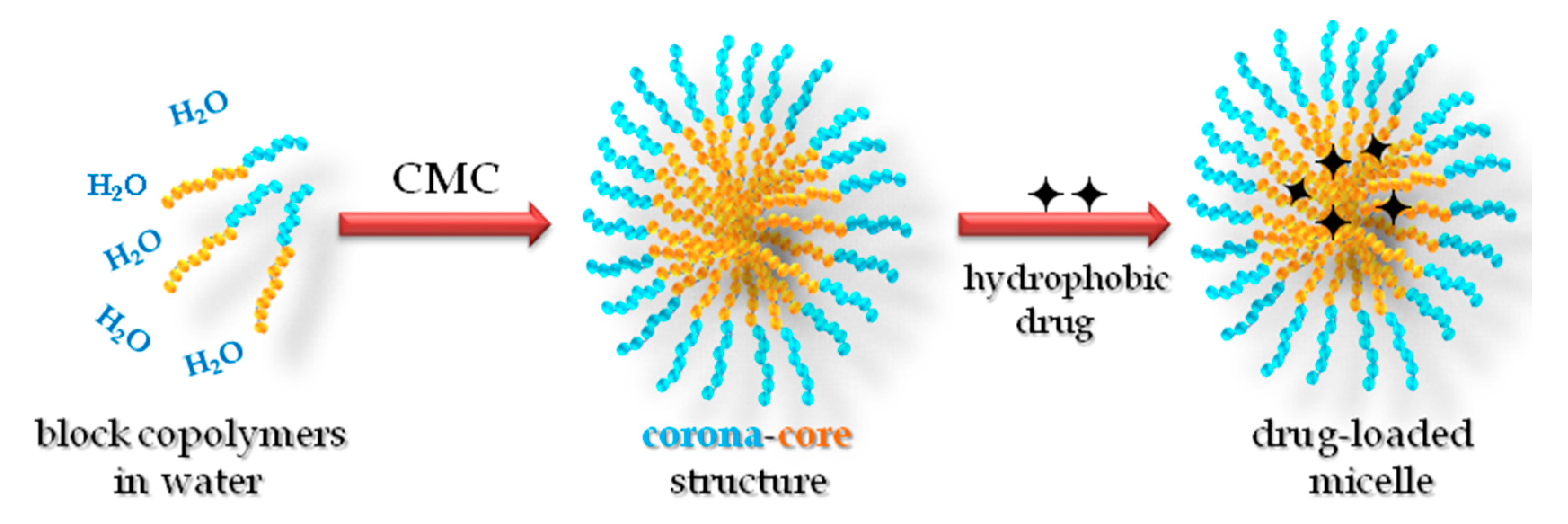
:1. Introduction
2. Characteristics of Diblock Copolymers
2.1. The Molecular Weight and Polydispersity of the Polymer
2.2. The Critical Micelle Concentration
2.3. The Hydrophilic (Corona-Forming) Blocks
2.4. The Hydrophobic (Core-Forming) Blocks
2.5. The Crystallinity of Core-Forming Blocks
3. Characteristics of Polymeric Micelles
3.1. The Drug Partition Coefficient
3.2. The Core–Drug Compatibility
3.3. The Drug/Polymer Ratio
3.4. The Drug Release Kinetics
3.5. The Micelles Preparation and Drug-Loading Methods
3.6. Real Drug Release Kinetics
4. Conclusions and Perspectives
- Enhancing the drug-loading capacity in polymeric micelles through selecting the appropriate polymer with its hydrophobic core-forming blocks to favorably solubilize many drug molecules based on a prior prediction of the compatibility between the micelle core and a particular drug through the calculation of the Flory–Huggins interaction parameter (χ).
- Investigation of the effect of the micelle preparation method and solvents used on the micelle size, morphology, polydispersity, and stability, as well as the drug-loading efficiency and release kinetics.
- The polymer compositions (i.e., the molecular weight and hydrophobic/hydrophilic ratio) should be put in narrow distribution in order to eliminate their effect on micelle size (and hence, the loading efficiency of micelles), as well as to produce micelles of size <100 nm. The drug/polymer ratio should be tuned, because there is no common optimum value that produces the highest drug-loading without polymer and drug precipitations.
- Beside the interaction strength between the core and the drug, the flow rate in the bloodstream might have strong influence on the drug release rate. This issue has not been studied so far. This is can be investigated in a microfluidic cell to mimic the flow rate in the blood capillaries in order to precisely account for the drug release profile in an environment simulating the blood circulatory system.
Acknowledgments
Conflicts of Interest
References
- Hadjichristidis, N.; Pispas, S.; Floudas, G. Block Copolymers: Synthetic Strategies, Physical Properties, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Patrício, T.; Domingos, M.; Gloria, A.; D’Amora, U.; Coelho, J.F.; Bártolo, P.J. Fabrication and characterisation of pcl and pcl/pla scaffolds for tissue engineering. Rapid Prototyp. J. 2014, 20, 145–156. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lo, C.L.; Hsiue, G.H. Multifunctional nanomicellar systems for delivering anticancer drugs. J. Biomed. Mater. Res. A 2014, 102, 2024–2038. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. Intermolecular and Surface Forces; Elsevier Inc.: Oxford, UK, 2011. [Google Scholar]
- Alexandridis, P.; Lindman, B. Block Copolymers: Self-Assembly and Applications; Elsevier Science B.V.: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 31, 1107–1170. [Google Scholar] [CrossRef]
- Disher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, M.; Khougaz, K.; Eisenberg, A. Micellization of ionic block copolymers. Acc. Chem. Res. 1996, 29, 95–102. [Google Scholar] [CrossRef]
- Zhang, L.; Eisenberg, A. Formation of crew-cut aggregates of various morphologies from amphiphilic block copolymers in solution. Polym. Adv. Technol. 1998, 9, 677–699. [Google Scholar] [CrossRef]
- La, S.B.; Okano, T.; Kataoka, K. Preparation and characterization of the micelle-forming polymeric drug indomethacin-incorporated poly(ethylene oxide)–poly(β-benzyl l-aspartate) block copolymer micelles. J. Pharm. Sci. 1996, 85, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yasugi, K.; Harada, A.; Nagasaki, Y.; Kataoka, K. Temperature-related change in the properties relevant to drug delivery of poly(ethylene glycol)-poly(d,l-lactide) block copolymer micelles in aqueous milieu. J. Control. Release 2002, 82, 359–371. [Google Scholar] [CrossRef]
- Kwon, G.; Naito, M.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Micelles based on ab block copolymers of poly(ethylene oxide) and poly(β-benzyl l-aspartate). Langmuir 1993, 9, 945–949. [Google Scholar] [CrossRef]
- Nishiyama, N.; Kataoka, K. Nanostructured devices based on block copolymer assemblies for drug delivery: Designing structures for enhanced drug function. In Polymer Therapeutics II; Satchi-Fainaro, R., Duncan, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 193, pp. 67–101. [Google Scholar]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Yokoyama, M.; Sugiyama, T.; Okano, T.; Sakurai, Y.; Naito, M.; Kataoka, K. Analysis of micelle formation of an adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer by gel permeation chromatography. Pharm. Res. 1993, 10, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R.; Chaiko, M.A.; Ruckenstein, E. Locus of solubilization of benzene in surfactant micelles. J. Phys. Chem. B 1984, 88, 2916–2922. [Google Scholar] [CrossRef]
- Nagarajan, R.; Ganesh, K. Block copolymer self-assembly in selective solvents: Theory of solubilization in spherical micelles. Macromolecules 1989, 22, 4312–4325. [Google Scholar] [CrossRef]
- Nagarajan, R. Solubilization of hydrophobic substances by block copolymer micelles in aqueous solution. In Solvents and Self-Organization of Polymer; Webber, S.E., Munk, P., Tuzar, Z., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1996; Volume 327. [Google Scholar]
- Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Improved synthesis of adriamycin-conjugated poly (ethylene oxide)-poly(aspartic acid) block copolymer and formation of unimodal micellar structure with controlled amount of physically entrapped Adriamycin. J. Control. Release 1994, 32, 269–277. [Google Scholar] [CrossRef]
- Torchilin, V.P. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell. Mol. Life Sci. 2004, 61, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Ohya, Y.; Takeda, S.; Shibata, Y.; Ouchi, T.; Kano, A.; Iwata, T.; Mochizuki, S.; Taniwaki, Y.; Maruyama, A. Evaluation of polyanion-coated biodegradable polymeric micelles as drug delivery vehicles. J. Control. Release 2011, 155, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Alkahtani, S.A.; Akhter, S.; Ahmad, F.J.; Ahmad, J.; Akhtar, M.S.; Mohsin, N.; Abdel-Wahab, B.A. Progress in nanotechnology-based drug carrier in designing of curcumin nanomedicines for cancer therapy: Current state-of-the-art. J. Drug Target. 2016, 24, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Kataoka, K. Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv. Drug Deliv. Rev. 2009, 61, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Liao, L.C.; Lu, P.L.; Lo, C.L.; Tsai, H.C.; Huang, C.Y.; Wei, K.C.; Yen, T.C.; Hsiue, G.H. The accumulation of dual pH and temperature responsive micelles in tumors. Biomaterials 2012, 33, 4576–4588. [Google Scholar] [CrossRef] [PubMed]
- Koutroumanis, K.P.; Holdich, R.G.; Georgiadou, S. Synthesis and micellization of a pH-sensitive diblock copolymer for drug delivery. Int. J. Pharm. 2013, 455, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dong, R.; Zhu, X.; Yan, D. Photo-responsive polymeric micelles. Soft Matter 2014, 10, 6121–6138. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, K.K.; Le Meins, J.F.; Misra, A.; Voisin, P.; Bouchaud, V.; Ibarboure, E.; Schatz, C.; Lecommandoux, S. Biomimetic doxorubicin loaded polymersomes from hyaluronan-block-poly(gamma-benzyl glutamate) copolymers. Biomacromolecules 2009, 10, 2802–2808. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Maysinger, D.; Eisenberg, A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf. B 1999, 16, 3–27. [Google Scholar] [CrossRef]
- Kataoka, K. Targetable polymeric drugs In Controlled Drug Delivery: The next Generation; Park, K., Ed.; ACS: Washington, DC, USA, 1996. [Google Scholar]
- Stolnik, S.; Illum, L.; Davis, S.S. Long circulating microparticulate drug carriers. Adv. Drug Deliv. Rev. 1995, 16, 195–214. [Google Scholar] [CrossRef]
- Seymour, L.W.; Duncan, R.; Strohalm, J.; Kopecek, J. Effect of molecular weight (mw) of N-(2-hydroxypropyl)methacrylamide copolymers on body distribution and rate of excretion after subcutaneous, intraperitoneal and intravenous administration to rats. J. Biomed. Mater. Res. 1987, 21, 1341–1358. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Whiteman, K.R.; Torchilin, V.P. Accumulation of protein-loaded long-circulating micelles and liposomes in subcutaneous lewis lung carcinoma in mice. Pharm. Res. 1998, 15, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Lukyanov, A.N.; Gao, Z.; Mazzola, L.; Torchilin, V.P. Polyethylene glycol-diacyllipid micelles demonstrate increased acculumation in subcutaneous tumors in mice. Pharm. Res. 2002, 19, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Yasugi, K.; Nagasaki, Y.; Kato, M.; Kataoka, K. Preparation and characterization of polymer micelles from poly(ethylene glycol)-poly(d,l-lactide) block copolymers as potential drug carrier. J. Control. Release 1999, 62, 89–100. [Google Scholar] [CrossRef]
- Bader, H.; Ringsdorf, H.; Schmidt, B. Watersoluble polymers in medicine. Macromol. Mater. Eng. 1984, 123, 457–485. [Google Scholar] [CrossRef]
- Pratten, M.K.; Lloyd, J.B.; Ringsdorf, H. Micelle-forming block copolymers: Pinocytosis by macrophages and interaction with model membranes. Makromol. Chem. 1985, 186, 725–733. [Google Scholar] [CrossRef]
- Yokoyama, M.; Inoue, S.; Sakurai, Y. Molecular design for missile drug: Synthesis of adriamycin conjugated with igg using poly(ethylene glycol)-poly (aspartic acid) block copolymer as intermediate carrier. Makromol. Chem. 1989, 190, 2041–2054. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Chekhonln, V.P.; Alakhov, V.Y.; Batrakova, E.V.; Lebedev, A.S.; Melik-Nubarov, N.S.; Arzhakov, S.A.; Levashov, A.V.; Morozov, G.V.; Severin, E.S.; et al. The neuroleptic activity of haloperidol increases after its solubilization in surfactant micelles micelles as microcontainers for drug targeting. FEBS Lett. 1989, 258, 343–345. [Google Scholar] [CrossRef]
- Kwon, G.S.; Suwa, S.; Yokoyama, M.; Okano, T.; Kataoka, K. Enhanced tumor accumulation and prolonged circulation times of micelle-forming poly(ethylene oxide-aspartate) block copolymer-adriamycin conjugates. J. Control. Release 1994, 29, 17–23. [Google Scholar] [CrossRef]
- Kwon, G.S.; Naito, M.; Kataoka, K.; Yokoyama, M.; Sakurai, Y.; Okano, T. Block copolymer micelles as vehicles for hydrophobic drugs. Colloids Surf. B 1994, 2, 429–434. [Google Scholar] [CrossRef]
- Kwon, G.S.; Kataoka, K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 1995, 16, 295–309. [Google Scholar] [CrossRef]
- Kwon, G.; Naito, M.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Block copolymer micelles for drug delivery: Loading and release of doxorubicin. J. Control. Release 1997, 48, 195–201. [Google Scholar] [CrossRef]
- Kwon, G.S.; Naito, M.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Physical entrapment of adriamycin in ab block copolymer micelles. Pharm. Res. 1995, 12, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jackson, J.K.; Burt, H.M. Development of amphiphilic diblock copolymers as micellar carriers of taxol. Int. J. Pharm. 1996, 132. [Google Scholar] [CrossRef]
- Allen, C.; Yu, Y.; Maysinger, D.; Eisenberg, A. Polycaprolactone-b-poly(ethylene oxide) block copolymer micelles as a novel drug delivery vehicle for neurotrophic agents fk506 and L-685,818. Bioconjugate Chem. 1998, 9, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.G.; Kim, S.Y.; Lee, Y.M.; Cho, C.S.; Sung, Y.K. Methoxy poly(ethylene glycol)/ϵ-caprolactone amphiphilic block copolymeric micelle containing indomethacin. I preparation and characterization. J. Control. Release 1998, 51, 1–11. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, I.G.; Lee, Y.M.; Cho, C.S.; Sung, Y.K. Methoxy poly(ethylene glycol) and ϵ-caprolactone amphiphilic block copolymeric micelle containing indomethacin ii. Micelle formation and drug release behaviours. J. Control. Release 1998, 51, 13–22. [Google Scholar] [CrossRef]
- De Jaeghere, F.; Allemann, E.; Leroux, J.-C.; Stevels, W.; Feijen, J.; Doelker, E.; Gurny, R. Formulation and lyoprotection of poly (lactic acid-co-ethyleneoxide) nanoparticles: Influence on physical stability and in vitro cell uptake. Pharm. Res. 1999, 16, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Emoto, K.; Lijima, M.; Nagasaki, Y.; Aoyagi, T.; Okano, T.; Sakurai, Y.; Kataoka, K. Core-stabilized polymeric micelle as potential drug carrier: Increased solubilization of taxol. Polym. Adv. Technol. 1999, 10, 647–654. [Google Scholar] [CrossRef]
- Yokoyama, M.; Okano, T.; Sakurai, Y.; Ekimoto, H.; Shibazaki, C.; Kataoka, K. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric drug and its extremely long circulation in blood. Cancer Res. 1991, 51, 3229–3236. [Google Scholar] [PubMed]
- Yokoyama, M.; Okano, T.; Sakurai, Y.; Fukushima, S.; Okamoto, K.; Kataoka, K. Selective delivery of adriamycin to a solid tumor using a polymeric micelle carrier system. J. Drug Target. 1999, 7, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Lavasanifar, A.; Samuel, J.; Kwon, G.S. Micelles of poly(ethylene oxide)-block-poly(N-alkyl stearate l-aspartamide): Synthetic analogues of lipoproteins for drug delivery. J. Biomed. Mater. Res. 2000, 52, 831–835. [Google Scholar] [CrossRef]
- Fernandez, A.-M.; Van Derpoorten, K.; Dasnois, L.; Lebtahi, K.; Dubois, V.; Lobl, T.J.; Gangwar, S.; Oliyai, C.; Lewis, E.R.; Shochat, D.; et al. N-succinyl-(β-alanyl-l-leucyl-l-alanyl-l-leucyl)doxorubicin: An extracellularly tumor-activated prodrug devoid of intravenous acute toxicity. J. Med. Chem. 2001, 44, 3750–3753. [Google Scholar] [CrossRef] [PubMed]
- Benahmed, A.M.; Ranger, M.; Leroux, J.-C. Novel polymeric micelles based on the amphiphilic diblock copolymer poly(N-vinyl-2-pyrrolidone)-block-poly(d,l-lactide). Pharm. Res. 2001, 18, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Lele, B.S.; Leroux, J.-C. Synthesis and micellar characterization of novel amphiphilic a-b-a triblock copolymers of N-(2-hydroxypropyl)methacrylamide or N-vinyl-2-pyrrolidone with poly(ε-caprolactone). Macromolecules 2002, 35, 6714–6723. [Google Scholar] [CrossRef]
- Soo, P.L.; Luo, L.; Maysinger, D.; Eisenberg, A. Incorporation and release of hydrophobic probes in biocompatible polycaprolactone-block-poly(ethylene oxide) micelles: Implications for drug delivery. Langmuir 2002, 18, 9996–10004. [Google Scholar] [CrossRef]
- Santa, V.P.; Smith, D.; Leroux, J.-C. Novel pH-sensitive supramolecular assemblies for oral delivery of poorly water soluble drugs: Preparation and characterization. J. Control. Release 2004, 97, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, H.M.; Mahmud, A.; Sharifabadi, A.D.; Lavasanifar, A. Micelles of methoxy poly(ethylene oxide)-b-poly(ɛ-caprolactone) as vehicles for the solubilization and controlled delivery of cyclosporine a. J. Control. Release 2005, 104, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Roby, A.; Erdogan, S.; Torchilin, V.P. Solubilization of poorly soluble pdt agent, meso-tetraphenylporphin, in plain or immunotargeted peg-pe micelles results in dramatically improved cancer cell killing in vitro. Eur. J. Pharm. Biopharm. 2006, 62, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Dwan’Isa, J.-P.L.; Rouxhet, L.; Brewster, M.E.; Préat, V.; Arien, A. Spontaneously self-assembled micelles from poly(ethylene glycol)-b-poly(ɛ-caprolactone-co-trimethylene carbonate) for drug solubilization. Pharmazie 2008, 63, 235–240. [Google Scholar] [CrossRef]
- Huh, K.M.; Lee, S.C.; Cho, Y.W.; Lee, J.; Jeong, J.H.; Park, K. Hydrotropic polymer micelle system for delivery of paclitaxel. J. Control. Release 2005, 101, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.M.; Min, H.S.; Lee, S.C.; Lee, H.J.; Kim, S.; Park, K. A new hydrotropic block copolymer micelle system for aqueous solubilization of paclitaxel. J. Control. Release 2008, 126, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Letchfordd, K.; Liggins, R.; Burt, H. Solubilization of hydrophobic drugs by methoxy poly(ethylene glycol)-block-polycaprolactone diblock copolymer micelles: Theoretical and experimental data and correlations. J. Pharm. Sci. 2008, 97, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Molavi, O.; Ma, Z.; Mahmud, A.; Alshamsan, A.; Samuel, J.; Lai, R.; Kwon, G.S.; Lavasanifar, A. Polymeric micelles for the solubilization and delivery of stat3 inhibitor cucurbitacins in solid tumors. Int. J. Pharm. 2008, 347, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Higuchi, Y.; Kawakami, S.; Hashida, M.; Kagechika, H.; Shudo, K.; Yokoyama, M. Encapsulation of the synthetic retinoids am80 and le540 into polymeric micelles and the retinoids’ release control. J. Control. Release 2009, 136, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.C.; Alani, A.W.; Rao, D.A.; Rockich, N.C.; Kwon, G.S. Multi-drug loaded polymeric micelles for simultaneous delivery of poorly soluble anticancer drugs. J. Control. Release 2009, 140, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Gong, C.; Gou, M.; Fu, S.; Guo, Q.; Shi, S.; Luo, F.; Guo, G.; Qiu, L.; Qian, Z. Biodegradable poly(epsilon-caprolactone)-poly(ethylene glycol) copolymers as drug delivery system. Int. J. Pharm. 2009, 381, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kuskov, A.N.; Voskresenskaya, A.A.; Goryachaya, A.V.; Artyukhov, A.A.; Shtilman, M.I.; Tsatsakis, A.M. Preparation and characterization of amphiphilic poly-N-vinylpyrrolidone nanoparticles containing indomethacin. J. Mater. Sci. Mater. Med. 2010, 21, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Olbrich, C.; Krause, M.; Kissel, T. Solubilization of sagopilone, a poorly water-soluble anticancer drug, using polymeric micelles for parenteral delivery. Int. J. Pharm. 2010, 389, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jing, X. Biodegradable amphiphilic polymer–drug conjugate micelles. Expert Opin. Drug Deliv. 2009, 6, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, K.K.; Agrawal, H.G.; Le Meins, J.F.; Misra, A.; Upadhyay, C.; Lecommandoux, S. Role of block copolymer nanoconstructs in cancer therapy. Crit. Rev. Ther. Drug Carrier Syst. 2009, 26, 157–205. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M. Polymeric micelles as a new drug carrier system and their required considerations for clinical trials. Expert Opin. Drug Deliv. 2010, 7, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Khemtong, C.; Yang, X.; Chang, X.; Gao, J. Nanonization strategies for poorly water-soluble drugs. Drug Discov. Today 2011, 16, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Soo, P.L.; Eisenberg, A. Preparation of block copolymer vesicles in solution. J. Polym. Sci. B 2004, 42, 923–938. [Google Scholar] [CrossRef]
- Lecommandoux, S.; Sandre, O.; Chécot, F.; Rodriguez-Hernandez, J.; Perzynski, R. Magnetic nanocomposite, micelles and vesicles. Adv. Mater. 2005, 17, 712–719. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, B.; Chen, S.; Du, J. Polymer vesicles: Mechanism, preparation, application, and responsive behavior. Prog. Polym. Sci. 2017, 64, 1–22. [Google Scholar] [CrossRef]
- Almgren, M.; Brown, W.; Hvidt, S. Self-aggregation and phase behavior of peo–ppo–peo block copolymers in aqueous solution. Colloid Polym. Sci. 1995, 27, 2–15. [Google Scholar] [CrossRef]
- Talelli, M.; Rijcken, C.J.; van Nostrum, C.F.; Storm, G.; Hennink, W.E. Micelles based on hpma copolymers. Adv. Drug Deliv. Rev. 2010, 62, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.G.; Okano, T.; Kataoka, K.; Kwon, G. Polymeric micelles for drug delivery: Solubilization and haemolytic activity of amphotericin b. J. Control. Release 1998, 53, 131–136. [Google Scholar] [CrossRef]
- Liaw, J.; Aoyagi, T.; Kataoka, K.; Sakurai, Y.; Okano, T. Visualization of peo-pbla-pyrene polymeric micelles by atomic force microscopy. Pharm. Res. 1998, 15, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Görner, T.; Gref, R.; Michenot, D.; Sommer, F.; Tran, M.N.; Dellacherie, E. Lidocaine-loaded biodegradable nanospheres. I. Optimization of the drug incorporation into the polymer matrix. J. Control. Release 1999, 57, 259–268. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Y.; Allen, C. Polymer-drug compatibility: A guide to the development of delivery systems for the anticancer agent, ellipticine. J. Pharm. Sci. 2004, 93. [Google Scholar] [CrossRef] [PubMed]
- Mosqueira, V.C.F.; Legrand, P.; Gulik, A.; Bourdon, O.; Gref, R.; Labarre, D.; Barratt, G. Relationship between complement activation, cellular uptake and surface physicochemical aspects of novel peg-modified nanocapsules. Biomaterials 2001, 22, 2967–2979. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, D.W.; Shim, Y.H.; Bang, J.S.; Oh, H.S.; Kim, S.W.; Seo, M.H. In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy. J. Control. Release 2001, 72, 191–202. [Google Scholar] [CrossRef]
- Kumari, P.; Swami, M.O.; Nadipalli, S.K.; Myneni, S.; Ghosh, B.; Biswas, S. Curcumin delivery by poly(lactide)-based co-polymeric micelles: An in vitro anticancer study. Pharm. Res. 2016, 33, 826–841. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, Y.; Okada, T.; Scholz, C.; Iijima, M.; Kato, M.; Kataoka, K. The reactive polymeric micelle based on an aldehyde-ended poly(ethylene glycol)/poly(lactide) block copolymer. Macromolecules 1998, 31, 1473–1479. [Google Scholar] [CrossRef]
- Iijima, M.; Nagasaki, Y.; Okada, T.; Kato, M.; Kataoka, K. Core-polymerized reactive micelles from heterotelechelic amphiphilic block copolymers. Macromolecules 1999, 32, 1140–1146. [Google Scholar] [CrossRef]
- Soleymani Abyaneh, H.; Vakili, M.R.; Zhang, F.; Choi, P.; Lavasanifar, A. Rational design of block copolymer micelles to control burst drug release at a nanoscale dimension. Acta Biomater. 2015, 24, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Eisenberg, A.; Mrsic, J.; Maysinger, D. Pcl-b-peo micelles as a delivery vehicle for fk506: Assessment of a functional recovery of crushed peripheral nerve. Drug Deliv. 2000, 7, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Deng, X.; Yang, H. Biodegradable poly(ε-caprolactone)-poly(ethylene glycol) block copolymers: Characterization and their use as drug carriers for a controlled delivery system. Biomaterials 2003, 24, 3563–3570. [Google Scholar] [CrossRef]
- Shuai, X.; Merdan, T.; Schaper, A.K.; Xi, F.; Kissel, T. Core-cross-linked polymeric micelles as paclitaxel carriers. Bioconjugate Chem. 2004, 15. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, H.M.; Brocks, D.R.; Lavasanifar, A. Polymeric micelles for the solubilization and delivery of cyclosporine A: Pharmacokinetics and biodistribution. Biomaterials 2005, 26, 7251–7259. [Google Scholar] [CrossRef] [PubMed]
- Soo, P.L.; Lovric, J.; Davidson, P.; Maysinger, D.; Eisenberg, A. Polycaprolactone-block-poly(ethylene oxide) micelles: A nanodelivery system for 17β-estradiol. Mol. Pharm. 2005, 2, 519–527. [Google Scholar] [CrossRef]
- Gou, M.; Zheng, X.; Men, K.; Zhang, J.; Wang, B.; Lv, L.; Wang, X.; Zhao, Y.; Luo, F.; Chen, L.; et al. Self-assembled hydrophobic honokiol loaded mpeg-pcl diblock copolymer micelles. Pharm. Res. 2009, 26, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Shahin, M.; Lavasanifar, A. Novel self-associating poly(ethylene oxide)-b-poly(epsilon-caprolactone) based drug conjugates and nano-containers for paclitaxel delivery. Int. J. Pharm. 2010, 389, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Gref, R.; Minamitake, Y.; Peracchia, M.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Ferruti, P.; Pence, M.; D’Addato, P.; Ranucci, E.; Deghenghi, R. Synthesis and properties of novel block copolymers containing poly(lactic-glycolic acid) and poly(ethyleneglycol) segments. Biomaterials 1995, 16, 1423–1428. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Cheon, J.-B.; Kim, S.-H.; Nah, J.-W.; Lee, Y.-M.; Sung, Y.-K.; Akaike, T.; Cho, C.-S. Clonazepam release from core-shell type nanoparticles in vitro. J. Control. Release 1998, 51, 169–178. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Nah, J.-W.; Lee, H.-C.; Kim, S.-H.; Cho, C.-S. Adriamycin release from flower-type polymeric micelle based on star-block copolymer composed of poly(γ-benzyl l-glutamate) as the hydrophobic part and poly(ethylene oxide) as the hydrophilic part. Int. J. Pharm. 1999, 188, 49–58. [Google Scholar] [CrossRef]
- Kataoka, K.; Matsumoto, T.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Fukushima, S.; Okamoto, K.; Kwon, G.S. Doxorubicin-loaded poly(ethylene glycol)-poly(beta-benzyl-l-aspartate) copolymer micelles: Their pharmaceutical characteristics and biological significance. J. Control. Release 2000, 64, 143–153. [Google Scholar] [CrossRef]
- Lee, H.; Zeng, F.; Dunne, M.; Allen, C. Methoxy poly(ethylene glycol)-block-poly(δ-valerolactone) copolymer micelles for formulation of hydrophobic drugs. Biomacromolecules 2005, 6, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chu, I.-M. Methoxy poly(ethylene glycol)-b-poly(valerolactone) diblock polymeric micelles for enhanced encapsulation and protection of camptothecin. Eur. Polym. J. 2008, 44, 3922–3930. [Google Scholar] [CrossRef]
- Shuai, X.; Ai, H.; Nasongkla, N.; Kim, S.; Gao, J. Micellar carriers based on block copolymers of poly(ε-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J. Control. Release 2004, 98, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, B.; Du, L.; Xu, H.; Xu, X.; Wang, C.; Fan, Y.; Cong, M.; Yin, J.; Li, H.; Guan, H. Self-assembled micelle loading cabazitaxel for therapy of lung cancer. Int. J. Pharm. 2016, 499, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lai, K.L.; Chan, P.S.; Leung, S.C.; Li, H.Y.; Fang, Y.; To, K.K.; Choi, C.H.; Gao, Q.Y.; Lee, T.W. Micellar delivery of dasatinib for the inhibition of pathologic cellular processes of the retinal pigment epithelium. Colloids Surf. B Biointerfaces 2016, 140, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Satoh, A.; Sakurai, Y.; Okano, T.; Matsumura, Y.; Kakizoe, T.; Kataoka, K. Incorporation of water-insoluble anticancer drug into polymeric micelles and control of their particle size. J. Control. Release 1998, 55, 219–229. [Google Scholar] [CrossRef]
- Gadelle, F.; Koros, W.J.; Schechter, R.S. Solubilization of aromatic solutes in block copolymers. Macromolecules 1995, 28, 4883–4892. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Nazarova, I.R.; Astafieva, I.V.; Batrakova, E.V.; Alakhov, V.Y.; Yaroslavov, A.A.; Kabanov, A.V. Micelle formation and solubilization of fluorescent probes in poly(oxyethylene-b-oxypropylene-b-oxyethylene) solutions. Macromolecules 1995, 28, 2303–2314. [Google Scholar] [CrossRef]
- Harris, J.M. Introduction to biotechnical and biomedical applications of poly(ethylene glycol). In Poly(ethylene glycol) Chemistry: Biotechnical and Biomedical Applications; Harris, J.M., Ed.; Springer: Boston, MA, USA, 1992; pp. 1–14. [Google Scholar] [CrossRef]
- Bae, Y.; Nishiyama, N.; Fukushima, S.; Koyama, H.; Yasuhiro, M.; Kataoka, K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: Tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjugate Chem. 2005, 16, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Wang, C.-H.; Hsiue, G.-H. Polymeric micelles with a pH-responsive structure as intracellular drug carriers. J. Control. Release 2005, 108, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Lecommandoux, S.; Sandre, O.; Chécot, F.; Perzynski, R. Smart hybrid magnetic self-assembled micelles and hollow capsules. Prog. Solid State Chem. 2006, 34, 171–179. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, J.H.; Bae, S.M.; Shin, H.; Kim, M.S.; Park, S.; Lee, H.; Park, R.W.; Kim, I.S.; Kim, K.; et al. Tumoral acidic pH-responsive mpeg-poly(beta-amino ester) polymeric micelles for cancer targeting therapy. J. Control. Release 2010, 144, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; De Gennes, P.G. Protein—Surface interactions in the presence of polyethylene oxide: I. Simplified theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Gabizon, A.; Papahadjopoulos, D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc. Natl. Acad. Sci. USA 1988, 85, 6949–6953. [Google Scholar] [CrossRef] [PubMed]
- Vittaz, M.; Bazile, D.; Spenlehauer, G.; Verrecchia, T.; Veillard, M.; Puisieux, F.; Labarre, D. Effect of peo surface density on long-circulating pla-peo nanoparticles which are very low complement activators. Biomaterials 1996, 17, 1575–1581. [Google Scholar] [CrossRef]
- Hurter, P.N.; Hatton, T.A. Solubilization of polycyclic aromatic hydrocarbons by poly(ethylene oxide-propylene oxide) block copolymer micelles: Effects of polymer structure. Langmuir 1992, 8, 1291–1299. [Google Scholar] [CrossRef]
- Lin, W.-J.; Juang, L.-W.; Lin, C.-C. Stability and release performance of a series of pegylated copolymeric micelles. Pharm. Res. 2003, 20, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Glavas, L.; Odelius, K.; Albertsson, A.-C. Tuning loading and release by modification of micelle core crystallinity and preparation. Polym. Adv. Technol. 2015, 26, 880–888. [Google Scholar] [CrossRef]
- Burt, H.M.; Zhang, X.; Toleikis, P.; Embree, L.; Hunter, W.L. Development of copolymers of poly(d,l-lactide) and methoxypolyethylene glycol as micellar carriers of paclitaxel. Colloids Surf. B 1999, 16, 161–171. [Google Scholar] [CrossRef]
- Klok, H.A.; Lecommandoux, S. Supramolecular materials via block copolymer self-assembly. Adv. Mater. 2001, 13, 1217–1229. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Coulembier, O.; Degée, P.; Hedrick, J.L.; Dubois, P. From controlled ring-opening polymerization to biodegradable aliphatic polyester: Especially poly(β-malic acid) derivatives. Prog. Polym. Sci. 2006, 31, 723–747. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of plga nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ogura, A.; Kaneko, T.; Murata, Y.; Akashi, M. Precise synthesis of aba triblock copolymers comprised of poly(ethylene oxide) and poly(β-benzyl-l-aspartate): A hierarchical structure inducing excellent elasticity. Macromolecules 2004, 37, 1370–1377. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, L.; Liang, Q.; Zhang, X.; Guan, H.; Xu, X.; Chen, X.; Jing, X. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J. Control. Release 2005, 105, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Van Krevelen, D.W.; Te Nijenhuis, K. Cohesive properties and solubility. In Properties of Polymers, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 189–227. [Google Scholar]
- Fedors, R.F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Hoy, K.L. New values of the solubility parameters from vapor pressure data. J. Paint Technol. 1970, 42, 115–118. [Google Scholar]
- Hansen, C.M. The three-dimensional solubility parameter-key to paint-component affinities i. Solvents, plasticizers, polymers and resins. J. Paint Technol. 1967, 39, 104–117. [Google Scholar]
- Harada, Y.; Yamamoto, T.; Sakai, M.; Saiki, T.; Kawano, K.; Maitani, Y.; Yokoyama, M. Effects of organic solvents on drug incorporation into polymeric carriers and morphological analyses of drug-incorporated polymeric micelles. Int. J. Pharm. 2011, 404, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Hoy Solubility Parameter Calculation with Solvent Database. Available online: http://www.compchemcons.com/products/index.html#Hoy (accessed on 20 April 2018).
- Hagan, S.A.; Coombes, A.G.A.; Garnett, M.C.; Dunn, S.E.; Davies, M.C.; Illum, L.; Davis, S.S.; Harding, S.E.; Purkiss, S.; Gellert, P.R. Polylactide-poly(ethylene glycol) copolymers as drug delivery systems. 1. Characterization of water dispersible micelle-forming systems. Langmuir 1996, 12, 2153–2161. [Google Scholar] [CrossRef]
- Gorshkova, M.Y.; Stotskaya, L.L. Micelle-like macromolecular systems for controlled release of daunomycin. Polym. Adv. Technol. 1998, 9, 362–367. [Google Scholar] [CrossRef]
- Vangeyte, P.; Gautier, S.; Jérôme, R. About the methods of preparation of poly(ethylene oxide)-b-poly(ε-caprolactone) nanoparticles in water: Analysis by dynamic light scattering. Colloids Surf. A 2004, 242, 203–211. [Google Scholar] [CrossRef]
- Zhao, J.; Pispas, S.; Zhang, G. Effect of sonication on polymeric aggregates formed by poly(ethylene oxide)-based amphiphilic block copolymers. Macromol. Chem. Phys. 2009, 210, 1026–1032. [Google Scholar] [CrossRef]
- Yokoyama, M.; Fukushima, S.; Uehara, R.; Okamoto, K. Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor. J. Control Release 1998, 50, 79–92. [Google Scholar] [CrossRef]
- Hiroshi, M.; Alexander, K.; Kazunori, K.; Teruo, O. Polymer Drugs in the Clinical Stage; Springer: New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Fournier, C.; Hecquet, B.; Bouffard, P.; Vert, M.; Caty, A.; Vilain, M.-O.; Vanseymortier, L.; Merle, S.; Krikorian, A.; Lefebvre, J.-L.; et al. Experimental studies and preliminary clinical trial of vinorelbine-loaded polymeric bioresorbable implants for the local treatment of solid tumors. Cancer Res. 1991, 51, 5384–5391. [Google Scholar] [PubMed]
- Feng, S.S.; Huang, G.F.; Mu, L. Nanospheres of biodegradable polymers: A system for clinical administration of an anticancer drug paclitaxel (taxol). Ann. Acad. Med. Singap. 2000, 29, 633–639. [Google Scholar] [PubMed]


| Requirement | Consequences in Polymeric Micelle Design |
|---|---|
| Protect drug from degradation | Encapsulation into the micellar core. |
| Intravenous injection | Sub-100 nm. |
| Obtain the desired micelle size | Adjust the physicochemical properties of the polymer with its constituents and apply an adequate preparation method. |
| Prevent opsonization | Coating with hydrophilic polymer (PEG). |
| Decrease the drug release rate | Adjust the core–drug compatibility (χ). |
| Control of biodistribution | Introduction of targeting moieties (antibodies, peptides, carbohydrates). |
| Control of pharmacokinetics and pharmacodynamics | All previous parameters. |
| Elimination | Use of biocompatible and biodegradable materials. |
| Factors | Performance Properties | ||||||
|---|---|---|---|---|---|---|---|
| Stability, CMC, Kd | Size | Surface Properties, Hydrophilicity | Morphology | LE | RP | ||
| Block copolymer | MW | ||||||
| C | |||||||
| HLB | |||||||
| PDI | |||||||
| Core blocks | Length | ||||||
| δpl | |||||||
| Tg | |||||||
| Biodegr-adibility | |||||||
| Corona blocks | Length | ||||||
| δph | |||||||
| Biocomp-atibility | |||||||
| Drug | δd | ||||||
| vm | |||||||
| C | |||||||
| Preparation method | |||||||
| Polymer | Core/Corona wt Ratio | Method | Size of Unloaded Micelles (nm) | Drug | Size of Loaded Micelles (nm) | LE% | Ref. |
|---|---|---|---|---|---|---|---|
| PEG5000-PCL5000 | 1.0 | Sonication | 69.0 | Saglopine | ND | 70 | [72] |
| PEG2000-PCL1400 | 0.7 | 55.0 | ND | 66 | |||
| MPEG5000-PCL5000 | 1.0 | Cosolvent evaporation | 87.5 | CsA | 100 | 52.2 | [97] |
| MPEG5000-PCL13000 | 2.6 | 78.7 | 98.6 | 63.8 | |||
| MPEG5000-PCL24000 | 4.8 | 99.8 | 102.3 | 49.5 | |||
| PEG2000-PCL2000 | 1.0 | Solvent displacement/sonication | 17.0 | Doxorubicin | 25.4 | 3.29 | [108] |
| MPEG5000-PCL2500 | 0.5 | 29.7 | 22.9 | 3.10 | |||
| MPEG5000-PCL5000 | 1.0 | 41.0 | 37.3 | 4.03 | |||
| MPEG5000-PCL8500 | 1.7 | 56.9 | 84.0 | 4.09 | |||
| MPEG5000-PCL24700 | 4.9 | 86.3 | 104.9 | 4.30 | |||
| MPEG2000-PCL1200 | 0.6 | Cosolvent evaporation | 29.4 | Paclitaxel | 31.3 | 3.3 | [96] |
| MPEG2000-PCL2700 | 1.4 | 37.3 | 42.6 | 13 | |||
| MPEG5000-PCL3800 | 0.7 | 71.8 | 65.3 | 23 | |||
| MPEG5000-PCL18000 | 3.6 | 97.7 | 91.9 | 38 | |||
| PEG5000-PCL4000 | 0.8 | Dialysis | ND | Ellipticine | 20 a | 75.9 a | [87] |
| Dry down | ND | 76 a | 65.3 a | ||||
| PEG2000-PCL900 | 0.5 | Dialysis | ND | FK506 | 50 | 21 | [94] |
| PEG1980-PCL1368 | 0.3 | Dialysis | ND | 17β-estradiol | ND | 10 | [98] |
| PEG1980-PCL2622 | 0.5 | 25 | 30 | 19 | |||
| PEG1980-PCL17328 | 3.4 | ND | ND | 90 | |||
| PEG2000-PCL2280 | 1.1 | Cosolvent evaporation | ND | Cabazitaxel | 28.8 | 99.3 | [109] |
| PEG5000-PCL5000 | 1.0 | Cosolvent evaporation | ND | Cucurbitacin B | 73.3 | 30.2 | [67] |
| PEG5000-PCL24000 | 4.8 | ND | 78.3 | 65.1 | |||
| PEG5000-PCL5000 | 1.0 | ND | Cucurbitacin I | 72.2 | 44.1 | ||
| PEG5000-PCL24000 | 4.8 | ND | 77.2 | 68.4 | |||
| PEG5000-PCL4790 | 1.0 | Dialysis | 62.5 | Paclitaxel | 69.2 | 24.7 | [100] |
| PEG5000-PCL10000 | 2.0 | Cosolvent evaporation | ND | Dasatinib | 54.3 | 95.4 | [110] |
| MPEG5333-PCL2638 | 0.5 | Dialysis | 54 | Indomethacin | ND | ND | [47] |
| MPEG5333-PCL4984 | 0.9 | 77 | ND | ND | |||
| MPEG5333-PCL8034 | 1.5 | 114 | 120–165 b | 16.8–42.2 b | |||
| MPEG5333-PCL9068 | 1.7 | 130 | ND | ND | |||
| MPEG5000-PCL2166 | 0.4 | Emulsion-solvent evaporation | 45.3 | Indomethacin, Curcumin, Plumbagin, Paclitaxel, Etoposide | See Ref. [66] | [66] | |
| MPEG2000-PCL1320 | 0.7 | 22.3 | |||||
| MPEG2000-PCL852 | 0.4 | 14.7 | |||||
| MPEG750-PCL464 | 0.6 | 12.4 | |||||
| MPEG750-PCL323 | 0.4 | 13.5 | |||||
| MPEG750-PCL197 | 0.3 | 11.1 | |||||
| MPEG-PCL | Direct dissolution assisted by ultrasound | 27 | Honokiol | 31 c | 65.4 c | [99] | |
| PEG5000-PDLLA4200 | 0.8 | Dialysis | ND | Ellipticine | 76 | 1.2 | [87] |
| Dry down | ND | 106 | 6.2 | ||||
| PEG5000-PDLLA45000 | 9.0 | Emulsion-solvent evaporation | ND | Lidocaine | 203 | 17 | [86] |
| MPEG2000-PDLLA2000 | 1.0 | Emulsion-solvent evaporation | ND | Paclitaxel | <50 nm | 25 | [45] |
| MPEG2000-PDLLA1333 | 0.7 | ND | 25 | ||||
| MPEG5000-PDLLA2143 | 0.4 | ND | 10 | ||||
| PEG52000-PDLLA56000 | 1.1 | Dialysis | 33 | ND | ND | ND | [91] |
| PEG91000-PDLLA56000 | 0.6 | 30 | ND | ND | |||
| PEG4100-PDLLA1200 | 0.3 | Dialysis | 154 | ND | ND | ND | [35] |
| PEG6000-PDLLA3000 | 0.5 | 28.1 | ND | ND | |||
| PEG5700-PDLLA5400 | 1.0 | 33.5 | ND | ND | |||
| PEG6100-PDLLA7800 | 1.3 | 35.0 | ND | ND | |||
| PEG5000-PBCL4700 | 0.9 | Cosolvent evaporation | ND | Cucurbitacin B | 76.3 | 92.9 | [67] |
| ND | Cucurbitacin B | 74.1 | 74.1 | ||||
| PEG5000-PBCL4470 | 0.9 | Dialysis | 64.3 | Paclitaxel | 61.0 | 36.4 | [100] |
| PEG12000-PBLA5000 | 0.4 | Dialysis d | 19 | Indomethacin | 29 | 20.4 | [10] |
| o/w emulsion | ND | 25 | 22.1 | ||||
| PEG12000-PBLA3000 | 0.3 | Dialysis | 20 | Amphotericin B | 25.8 | 27–30 e | [84] |
| PEG-PBLA | Dialysis | >100 | KRN 5500 | [111] | |||
| PEG12000-PBLA5000 | 0.4 | o/w emulsion | 19 | Doxorubicin | 37 | 65 | [43] |
| MPEG2000-PVL1000 | 0.5 | Emulsion-solvent evaporation | ND | Paclitaxel | 200 | 37 | [106] |
| MPEG2000-PVL2000 | 1.0 | ND | 31 | 92 | |||
| MPEG5000-PVL2600 | 0.5 | ND | 225 | 10 | |||
| MPEG5000-PVL4900 | 1.0 | ND | 138 | 3 | |||
| Polymer | Core/Corona Ratio | CMC (mg/L) | Ref. |
|---|---|---|---|
| PEG5000-PCL5000 | 1.0 | 1.8 | [97] |
| PEG5000-PCL13000 | 2.6 | 0.8 | |
| PEG5000-PCL24000 | 4.8 | 0.5 | |
| MPEG5000-PCL2166 | 0.4 | 4.5 | [66] |
| MPEG2000-PCL852 | 0.4 | 21.0 | |
| MPEG750-PCL323 | 0.4 | 122.1 | |
| MPEG750-PCL464 | 0.6 | 71.5 | |
| PEG12000-PBLG8400 | 0.7 | 2.7 | [103] |
| PEG12000-PBLG39800 | 3.3 | 2.2 | |
| PEG12000-PBLG91700 | 7.6 | 2.0 | |
| PEG5000-PBLA2381 | 0.5 | 10 | [12] |
| PEG5000-PBLA4762 | 1.0 | 5 | |
| PEG12000-PBLA4762 | 0.4 | 10 | |
| PEG12000-PBLA5000 | 0.4 | 18 | [10] |
| PEG6000-PDLLA3000 | 0.5 | 4.5 | [35] |
| PEG6100-PDLLA7800 | 1.3 | 2.5 | |
| MPEG2000-PVL550 | 0.3 | 176 | [106] |
| MPEG2000-PVL1000 | 0.5 | 80.4 | |
| MPEG2000-PVL2000 | 1.0 | 23.3 |
| Polymer | Tg (°C) | State | Ref. |
|---|---|---|---|
| PCL | −60 | Semicrystalline | [128] |
| PVL | −47 to −70 | Semicrystalline | [106] |
| PDLLA | 34.5 | Amorphous | [35] |
| PLGA | 40–60 | Amorphous | [129] |
| PBLA | - | Amorphous | [87] |
| PBLG | 50 | - | [130] |
| Drug | χPCL-drug | χPDLLA-drug | χPBLG-drug | χPVL-drug | χPLGA-drug |
|---|---|---|---|---|---|
| Fenofibrate | 0.002 | 5.380 | 0.563 | 2.222 | 3.983 |
| Curcumin | 6.563 | 0.017 | 3.047 | 0.969 | 0.216 |
| Cyclosporine A | 1.942 | 10.455 | 0.026 | 2.591 | 6.756 |
| Indomethacin | 2.872 | 0.422 | 0.823 | 0.030 | 0.108 |
| Paclitaxel | 8.908 | 0.470 | 3.069 | 0.363 | 0.033 |
| Camptothecin | 28.691 | 9.338 | 21.011 | 14.924 | 11.365 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, Y.H.A.; Youssry, M. Polymeric Micelles of Biodegradable Diblock Copolymers: Enhanced Encapsulation of Hydrophobic Drugs. Materials 2018, 11, 688. https://doi.org/10.3390/ma11050688
Hussein YHA, Youssry M. Polymeric Micelles of Biodegradable Diblock Copolymers: Enhanced Encapsulation of Hydrophobic Drugs. Materials. 2018; 11(5):688. https://doi.org/10.3390/ma11050688
Chicago/Turabian StyleHussein, Yasser H. A., and Mohamed Youssry. 2018. "Polymeric Micelles of Biodegradable Diblock Copolymers: Enhanced Encapsulation of Hydrophobic Drugs" Materials 11, no. 5: 688. https://doi.org/10.3390/ma11050688





