Synthesis and Luminescent Properties of Europium Complexes Covalently Bonded to Hybrid Materials Based on MCM-41 and Poly(Ionic Liquids)
Abstract
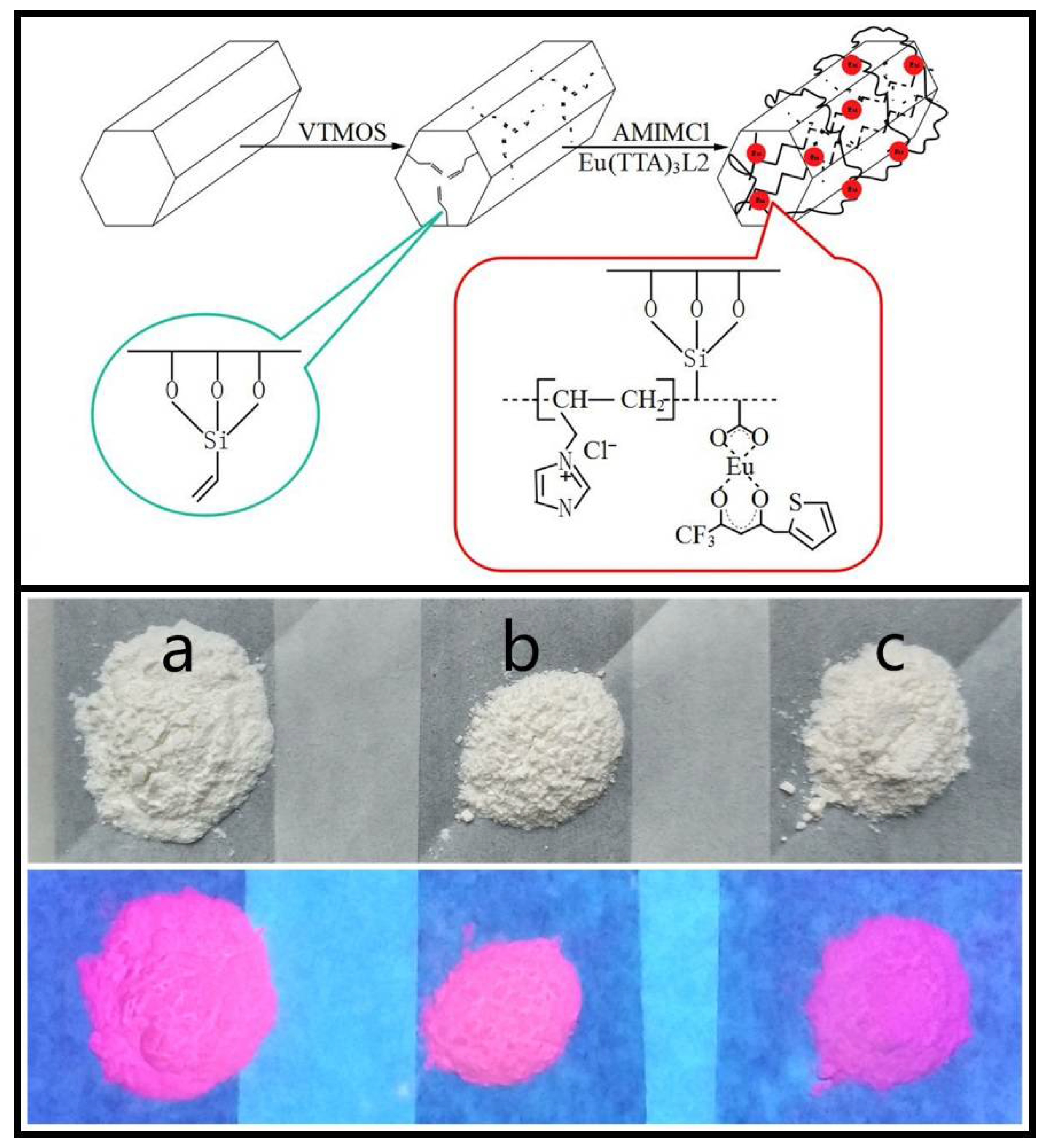
:1. Introduction
2. Materials and Methods
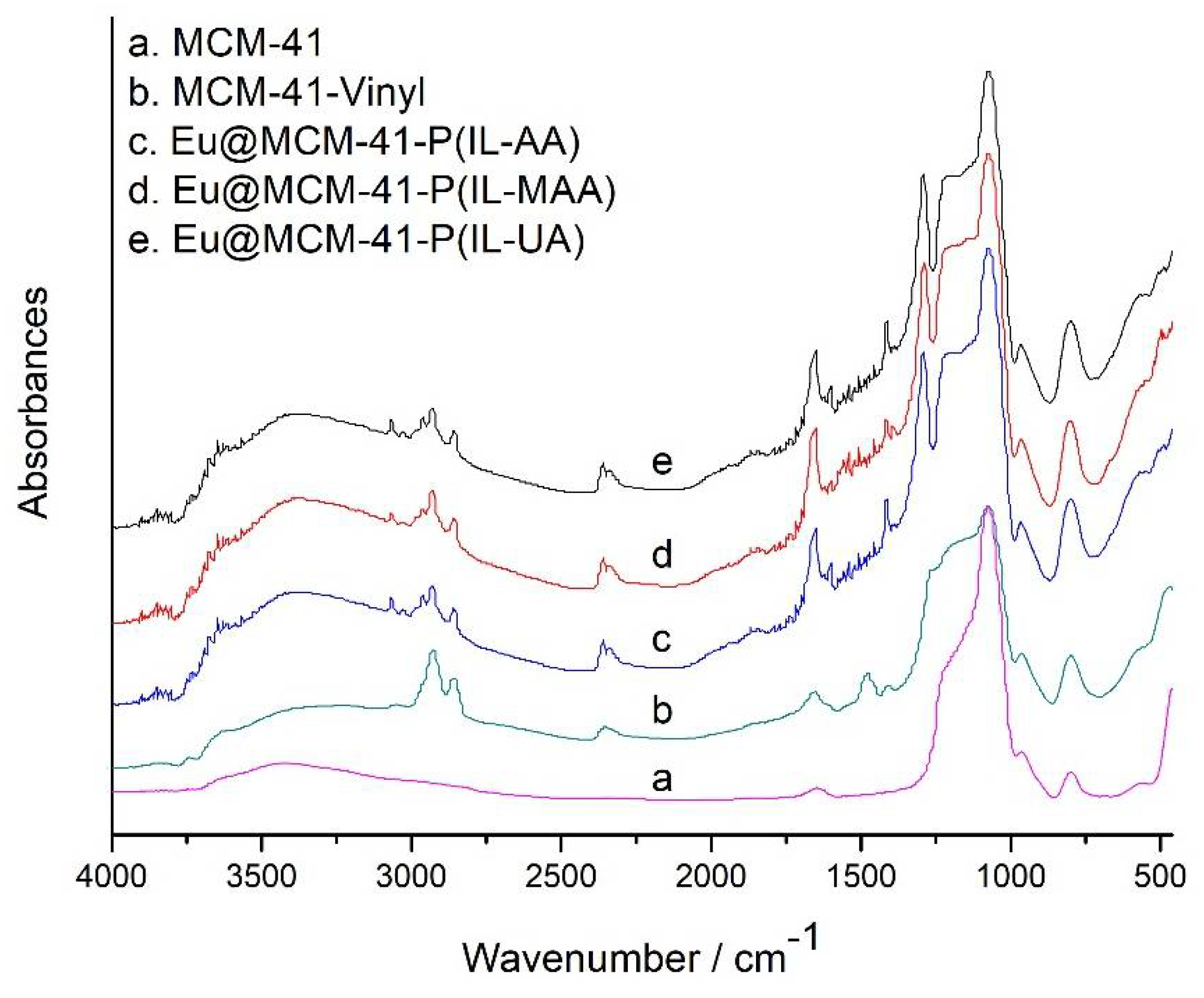
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Han, B.; Dai, Y.; Zhang, J.; Shi, H. Luminescence properties of a novel yellow-emitting phosphor NaLaMgWO6:Dy3+. Mater. Lett. 2017, 204, 145–148. [Google Scholar] [CrossRef]
- Liao, X.; Jiang, X.; Yang, Q.; Wang, L.; Chen, D. Spectral Properties of Er3+/Tm3+ Co-Doped ZBLAN Glasses and Fibers. Materials 2017, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Lönnrot, M.; Sjöroos, M.; Salminen, K.; Maaronen, M.; Hyypiä, T.; Hyöty, H. Diagnosis of enterovirus and rhinovirus infections by RT-PCR and time-resolved fluorometry with lanthanide chelate labeled probes. J. Med. Virol. 2016, 59, 378–384. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Coluccini, C.; Griend, D.A.V.; Forni, A.; Pasini, D. From red to blue shift: Switching the binding affinity from the acceptor to the donor end by increasing the π-bridge in push–pull chromophores with coordinative ends. New J. Chem. 2013, 37, 2792–2799. [Google Scholar] [CrossRef]
- Aguiar, F.P.; Costa, I.F.; Espínola, J.G.P.; Faustino, W.M.; Moura, J.L.; Brito, H.F.; Paolini, T.B.; Felinto, M.C.F.; Teotonio, E.E. Luminescent hybrid materials functionalized with lanthanide ethylenodiaminotetraacetate complexes containing β-diketonate as antenna ligands. J. Lumin. 2016, 170, 538–546. [Google Scholar] [CrossRef]
- Pacini, A.; Caricato, M.; Ferrari, S.; Capsoni, D.; Antxon, M.D.I.; Muñoz-Gerra, S.; Pasini, D. Poly(γ-glutamic acid) esters with reactive functional groups suitable for orthogonal conjugation strategies. J. Polym. Sci. Part A Polym. Chem. 2015, 50, 4790–4799. [Google Scholar] [CrossRef]
- Yan, B. Recent Progress in Photofunctional Lanthanide Hybrid Materials. RSC Adv. 2012, 2, 9304–9324. [Google Scholar] [CrossRef]
- Machado, K.; Mukhopadhyay, S.; Videira, R.A.; Mishra, J.; Mobin, S.M.; Mishra, G.S. Polymer encapsulated scorpionate Eu3+ complexes as novel hybrid materials for high performance luminescence applications. RSC Adv. 2015, 5, 35675–35682. [Google Scholar] [CrossRef]
- Li, W.X.; Zheng, Y.S.; Cao, X.F.; Bai, J.; Fu, Z.F.; Bao, J.R.; Li, Y.L. Preparation, characterization, and luminescence properties of dysprosium perchlorate with MABA-Si and phen or dipy complexes as well as SiO2@Dy(MABA-Si)core-shell structure nanometermeter luminescent composites. J. Lumin. 2016, 178, 470–478. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Zhang, W.; Liu, B. Luminescence properties of nanozeolite L grafted with terbium organic complex. Mater. Lett. 2008, 62, 3167–3170. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, H. Hybrid materials based on lanthanide organic complexes: A review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Gao, M. R.; Yuan, J.; Antonietti, M. Ionic liquids and poly(ionic liquid)s for morphosynthesis of inorganic materials. Chemistry 2016, 23, 5391–5403. [Google Scholar] [CrossRef] [PubMed]
- Lunstroot, K.; Driesen, K.; Nockemann, P. Luminescent Ionogels Based on Europium-Doped Ionic Liquids Confined within Silica-Derived Networks. Chem. Mater. 2006, 18, 5711–5715. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, T.; Li, Z.; Wang, Y. Transparent and luminescent ionogels composed of Eu3+ coordinated ionic liquids and poly(methyl methacrylate). Luminescence 2015, 30, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, J.; Chen, M.; Wang, Y. Lanthanide Luminescence Improvement by Using a Functional Poly(Ionic Liquid) as Matrix and Co-ligand. Chem. Asian J. 2016, 11, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Xue, Z.; Wang, Y.; Liu, Y.; Li, H. Luminescent Materials of Europium(III) Coordinated by a Terpyridine-Functionalized Poly(Ionic Liquid). Eur. J. Inorg. Chem. 2013, 2014, 469–474. [Google Scholar] [CrossRef]
- Wang, H.F.; Wang, Y.G.; Zhang, L.; Li, H.R. Transparent and luminescent ionogels based on lanthanide-containing ionic liquids and poly(methyl methacrylate) prepared through an environmentally friendly method. RSC Adv. 2013, 3, 8535–8540. [Google Scholar] [CrossRef]
- Yuan, J.Y.; Antonietti, M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef]
- Melby, L.R.; Rose, N.J.; Abramson, E.; Caris, J.C. Synthesis and Fluorescence of Some Trivalent Lanthanide Complexes. J. Am. Chem. Soc. 1964, 86, 5117–5125. [Google Scholar] [CrossRef]
- Hehlen, M.P.; Brik, M.G.; Krämer, K.W. 50th anniversary of the Judd–Ofelt theory: An experimentalist’s view of the formalism and its application. J. Lumin. 2013, 136, 221–239. [Google Scholar] [CrossRef]


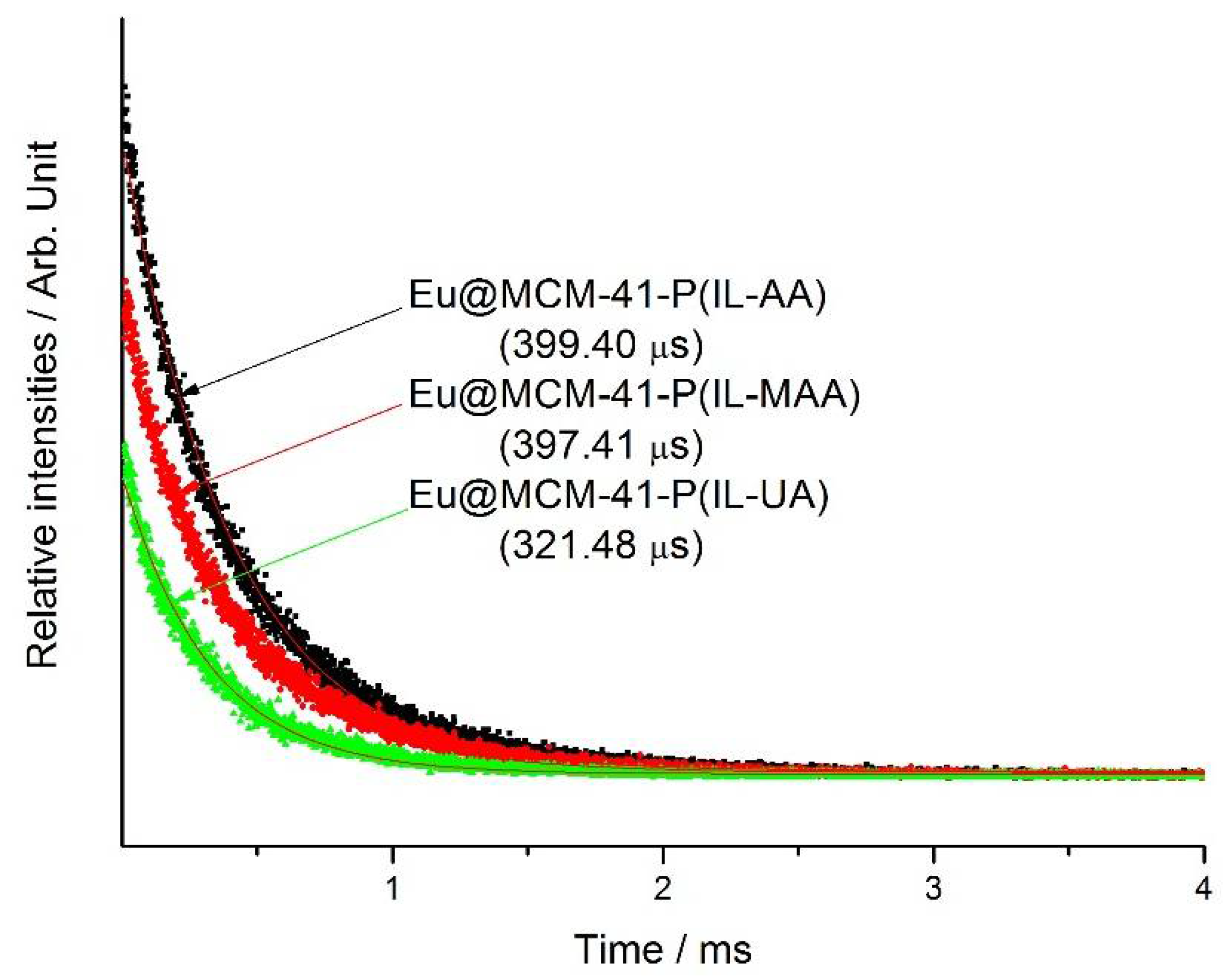
| τ (μs) a | Ar (s−1) | Anr (s−1) | η (%) b | |
|---|---|---|---|---|
| Eu@MCM-41-P (IL-AA) | 399.4 | 928.0 | 1573.3 | 37.1 |
| Eu@MCM-41-P (IL-MAA) | 397.4 | 907.1 | 1605.6 | 36.1 |
| Eu@MCM-41-P (IL-UA) | 321.5 | 1031.7 | 2075.8 | 33.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Wang, M.; Li, Q. Synthesis and Luminescent Properties of Europium Complexes Covalently Bonded to Hybrid Materials Based on MCM-41 and Poly(Ionic Liquids). Materials 2018, 11, 677. https://doi.org/10.3390/ma11050677
Zheng X, Wang M, Li Q. Synthesis and Luminescent Properties of Europium Complexes Covalently Bonded to Hybrid Materials Based on MCM-41 and Poly(Ionic Liquids). Materials. 2018; 11(5):677. https://doi.org/10.3390/ma11050677
Chicago/Turabian StyleZheng, Xiaolong, Meiyu Wang, and Qiuping Li. 2018. "Synthesis and Luminescent Properties of Europium Complexes Covalently Bonded to Hybrid Materials Based on MCM-41 and Poly(Ionic Liquids)" Materials 11, no. 5: 677. https://doi.org/10.3390/ma11050677





