Facile Synthesis of Nitrogen and Oxygen Co-Doped Clews of Carbon Nanobelts for Supercapacitors with Excellent Rate Performance
Abstract
:1. Introduction
2. Experiment
2.1. Synthesis of LPHF
2.2. NH3·H2O Treatment
2.3. Physicochemical Characterization
2.4. Characterization of Electrochemical Performance
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gogotsi, Y.; Simon, P. True performance metrics in electrochemical energy storage. Science 2011, 334, 917–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Li, L.; Li, Z.; Liao, H.; Zhang, H. Ultrathin carbon gauze for high-rate supercapacitor. Electrochim. Acta 2016, 222, 990–998. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.; Xu, H.; Xiong, X.; Jiang, Y.; Zou, F.; Hu, X.; Xin, Y.; Zhang, Z.; Huang, Y. Synthesis of functionalized 3D hierarchical porous carbon for high-performance supercapacitors. Energy Environ. Sci. 2013, 6, 2497. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, R.; Sundaram, M.M. Electrochemical synthesis of polyaniline cross-linked NiMoO4 nanofibre dendrites for energy storage devices. New J. Chem. 2016, 40, 7456–7464. [Google Scholar] [CrossRef]
- Bao, L.; Zang, J.; Li, X. Flexible Zn2SnO4/MnO2 core/shell nanocable-carbon microfiber hybrid composites for high-performance supercapacitor electrodes. Nano Lett. 2011, 11, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, G.; Zhu, J.; Zeng, Z.; Zhu, X. NiO/LaNiO3 film electrode with binder-free for high performance supercapacitor. Appl. Surf. Sci. 2016, 384, 92–98. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Kodama, M.; Shiraishi, S.; Hatori, H.; Zhu, Z.H.; Lu, G.Q. Nitrogen-enriched nonporous carbon electrodes with extraordinary supercapacitance. Adv. Funct. Mater. 2009, 19, 1800–1809. [Google Scholar] [CrossRef]
- Inal, I.I.G.; Holmes, S.M.; Banford, A.; Aktas, Z. The performance of supercapacitor electrodes developed from chemically activated carbon produced from waste tea. Appl. Surf. Sci. 2015, 357, 696–703. [Google Scholar] [CrossRef]
- Faraji, S.; Ani, F.N. The development supercapacitor from activated carbon by electroless plating—A review. Renew. Sustain. Energy Rev. 2015, 42, 823–834. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, H.H.; Jin, H.J. Pseudocapacitive effects of N-doped carbon nanotube electrodes in supercapacitors. Materials 2012, 5, 1258–1266. [Google Scholar] [CrossRef]
- Talapatra, S.; Kar, S.; Pal, S.K.; Vajtai, R.; Ci, L.; Victor, P.; Shaijumon, M.M.; Kaur, S.; Nalamasu, O.; Ajayan, P.M. Direct growth of aligned carbon nanotubes on bulk metals. Nat. Nanotechnol. 2006, 1, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Frackowiak, E.; Béguin, F. Electrochemical storage of energy in carbon nanotubes and nanostructured carbons. Carbon N. Y. 2002, 40, 1775–1787. [Google Scholar] [CrossRef]
- Jiang, L.; Sheng, L.; Long, C.; Wei, T.; Fan, Z. Functional Pillared Graphene Frameworks for Ultrahigh Volumetric Performance Supercapacitors. Adv. Energy Mater. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Lee, J.H.; Avsar, A.; Jung, J.; Tan, J.Y.; Watanabe, K.; Taniguchi, T.; Natarajan, S.; Eda, G.; Adam, S.; Castro Neto, A.H.; Özyilmaz, B. Van der Waals force: A dominant factor for reactivity of graphene. Nano Lett. 2015, 15, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Sun, J.; Huang, Y.; Zhu, M.; Pei, Z.; Li, H.; Wang, Y.; Li, N.; Zhang, H.; Zhi, C. Recent Progress on Flexible and Wearable Supercapacitors. Small 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhi, J.; Reiser, O.; Wang, Y.; Hu, A. From natural cotton thread to sewable energy dense supercapacitors. Nanoscale 2017, 9, 6406–6416. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, A.B.; Pico, F.; Rojo, J.M. Influence of pore structure on electric double-layer capacitance of template mesoporous carbons. J. Power Sources 2004, 133, 329–336. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Wang, S.; Ren, Z.; Li, J.; Ren, Y.; Zhao, L.; Yu, J. Cotton-based hollow carbon fibers with high specific surface area prepared by ammonia etching for supercapacitor application. RSC Adv. 2014, 4, 31300–31307. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Ke, Q.; Ho, K.H.; Hu, Y.; Wang, J. Tuning the porous texture and specific surface area of nanoporous carbons for supercapacitor electrodes by adjusting the hydrothermal synthesis temperature. J. Mater. Chem. A 2013, 1, 12962–12970. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors. Science 2011, 332, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhong, Q.; Kim, N.D.; Ruan, G.; Yang, Y.; Gao, C.; Fei, H.; Li, Y.; Ji, Y.; Tour, J.M. Nitrogen-doped carbonized cotton for highly flexible supercapacitors. Carbon N. Y. 2016, 105, 260–267. [Google Scholar] [CrossRef]
- Kiciński, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Carbon N. Y. 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, F.; Zhang, T.; Leng, K.; Zhang, L.; Yang, X.; Ma, Y.; Huang, Y.; Zhang, M.; Chen, Y. Synthesis and supercapacitor performance studies of N-doped graphene materials using o-phenylenediamine as the double-N precursor. Carbon N. Y. 2013, 63, 508–516. [Google Scholar] [CrossRef]
- Wang, D.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H. 3D Aperiodic Hierarchical Porous Graphitic Carbon Material for High-Rate Electrochemical Capacitive Energy Storage. Angew. Chem. 2008, 47, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, L.; Cheng, J.; Hu, H. Hierarchical porous carbons prepared from direct coal liquefaction residue and coal for supercapacitor electrodes. Carbon N. Y. 2013, 55, 221–232. [Google Scholar] [CrossRef]
- Zeng, S.-Z.; Jin, N.-Z.; Zhang, H.-L.; Hai, B.; Chen, X.-H.; Shi, J. High-modulus all-carbon ladder polymer of hydroquinone and formaldehyde that bridges the gap between single-strand polymers and graphene nanoribbons. RSC Adv. 2014, 4, 18676–18682. [Google Scholar] [CrossRef]
- He, L.; Zhang, Y.; Xin, J.H. Highly conjugated graphitic 3D carbon frameworks for supercapacitors with long cycling stability. Carbon N. Y. 2016, 109, 650–657. [Google Scholar] [CrossRef]
- He, X.; Ma, H.; Wang, J.; Xie, Y.; Xiao, N.; Qiu, J. Porous carbon nanosheets from coal tar for high-performance supercapacitors. J. Power Sources 2017, 357, 41–46. [Google Scholar] [CrossRef]
- Wu, X.L.; Chen, L.L.; Xin, S.; Yin, Y.X.; Guo, Y.G.; Kong, Q.S.; Xia, Y.Z. Preparation and Li storage properties of hierarchical porous carbon fibers derived from alginic acid. ChemSusChem 2010, 3, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xu, Y.; Zhang, Y.; Feng, T.; Wang, J.; Mao, S.; Xiong, L. Porous and high electronic conductivity nitrogen-doped nano-sheet carbon derived from polypyrrole for high-power supercapacitors. Carbon N. Y. 2016, 107, 638–645. [Google Scholar] [CrossRef]
- Minakshi, M.; Barmi, M.J.; Jones, R.T. Rescaling metal molybdate nanostructures with biopolymer for energy storage having high capacitance with robust cycle stability. Dalt Trans. 2017, 46, 3588–3600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, N.; Zhao, P.; Yao, M.; Hu, W. Azide-assisted hydrothermal synthesis of N-doped mesoporous carbon cloth for high-performance symmetric supercapacitor employing LiClO4 as electrolyte. Compos. Part A Appl. Sci. Manuf. 2017, 98, 58–65. [Google Scholar] [CrossRef]
- Zeng, S.Z.; Yao, Y.; Zeng, X.; He, Q.; Zheng, X.; Chen, S.; Tu, W.; Zou, J. A composite of hollow carbon nanospheres and sulfur-rich polymers for lithium-sulfur batteries. J. Power Sources 2017, 357, 11–18. [Google Scholar] [CrossRef]
- Kim, W.J.; Ko, T.H.; Seo, M.K.; Chung, Y.S.; Kim, H.Y.; Kim, B.S. Engineered carbon fiber papers as flexible binder-free electrodes for high-performance capacitive energy storage. J. Ind. Eng. Chem. 2018, 59, 277–285. [Google Scholar] [CrossRef]
- Li, K.B.; Shi, D.W.; Cai, Z.Y.; Zhang, G.L.; Huang, Q.A.; Liu, D.; Yang, C.P. Studies on the equivalent serial resistance of carbon supercapacitor. Electrochim. Acta 2015, 174, 596–600. [Google Scholar] [CrossRef]
- Zeng, S.; Zeng, X.; Tu, W.; Yao, Y.; Yu, L.; Wu, H.; Jin, W.; Huang, H.; Zou, J. Facile and tailored synthesis of ultrahigh-surface- area clews of carbon nanobelts for high-rate. J. Mater. Chem. A Mater. Energy Sustain. 2017, 5, 23209–23220. [Google Scholar] [CrossRef]
- Xing, W.; Huang, C.C.; Zhuo, S.P.; Yuan, X.; Wang, G.Q.; Hulicova-Jurcakova, D.; Yan, Z.F.; Lu, G.Q. Hierarchical porous carbons with high performance for supercapacitor electrodes. Carbon N. Y. 2009, 47, 1715–1722. [Google Scholar] [CrossRef]
- Hasegawa, G.; Kanamori, K.; Kiyomura, T.; Kurata, H.; Abe, T.; Nakanishi, K. Hierarchically Porous Carbon Monoliths Comprising Ordered Mesoporous Nanorod Assemblies for High-Voltage Aqueous Supercapacitors. Chem. Mater. 2016, 28, 3944–3950. [Google Scholar] [CrossRef]
- John, A.R.; Arumugam, P. Open ended nitrogen-doped carbon nanotubes for the electrochemical storage of energy in a supercapacitor electrode. J. Power Sources 2015, 277, 387–392. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, C.; Chen, G.; Liu, Z.; Ma, M.; Xie, Q.; Zheng, N.; Yao, S. Synthesis of ultrathin nitrogen-doped graphitic carbon nanocages as advanced electrode materials for supercapacitor. ACS Appl. Mater. Interfaces 2013, 5, 2241–2248. [Google Scholar] [CrossRef] [PubMed]


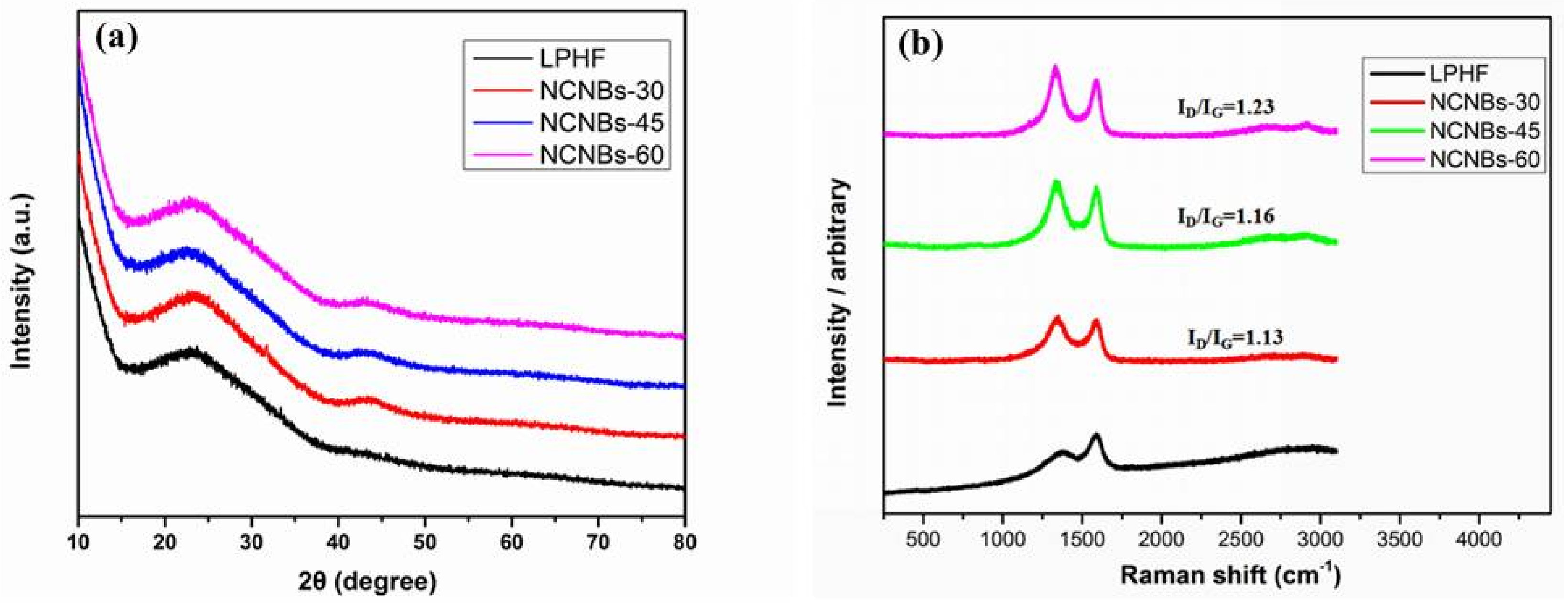
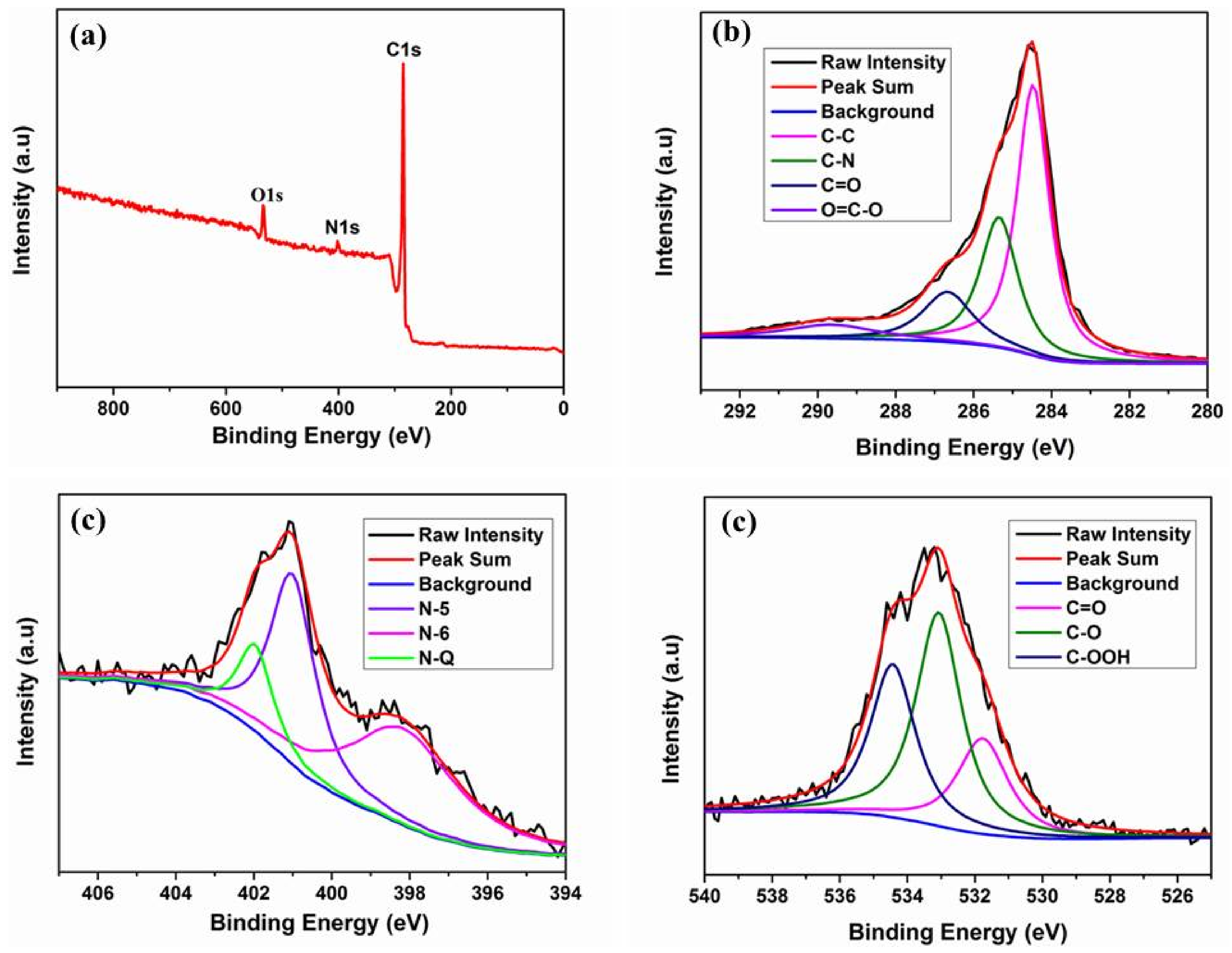

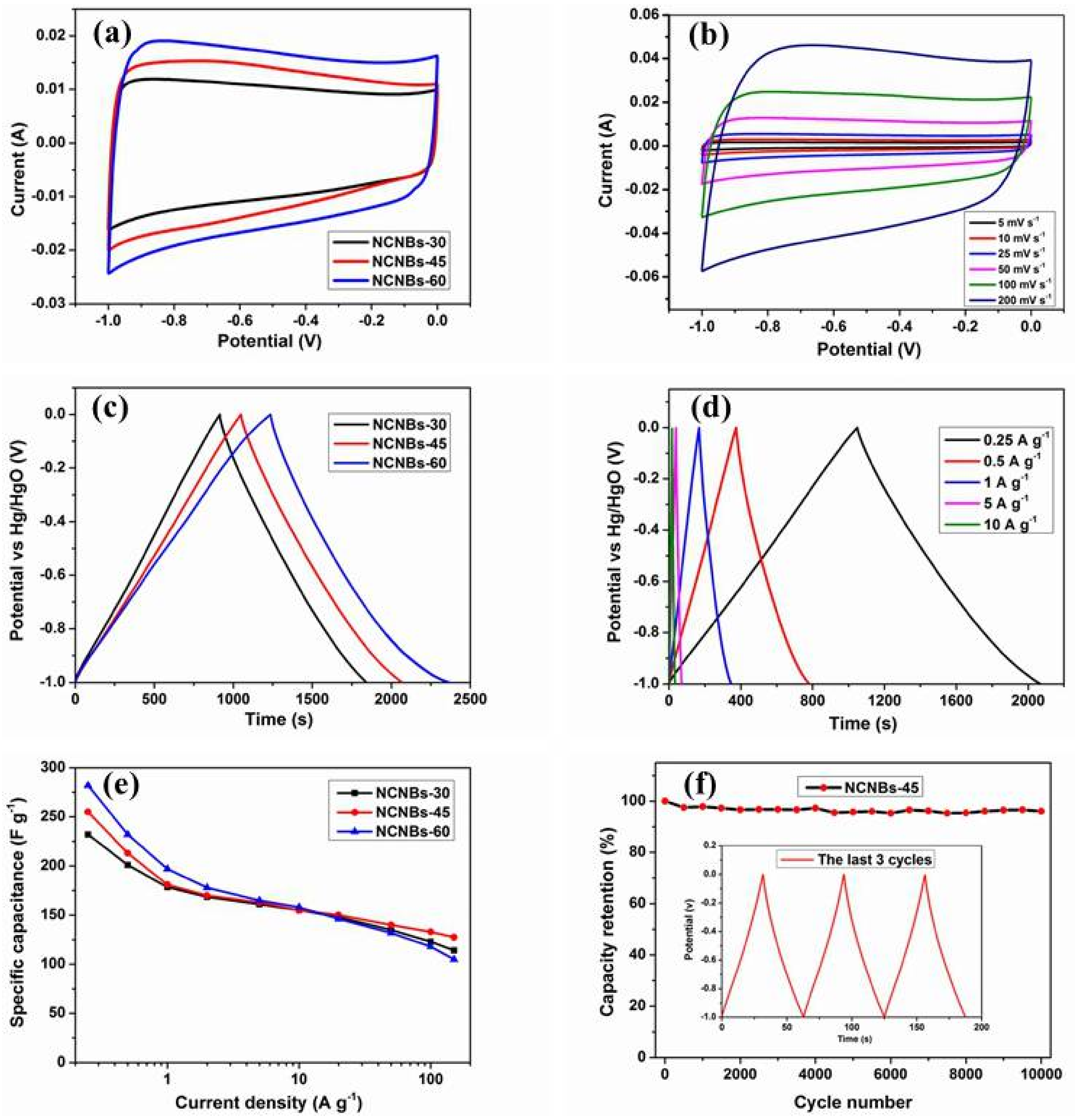
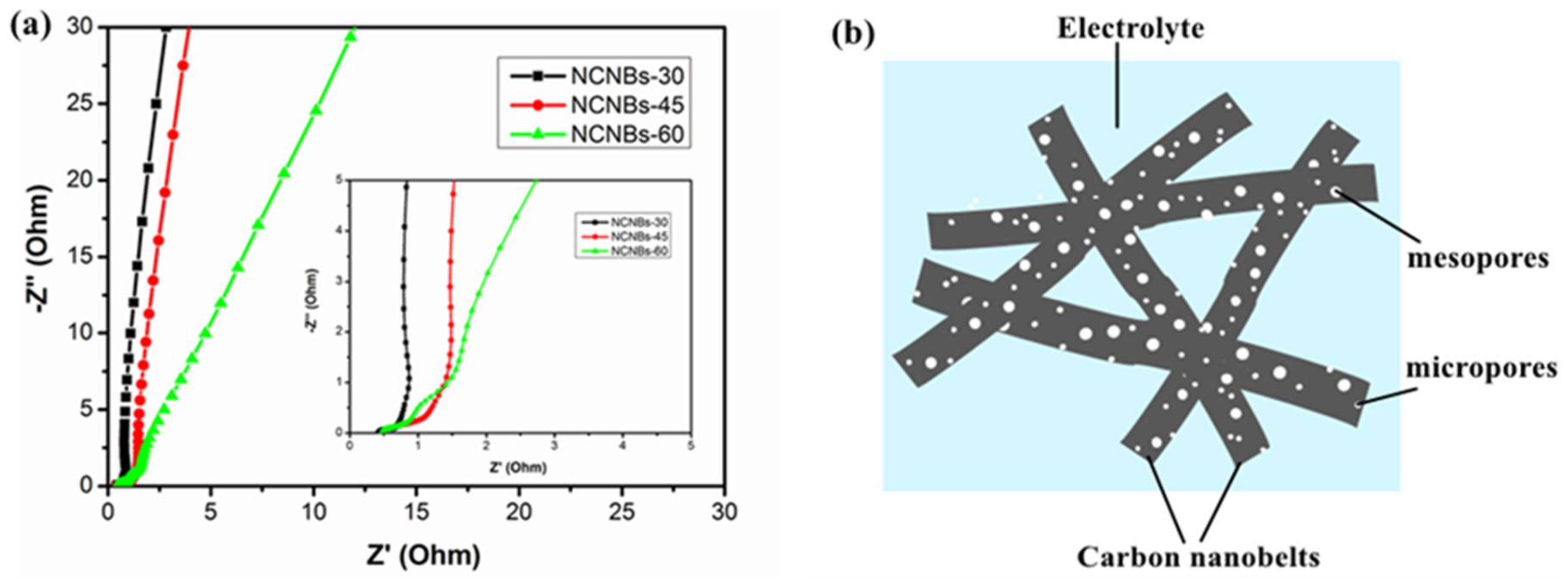
| Sample | SBET (m2 g−1) | SDFT (m2 g−1) | Pore Volume Fraction (cm3 g−1) | Element Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| Vtotal | Vmic | Vmes | C (wt %) | N (wt %) | O (wt %) | |||
| LPHF | 137 | 82 | 0.19 | 0.03 | 0.06 | 66.49 | -- | 24.36 |
| NCNBs-30 | 1804 | 1421 | 0.83 | 0.52 | 0.24 | 93.34 | 3.02 | 7.93 |
| NCNBs-45 | 2330 | 1620 | 1.10 | 0.53 | 0.40 | 92.50 | 2.65 | 6.64 |
| NCNBs-60 | 2994 | 1875 | 1.47 | 0.54 | 0.83 | 92.61 | 2.36 | 5.57 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Zeng, S.; Zeng, X.; Li, X.; Wu, H.; Yao, Y.; Tu, W.; Zou, J. Facile Synthesis of Nitrogen and Oxygen Co-Doped Clews of Carbon Nanobelts for Supercapacitors with Excellent Rate Performance. Materials 2018, 11, 556. https://doi.org/10.3390/ma11040556
Yu L, Zeng S, Zeng X, Li X, Wu H, Yao Y, Tu W, Zou J. Facile Synthesis of Nitrogen and Oxygen Co-Doped Clews of Carbon Nanobelts for Supercapacitors with Excellent Rate Performance. Materials. 2018; 11(4):556. https://doi.org/10.3390/ma11040556
Chicago/Turabian StyleYu, Liang, Shaozhong Zeng, Xierong Zeng, Xiaohua Li, Hongliang Wu, Yuechao Yao, Wenxuan Tu, and Jizhao Zou. 2018. "Facile Synthesis of Nitrogen and Oxygen Co-Doped Clews of Carbon Nanobelts for Supercapacitors with Excellent Rate Performance" Materials 11, no. 4: 556. https://doi.org/10.3390/ma11040556




