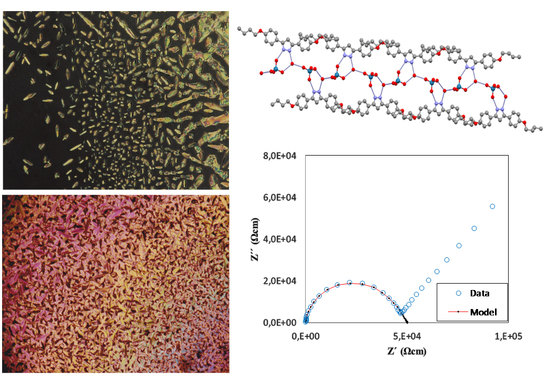
New Pyrazolium Salts as a Support for Ionic Liquid Crystals and Ionic Conductors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Physical Measurements
2.2. Preparation of 3-(4-Alkyloxyphenyl)-5-(4-alkyloxyphenyl)pyrazole
2.3. Preparation of 3-(4-Alkyloxyphenyl)-5-(4-alkyloxyphenyl)pyrazolium Salts of the Type Cl-n,m (n = m = 4, 8, 12; n = 4, 8 and m =12) and BF4-n,m (n = m = 4, 8, 12; n = 4, 8 and m =12)
2.4. Preparation of 3-(4-Alkyloxyphenyl)-5-(4-alkyloxyphenyl)pyrazolium Salts of the Type ReO4-n,m (n = m = 4, 8; n = 4, 8 and m =12), PTS-n,m (n = m = 4, 8, 12; n = 4, 8 and m =12) and OTf-n,m (n = m = 4, 8; n = 4, 8 and m =12)
3. Results and Discussion
3.1. Synthesis and Characterization
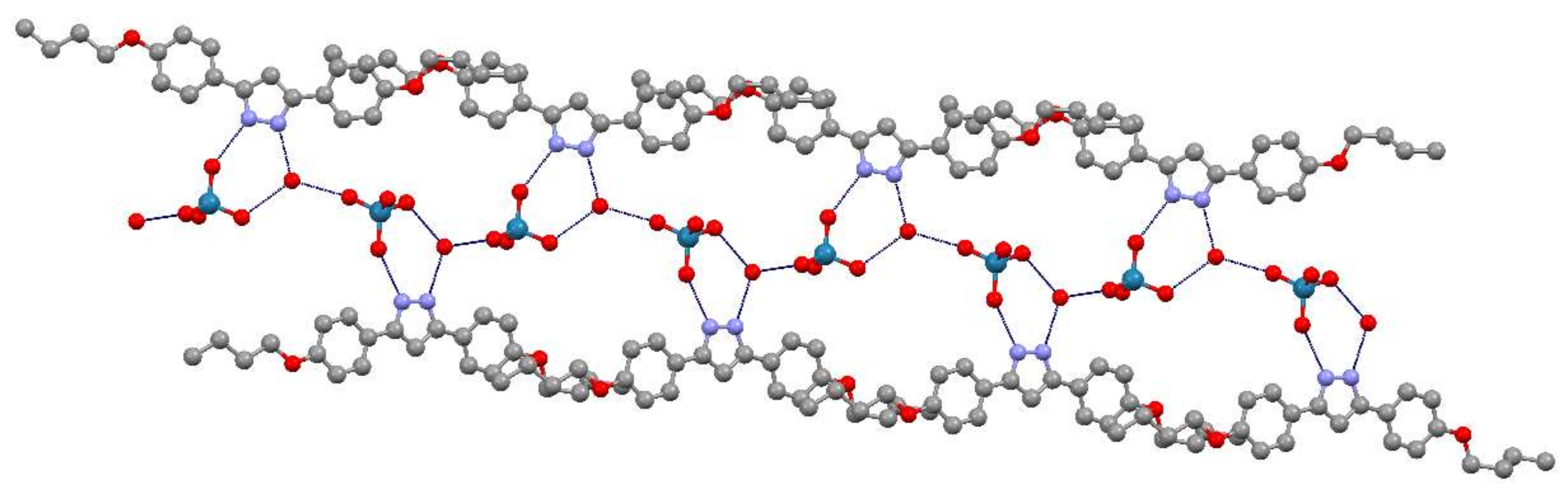
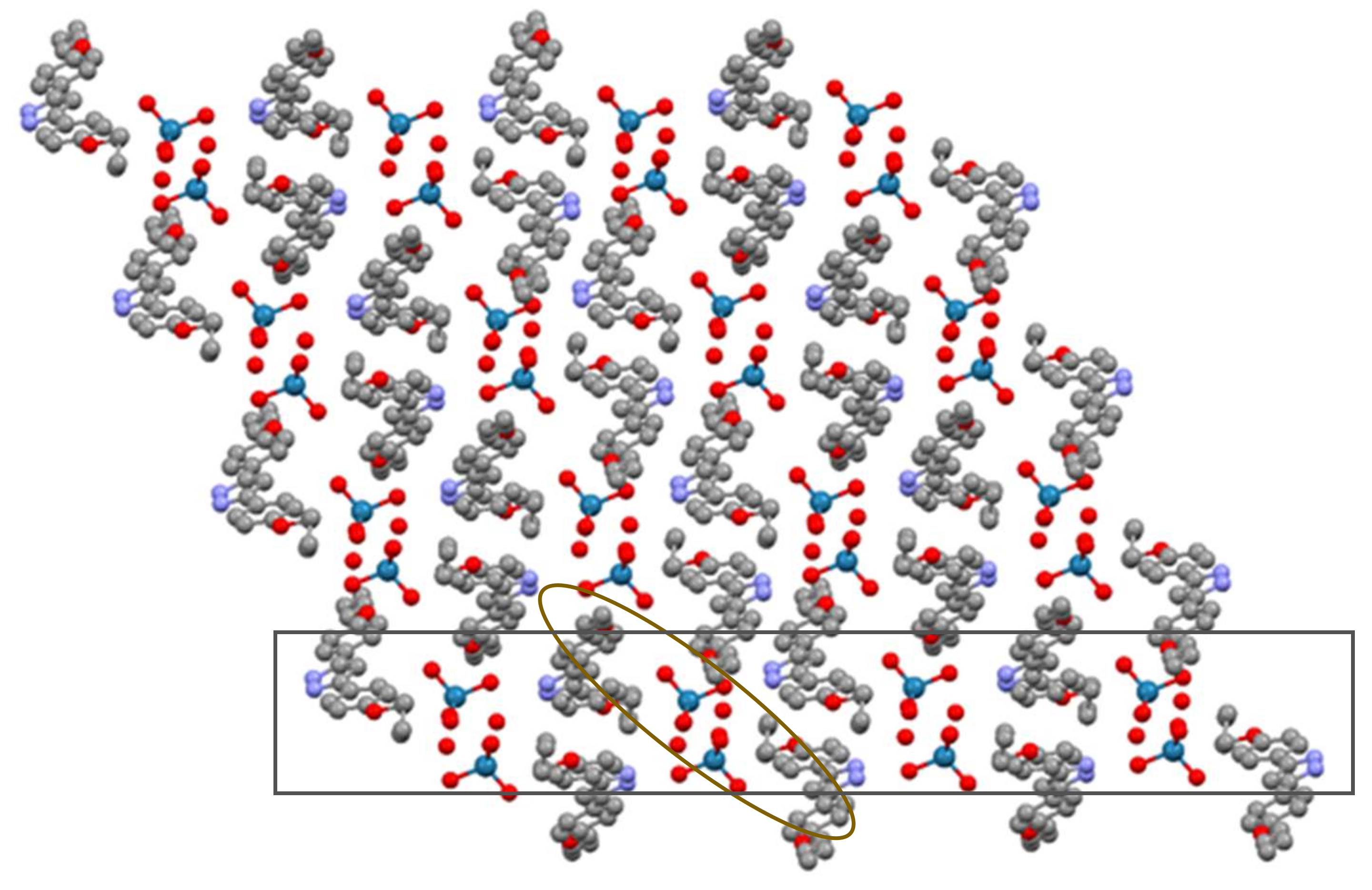
3.2. X-Ray Crystal Structure of [H2pzR(4),R(4)][ReO4]·H2O, (ReO4-4,4·H2O), (11·H2O)
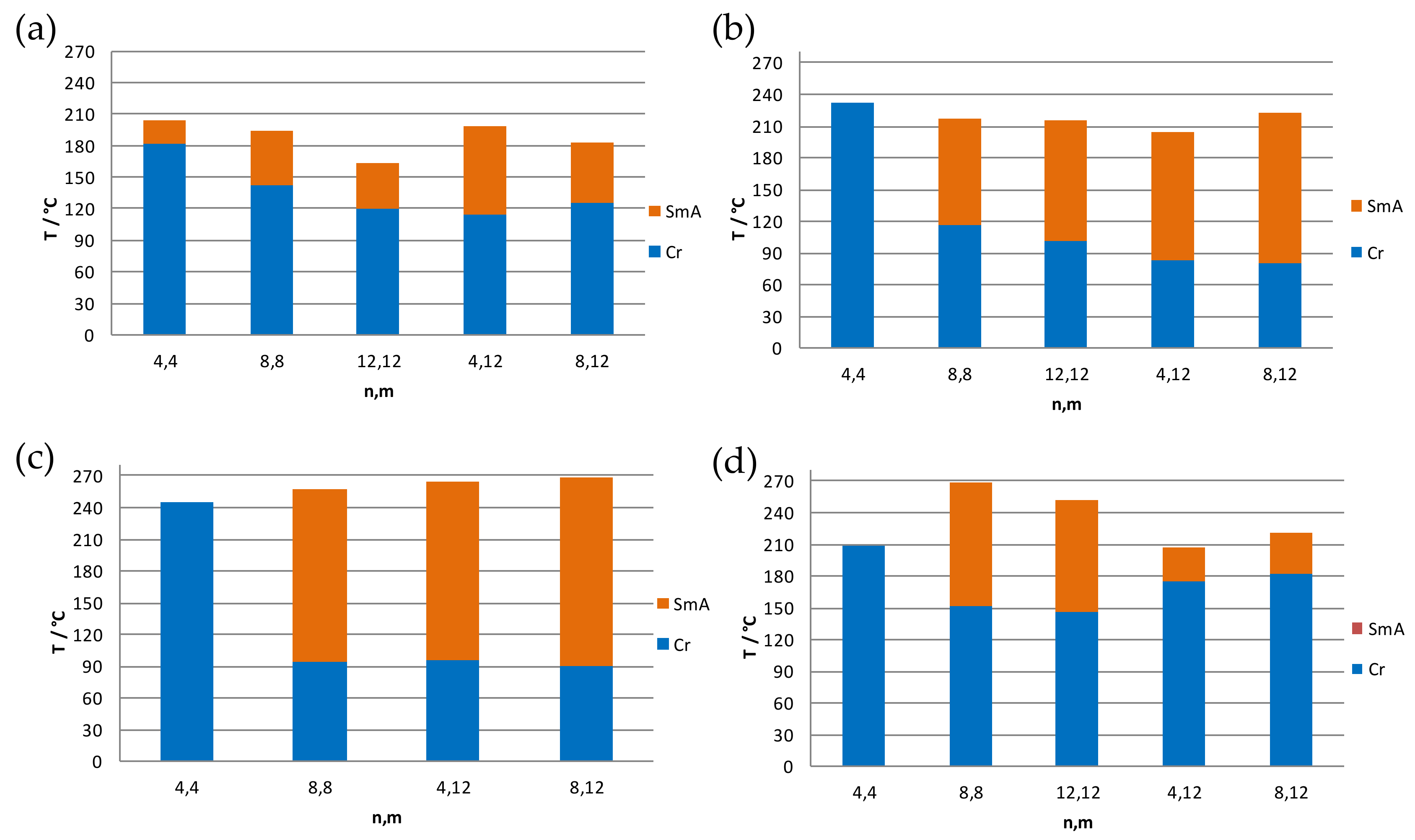
3.3. Thermal Behavior
3.4. Variable Temperature Low Angle Powder X-Ray Diffraction Studies
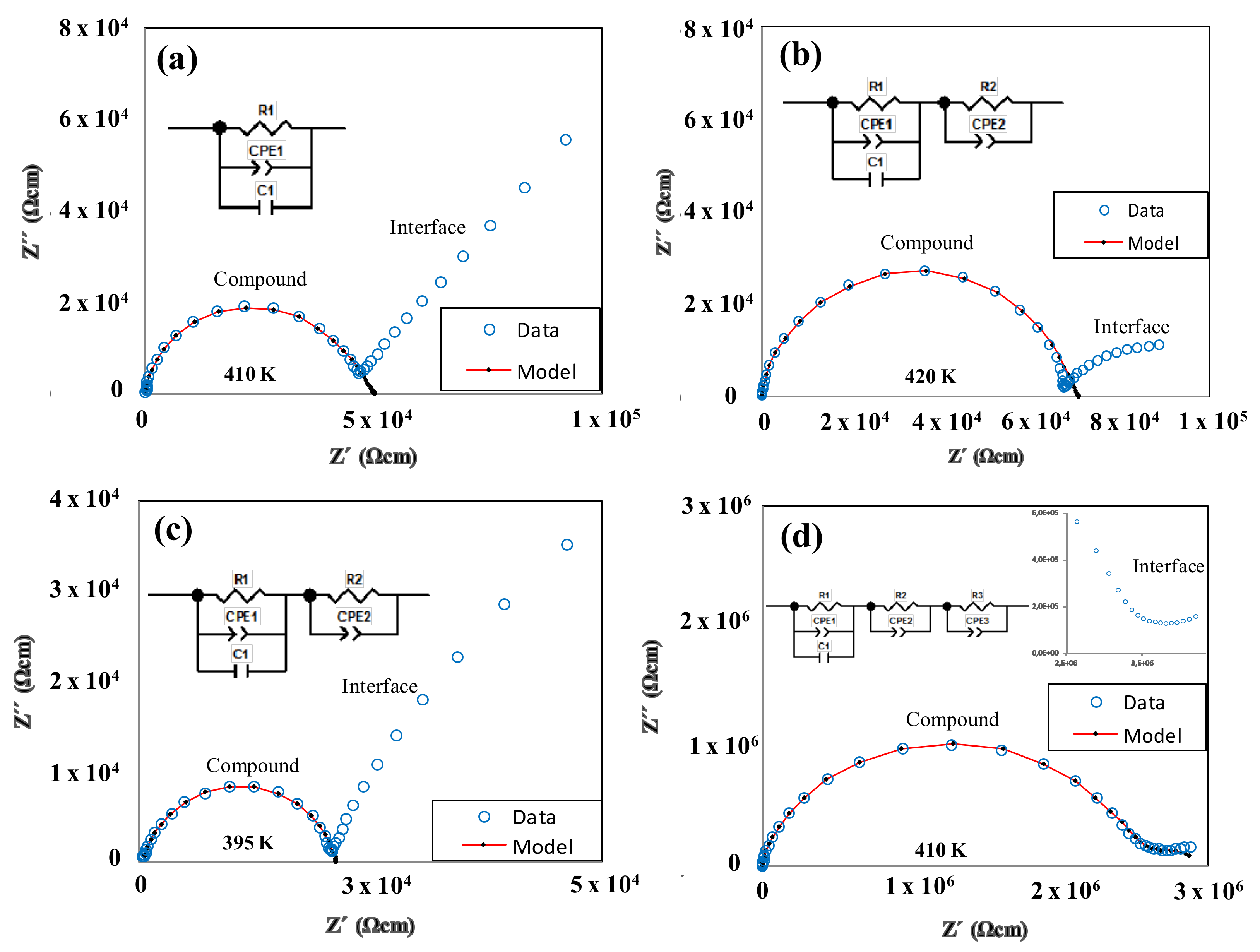
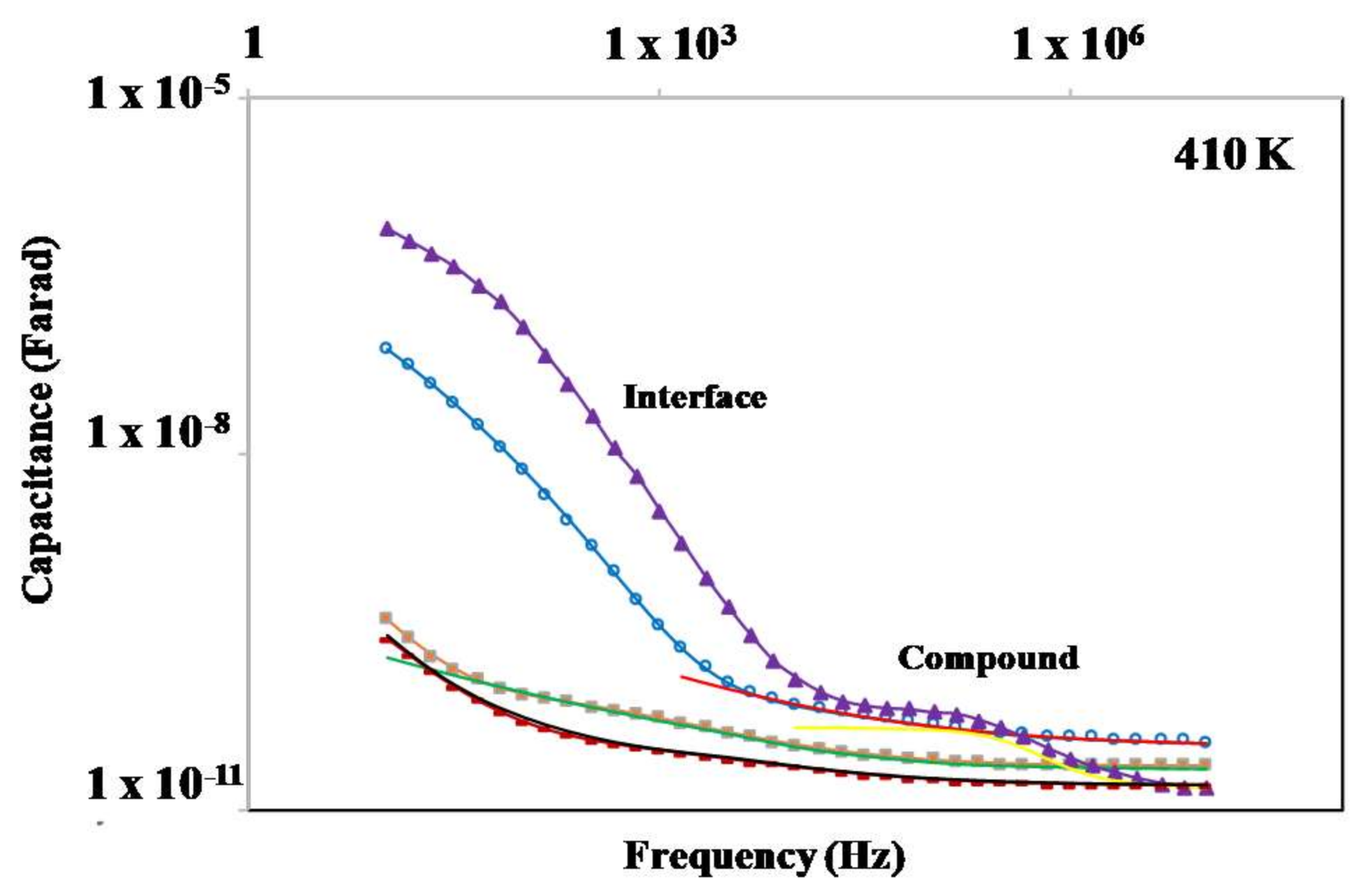
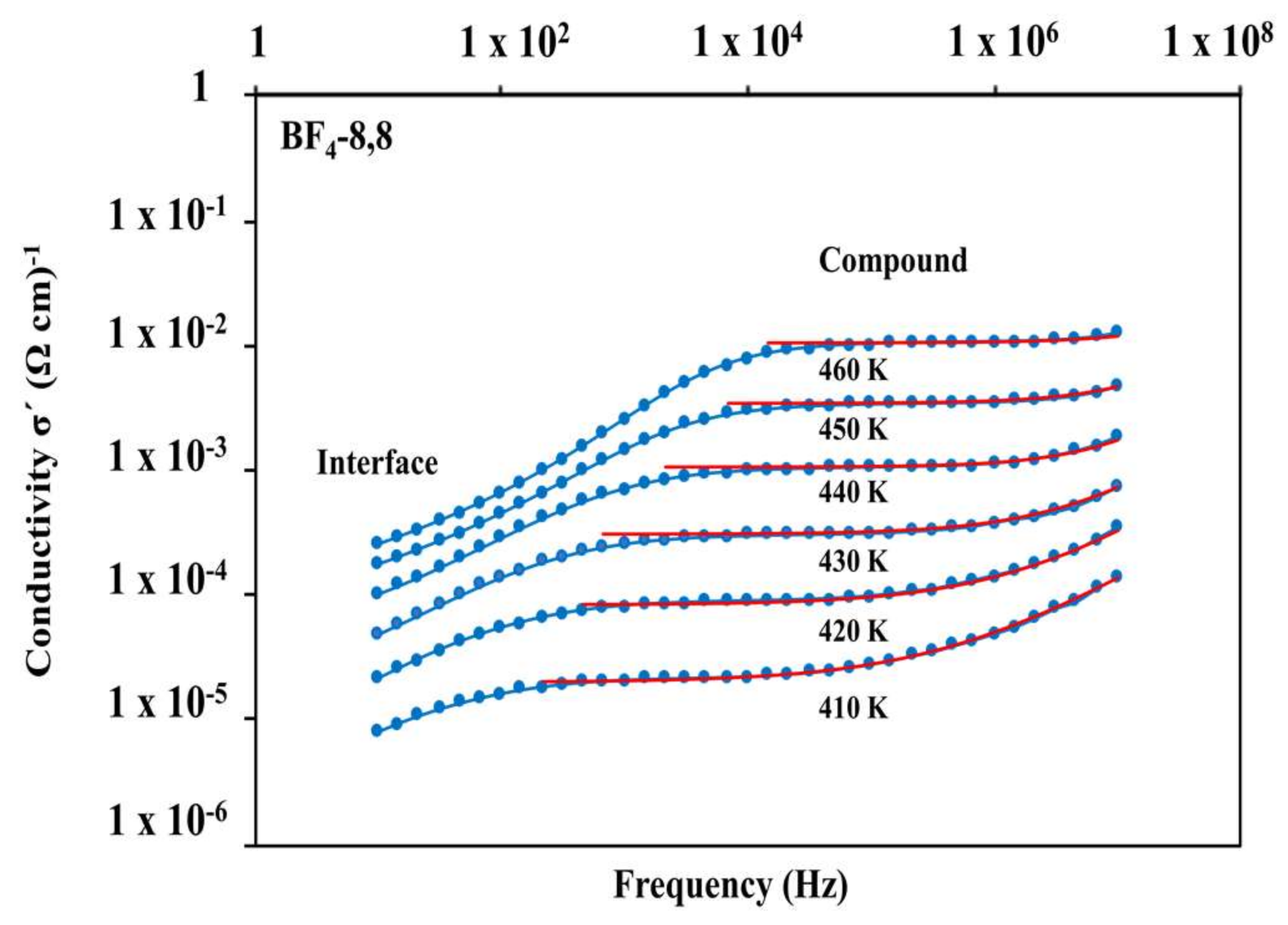
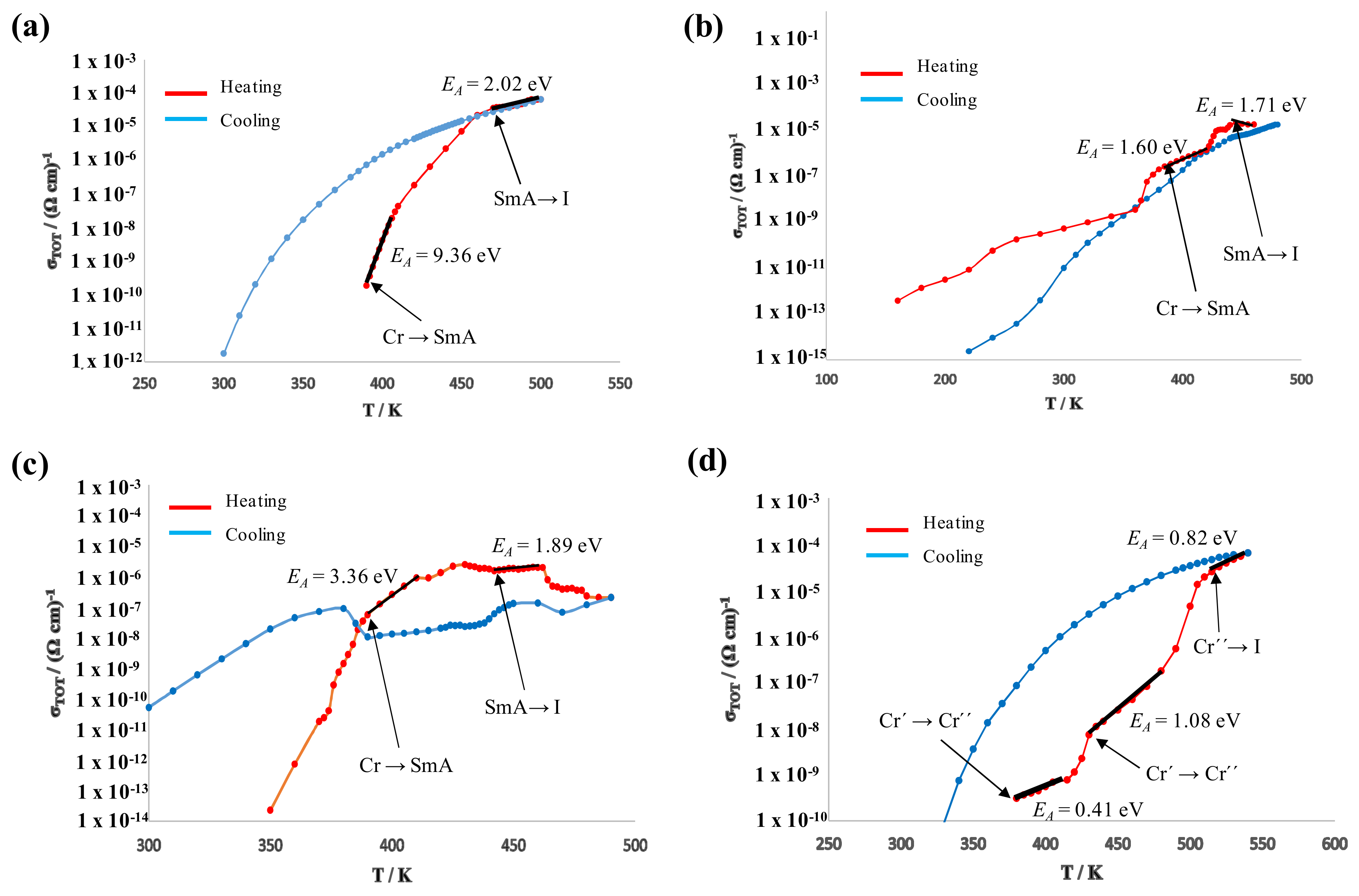
3.5. Dielectric Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IL | Ionic Liquid |
| ILC | Ionic Liquid Crystal |
| IR | Infrared |
| LC | Liquid Crystal |
| LCD | Liquid Crystal Display |
| I | Isotropic Liquid |
| Cr, Cr′, Cr″ | Solid Phases |
| Sm | Smectic Mesophase |
| POM | Polarized light Optical Microscopy |
| DSC | Differential Scanning Calorimetry |
| XRD | X-ray Diffraction |
| CPE | Constant Phase Element |
| RC | Resistor-Capacitor |
| NMR | Nuclear Magnetic Resonance |
References
- Binnemans, K. Ionic Liquid Crystals. Chem. Rev. 2005, 105, 4148–4204. [Google Scholar] [CrossRef] [PubMed]
- Goosens, K.; Lava, K.; Bielawski, C.W.; Binnemans, K. Ionic Liquid Crystals: Versatile Materials. Chem. Rev. 2016, 116, 4643–4807. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Tousley, M.E.; Cowan, M.G.; Wiesenauer, B.R.; Nejati, S.; Choo, Y.; Noble, R.D.; Elimelech, M.; Gin, D.L.; Osuji, C.O. Scalable Fabrication of Polymer Membranes with Vertically Aligned 1 nm Pores by Magnetic Field Directed Self-Assembly. ACS Nano 2014, 8, 11977–11986. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.R.; Yang, M.; Choi, S.-I.; Chi, M.; Luo, M.; Zhang, C.; Li, Z.-Y.; Camargo, P.H.C.; Ribeiro, S.J.L.; Xia, Y. Facile Synthesis of Sub-20 nm Silver Nanowires through a Bromide-Mediated Polyol Method. ACS Nano 2016, 10, 7892–7900. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Kawabata, K.; Whang, D.M.; Osuji, C.O. Polymer Nanosheets from Supramolecular Assemblies of Conjugated Linoleic Acid−High Surface Area Adsorbents from Renewable Materials. Langmuir 2017, 33, 10690–10697. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Ionic liquids in catalysis. Coord. Chem. Rev. 2004, 248, 2459–2477. [Google Scholar] [CrossRef]
- Yoshio, M.; Mukai, T.; Ohno, H.; Kato, T. One-Dimensional Ion Transport in Self-Organized Columnar Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 994–995. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional Liquid-Crystalline Assemblies: Self-Organized Soft Materials. Angew. Chem. Int. Ed. 2006, 45, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Masafumi, Y.; Takahiro, I.; Harutoki, S.; Takayoshi, K.; Atsushi, H.; Tomohiro, M.; Hiroyuki, O.; Takashi, K. Columnar Liquid-Crystalline Imidazolium Salts. Effects of Anions and Cations on Mesomorphic Properties and Ionic Conductivities. Bull. Chem. Soc. Jpn. 2007, 80, 1836–1841. [Google Scholar]
- Dobbs, W.; Suisse, J.-M.; Douce, L.; Welter, R. Electrodeposition of Silver Particles and Gold Nanoparticles from Ionic Liquid-Crystal Precursors. Angew. Chem. Int. Ed. 2006, 45, 4179–4182. [Google Scholar] [CrossRef] [PubMed]
- Taubert, A.; Li, Z. Inorganic materials from ionic liquids. Dalton Trans. 2007, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Taubert, A.; Palivan, C.; Casse, O.; Gozzo, F.; Schmitt, B. Ionic Liquid-Crystal Precursors (ILCPs) for CuCl Platelets: The Origin of the Exothermic Peak in the DSC Curves. J. Phys. Chem. C 2007, 111, 4077–4082. [Google Scholar] [CrossRef]
- Wang, Q.-T.; Wang, X.-B.; Lou, W.-J.; Hao, J.-C. Stable Blue- and Green-Emitting Zinc Oxide from Ionic Liquid Crystal Precursors. ChemPhysChem 2009, 10, 3201–3203. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, W.; Heinrich, B.; Bourgogne, C.; Donnio, B.; Terazzi, E.; Bonnet, M.-E.; Stock, F.; Erbacher, P.; Bolcato-Bellemin, A.-L.; Douce, L. Mesomorphic Imidazolium Salts: New Vectors for Efficient siRNA Transfection. J. Am. Chem. Soc. 2009, 131, 13338–13346. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, W.; Douce, L.; Heinrich, B. 1-(4-Alkyloxybenzyl)-3-methyl-1H-imidazol-3-ium organic backbone: A versatile smectogenic moiety. Beilstein J. Org. Chem. 2009, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Matsumoto, T.; Akita, T.; Nishiyama, N. Ion Conductive Properties in Ionic Liquid Crystal Confined in Porous Membrane. J. Mater. Chem. C 2015, 3, 6144–6147. [Google Scholar] [CrossRef]
- Hernandez Rueda, J.J.; Zhang, H.; Rosenthal, M.; Möller, M.; Zhu, X.; Ivanov, D.A. Polymerizable Wedge-Shaped Ionic Liquid Crystals for Fabrication of Ion-Conducting Membranes: Impact of the Counterion on the Phase Structure and Conductivity. Eur. Polym. J. 2016, 81, 674–685. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, Q.; Li, D.; Deng, Y. Silica-Gel-Confined Ionic Liquids: A New Attempt for the Development of Supported Nanoliquid Catalysis. Chem. Eur. J. 2005, 12, 5279–5288. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ishiguro, F.; Jeon, J.-H.; Hiraoka, T.; Chujo, Y. POSS Ionic Liquid Crystals. NPG Asia Mater. 2015, 7, e174. [Google Scholar] [CrossRef]
- Goossens, K.; Lava, K.; Nockemann, P.; Van Hecke, K.; Van Meervelt, L.; Driesen, K.; Görller-Walrand, C.; Binnemans, K.; Cardinaels, T. Pyrrolidinium Ionic Liquid Crystals. Chem. Eur. J. 2009, 15, 656–674. [Google Scholar] [CrossRef] [PubMed]
- Lava, K.; Binnemans, K.; Cardinaels, T. Piperidinium, Piperazinium and Morpholinium Ionic Liquid Crystals. J. Phys. Chem. B 2009, 113, 9506–9511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, S.; Ichikawa, T.; Kato, T.; Ohno, H. Nano-biphasic ionic liquid systems composed of hydrophobic phosphonium salts and a hydrophilic ammonium salt. Chem. Commun. 2012, 48, 5271–5273. [Google Scholar] [CrossRef] [PubMed]
- Stappert, K.; Mudring, A.V. Triazolium based ionic liquid crystals: Effect of asymmetric substitution. RSC Adv. 2015, 5, 16886–16896. [Google Scholar] [CrossRef]
- Kana, T.; Takuma, Y.; Takashi, K. Luminescent Ionic Liquid Crystals Based on Tripodal Pyridinium Salts. Chem. Lett. 2008, 37, 1208–1209. [Google Scholar]
- Mayoral, M.J.; Ovejero, P.; Campo, J.A.; Heras, J.V.; Pinilla, E.; Torres, M.R.; Cano, M. Ionic liquid crystals from β-diketonyl containing pyridinium cations and tetrachlorozincate anions. Inorg. Chem. Commun. 2009, 12, 214–218. [Google Scholar] [CrossRef]
- Westphal, E.; Silva, D.H.d.; Molin, F.; Gallardo, H. Pyridinium and imidazolium 1,3,4-oxadiazole ionic liquid crystals: A thermal and photophysical systematic investigation. RSC Adv. 2013, 3, 6442–6454. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Seddon, K.R. The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic liquid crystals. J. Chem. Soc. Dalton Trans. 1999, 13, 2133–2140. [Google Scholar] [CrossRef]
- Downard, A.; Earle, M.J.; Hardacre, C.; McMath, S.E.J.; Nieuwenhuyzen, M.; Teat, S.J. Structural Studies of Crystalline 1-Alkyl-3-Methylimidazolium Chloride Salts. Chem. Mater. 2004, 16, 43–48. [Google Scholar] [CrossRef]
- Getsis, A.; Mudring, A.-V. 1-Dodecyl-3-methylimidazolium bromide monohydrate. Acta Crystallogr. Sect. E 2005, 61, o2945–o2946. [Google Scholar] [CrossRef]
- Dobbs, W.; Douce, L.; Allouche, L.; Louati, A.; Malbosc, F.; Welter, R. New ionic liquid crystals based on imidazolium salts. New J. Chem. 2006, 30, 528–532. [Google Scholar] [CrossRef]
- Luo, S.-C.; Sun, S.; Deorukhkar, A.R.; Lu, J.-T.; Bhattacharyya, A.; Lin, I.J.B. Ionic liquids and ionic liquid crystals of vinyl functionalized imidazolium salts. J. Mater. Chem. 2011, 21, 1866–1873. [Google Scholar] [CrossRef]
- Sakuda, J.; Yoshio, M.; Ichikawa, T.; Ohno, H.; Kato, T. 2D assemblies of ionic liquid crystals based on imidazolium moieties: Formation of ion-conductive layers. New J. Chem. 2015, 39, 4471–4477. [Google Scholar] [CrossRef]
- Sanchez, I.; Campo, J.A.; Heras, J.V.; Rosario Torres, M.; Cano, M. Pyrazolium salts as a new class of ionic liquid crystals. J. Mater. Chem. 2012, 22, 13239–13251. [Google Scholar] [CrossRef]
- Yoshio, M.K.T. Liquid Crystals as Ion Conductors. In Handbook of Liquid Crystals: Applications of Liquid Crystals, 2nd ed.; Wiley: Hoboken, NJ, USA, 2014; Volume 8. [Google Scholar]
- Goodby, J.W.; Collings, P.J.; Kato, T.; Tschierske, C.; Gleeson, H.; Raynes, P. Handbook of Liquid Crystals; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Gainaru, C.; Rivera, A.; Putselyk, S.; Eska, G.; Rössler, E.A. Low-temperature dielectric relaxation of molecular glasses: Crossover from the nearly constant loss to the tunneling regime. Phys. Rev. B 2005, 72, 174203. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX97, Program for Refinement of Crystal Structure; Universidad de Göttingen: Göttingen, Alemania, 1997. [Google Scholar]
- Torralba, M.C.; Cano, M.; Campo, J.A.; Heras, J.V.; Pinilla, E.; Torres, M.R. Chemistry of Rh(I) complexes based on mesogenic 3,5-disubstituted pyrazole ligands. X-ray crystal structures of 3,5-di(4-n-butoxyphenyl)pyrazole (Hpzbp2) and [Rh(μ-pzR2)(CO)2]2 (R=C6H4OCnH2n+1, n=10, 12) compounds. Part II. J. Organomet. Chem. 2002, 654, 150–161. [Google Scholar] [CrossRef]
- Ignat’ev, N.V.; Barthen, P.; Kucheryna, A.; Willner, H.; Sartori, P. A Convenient Synthesis of Triflate Anion Ionic Liquids and Their Properties. Molecules 2012, 17, 5319–5338. [Google Scholar] [CrossRef] [PubMed]
- Ernö, P.; Bülhmann, P.; DBadertscher, M. Structure Determination of Organic Compounds. Tables of Spetral Data, 4th ed.; Springer: Berlin, Germany, 2009. [Google Scholar]
- Barbera, J.; Cativiela, C.; Serrano, J.L.; Zurbano, M.M. Mesogenic behaviour in some pyrazole and isoxazole derivatives. Liq. Cryst. 1992, 11, 887–897. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Barsukov, E.M.J. Impedance Spectroscopy: Theory, Experiment and Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Prado-Gonjal, J.; Schmidt, R.; Espíndola-Canuto, J.; Ramos-Alvarez, P.; Morán, E. Increased ionic conductivity in microwave hydrothermally synthesized rare-earth doped ceria Ce1−xRExO2−(x/2). J. Power Sources 2012, 209, 163–171. [Google Scholar] [CrossRef]
- Prado-Gonjal, J.; Heuguet, R.; Muñoz-Gil, D.; Rivera-Calzada, A.; Marinel, S.; Morán, E.; Schmidt, R. Microwave synthesis & sintering of Sm and Ca co-doped ceria ceramics. Int. J. Hydrog. Energy 2015, 40, 15640–15651. [Google Scholar] [Green Version]
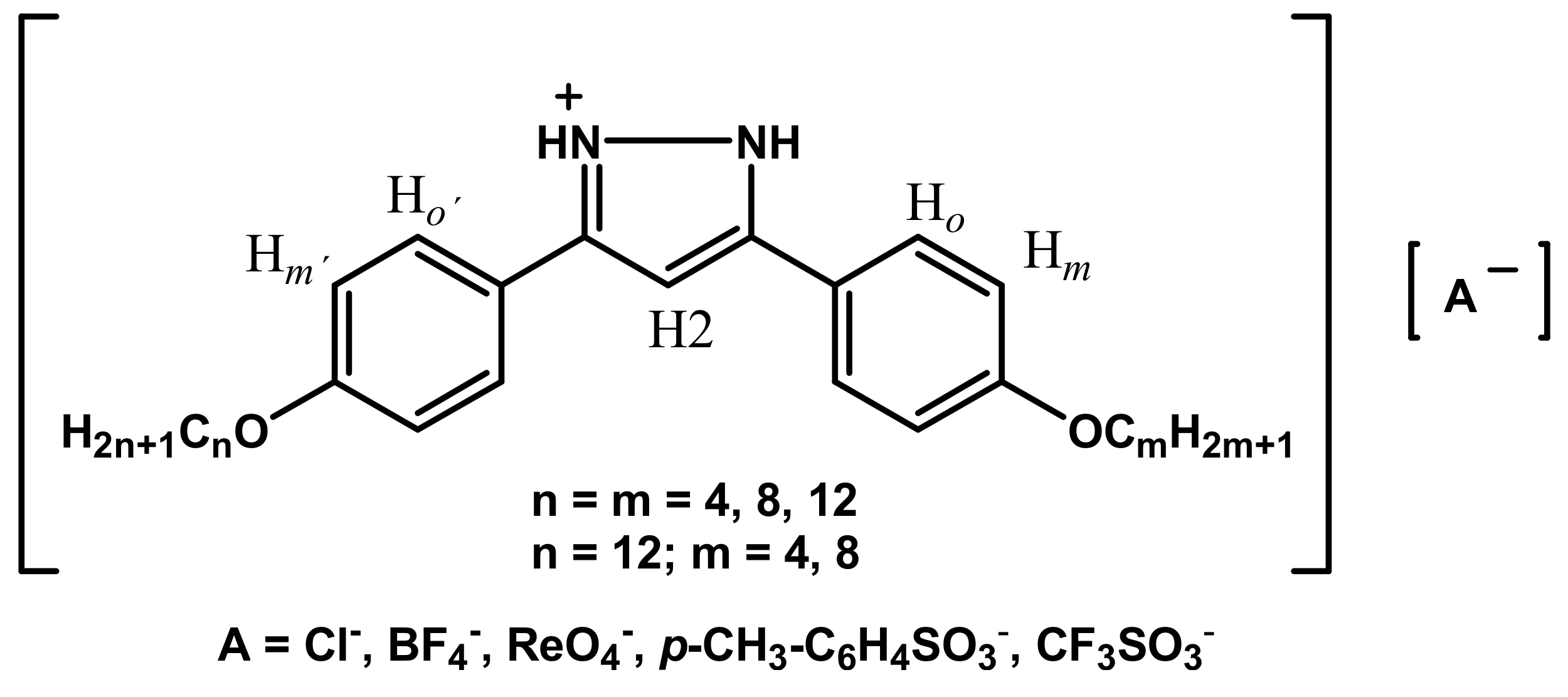
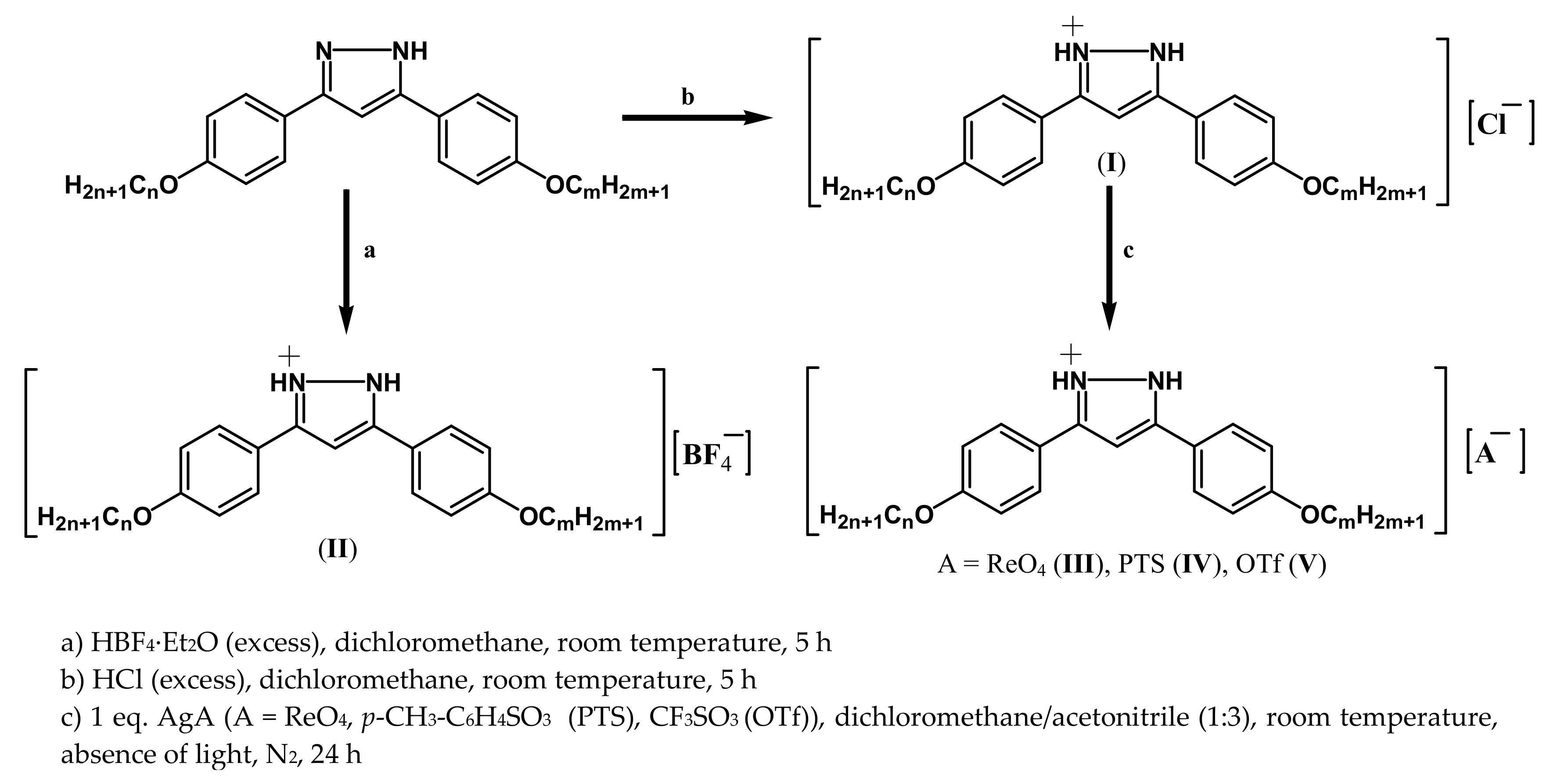
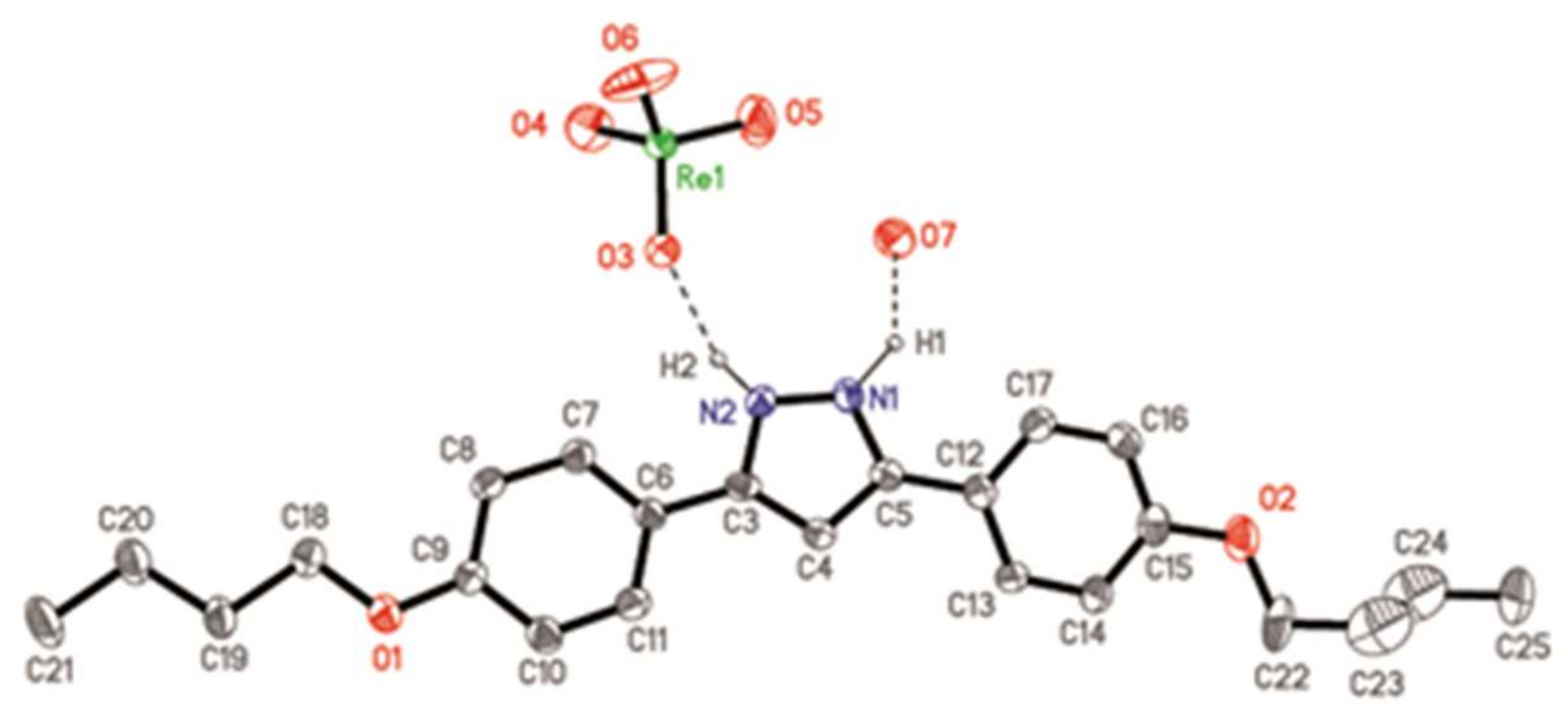


| Family | I | II | III | IV | V |
|---|---|---|---|---|---|
| n,m | Cl-n,m | BF4-n,m | ReO4-n,m | PTS-n,m | OTf-n,m |
| Label (No.) | Label (No.) | Label (No.) | Label (No.) | Label (No.) | |
| 4,4 | Cl-4,4 (1) | BF4-4,4 (6) | ReO4-4,4 (11) | PTS-4,4 (15) | OTf-4,4 (20) |
| 8,8 | Cl-8,8 (2) | BF4-8,8 (7) | ReO4-8,8 (12) | PTS-8,8 (16) | OTf-8,8 (21) |
| 12,12 | Cl-12,12 (3) | BF4-12,12 (8) | - | PTS-12,12 (17) | - |
| 4,12 | Cl-4,12 (4) | BF4-4,12 (9) | ReO4-4,12 (13) | PTS-4,12 (18) | OTf-4,12 (22) |
| 8,12 | Cl-8,12 (5) | BF4-8,12 (10) | ReO4-8,12 (14) | PTS-8,12 (19) | OTf-8,12 (23) |
| Comp. | Transition a | T b/°C | ΔH/kJ mol−1 | Comp. | Transition a | T b/°C | ΔH/kJ mol−1 |
|---|---|---|---|---|---|---|---|
| Cl-4,4 (1) | Cr → SmA | 181 d | 15.4 | ReO4-4,4 (11) | Cr → Cr′ | 138 | 13.0 |
| SmA → I | 200 d | 2.8 | Cr′ → I | 245 | 9.8 | ||
| I → SmA | 199 d | −2.5 | |||||
| SmA → Cr | 171 d | −16.0 | |||||
| Cl-8,8 (2) | Cr → SmA | 143 d | 11.8 | ReO4-8,8 (12) | Cr → SmA | 94 | 39.6 |
| SmA → I | 199 d | 6.1 | SmA → I | 257 f | 2.7 | ||
| I → SmA | 198 d | −6.0 | I → SmA | 245 e | |||
| SmA → Cr | 139 d | −12.6 | |||||
| Cl-12,12 (3) | Cr → Cr′ | 67 d | 16.4 | ReO4-4,12 (13) | Cr → Cr′ | 85 c | 30.0 g |
| Cr′ → Cr″ | 92 d | 23.4 | Cr′→ SmA | 96 c | |||
| Cr″ → SmA | 118 d | 13.1 | SmA → I | 265 c | 4.0 | ||
| SmA → I | 174 d | 7.3 | I → SmA | 235 e | |||
| I → SmA | 173 d | −6.9 | |||||
| SmA → Cr | 122 d | −12.6 | |||||
| Cr → Cr′ | 66 d | −31.0 | |||||
| Cl-4,12 (4) | Cr → SmA | 114 c,d | 5.5 | ReO4-8,12 (14) | Cr → SmA | 90 c | 39.0 |
| SmA → I | 199 c,d | 12.6 | SmA → I | 268 c | 9.4 | ||
| I → SmA | 182 c,d | −5.4 | I → SmA | 250 e | |||
| SmA → Cr | 125 c,d | −8.3 | |||||
| Cl-8,12 (5) | Cr → SmA | 126 d | 11.8 | PTS-4,4 (15) | Cr → Cr′ | 88 | 1.2 |
| SmA → I | 184 d | 7.0 | Cr′ → Cr″ | 147 | 3.9 | ||
| I → SmA | 183 d | −7.0 | Cr″ → I | 208 | 26.1 | ||
| SmA → Cr | 124 d | −11.7 | I → Cr | 203 | −22.0 | ||
| BF4-4,4 (6) | Cr → Cr′ | 59 | 13.6 | PTS-8,8 (16) | Cr → Cr′ | 110 c | 8.3 |
| Cr′ → Cr″ | 154 | 3.3 | Cr′ → Cr″ | 131 c | 1.7 | ||
| Cr″ → Cr″′ | 194 | 19.0 | Cr″ → SmA | 151 c | 7.2 | ||
| Cr″′ → I | 232 | 1.2 | SmA → I | 269 c,f | 27.1 | ||
| I → Cr | 160 | −7.7 | |||||
| BF4-8,8 (7) | Cr → SmA | 116 | 32.4 | PTS-12,12 (17) | Cr → Cr′ | 115 | 24.6 |
| SmA → I | 217 | 5.8 | Cr′ → SmA | 146 | 15.9 | ||
| I → SmA | 163 | −2.2 | SmA → I | 252 e,f | |||
| BF4-12,12 (8) | Cr → SmA | 101 | 24.7 | PTS-4,12 (18) | Cr → Cr′ | 108 | 16.2 |
| SmA → I | 215 | 7.3 | Cr′ → Cr″ | 132 | 8.3 | ||
| I → SmA | 211 | −5.6 | Cr″ → SmA | 175 | 17.9 | ||
| SmA → Cr | 63 | −8.1 | SmA → I | 207 | 5.7 | ||
| I → SmA | 204 | −5.1 | |||||
| SmA → Cr | 164 | −4.7 | |||||
| Cr′ → Cr″ | 139 | −8.1 | |||||
| BF4-4,12 (9) | Cr → Cr′ | 70 | 27.4 g | PTS-8,12 (19) | Cr → Cr′ | 99 | 39.5 |
| Cr′ → SmA | 83 | Cr′ → Cr″ | 172 | 14.1 g | |||
| SmA → I | 205 | 3.7 | Cr″→ SmA | 182 | |||
| I → SmA | 199 | −2.7 | SmA → I | 221 | 7.4 | ||
| I → SmA | 220 | −6.2 | |||||
| SmA → Cr | 175 | −3.7 | |||||
| Cr′ → Cr″ | 152 | −8.2 | |||||
| BF4-8,12 (10) | Cr → Cr′ | 71 | 37.5 g | ||||
| Cr′ → SmA | 80 | ||||||
| SmA → I | 223 | 7.6 | |||||
| I → SmA | 222 | −6.1 | |||||
| SmA → Cr | 112 e |
| Compound | T/°C | 2θ/° | dobs a/Å | dcal a/Å | [hkl] b | Lattice Constant/Å |
|---|---|---|---|---|---|---|
| Cl-8,8 (2) | 170 c | 3.0 | 29.0 | 29.0 | (001) | c = 29.2 |
| 6.0 | 14.7 | 14.5 | (002) | |||
| 17.0 | 5.2 | - | halo | |||
| Cl-8,12 (5) | 160 c | 2.7 | 32.1 | 32.1 | (001) | c = 32.5 |
| 5.4 | 16.4 | 16.1 | (002) | |||
| 17.0 | 5.2 | - | halo | |||
| BF4-4,12 (9) | 100 | 3.6 | 24.4 | 24.4 | (001) | c = 24.8 |
| 7.2 | 12.3 | 12.2 | (002) | |||
| 10.4 | 8.5 | 8.1 | (003) | |||
| 17.0 | 5.2 | - | halo | |||
| BF4-8,12 (10) | 80 | 2.6 | 33.5 | 33.5 | (001) | c = 34.0 |
| 5.1 | 17.2 | 16.8 | (002) | |||
| 17.0 | 5.2 | - | halo | |||
| ReO4-8,12 (14) | 110 | 3.5 | 25.2 | 25.2 | (001) | c = 25.5 |
| 6.9 | 12.8 | 12.6 | (002) | |||
| 10.3 | 8.6 | 8.4 | (003) | |||
| 18.9 | 4.7 | - | halo | |||
| PTS-8,8 (16) | 155 | 4.2 | 20.8 | 20.8 | (001) | c = 20.9 |
| 8.4 | 10.5 | 10.4 | (002) | |||
| 12.6 | 7.0 | 6.9 | (003) | |||
| 17.0 | 5.2 | - | halo | |||
| PTS-8,12 (19) | 155 | 3.3 | 26.7 | 26.7 | (001) | c = 26.8 |
| 6.6 | 13.3 | 13.3 | (002) | |||
| 17.0 | 5.2 | - | halo |
| Compound | EA (Solid) (eV) | EA (Mesophase) (eV) | σ460K (Ωcm)−1 |
|---|---|---|---|
| BF4-8,8 (7) | 9.36 | 2.02 | 2.2 × 10−5 |
| Cl-8,8 (2) | 1.60 | 1.71 | 7.0 × 10−6 |
| Cl-12,12 (3) | 3.36 | 1.89 | 1.9 × 10−6 |
| OTf-8,8 (21) | 1.08 | - | 4.3 × 10−8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastor, M.J.; Sánchez, I.; Campo, J.A.; Schmidt, R.; Cano, M. New Pyrazolium Salts as a Support for Ionic Liquid Crystals and Ionic Conductors. Materials 2018, 11, 548. https://doi.org/10.3390/ma11040548
Pastor MJ, Sánchez I, Campo JA, Schmidt R, Cano M. New Pyrazolium Salts as a Support for Ionic Liquid Crystals and Ionic Conductors. Materials. 2018; 11(4):548. https://doi.org/10.3390/ma11040548
Chicago/Turabian StylePastor, María Jesús, Ignacio Sánchez, José A. Campo, Rainer Schmidt, and Mercedes Cano. 2018. "New Pyrazolium Salts as a Support for Ionic Liquid Crystals and Ionic Conductors" Materials 11, no. 4: 548. https://doi.org/10.3390/ma11040548