Advances in Materials for Recent Low-Profile Implantable Bioelectronics
Abstract
:1. Introduction
2. Traditional Materials Used in Implantable Bioelectronics
2.1. Examples of Traditional Electronic Materials
2.2. Challenges and Limitations of Traditional Materials
3. New Materials for Soft and Flexible Electronics
3.1. Organic Materials
3.2. Inorganic Materials
4. Biodegradable Materials for Transient Electronics
4.1. Metallic Biodegradable Materials
4.2. Polymeric Biodegradable Materials
5. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Joung, Y.-H. Development of implantable medical devices: From an engineering perspective. Int. Neurourol. J. 2013, 17, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Fiandra, O. The first pacemaker implant in america. Pacing Clin. Electrophysiol. 1988, 11, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.S.; Dorman, M.F. Cochlear implants: A remarkable past and a brilliant future. Hear. Res. 2008, 242, 3–21. [Google Scholar] [CrossRef] [PubMed]
- DiMarco, J.P. Implantable cardioverter—Defibrillators. N. Engl. J. Med. 2003, 349, 1836–1847. [Google Scholar] [CrossRef] [PubMed]
- Rijkhoff, N.; Wijkstra, H.; Van Kerrebroeck, P.; Debruyne, F. Urinary bladder control by electrical stimulation: Review of electrical stimulation techniques in spinal cord injury. Neurourol. Urodyn. 1997, 16, 39–53. [Google Scholar] [CrossRef]
- Howe, C.; Lee, Y.; Chen, Y.; Chun, Y.; Yeo, W.-H. An implantable, stretchable microflow sensor integrated with a thin-film nitinol stent. In Proceedings of the IEEE 66th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 31 May–3 June 2016; pp. 1638–1643. [Google Scholar]
- Onuki, Y.; Bhardwaj, U.; Papadimitrakopoulos, F.; Burgess, D.J. A Review of the Biocompatibility of Implantable Devices: Current Challenges to Overcome Foreign Body Response. J. Diabetes Sci. Technol. 2008, 2, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Helmus, M.N.; Gibbons, D.F.; Cebon, D. Biocompatibility: Meeting a key functional requirement of next-generation medical devices. Toxicol. Pathol. 2008, 36, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Herbert, R.; Kim, J.-H.; Kim, Y.S.; Lee, H.M.; Yeo, W.-H. Soft material-enabled, flexible hybrid electronics for medicine, healthcare, and human-machine interfaces. Materials 2018, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Bäcklund, Y.; Rosengren, L.; Hök, B.; Svedbergh, B. Passive silicon transensor intended for biomedical, remote pressure monitoring. Sens. Actuators A Phys. 1990, 21, 58–61. [Google Scholar] [CrossRef]
- Rosengren, L.; Rangsten, P.; Bäcklund, Y.; Hök, B.; Svedbergh, B.; Selén, G. A system for passive implantable pressure sensors. Sens. Actuators A Phys. 1994, 43, 55–58. [Google Scholar] [CrossRef]
- Walter, P.; Schnakenberg, U.; vom Bögel, G.; Ruokonen, P.; Krüger, C.; Dinslage, S.; Handjery, H.C.L.; Richter, H.; Mokwa, W.; Diestelhorst, M.; et al. Development of a completely encapsulated intraocular pressure sensor. Ophthalmic Res. 2000, 32, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Stangel, K.; Kolnsberg, S.; Hammerschmidt, D.; Hosticka, B.; Trieu, H.; Mokwa, W. A programmable intraocular CMOS pressure sensor system implant. IEEE J. Solid-State Circ. 2001, 36, 1094–1100. [Google Scholar] [CrossRef]
- Ganji, B.A.; Shahiri-Tabarestani, M. A novel high sensitive mems intraocular capacitive pressure sensor. Microsyst. Technol. 2013, 19, 187–194. [Google Scholar] [CrossRef]
- Chitnis, G.; Maleki, T.; Samuels, B.; Cantor, L.B.; Ziaie, B. A minimally invasive implantable wireless pressure sensor for continuous IOP monitoring. IEEE Trans. Biomed. Eng. 2013, 60, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Chatzandroulis, S.; Tsoukalas, D.; Neukomm, P.A. A miniature pressure system with a capacitive sensor and a passive telemetry link for use in implantable applications. J. Microelectromech. Syst. 2000, 9, 18–23. [Google Scholar] [CrossRef]
- Yoon, H.J.; Jung, J.M.; Jeong, J.S.; Yang, S.S. Micro devices for a cerebrospinal fluid (CSF) shunt system. Sens. Actuators A Phys. 2004, 110, 68–76. [Google Scholar] [CrossRef]
- Murphy, O.H.; Bahmanyar, M.R.; Borghi, A.; McLeod, C.N.; Navaratnarajah, M.; Yacoub, M.H.; Toumazou, C. Continuous in vivo blood pressure measurements using a fully implantable wireless saw sensor. Biomed. Microdevices 2013, 15, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Fang, L.; Liang, B.; Wang, Q.; Wang, X.; He, L.; Bei, W.; Ko, W.H. Studies of a high-sensitive surface acoustic wave sensor for passive wireless blood pressure measurement. Sens. Actuators A Phys. 2011, 169, 74–82. [Google Scholar] [CrossRef]
- Liang, B.; Fang, L.; Tu, C.; Zhou, C.; Wang, X.; Wang, Q.; Wang, P.; Ye, X. A novel implantable saw sensor for blood pressure monitoring. In Proceedings of the 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference (TRANSDUCERS), Beijing, China, 5–9 June 2011; pp. 2184–2187. [Google Scholar]
- Bal, B.S.; Rahaman, M. Orthopedic applications of silicon nitride ceramics. Acta Biomater. 2012, 8, 2889–2898. [Google Scholar] [CrossRef]
- Melik, R.; Perkgoz, N.K.; Unal, E.; Puttlitz, C.; Demir, H.V. Bio-implantable passive on-chip RF-mems strain sensing resonators for orthopaedic applications. J. Micromech. Microeng. 2008, 18, 115017. [Google Scholar] [CrossRef]
- McGilvray, K.C.; Unal, E.; Troyer, K.L.; Santoni, B.G.; Palmer, R.H.; Easley, J.T.; Demir, H.V.; Puttlitz, C.M. Implantable microelectromechanical sensors for diagnostic monitoring and post-surgical prediction of bone fracture healing. J. Orthop. Res. 2015, 33, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Lakshmanan, S.; Apigo, D.; Kanwal, A.; Liu, S.; Russell, T.; Madsen, J.R.; Thomas, G.A.; Farrow, R.C. Demonstration that a new flow sensor can operate in the clinical range for cerebrospinal fluid flow. Sens. Actuators A Phys. 2015, 234, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Apigo, D.J.; Bartholomew, P.L.; Russell, T.; Kanwal, A.; Farrow, R.C.; Thomas, G.A. An angstrom-sensitive, differential mems capacitor for monitoring the milliliter dynamics of fluids. Sens. Actuators A Phys. 2016, 251, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Apigo, D.J.; Bartholomew, P.L.; Russell, T.; Kanwal, A.; Farrow, R.C.; Thomas, G.A. Evidence of an application of a variable mems capacitive sensor for detecting shunt occlusions. Sci. Rep. 2017, 7, 46039. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Brox, D.; Assadsangabi, B.; Hsiang, Y.; Takahata, K. Intelligent telemetric stent for wireless monitoring of intravascular pressure and its in vivo testing. Biomed. Microdevices 2014, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Brox, D.; Assadsangabi, B.; Ali, M.S.M.; Takahata, K. A stainless-steel-based implantable pressure sensor chip and its integration by microwelding. Sens. Actuators A Phys. 2017, 257, 134–144. [Google Scholar] [CrossRef]
- Scholten, K.; Meng, E. Materials for microfabricated implantable devices: A review. Lab Chip 2015, 15, 4256–4272. [Google Scholar] [CrossRef] [PubMed]
- Kalvesten, E.; Smith, L.; Tenerz, L.; Stemme, G. The first surface micromachined pressure sensor for cardiovascular pressure measurements. In Proceedings of the 1998 Eleventh Annual International Workshop on Micro Electro Mechanical Systems (MEMS 98), Heidelberg, Germany, 25–29 January 1998; pp. 574–579. [Google Scholar]
- Ziaie, B.; Najafi, K. An implantable microsystem for tonometric blood pressure measurement. Biomed. Microdevices 2001, 3, 285–292. [Google Scholar] [CrossRef]
- Park, J.; Lee, N.-E.; Lee, J.; Park, J.; Park, H. Deep dry etching of borosilicate glass using sf6 and sf6/Ar inductively coupled plasmas. Microelectron. Eng. 2005, 82, 119–128. [Google Scholar] [CrossRef]
- Kolari, K.; Saarela, V.; Franssila, S. Deep plasma etching of glass for fluidic devices with different mask materials. J. Micromech. Microeng. 2008, 18, 064010. [Google Scholar] [CrossRef]
- Theodor, M.; Ruh, D.; Fiala, J.; Förster, K.; Heilmann, C.; Manoli, Y.; Beyersdorf, F.; Zappe, H.; Seifert, A. Subcutaneous blood pressure monitoring with an implantable optical sensor. Biomed. Microdevices 2013, 15, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Fiala, J.; Bingger, P.; Ruh, D.; Foerster, K.; Heilmann, C.; Beyersdorf, F.; Zappe, H.; Seifert, A. An implantable optical blood pressure sensor based on pulse transit time. Biomed. Microdevices 2013, 15, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Theodor, M.; Fiala, J.; Ruh, D.; Foerster, K.; Heilmann, C.; Beyersdorf, F.; Manoli, Y.; Zappe, H.; Seifert, A. Implantable accelerometer system for the determination of blood pressure using reflected wave transit time. Sens. Actuators A Phys. 2014, 206, 151–158. [Google Scholar] [CrossRef]
- Grayson, A.C.R.; Shawgo, R.S.; Johnson, A.M.; Flynn, N.T.; Li, Y.; Cima, M.J.; Langer, R. A biomems review: Mems technology for physiologically integrated devices. Proc. IEEE 2004, 92, 6–21. [Google Scholar] [CrossRef]
- Potkay, J.A. Long term, implantable blood pressure monitoring systems. Biomed. Microdevices 2008, 10, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bellamkonda, R.V.; Sun, W.; Levenston, M.E. Biomechanical analysis of silicon microelectrode-induced strain in the brain. J. Neural Eng. 2005, 2, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Norton, J.J.; Lee, Y.; Lee, D.S.; Agee, N.; Chen, Y.; Chun, Y.; Yeo, W.-H. Soft, conformal bioelectronics for a wireless human-wheelchair interface. Biosens. Bioelectron. 2017, 91, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Nicholls, B.; Lee, D.S.; Chen, Y.; Chun, Y.; Ang, C.S.; Yeo, W.-H. Soft electronics enabled ergonomic human-computer interaction for swallowing training. Sci. Rep. 2017, 7, 46697. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.J.; Lee, D.S.; Lee, J.W.; Lee, W.; Kwon, O.; Won, P.; Jung, S.-Y.; Cheng, H.; Jeong, J.-W.; Akce, A.; et al. Soft, curved electrode systems capable of integration on the auricle as a persistent brain–computer interface. Proc. Natl. Acad. Sci. USA 2015, 112, 3920–3925. [Google Scholar] [CrossRef] [PubMed]
- Araci, I.E.; Su, B.; Quake, S.R.; Mandel, Y. An implantable microfluidic device for self-monitoring of intraocular pressure. Nat. Med. 2014, 20, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Yang, S. Highly stable liquid metal-based pressure sensor integrated with a microfluidic channel. Sensors 2015, 15, 11823–11835. [Google Scholar] [CrossRef] [PubMed]
- Koley, G.; Liu, J.; Nomani, M.W.; Yim, M.; Wen, X.; Hsia, T.-Y. Miniaturized implantable pressure and oxygen sensors based on polydimethylsiloxane thin films. Mater. Sci. Eng. C 2009, 29, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-C.; Lin, C.-C.K.; Ju, M.-S. An implantable capacitive pressure sensor for biomedical applications. Sens. Actuators A Phys. 2007, 134, 382–388. [Google Scholar] [CrossRef]
- Adrega, T.; Lacour, S. Stretchable gold conductors embedded in PDMS and patterned by photolithography: Fabrication and electromechanical characterization. J. Micromech. Microeng. 2010, 20, 055025. [Google Scholar] [CrossRef]
- Lacour, S.P.; Benmerah, S.; Tarte, E.; FitzGerald, J.; Serra, J.; McMahon, S.; Fawcett, J.; Graudejus, O.; Yu, Z.; Morrison, B. Flexible and stretchable micro-electrodes for in vitro and in vivo neural interfaces. Med. Biol. Eng. Comput. 2010, 48, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Y.; Zhong, X.; Wang, W.; Miao, Q.; Zhu, J.-J. Flexible PDMS-based three-electrode sensor. Electrochem. Commun. 2010, 12, 1600–1604. [Google Scholar] [CrossRef]
- Aquilina, K.; Thoresen, M.; Chakkarapani, E.; Pople, I.K.; Coakham, H.B.; Edwards, R.J. Preliminary evaluation of a novel intraparenchymal capacitive intracranial pressure monitor. J. Neurosurg. 2011, 115, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Bingger, P.; Zens, M.; Woias, P. Highly flexible capacitive strain gauge for continuous long-term blood pressure monitoring. Biomed. Microdevices 2012, 14, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Z.; Chan, I.-S.; Lam, D.C. Capacitive contact lens sensor for continuous non-invasive intraocular pressure monitoring. Sens. Actuators A Phys. 2013, 203, 112–118. [Google Scholar] [CrossRef]
- Chen, G.-Z.; Chan, I.-S.; Leung, L.K.; Lam, D.C. Soft wearable contact lens sensor for continuous intraocular pressure monitoring. Med. Eng. Phys. 2014, 36, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Farandos, N.M.; Yetisen, A.K.; Monteiro, M.J.; Lowe, C.R.; Yun, S.H. Contact lens sensors in ocular diagnostics. Adv. Healthc. Mater. 2015, 4, 792–810. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Rodger, D.C.; Agrawal, R.; Saati, S.; Meng, E.; Varma, R.; Humayun, M.S.; Tai, Y.-C. Implantable micromechanical parylene-based pressure sensors for unpowered intraocular pressure sensing. J. Micromech. Microeng. 2007, 17, 1931. [Google Scholar] [CrossRef]
- Chen, P.-J.; Rodger, D.C.; Saati, S.; Humayun, M.S.; Tai, Y.-C. Microfabricated implantable parylene-based wireless passive intraocular pressure sensors. J. Microelectromech. Syst. 2008, 17, 1342–1351. [Google Scholar] [CrossRef]
- Chen, P.-J.; Saati, S.; Varma, R.; Humayun, M.S.; Tai, Y.-C. Wireless intraocular pressure sensing using microfabricated minimally invasive flexible-coiled LC sensor implant. J. Microelectromech. Syst. 2010, 19, 721–734. [Google Scholar] [CrossRef]
- Ha, D.; de Vries, W.N.; John, S.W.; Irazoqui, P.P.; Chappell, W.J. Polymer-based miniature flexible capacitive pressure sensor for intraocular pressure (IOP) monitoring inside a mouse eye. Biomed. Microdevices 2012, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Jin, W.; Baldwin, A.; Yu, L.; Christian, E.; Krieger, M.D.; McComb, J.G.; Meng, E. Parylene mems patency sensor for assessment of hydrocephalus shunt obstruction. Biomed. Microdevices 2016, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kuo, J.T.; Hara, S.A.; Lee, C.D.; Yu, L.; Gutierrez, C.; Hoang, T.; Pikov, V.; Meng, E. 3d parylene sheath neural probe for chronic recordings. J. Neural Eng. 2013, 10, 045002. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.T.; Kim, B.J.; Hara, S.A.; Lee, C.D.; Gutierrez, C.A.; Hoang, T.Q.; Meng, E. Novel flexible parylene neural probe with 3d sheath structure for enhancing tissue integration. Lab Chip 2013, 13, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Rodger, D.C.; Fong, A.J.; Li, W.; Ameri, H.; Ahuja, A.K.; Gutierrez, C.; Lavrov, I.; Zhong, H.; Menon, P.R.; Meng, E.; et al. Flexible parylene-based multielectrode array technology for high-density neural stimulation and recording. Sens. Actuators B Chem. 2008, 132, 449–460. [Google Scholar] [CrossRef]
- Yu, L.; Gutierrez, C.A.; Meng, E. An electrochemical microbubble-based mems pressure sensor. J. Microelectromech. Syst. 2016, 25, 144–152. [Google Scholar] [CrossRef]
- Zhao, Z.; Kim, E.; Luo, H.; Zhang, J.; Xu, Y. Flexible deep brain neural probes based on a parylene tube structure. J. Micromech. Microeng. 2017, 28, 015012. [Google Scholar] [CrossRef]
- Chen, L.Y.; Tee, B.C.-K.; Chortos, A.L.; Schwartz, G.; Tse, V.; Lipomi, D.J.; Wong, H.-S.P.; McConnell, M.V.; Bao, Z. Continuous wireless pressure monitoring and mapping with ultra-small passive sensors for health monitoring and critical care. Nat. Commun. 2014, 5, 5028. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Lai, H.-Y.; Lin, S.-H.; Cho, C.-W.; Chao, W.-H.; Liao, C.-H.; Tsang, S.; Chen, Y.-F.; Lin, S.-Y. Design and fabrication of a polyimide-based microelectrode array: Application in neural recording and repeatable electrolytic lesion in rat brain. J. Neurosci. Methods 2009, 182, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bhandari, R.; Klein, M.; Negi, S.; Rieth, L.; Tathireddy, P.; Toepper, M.; Oppermann, H.; Solzbacher, F. Integrated wireless neural interface based on the utah electrode array. Biomed. Microdevices 2009, 11, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Rousche, P.J.; Pellinen, D.S.; Pivin, D.P.; Williams, J.C.; Vetter, R.J.; Kipke, D.R. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans. Biomed. Eng. 2001, 48, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Viventi, J.; Kim, D.-H.; Vigeland, L.; Frechette, E.S.; Blanco, J.A.; Kim, Y.-S.; Avrin, A.E.; Tiruvadi, V.R.; Hwang, S.-W.; Vanleer, A.C.; et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 2011, 14, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Hwang, H.; Lee, S.H.; Kang, J.Y.; Park, J.-H.; Seo, C.; Park, C. A wireless intraocular pressure sensor with variable inductance using a ferrite material. J. Semicond. Technol. Sci. 2013, 13, 355–360. [Google Scholar] [CrossRef]
- Shin, K.-S.; Jang, C.-I.; Kim, M.J.; Yun, K.-S.; Park, K.H.; Kang, J.Y.; Lee, S.H. Development of novel implantable intraocular pressure sensors to enhance the performance in in vivo tests. J. Microelectromech. Syst. 2015, 24, 1896–1905. [Google Scholar] [CrossRef]
- Chiu, Y.-Y.; Lin, W.-Y.; Wang, H.-Y.; Huang, S.-B.; Wu, M.-H. Development of a piezoelectric polyvinylidene fluoride (PVDF) polymer-based sensor patch for simultaneous heartbeat and respiration monitoring. Sens. Actuators A Phys. 2013, 189, 328–334. [Google Scholar] [CrossRef]
- Li, C.; Wu, P.-M.; Shutter, L.A.; Narayan, R.K. Dual-mode operation of flexible piezoelectric polymer diaphragm for intracranial pressure measurement. Appl. Phys. Lett. 2010, 96, 053502. [Google Scholar] [CrossRef]
- Sharma, T.; Aroom, K.; Naik, S.; Gill, B.; Zhang, J.X. Flexible thin-film PVDF-TRFE based pressure sensor for smart catheter applications. Ann. Biomed. Eng. 2013, 41, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Je, S.-S.; Gill, B.; Zhang, J.X. Patterning piezoelectric thin film PVDF–TRFE based pressure sensor for catheter application. Sens. Actuators A Phys. 2012, 177, 87–92. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, S.W.; Min, K.S.; Kim, S.J. A novel multilayered planar coil based on biocompatible liquid crystal polymer for chronic implantation. Sens. Actuators A Phys. 2013, 197, 38–46. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, S.W.; Min, K.S.; Shin, S.; Jun, S.B.; Kim, S.J. Liquid crystal polymer (LCP), an attractive substrate for retinal implant. Sens. Mater. 2012, 24, 189–203. [Google Scholar]
- Lee, S.Y.; Park, K.-I.; Huh, C.; Koo, M.; Yoo, H.G.; Kim, S.; Ah, C.S.; Sung, G.Y.; Lee, K.J. Water-resistant flexible gan led on a liquid crystal polymer substrate for implantable biomedical applications. Nano Energy 2012, 1, 145–151. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.-C.; Durand, D.M. Flexible nerve stimulation electrode with iridium oxide sputtered on liquid crystal polymer. IEEE Trans. Biomed. Eng. 2009, 56, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.Y.; Chlebowski, A.L.; Irazoqui, P.P. A miniature-implantable RF-wireless active glaucoma intraocular pressure monitor. IEEE Trans. Biomed. Circ. Syst. 2010, 4, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-W.; Choi, Y.-S. A novel pressure sensor with a PDMS diaphragm. Microelectron. Eng. 2008, 85, 1054–1058. [Google Scholar] [CrossRef]
- Lötters, J.C.; Olthuis, W.; Veltink, P.; Bergveld, P. The mechanical properties of the rubber elastic polymer polydimethylsiloxane for sensor applications. J. Micromech. Microeng. 1997, 7, 145–147. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Larmagnac, A.; Eggenberger, S.; Janossy, H.; Vörös, J. Stretchable electronics based on Ag-PDMS composites. Sci. Rep. 2014, 4, 7254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varel, Ç.; Shih, Y.-C.; Otis, B.P.; Shen, T.S.; Böhringer, K.F. A wireless intraocular pressure monitoring device with a solder-filled microchannel antenna. J. Micromech. Microeng. 2014, 24, 045012. [Google Scholar] [CrossRef]
- Pal, R.K.; Pradhan, S.; Narayanan, L.; Yadavalli, V.K. Micropatterned conductive polymer biosensors on flexible pdms films. Sens. Actuators B Chem. 2018, 259, 498–504. [Google Scholar] [CrossRef]
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D.H. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef] [PubMed]
- Carl Ward, T.; Timothy Perry, J. Dynamic mechanical properties of medical grade silicone elastomer stored in simulated body fluids. J. Biomed. Mater. Res. Part A 1981, 15, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Puskas, J.E.; Chen, Y. Biomedical application of commercial polymers and novel polyisobutylene-based thermoplastic elastomers for soft tissue replacement. Biomacromolecules 2004, 5, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Hassler, C.; von Metzen, R.P.; Ruther, P.; Stieglitz, T. Characterization of Parylene C as an encapsulation material for implanted neural prostheses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, A.; Degache, A.; Descamps, E.; Dahan, L.; Bergaud, C. In vitro and in vivo biostability assessment of chronically-implanted Parylene C neural sensors. Sens. Actuators B Chem. 2017, 251, 1001–1008. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Starr, P.; Bartels, K.; Agrawal, C.M.; Bailey, S. A thin-film pressure transducer for implantable and intravascular blood pressure sensing. Sens. Actuators A Phys. 2016, 248, 38–45. [Google Scholar] [CrossRef]
- Hasenkamp, W.; Forchelet, D.; Pataky, K.; Villard, J.; Van Lintel, H.; Bertsch, A.; Wang, Q.; Renaud, P. Polyimide/su-8 catheter-tip mems gauge pressure sensor. Biomed. Microdevices 2012, 14, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, H.; Poh, M.; Xia, F.; Cheng, Z.-Y.; Xu, H.; Huang, C. An all-organic composite actuator material with a high dielectric constant. Nature 2002, 419, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cheng, Z.-Y.; Olson, D.; Mai, T.; Zhang, Q.; Kavarnos, G. Ferroelectric and electromechanical properties of poly (vinylidene-fluoride–trifluoroethylene–chlorotrifluoroethylene) terpolymer. Appl. Phys. Lett. 2001, 78, 2360–2362. [Google Scholar] [CrossRef]
- Wang, X.; Engel, J.; Liu, C. Liquid crystal polymer (LCP) for mems: Processes and applications. J. Micromech. Microeng. 2003, 13, 628–633. [Google Scholar] [CrossRef]
- Kim, D.H.; Xiao, J.; Song, J.; Huang, Y.; Rogers, J.A. Stretchable, curvilinear electronics based on inorganic materials. Adv. Mater. 2010, 22, 2108–2124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Seo, J.-H.; Paskiewicz, D.M.; Zhu, Y.; Celler, G.K.; Voyles, P.M.; Zhou, W.; Lagally, M.G.; Ma, Z. Fast flexible electronics with strained silicon nanomembranes. Sci. Rep. 2013, 3, 1291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Seo, J.-H.; Zhou, W.; Ma, Z. Fast flexible electronics using transferrable silicon nanomembranes. J. Phys. D Appl. Phys. 2012, 45, 143001. [Google Scholar] [CrossRef]
- Kim, D.-H.; Ghaffari, R.; Lu, N.; Rogers, J.A. Flexible and stretchable electronics for biointegrated devices. Annu. Rev. Biomed. Eng. 2012, 14, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Lagally, M.; Nuzzo, R. Synthesis, assembly and applications of semiconductor nanomembranes. Nature 2011, 477, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Viventi, J.; Kim, D.-H.; Moss, J.D.; Kim, Y.-S.; Blanco, J.A.; Annetta, N.; Hicks, A.; Xiao, J.; Huang, Y.; Callans, D.J.; et al. A conformal, bio-interfaced class of silicon electronics for mapping cardiac electrophysiology. Sci. Transl. Med. 2010, 2, 24ra22. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhang, Y.; Lai, W.Y.; Huang, W. Stretchable thin-film electrodes for flexible electronics with high deformability and stretchability. Adv. Mater. 2015, 27, 3349–3376. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Sulkin, M.S.; Kim, J.S.; Goudeseune, C.; Chao, H.Y.; Song, J.W.; Yang, S.Y.; Hsu, Y.Y.; Ghaffari, R.; Efimov, I.R.; et al. Stretchable, multiplexed ph sensors with demonstrations on rabbit and human hearts undergoing ischemia. Adv. Healthc. Mater. 2014, 3, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.J.; Owh, C.; Chee, P.L.; Kyaw, A.K.K.; Kai, D.; Loh, X.J. Biodegradable electronics: Cornerstone for sustainable electronics and transient applications. J. Mater. Chem. C 2016, 4, 5531–5558. [Google Scholar] [CrossRef]
- Yin, L.; Cheng, H.; Mao, S.; Haasch, R.; Liu, Y.; Xie, X.; Hwang, S.W.; Jain, H.; Kang, S.K.; Su, Y. Dissolvable metals for transient electronics. Adv. Funct. Mater. 2014, 24, 645–658. [Google Scholar] [CrossRef]
- Wang, H.; Shi, Z. In vitro biodegradation behavior of magnesium and magnesium alloy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Tao, H.; Kim, D.-H.; Cheng, H.; Song, J.-K.; Rill, E.; Brenckle, M.A.; Panilaitis, B.; Won, S.M.; Kim, Y.-S.; et al. A physically transient form of silicon electronics. Science 2012, 337, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Martinez, A.W.; Song, C.; Herrault, F.; Allen, M.G. A microfabricated wireless RF pressure sensor made completely of biodegradable materials. J. Microelectromech. Syst. 2014, 23, 4–13. [Google Scholar] [CrossRef]
- Kang, S.K.; Hwang, S.W.; Yu, S.; Seo, J.H.; Corbin, E.A.; Shin, J.; Wie, D.S.; Bashir, R.; Ma, Z.; Rogers, J.A. Biodegradable thin metal foils and spin-on glass materials for transient electronics. Adv. Funct. Mater. 2015, 25, 1789–1797. [Google Scholar] [CrossRef]
- Curry, E.J.; Ke, K.; Chorsi, M.T.; Wrobel, K.S.; Miller, A.N.; Patel, A.; Kim, I.; Feng, J.; Yue, L.; Wu, Q.; et al. Biodegradable piezoelectric force sensor. Proc. Natl. Acad. Sci. USA 2018, 115, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-K.; Murphy, R.K.; Hwang, S.-W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.J.; Kuzum, D.; Hwang, S.-W.; Kim, B.H.; Juul, H.; Kim, N.H.; Won, S.M.; Chiang, K.; Trumpis, M.; Richardson, A.G.; et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 2016, 15, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Boutry, C.M.; Nguyen, A.; Lawal, Q.O.; Chortos, A.; Bao, Z. Fully biodegradable pressure sensor, viscoelastic behavior of PGS dielectric elastomer upon degradation. In Proceedings of the 2015 IEEE SENSORS, Busan, Korea, 1–4 November 2015; pp. 1–4. [Google Scholar]
- Kim, D.-H.; Kim, Y.-S.; Amsden, J.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G.; Zakin, M.R.; Rogers, J.A. Silicon electronics on silk as a path to bioresorbable, implantable devices. Appl. Phys. Lett. 2009, 95, 133701. [Google Scholar] [CrossRef]
- Pal, R.K.; Farghaly, A.A.; Wang, C.; Collinson, M.M.; Kundu, S.C.; Yadavalli, V.K. Conducting polymer-silk biocomposites for flexible and biodegradable electrochemical sensors. Biosens. Bioelectron. 2016, 81, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Kaplan, D.L.; Omenetto, F.G. Silk materials—A road to sustainable high technology. Adv. Mater. 2012, 24, 2824–2837. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Song, J.K.; Huang, X.; Cheng, H.; Kang, S.K.; Kim, B.H.; Kim, J.H.; Yu, S.; Huang, Y.; Rogers, J.A. High-performance biodegradable/transient electronics on biodegradable polymers. Adv. Mater. 2014, 26, 3905–3911. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Boutry, C.M.; Nguyen, A.; Lawal, Q.O.; Chortos, A.; Rondeau-Gagné, S.; Bao, Z. A sensitive and biodegradable pressure sensor array for cardiovascular monitoring. Adv. Mater. 2015, 27, 6954–6961. [Google Scholar] [CrossRef] [PubMed]
- Lewitus, D.; Smith, K.L.; Shain, W.; Kohn, J. Ultrafast resorbing polymers for use as carriers for cortical neural probes. Acta Biomater. 2011, 7, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.-S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D.; et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511–517. [Google Scholar] [CrossRef] [PubMed]

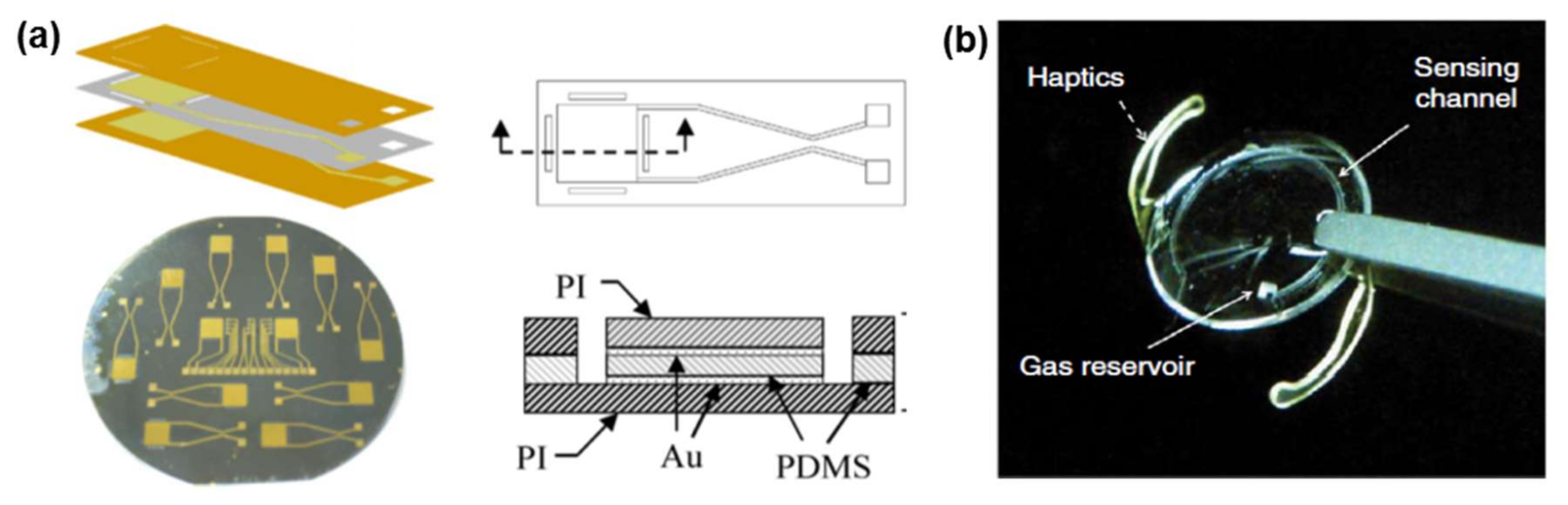
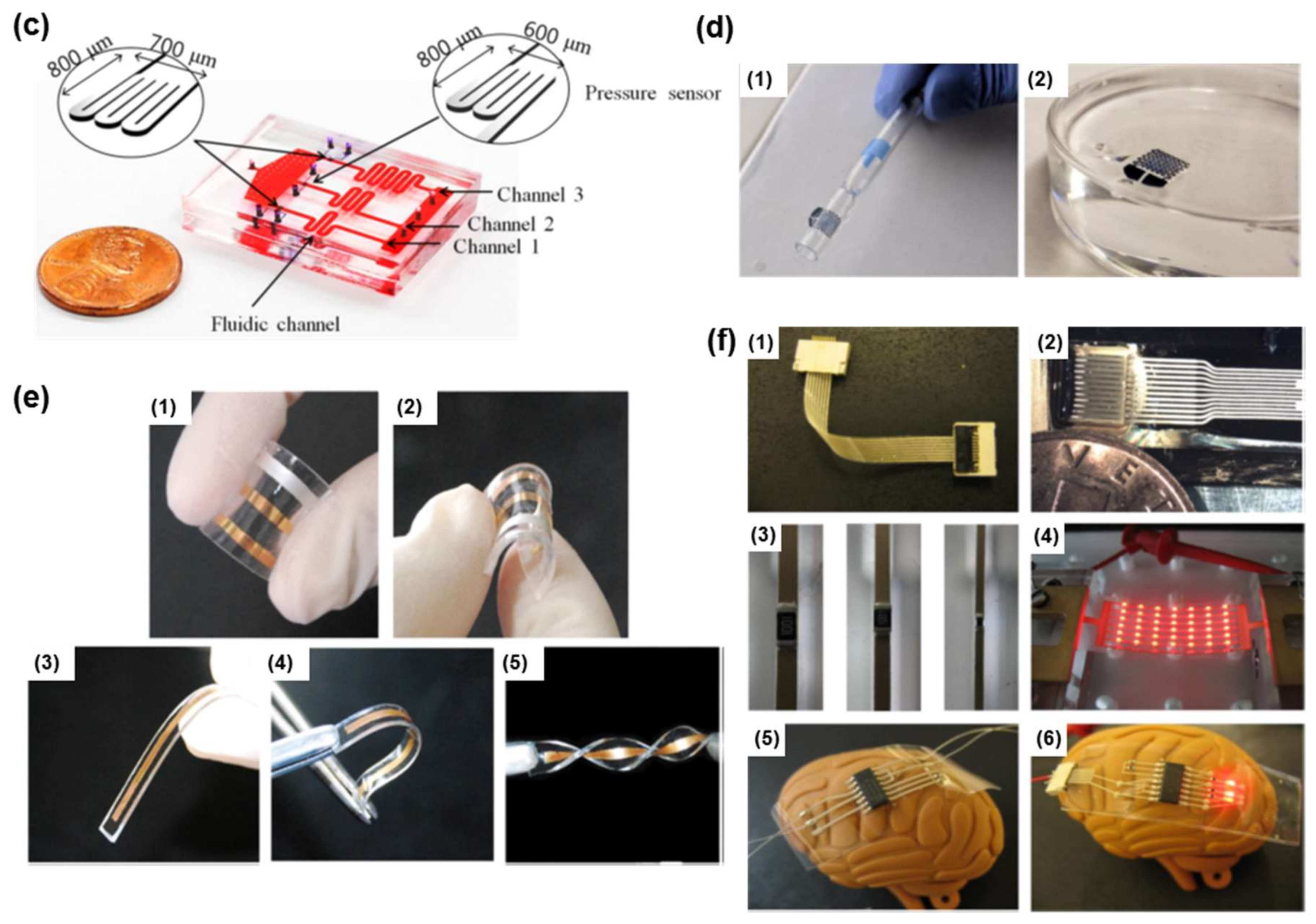


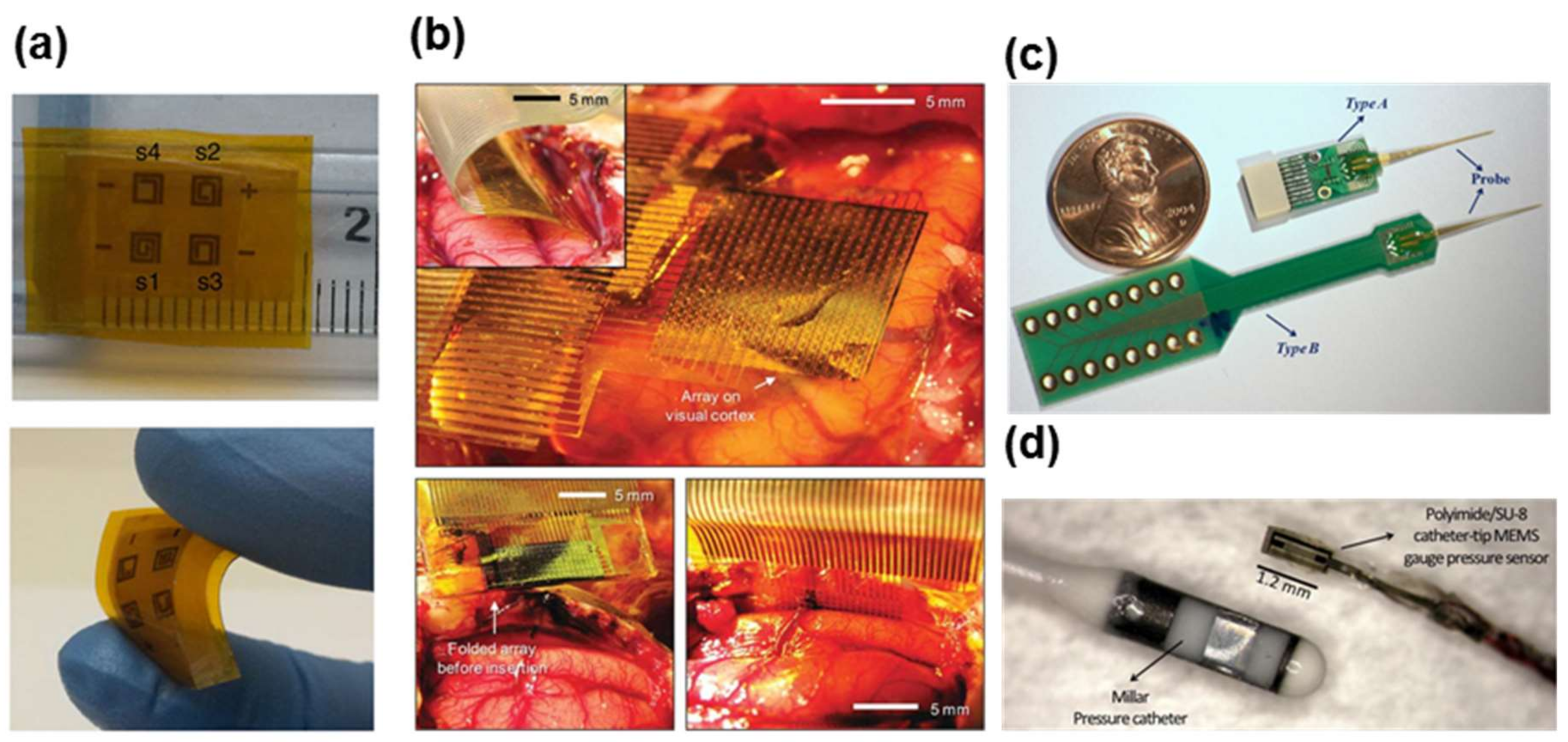



| Materials | Properties | Device Component | Applications | References |
|---|---|---|---|---|
| Silicon | Compatible with microfabrication | Substrate | Intraocular pressure and cardiovascular monitoring | [10,11,12,13,14,15] |
| Silicon | Compatible with microfabrication | Structural diaphragm | Blood pressure and shunt pressure sensor | [16,17] |
| Silicon oxide | High-quality factor | Structural diaphragm and substrate | Surface acoustic wave blood pressure sensor | [18,19,20] |
| Silicon nitride | Thermally stable | Dielectric layer | Orthopedic sensor | [21,22,23] |
| Silicon nitride | Thermally stable | Insulation layer | Cerebrospinal fluid flow monitoring | [24,25,26] |
| Stainless steel | Compatible with stents | Substrate | Capacitive pressure sensor | [27,28] |
| Materials | Properties | Device Component | Applications | References |
|---|---|---|---|---|
| PDMS | Low modulus, high dielectric strength, low chemical reactivity | Microfluidic channel | Pressure monitoring | [43,44] |
| Dielectric layer | Pressure and oxygen sensor in blood | [45,46] | ||
| Substrate layer | Physiological recording | [47,48,49] | ||
| Medical grade silicone | High tear strength and elasticity, transparency | Encapsulation layer | Soft contact lens sensor, intracranial and blood pressure monitoring | [50,51,52,53,54] |
| Parylene C | Chemical and biological inert, low water permeability and absorption | Structural diaphragm | Intraocular pressure monitoring | [55,56,57,58] |
| Substrate layer | Neural electrode probe, hydrocephalus shunt occlusion detection | [59,60,61,62,63,64] | ||
| Polyimide | High heat resistance | Substrate layer | Intraocular and cardiovascular pressure monitoring | [15,65,66,67,68,69] |
| Structural diaphragm | Intraocular pressure monitoring | [70,71] | ||
| PVDF | Piezoelectricity | Structural diaphragm | Intracranial and endovascular pressure monitoring | [72,73,74,75] |
| LCP | Low dielectric constant and low moisture absorption rate | Substrate | Intraocular pressure monitoring | [76,77,78,79] |
| Encapsulation | Active intraocular pressure monitoring | [80] |
| Materials | Degradation Rate | Device Component | Applications | References |
|---|---|---|---|---|
| Magnesium | High reactivity | Electrode | Thermal therapy | [110] |
| Zinc and Iron | 0.46 mg/(cm2·h) | Conductor | RF pressure sensor | [111] |
| Polylactic-co-Glycolic Acid (PLGA) | Several weeks | Substrate | Brain monitoring, wound healing, pressure monitoring | [113,114,115] |
| poly(glycerol-sebacate) (PGS) | A few months | Substrate | Cardiovascular monitoring | [116] |
| Silk | Several weeks | Substrate | Neural recording, drug delivery device | [86,117,118,119] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Kim, Y.-S.; Tillman, B.W.; Yeo, W.-H.; Chun, Y. Advances in Materials for Recent Low-Profile Implantable Bioelectronics. Materials 2018, 11, 522. https://doi.org/10.3390/ma11040522
Chen Y, Kim Y-S, Tillman BW, Yeo W-H, Chun Y. Advances in Materials for Recent Low-Profile Implantable Bioelectronics. Materials. 2018; 11(4):522. https://doi.org/10.3390/ma11040522
Chicago/Turabian StyleChen, Yanfei, Yun-Soung Kim, Bryan W. Tillman, Woon-Hong Yeo, and Youngjae Chun. 2018. "Advances in Materials for Recent Low-Profile Implantable Bioelectronics" Materials 11, no. 4: 522. https://doi.org/10.3390/ma11040522






