Preparation of Hierarchical Highly Ordered Porous Films of Brominated Poly(phenylene oxide) and Hydrophilic SiO2/C Membrane via the Breath Figure Method
Abstract
:1. Introduction
2. Results and Discussion
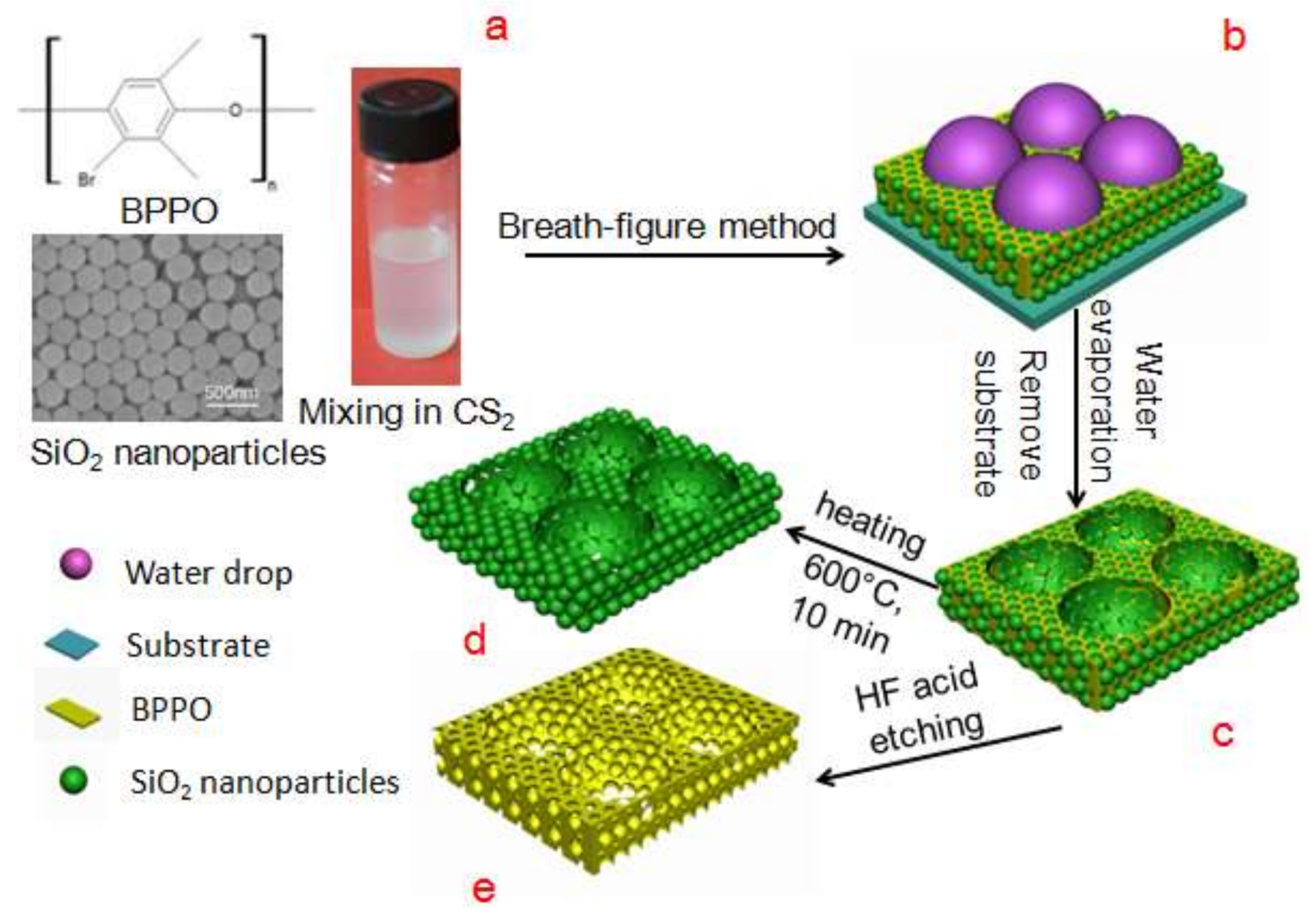
2.1. Morphology of Microfiltration Membrane
2.2. Analysis of Particle Assisted Membrane Formation
2.3. SiO2/C Membrane and HPP Microfiltration Membrane
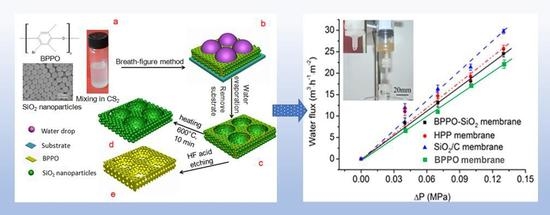
2.4. Application in Microfiltration
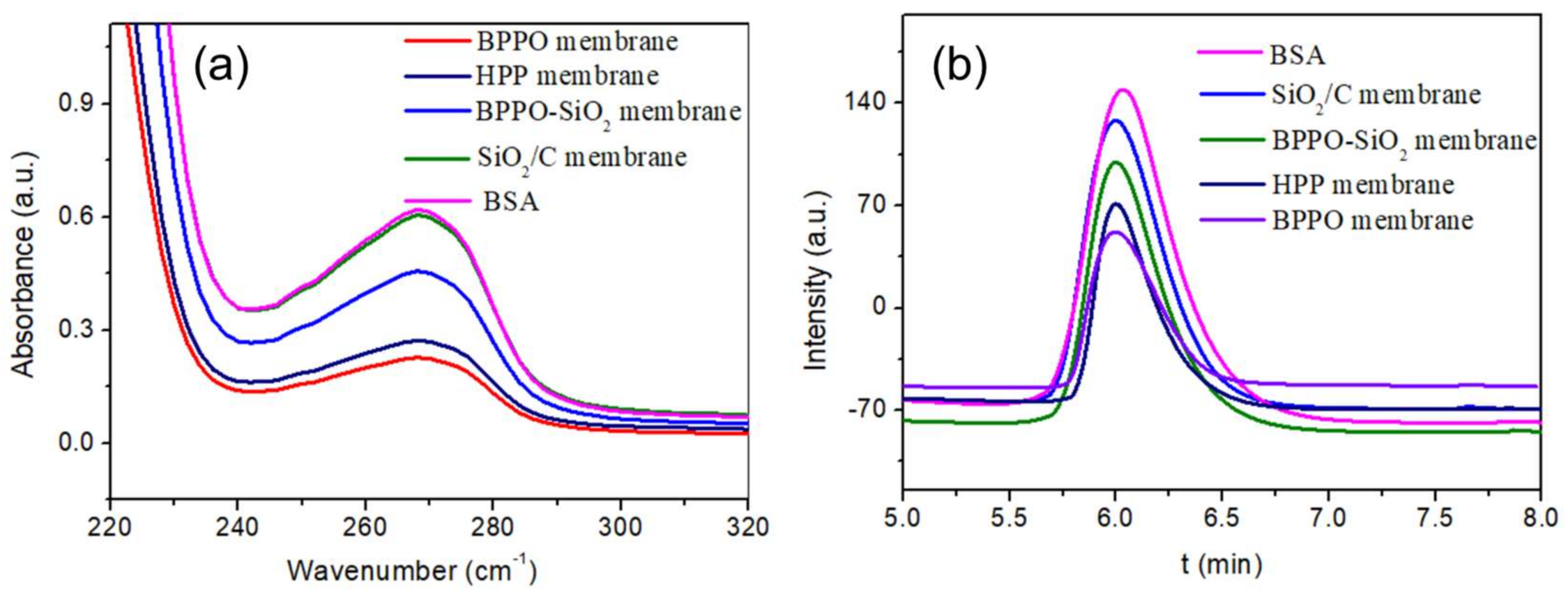
2.5. Anti-Fouling Performance
3. Materials and Methods
3.1. Materials
3.2. The Synthesis of Silica Microspheres
3.3. Preparation of Ordered Porous Membranes
3.4. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, X.; Zhuang, J.; Wang, X. SnO2 quantum dots and quantum wires: Controllable synthesis, self-assembled 2D architectures, and gas-sensing properties. J. Am. Chem. Soc. 2008, 130, 12527–12535. [Google Scholar] [CrossRef] [PubMed]
- Chari, K.; Lander, C.W.; Sudol, R.J. Anamorphic microlens arrays based on breath-figure template with adaptive surface reconstruction. Appl. Phys. Lett. 2008, 92, 111916. [Google Scholar] [CrossRef]
- Yu, B.; Liu, X.; Cong, H.; Yuan, H.; Wang, D.; Li, Z. Graphene-based multilayers constructed from layer-by-layer self-assembly techniques. J. Nanosci. Nanotechnol. 2014, 14, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C. Micropatterning of Proteins on 3D Porous Polymer Film Fabricated by Using the Breath-Figure Method. Adv. Mater. 2007, 19, 913–916. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, B.; Cong, H.; Peng, Q.; Yang, R.; Yang, S.; Yang, Z.; Luo, Y.; Xu, T.; Zhang, H.; et al. MODIFICATION PROGRESS OF POLYMER MEMBRANES FOR GAS SEPARATION. Rev. Adv. Mater. Sci. 2016, 44, 207–220. [Google Scholar]
- Vohra, V.; Yunus, S.; Attout, A.; Giovanella, U.; Scavia, G.; Tubino, R.; Botta, C.; Bolognesi, A. Bifunctional microstructured films and surfaces obtained by soft lithography from breath figure arrays. Soft Matter 2009, 5, 1656–1661. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Aceto, M.C.; Macedonio, F.; Drioli, E. Water droplets as template for next-generation self-assembled poly-(etheretherketone) with cardo membranes. J. Phys. Chem. B 2008, 112, 10483–10496. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gao, S.; Jing, J.; Lu, Q. Designing 3D Biological Surfaces via the Breath-Figure Method. Adv. Healthc. Mater. 2018, 1701043–1701061. [Google Scholar] [CrossRef] [PubMed]
- Bunz, U.H.F. Breath Figures as a Dynamic Templating Method for Polymers and Nanomaterials. Adv. Mater. 2010, 18, 973–989. [Google Scholar] [CrossRef]
- Male, U.; Shin, B.K. Coupling of breath figure method with interfacial polymerization: Bottom-surface functionalized honeycomb-patterned porous films. Polymer 2017, 119, 206–211. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Maniglio, D.; Sousa, N.; Mano, J.F.; Reis, R.L.; Migliaresi, C. From honeycomb- to microsphere-patterned surfaces of poly(lactic acid) and a starch-poly(lactic acid) blend via the breath figure method. J. Appl. Biomater. Funct. Mater. 2017, 15, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yu, B.; Cong, H.; Peng, Q.; Yang, Z.; Luo, Y.; Chi, M. Preparation of highly permeable BPPO microfiltration membrane with binary porous structures on a colloidal crystal substrate by the breath figure method. J. Colloid Interface Sci. 2016, 461, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, T.; Chai, S.; Zhang, L.; Wu, L.; Li, H. Supramolecular star polymer films with tunable honeycomb structures templated by breath figures. Polymer 2017, 117, 306–314. [Google Scholar] [CrossRef]
- Zhang, A.; Bai, H.; Li, L. Breath Figure: A Nature-Inspired Preparation Method for Ordered Porous Films. Chem. Rev. 2015, 115, 9801–9868. [Google Scholar] [CrossRef] [PubMed]
- Barbari, T.A.; Koros, W.J.; Paul, D.R. Polymeric membranes based on bisphenol-A for gas separations. J. Membr. Sci. 1989, 42, 69–86. [Google Scholar] [CrossRef]
- Chen, J.; Yan, X.; Zhao, Q.; Li, L.; Huang, F. Adjustable supramolecular polymer microstructures fabricated by the breath figure method. Polym. Chem. 2012, 3, 458–462. [Google Scholar] [CrossRef]
- Kon, K.; Brauer, C.N.; Hidaka, K.; Löhmannsröben, H.G.; Karthaus, O. Preparation of patterned zinc oxide films by breath figure templating. Langmuir ACS J. Surf. Colloids 2010, 26, 12173–12176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shen, Y.; Zhang, S.; Zhang, H. A vapor phase hydrothermal modification method converting a honeycomb structured hybrid film into photoactive TiO2 film. Langmuir ACS J. Surf. Colloids 2009, 25, 11032–11037. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Cui, J.; Song, A.; Hao, J. Fabrication of freestanding honeycomb films with through-pore structures via air/water interfacial self-assembly. Chem. Commun. 2011, 47, 1154–1156. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, Q.; Han, F.; Li, C.; Sun, J.; Lu, Y. Fabrication of conducting polypyrrole film with microlens arrays by combination of breath figures and replica molding methods. Polymer 2012, 53, 2599–2603. [Google Scholar] [CrossRef]
- Englert, B.C.; Scholz, S.; Leech, P.J.; Srinivasarao, M.; Bunz, U.H. Templated Ceramic Microstructures by Using the Breath-Figure Method. Chemistry 2005, 11, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.; Chen, Y. Robust Organic/Inorganic Hybrid Porous Thin Films via Breath-Figure Method and Gelation Process. Macromol. Rapid Commun. 2007, 28, 2024–2028. [Google Scholar] [CrossRef]
- Yang, H.; Fu, L.; Wei, L.; Liang, J.; Binks, B.P. Compartmentalization of incompatible reagents within Pickering emulsion droplets for one-pot cascade reactions. J. Am. Chem. Soc. 2015, 137, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Male, U.; Shin, B.K. Graphene oxide incorporated poly(ε-caprolactone) honeycomb-patterned porous polymer films by the breath figure method. Macromol. Res. 2017, 25, 297–302. [Google Scholar] [CrossRef]
- Villalobos, L.F.; Neelakanda, P.; Karunakaran, M.; Cha, D.; Peinemann, K.V. Poly-thiosemicarbazide/gold nanoparticles catalytic membrane: In-situ growth of well-dispersed, uniform and stable gold nanoparticles in a polymeric membrane. Catal. Today 2014, 236, 92–97. [Google Scholar] [CrossRef]
- White, C.M.; Strazisar, B.R.; Granite, E.J.; Hoffman, J.S.; Pennline, H.W. Separation and capture of CO2 from large stationary sources and sequestration in geological formations—Coalbeds and deep saline aquifers. J. Air Waste Manag. Assoc. 2003, 53, 645–715. [Google Scholar] [CrossRef] [PubMed]
- Pieranski, P. Two-dimensional interfacial colloidal crystals. Phys. Rev. Lett. 1980, 45, 569–572. [Google Scholar] [CrossRef]
- Horozov, T.S.; Binks, B.P. Particle-Stabilized Emulsions: A Bilayer or a Bridging Monolayer? Angew. Chem. Int. Ed. 2006, 45, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shao, Z.; Ji, J. Particle-assisted fabrication of honeycomb-structured hybrid films via breath figures method. Polymer 2010, 51, 4169–4175. [Google Scholar] [CrossRef]
- Cong, H.; Wang, J.; Yu, B.; Tang, J. Preparation of a highly permeable ordered porous microfiltration membrane of brominated poly (phenylene oxide) on an ice substrate by the breath figure method. Soft Matter 2012, 8, 8835–8839. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Wu, D.; Fu, R.; Xu, T. A novel proton-conductive membrane with reduced methanol permeability prepared from bromomethylated poly (2,6-dimethyl-1,4-phenylene oxide) (BPPO). J. Membr. Sci. 2008, 310, 522–530. [Google Scholar] [CrossRef]
- Xu, T.; Yang, W. Fundamental studies of a new series of anion exchange membranes: Membrane preparation and characterization. J. Membr. Sci. 2001, 190, 159–166. [Google Scholar]
- Angle, S.U.C. Fabrication of Polymer-Ag Honeycomb Hybrid Film by Metal Complexation Induced Phase Separation at the Air/Water Interface. Macromol. Mater. Eng. 2016, 301, 1011–1015. [Google Scholar]
- Yu, B.; Cong, H.; Zhao, X. Hybrid brominated sulfonated poly(2,6-diphenyl-1,4-phenylene oxide) and SiO2 nanocomposite membranes for CO2/N2 separation. Prog. Nat. Sci. 2012, 22, 662–667. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Xin, Z.; Deng, M.; Wen, Y.; Song, Y. Controlled inkjetting of a conductive pattern of silver nanoparticles based on the coffee-ring effect. Adv. Mater. 2013, 25, 6714–6718. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Yu, B.; Cong, H.; Chi, M.; Cheng, Y.; Lv, C. Preparation of Hierarchical Highly Ordered Porous Films of Brominated Poly(phenylene oxide) and Hydrophilic SiO2/C Membrane via the Breath Figure Method. Materials 2018, 11, 481. https://doi.org/10.3390/ma11040481
Yuan H, Yu B, Cong H, Chi M, Cheng Y, Lv C. Preparation of Hierarchical Highly Ordered Porous Films of Brominated Poly(phenylene oxide) and Hydrophilic SiO2/C Membrane via the Breath Figure Method. Materials. 2018; 11(4):481. https://doi.org/10.3390/ma11040481
Chicago/Turabian StyleYuan, Hua, Bing Yu, Hailin Cong, Ming Chi, Yuanzhe Cheng, and Chunxin Lv. 2018. "Preparation of Hierarchical Highly Ordered Porous Films of Brominated Poly(phenylene oxide) and Hydrophilic SiO2/C Membrane via the Breath Figure Method" Materials 11, no. 4: 481. https://doi.org/10.3390/ma11040481