Thermal Properties of Zeolite-Containing Composites
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Mordenite–Pore–Phenol Resin Composites
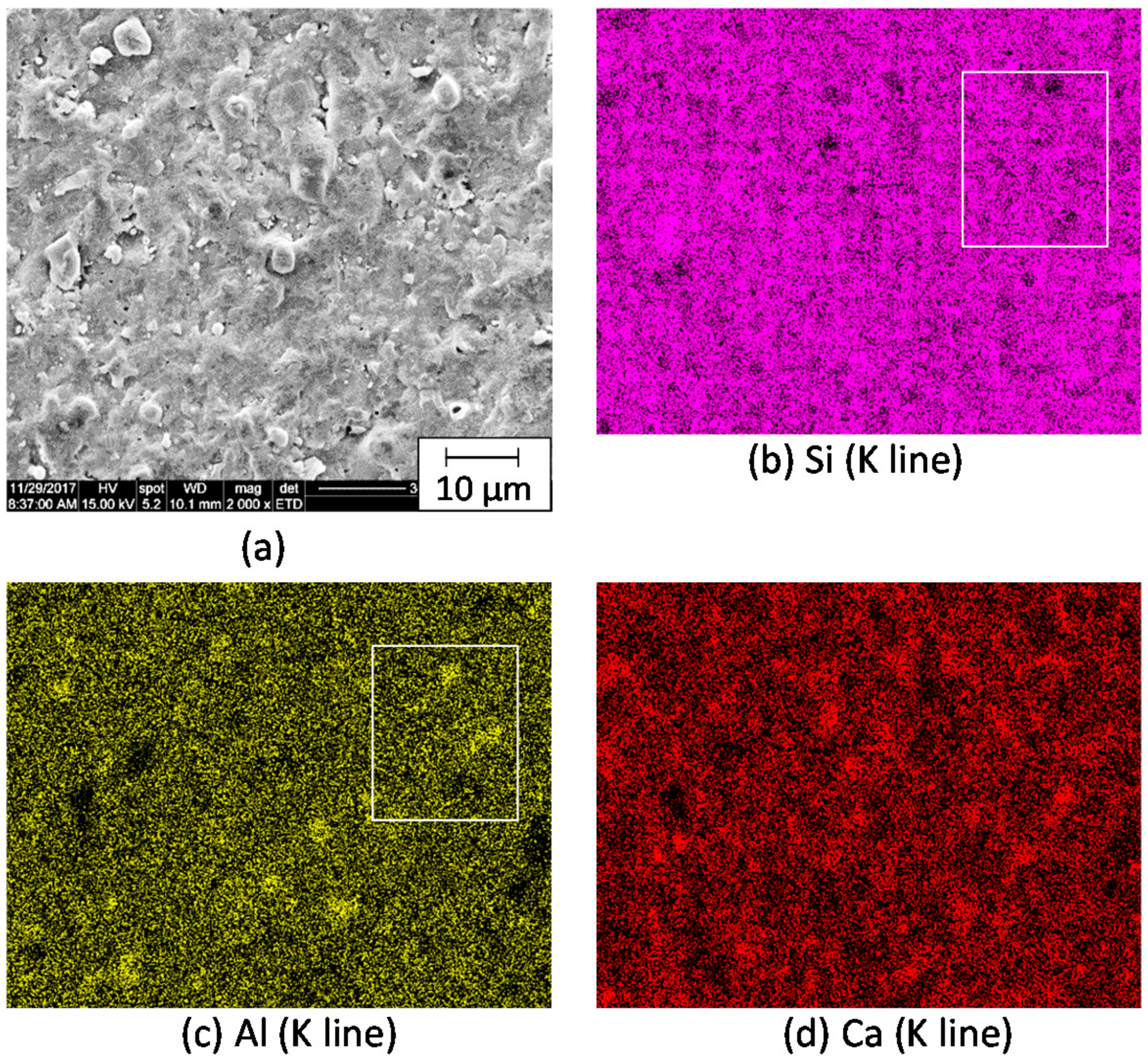
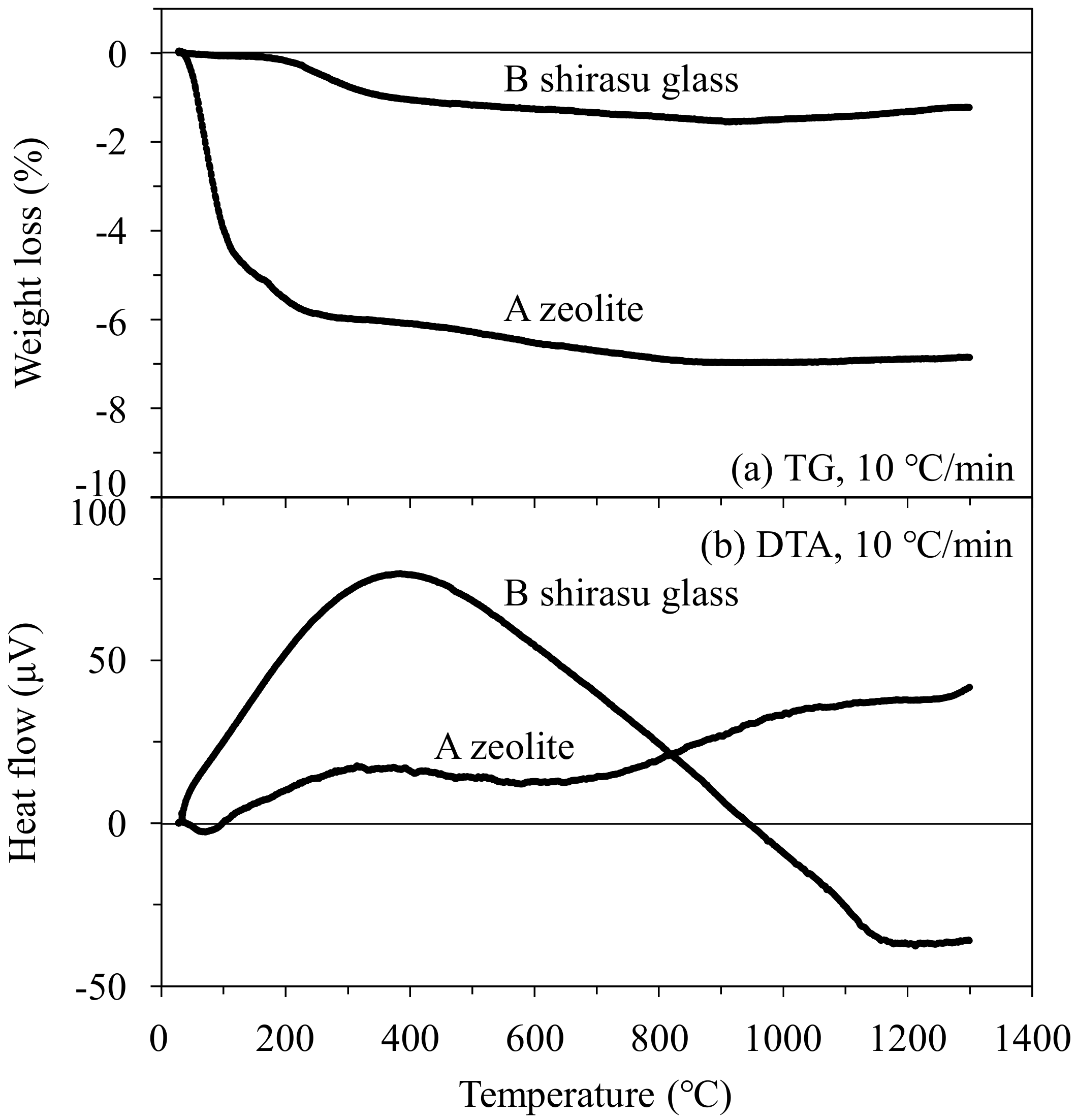
3.2. Microstructure of the Mordenite–Pore–Shirasu Glass Composites
3.3. Thermal Properties of the Mordenite–Shirasu Glass Composites
3.4. Temperature Dependence of Thermal Properties of the Zeolite–Pore–Shirasu Glass Composites
4. Conclusions
- (1)
- The thermal conductivity of mordenite was calculated to be 3.63 W/mK at room temperature based on the thermal conductivities of a dense monolithic phenol resin and the dense 12.4 vol % zeolite–3.1 vol % pore–phenol resin composite. The relative density of the composite decreased at higher zeolite contents (>15 vol %). The thermal conductivities of the composites with 0–21.7 vol % zeolite and 0.7–23.0 vol % pores were almost constant and were in the range of 0.23–0.30 W/mK.
- (2)
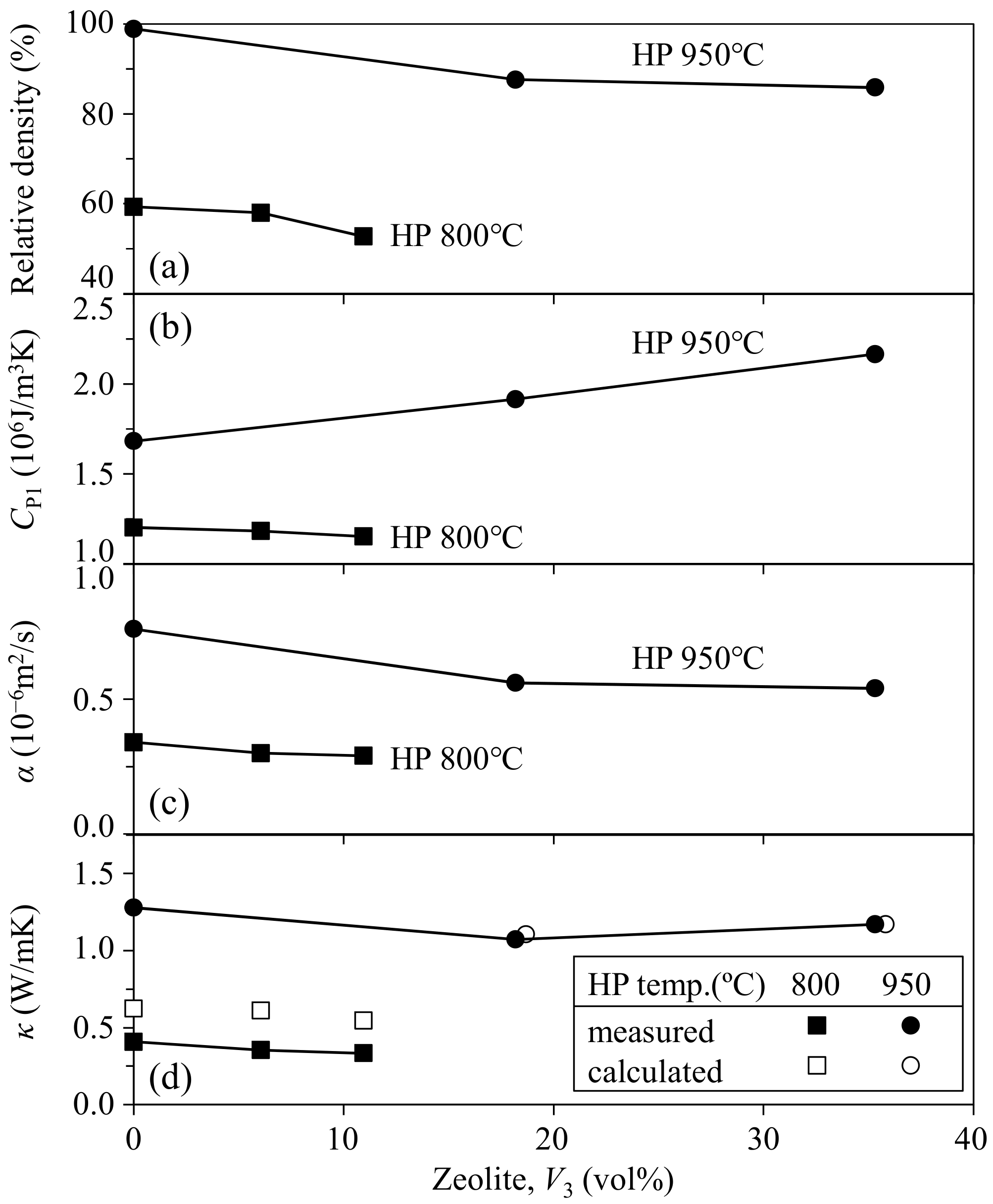
- In the zeolite–pore–shirasu glass composite, no change in the crystalline phases was observed after hot-pressing at 800–950 °C. The relative density of the composite decreased with increasing zeolite content and at a lower hot-pressing temperature. The thermal conductivities at room temperature were almost constant (1.07–1.28 W/mK) in the range from 0 to 35.3 vol % of zeolite content. This result is explained by the compensation effect between the zeolite with a higher thermal conductivity and air with a lower thermal conductivity.
- (3)
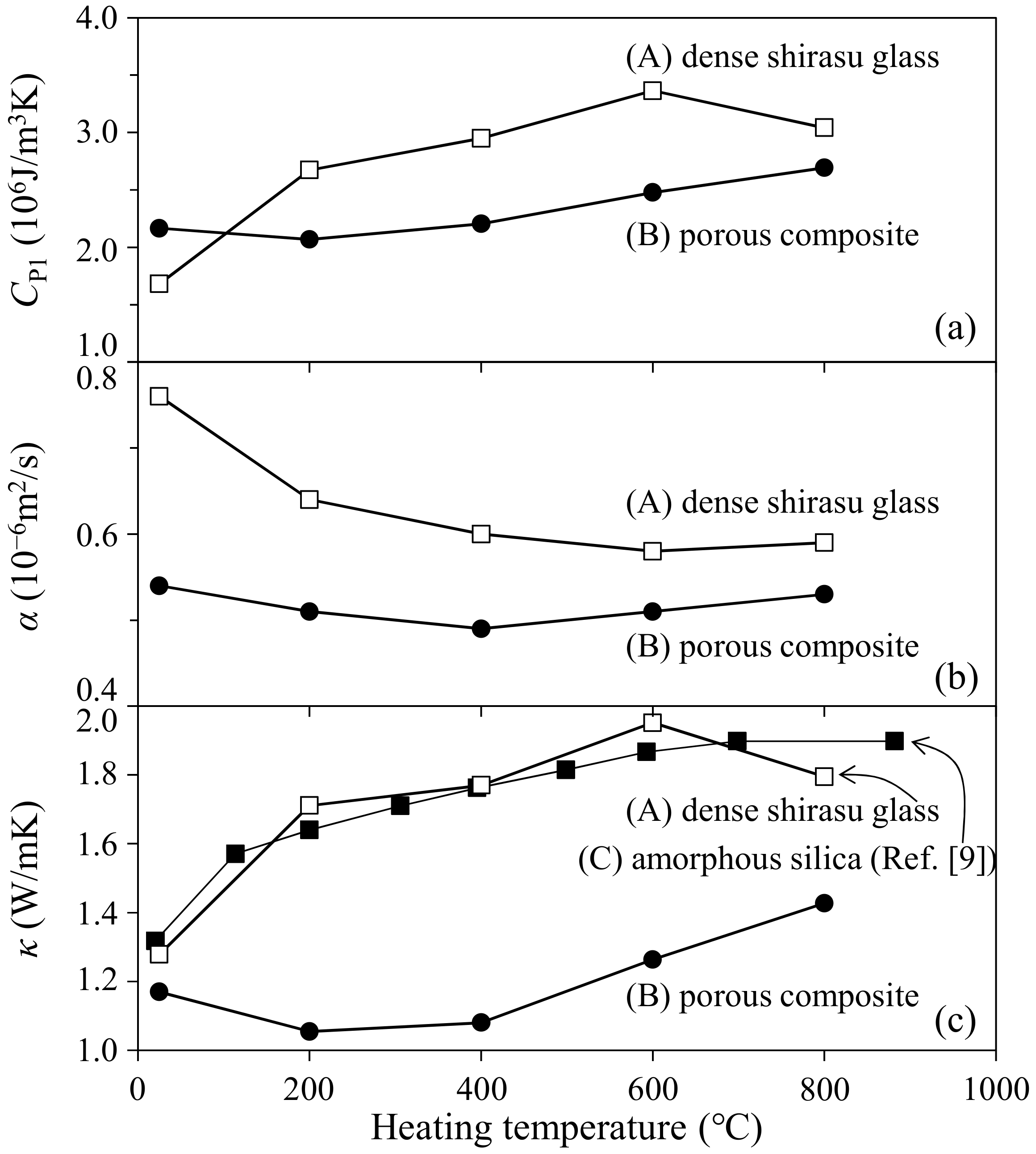
- The thermal conductivity of the zeolite–pore–shirasu glass composite with less than 15 vol % porosity was in good agreement with the calculated thermal conductivity for the shirasu glass continuous structure (structure A). However, the thermal conductivity of the composite decreased from the calculated value for structure A in the porosity range higher than 40 vol % and approached the value calculated for structure B with a pore continuous phase structure.
- (4)
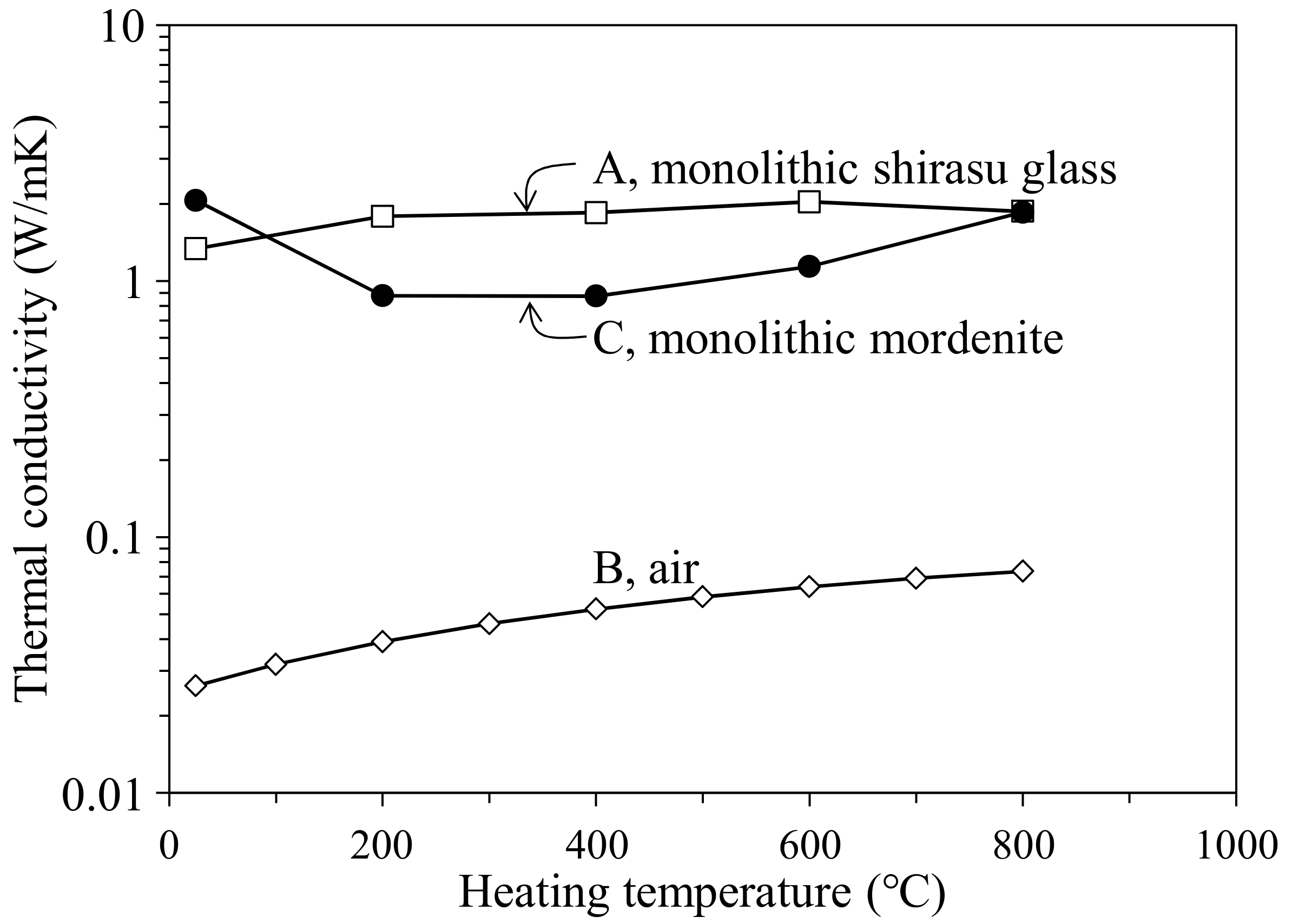
- The thermal conductivity of the monolithic mordenite was calculated for the composite hot-pressed at 950 °C to be 1.70–2.07 W/mK, and these values were smaller than the thermal conductivity (3.63 W/mK) of the zeolite processed at 150 °C. The decreased thermal conductivity of mordenite processed at high temperatures may reflect the partial decomposition of zeolite.
- (5)
- The thermal conductivity of the 35.3 vol % zeolite–14.2 vol % pore–shirasu glass composite exhibited the minimum value of 1.06 W/mK at 200 °C and then increased to 1.43 W/mK at 800 °C. The above result is strongly related to the change of the thermal conductivity of monolithic mordenite with heating. With heating, H2O, O2, and N2 molecules captured in the cage structure of mordenite are released, leading to the decrease in the thermal conductivity due to the formation of vacuum spaces in the mordenite.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coquil, T.; Lew, C.M.; Yan, Y.; Pilon, L. Thermal conductivity of pure silica MEL and MFI zeolite thin films. J. Appl. Phys. 2010, 108, 44902. [Google Scholar] [CrossRef]
- McGaughey, A.J.H.; Kaviany, M. Thermal conductivity decomposition and analysis using molecular dynamics simulations Part II. complex silica structures. Int. J. Heat Mass Trans. 2004, 47, 1799–1816. [Google Scholar] [CrossRef]
- Griesinger, A.; Spindler, K.; Hahne, E. Measurements and theoretical modelling of the effective thermal conductivity of zeolites. Int. J. Heat Mass Trans. 1999, 42, 4363–4374. [Google Scholar] [CrossRef]
- Hirata, Y. Representation of thermal conductivity of solid material with particulate inclusion. Ceram. Int. 2009, 35, 2921–2926. [Google Scholar] [CrossRef]
- Hirata, Y.; Fukushige, Y.; Kuwazuru, H.; Yamashita, R.; Sameshima, S.; Kamino, Y. Electrical properties of carbon fiber/shirasu glass composite. J. Phys. Chem. Solids 1997, 58, 1443–1449. [Google Scholar] [CrossRef]
- Itoh, S.; Hirata, Y.; Shimonosono, T.; Sameshima, S. Theoretical and experimental analyses of thermal conductivity of the alumina–mullite system. J. Eur. Ceram. Soc. 2015, 35, 605–612. [Google Scholar] [CrossRef]
- Hirata, Y.; Kinoshita, Y.; Shimonosono, T.; Chaen, T. Theoretical and experimental analyses of thermal properties of porous polycrystalline mullite. Ceram. Int. 2017, 43, 9973–9978. [Google Scholar] [CrossRef]
- Hata, K. (Ed.) Chemical Handbook, 3rd ed.; The Chemical Society of Japan: Tokyo, Japan, 1984; p. 72. [Google Scholar]
- Freeman, J.J.; Anderson, A.C. Thermal conductivity of amorphous solids. Phys. Rev. B 1986, 34, 5684–5690. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimonosono, T.; Hirata, Y.; Nishikawa, K.; Sameshima, S.; Sodeyama, K.; Masunaga, T.; Yoshimura, Y. Thermal Properties of Zeolite-Containing Composites. Materials 2018, 11, 420. https://doi.org/10.3390/ma11030420
Shimonosono T, Hirata Y, Nishikawa K, Sameshima S, Sodeyama K, Masunaga T, Yoshimura Y. Thermal Properties of Zeolite-Containing Composites. Materials. 2018; 11(3):420. https://doi.org/10.3390/ma11030420
Chicago/Turabian StyleShimonosono, Taro, Yoshihiro Hirata, Kyohei Nishikawa, Soichiro Sameshima, Kenichi Sodeyama, Takuro Masunaga, and Yukio Yoshimura. 2018. "Thermal Properties of Zeolite-Containing Composites" Materials 11, no. 3: 420. https://doi.org/10.3390/ma11030420



