Efficiency Evaluation of Food Waste Materials for the Removal of Metals and Metalloids from Complex Multi-Element Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Food Waste Adsorbents
2.2 Preparation of Aqueous Solutions and Synthetic Multi-Element Solutions
2.3. Preparation of Wastewater
2.4. Characterization of the Food Waste Adsorbents
2.5. Adsorption Experiments
2.6. Elements’ Adsorption Percentages
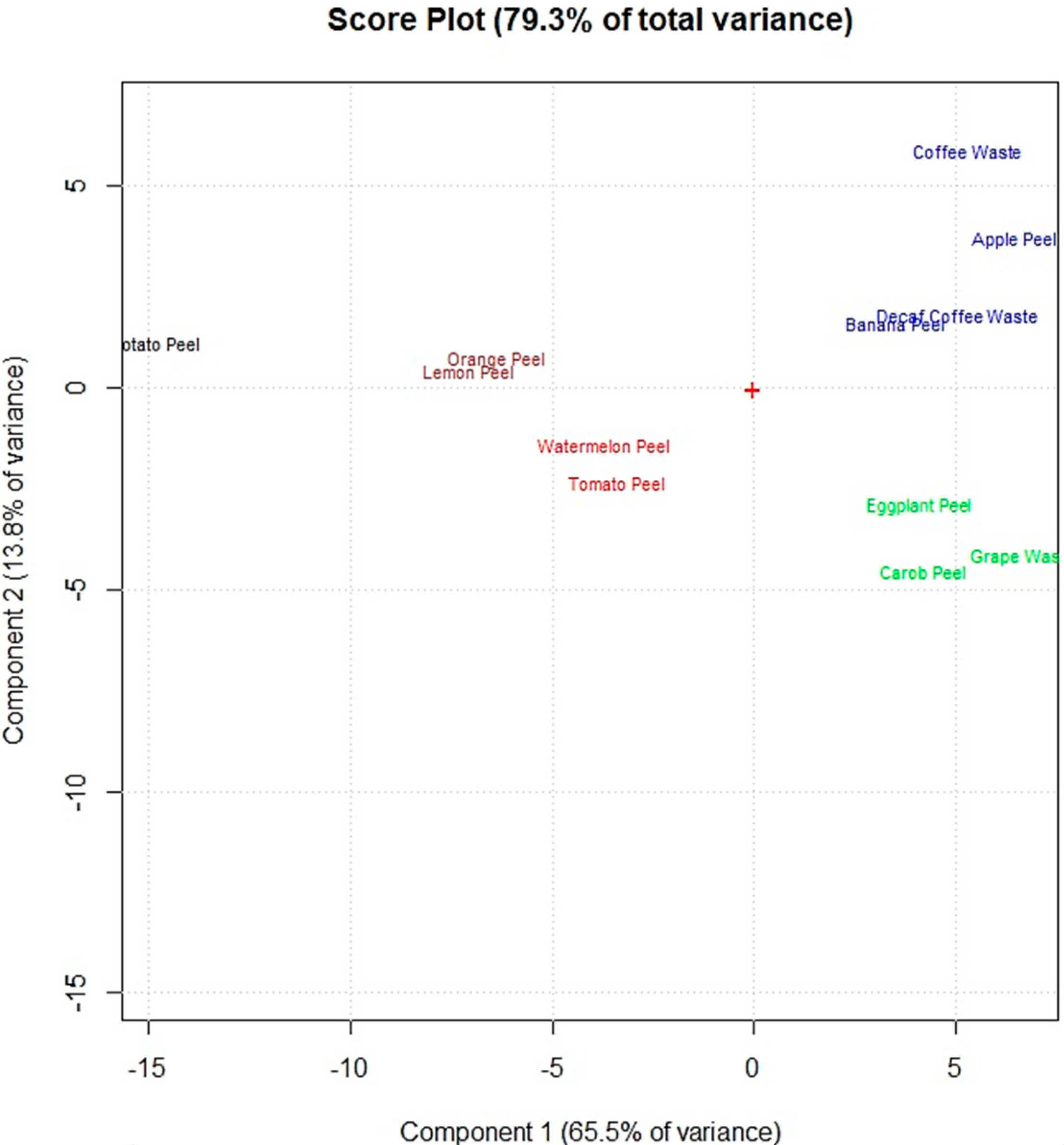
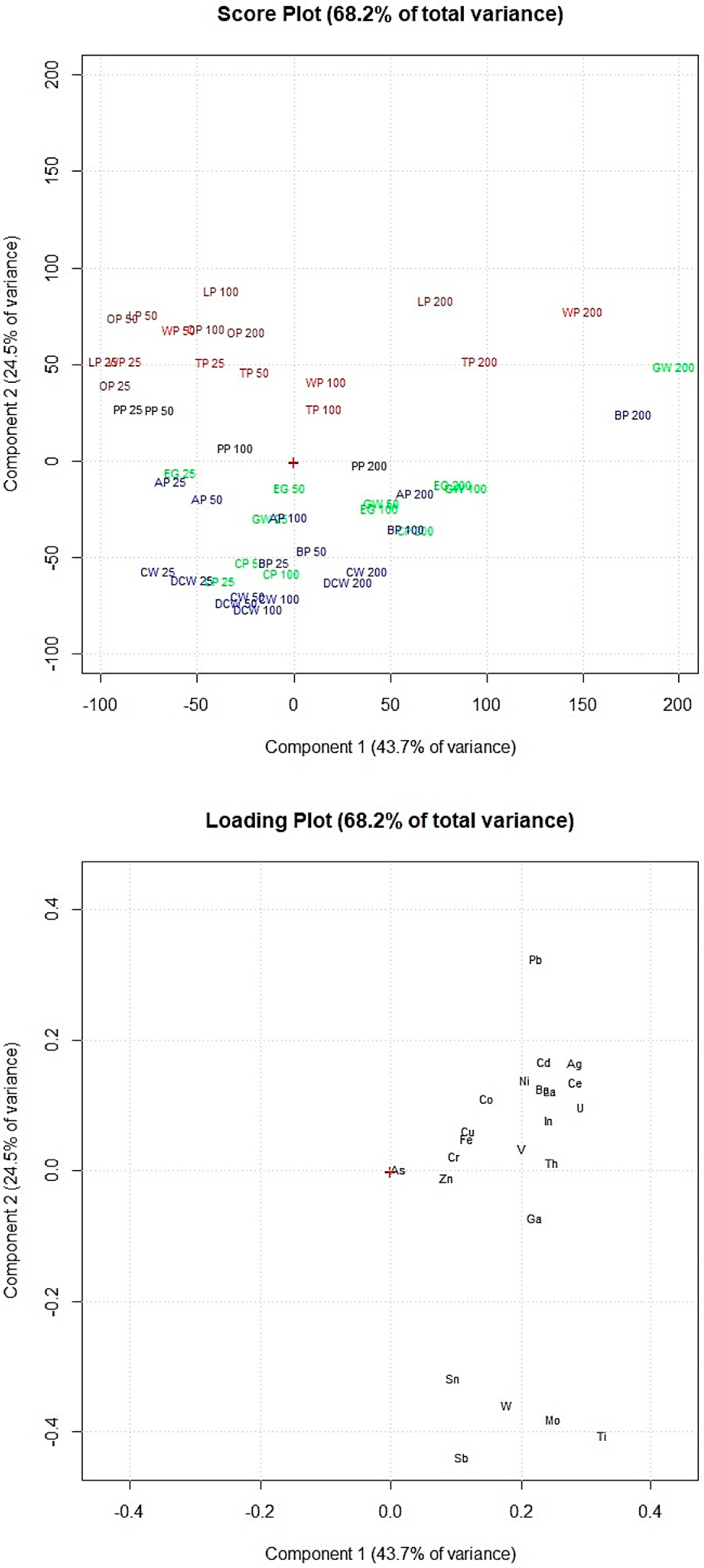
2.7. Statistical Analyses
3. Results and Discussion
3.1. SEM Micrographs
3.2. FTIR Spectra
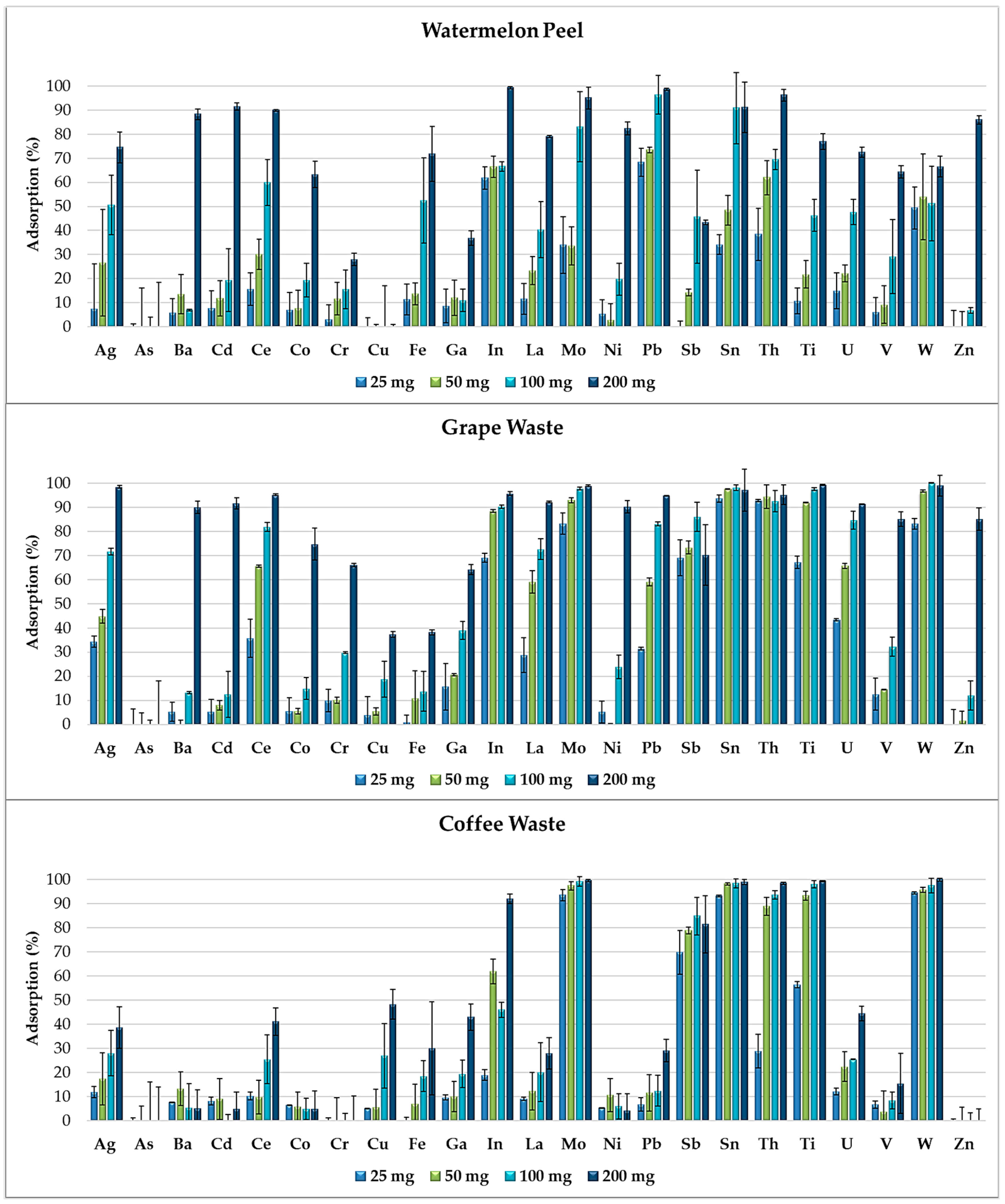
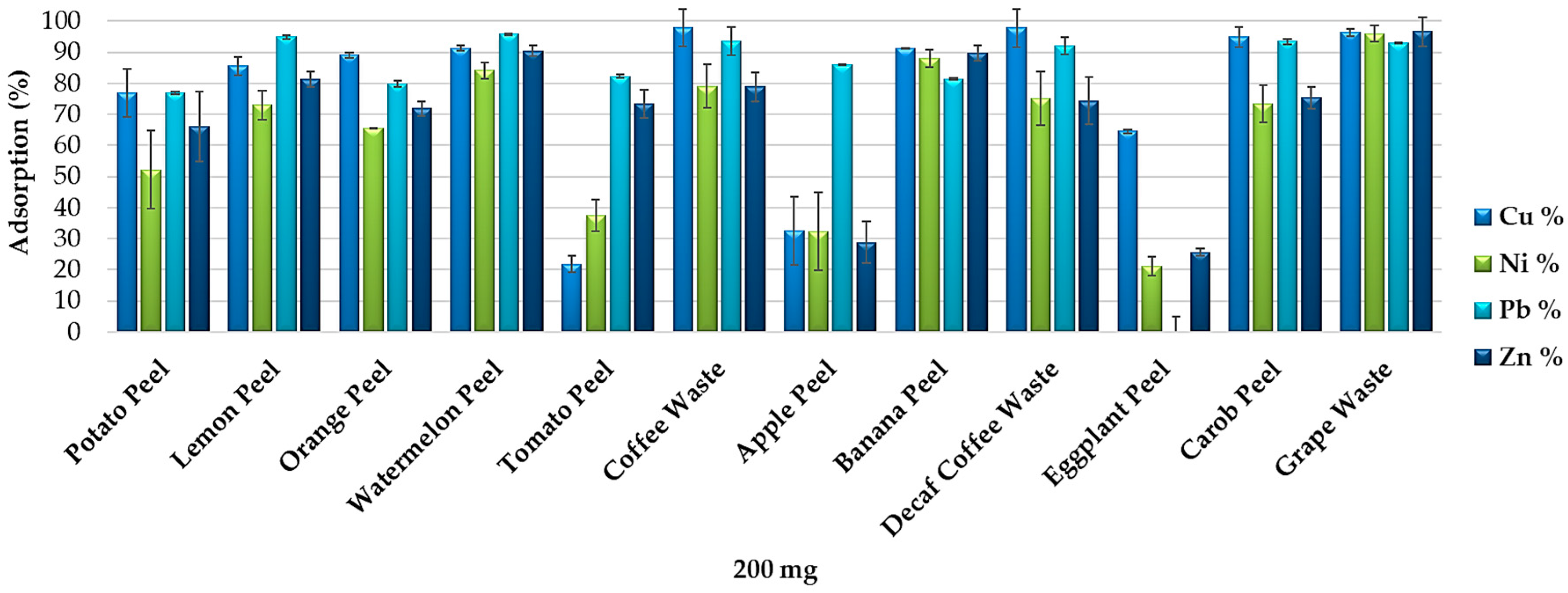
3.3. Removal Efficiency of Food Waste Adsorbents from Synthetic Multi-Element Solutions
3.4. Elements′ Removal Efficiency of Food Waste Adsorbents in Wastewater
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, H.A. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of hexavalent chromium-contaminated water and wastewater: A review. Water Air Soil Pollut. 2009, 200, 59–77. [Google Scholar] [CrossRef]
- Kamar, F.H.; Craciun, M.E.; Nechifor, A.C. Heavy metals: Sources, health effects, environmental effects, removal methods and natural adsorbent material as low-cost adsorbent: Short review. Int. J. Sci. Technol. Res. 2014, 3, 2974–2979. [Google Scholar]
- Aggarwal, D.; Goyal, M.; Bansal, R.C. Adsorption of chromium by activated carbon from aqueous solution. Carbon 1999, 37, 1989–1997. [Google Scholar] [CrossRef]
- Singh, S.P.; Ma, L.Q.; Harris, W.G. Heavy metal interactions with phosphatic clay. J. Environ. Qual. 2001, 30, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Stathi, P.; Litina, K.; Gournis, D.; Giannopoulos, T.S.; Deligiannakis, Y. Physicochemical study of novel organoclays as heavy metal ion adsorbents for environmental remediation. J. Colloid Interface Sci. 2007, 316, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Covelo, E.F.; Vega, F.A.; Andrade, M.L. Heavy metal adsorption and desorption by a Eutric Regosol and a District Regosol. Geophys. Res. Abstr. 2006, 8, 04553. [Google Scholar]
- Mataka, L.M.; Henry, E.M.T.; Masamba, W.R.L.; Sajidu, S.M. Lead remediation of contaminated water using Moringa Stenopetala and Moringa oleifera seed powder. Int. J. Environ. Sci. Technol. 2006, 3, 131–139. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P. Single-and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—An agricultural waste. Water Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef]
- Tan, W.T. Copper (II) adsorption by waste tea leaves and coffee powder. Pertanika 1985, 8, 223–230. [Google Scholar]
- Tee, T.W.; Khan, A.R.M. Removal of lead, cadmium and zinc by waste tea leaves. Environ. Technol. 1988, 9, 1223–1232. [Google Scholar] [CrossRef]
- Minamisawa, M.; Minamisawa, H.; Yoshida, S.; Takai, N. Adsorption behavior of heavy metals on biomaterials. J. Agric. Food Chem. 2004, 52, 5606–5611. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, S.S.; Goyal, D. Removal of heavy metals by waste tea leaves from aqueous solution. Eng. Life Sci. 2005, 5, 158–162. [Google Scholar] [CrossRef]
- Agwaramgbo, L.; Lathan, N.; Edwards, S.; Nunez, S. Assessing lead removal from contaminated water using solid biomaterials: Charcoal, coffee, tea, fishbone, and caffeine. J. Environ. Prot. 2013, 4, 741. [Google Scholar] [CrossRef]
- Ayub, S.; Ali, S.I.; Khan, N.A. Efficiency evaluation of neem (Azadirachta indica) bark in treatment of industrial wastewater. Environ. Pollut. Control J. 2001, 4, 34–38. [Google Scholar]
- Zvinowanda, C.M.; Okonkwo, J.O.; Shabalala, P.N.; Agyei, N.M. A novel adsorbent for heavy metal remediation in aqueous environments. Int. J. Environ. Sci. Technol. 2009, 6, 425–434. [Google Scholar] [CrossRef]
- Igwe, J.C.; Abia, A.A.; Ibeh, C.A. Adsorption kinetics and intraparticulate diffusivities of Hg, As and Pb ions on unmodified and thiolated coconut fiber. Int. J. Environ. Sci. Technol. 2008, 5, 83–92. [Google Scholar] [CrossRef]
- Tan, W.T.; Ooi, S.T.; Lee, C.K. Removal of chromium (VI) from solution by coconut husk and palm pressed fibres. Environ. Technol. 1993, 14, 277–282. [Google Scholar] [CrossRef]
- Srinivasan, K.; Balasubramanian, N.; Ramakrishna, T.V. Studies on chromium removal by rice husk carbon. Indian J. Environ. Health 1988, 30, 376–387. [Google Scholar]
- Khan, N.A.; Shaaban, M.G.; Hassan, M.A. Removal of heavy metal using an inexpensive adsorbent. Proceedings of UM Research Seminar, University of Malaya, Kuala Lumpur, Malaysia, 11–12 March 1988; pp. 1–5. [Google Scholar]
- Ajmal, M.; Rao, R.A.K.; Siddiqui, B.A. Studies on removal and recovery of Cr (VI) from electroplating wastes. Water Res. 1996, 30, 1478–1482. [Google Scholar] [CrossRef]
- Xirokostas, N.; Korkolis, A.; Diamantopoulou, L.; Moutsatsou, A. Characterisation of metal retention agents and study of their application in liquid wastes. Glob. Nest Int. J. 2003, 5, 29–37. [Google Scholar]
- Aliabadi, M.; Morshedzadeh, K.; Soheyli, H. Removal of hexavalent chromium from aqueous solution by lignocellulosic solid wastes. Int. J. Environ. Sci. Technol. 2006, 3, 321–325. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Shitu, A.; Tadda, M.A.; Ngabura, M. Utilization of various agricultural waste materials in the treatment of industrial wastewater containing heavy metals: A Review. Int. Res. J. Environ. Sci. 2014, 3, 62–71. [Google Scholar]
- Gustafsson, J.; Cederberg, C.; Sonesson, U.; Emanuelsson, A. The Methodology of the FAO Study: Global Food Losses and Food Waste-Extent, Causes and Prevention”—FAO, 2011. Available online: http://www.diva-portal.org/smash/get/diva2:944159/FULLTEXT01.pdf (accessed on 23 February 2018).
- Castro, R.S.; Caetano, L.; Ferreira, G.; Padilha, P.M.; Saeki, M.J.; Zara, L.F.; Martines, M.A.U.; Castro, G.R. Banana peel applied to the solid phase extraction of copper and lead from river water: Preconcentration of metal ions with a fruit waste. Ind. Eng. Chem. Res. 2011, 50, 3446–3451. [Google Scholar] [CrossRef]
- Mallampati, R.; Valiyaveettil, S. Apple peels—A versatile biomass for water purification? ACS Appl. Mater. Interfaces 2013, 5, 4443–4449. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.H.; Babar, Z.B.; Khamis, M.I. Removal of Lead (II) Ions from Aqueous Solution Using Eggplant Peels Activated Charcoal. Sep. Sci. Technol. 2015, 50, 91–98. [Google Scholar] [CrossRef]
- Aman, T.; Kazi, A.A.; Sabri, M.U.; Bano, Q. Potato peels as solid waste for the removal of heavy metal copper (II) from waste water/industrial effluent. Colloids Surf. B 2008, 63, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Ugbe, F.A.; Pam, A.A.; Ikudayisi, A.V. Thermodynamic properties of chromium (III) ion adsorption by sweet orange (Citrus sinensis) peels. Am. J. Anal. Chem. 2014, 5, 666. [Google Scholar] [CrossRef]
- Razafsha, A.; Ziarati, P. Removal of Heavy Metals from Oryza sativa Rice by Sour Lemon Peel as Bio-sorbent. Biomed. Pharmacol. J. 2016, 9, 543–553. [Google Scholar] [CrossRef]
- Arslanoglu, H.; Altundogan, H.S.; Tumen, F. Heavy metals binding properties of esterified lemon. J. Hazard. Mater. 2009, 164, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Lakshmipathy, R.; Sarada, N.C. Application of watermelon rind as sorbent for removal of nickel and cobalt from aqueous solution. Int. J. Miner. Process. 2013, 122, 63–65. [Google Scholar] [CrossRef]
- Lakshmipathy, R.; Vinod, A.V.; Sarada, N.C. Watermelon rind as biosorbent for removal of Cd2+ from aqueous solution: FTIR, EDX, and Kinetic studies. J. Indian Chem. Soc. 2013, 90, 1147–1154. [Google Scholar]
- Mallampati, R.; Valiyaveettil, S. Application of tomato peel as an efficient adsorbent for water purification—Alternative biotechnology? RSC Adv. 2012, 2, 9914–9920. [Google Scholar] [CrossRef]
- Kyzas, G.Z. Commercial coffee wastes as materials for adsorption of heavy metals from aqueous solutions. Materials 2012, 5, 1826–1840. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Kostoglou, M.; Lazaridis, N.K. Copper removal from aqueous systems with coffee wastes as low-cost materials. In Proceedings of the E3S Web of Conferences, Torino, Italy, 28–31 October 2013. [Google Scholar]
- Polat, S.; Putun, E.; Kilic, M.; Putun, A.E. Biosorption of Cu (II) from aqueous solution by grape waste: Equilibrium, Kinetics and Thermodynamics. J. Selcuk Univ. Nat. Appl. Sci. 2013, 2, 108–119. [Google Scholar]
- Dwyer, K.; Hosseinian, F.; Rod, M. The market potential of grape waste alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- Canepari, S.; Astolfi, M.L.; Marconi, E.; Farao, C. Caratterizzazione del Refluo Prodotto nel Processo Idrometallurgico per il Recupero di Elementi Pregiati da Schede Elettroniche (RAEE) Sviluppato da ENEA. Available online: http://www.enea.it/it/Ricerca_sviluppo/documenti/ricerca-di-sistema-elettrico/risparmio-energia-settore-civile/2014/rds-par2014-041.pdf (accessed on 23 February 2018).
- Canepari, S.; Cardarelli, E.; Giuliano, A.; Pietrodangelo, A. Determination of metals, metalloids and non-volatile ions in airborne particulate matter by a new two-step sequential leaching procedure: Part A: Experimental design and optimisation. Talanta 2006, 69, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Astolfi, M.L.; Canepari, S.; Vitali, M. Urinary levels of trace elements among primary school-aged children from Italy: The contribution of smoking habits of family members. Sci. Total Environ. 2016, 557, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.À.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Lyman, D.J.; Benck, R.; Dell, S.; Merle, S.; Murray-Wijelath, J. FTIR-ATR analysis of brewed coffee: effect of roasting conditions. J. Agric. Food Chem. 2003, 51, 3268–3272. [Google Scholar] [CrossRef] [PubMed]
- Kemsley, E.K.; Ruault, S.; Wilson, R.H. Discrimination between Coffea arabica and Coffea canephora variant robusta beans using infrared spectroscopy. Food Chem. 1995, 54, 321–326. [Google Scholar] [CrossRef]
- Wang, N.; Lim, L.T. Fourier transform infrared and physicochemical analyses of roasted coffee. J. Agric. Food Chem. 2012, 60, 5446–5453. [Google Scholar] [CrossRef] [PubMed]
- Haussard, M.; Gaballah, I.; Kanari, N.; De Donato, P.; Barrès, O.; Villieras, F. Separation of hydrocarbons and lipid from water using treated bark. Water Res. 2003, 37, 362–374. [Google Scholar] [CrossRef]
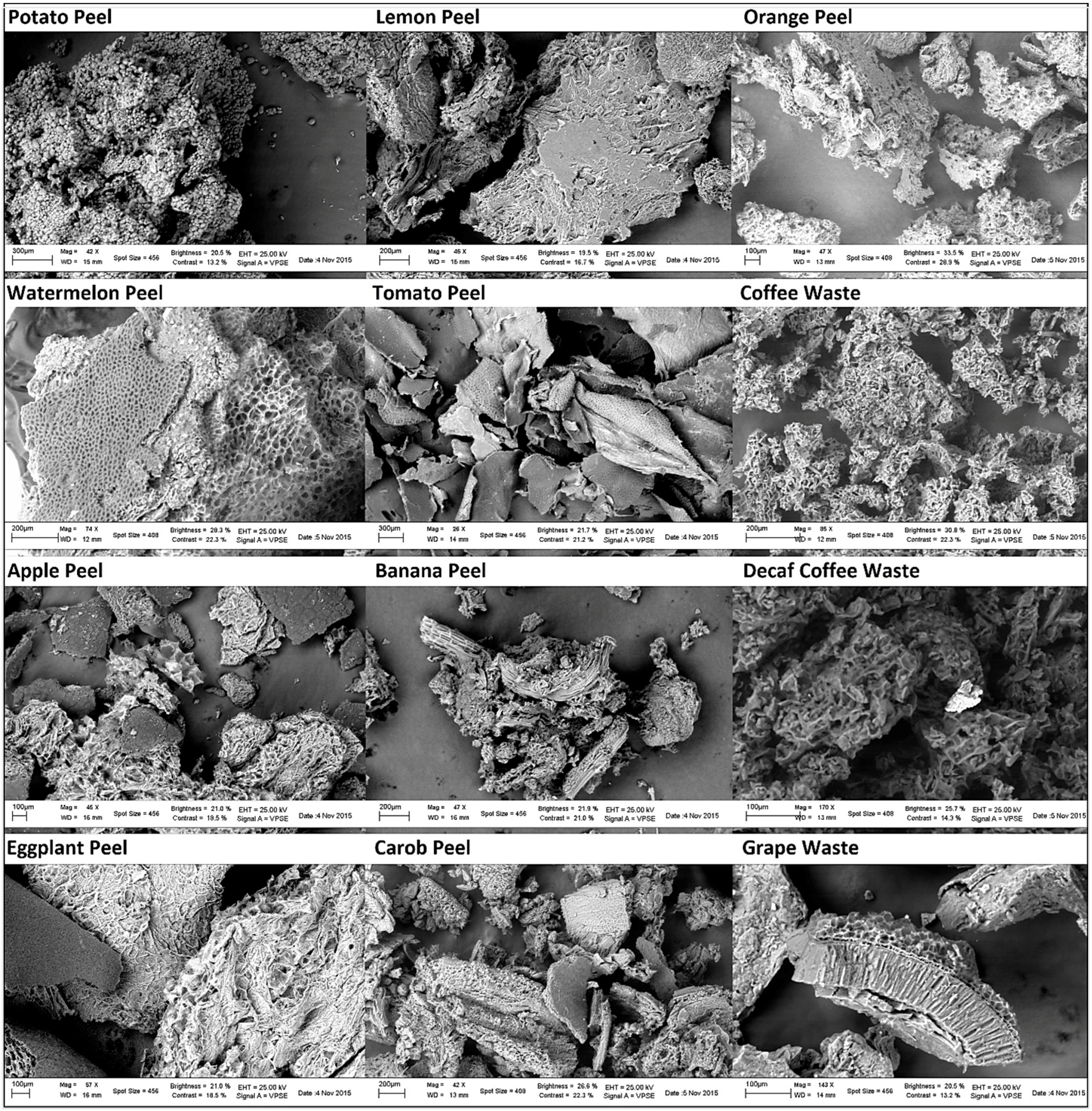

| Potato Peel | Lemon Peel | Orange Peel | Watermel. Peel | Tomato Peel | Coffee Waste | Apple Peel | Banana Peel | Decaf C. Waste | Eggplant Peel | Carob Peel | Grape Peel | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adsorption (%) | pH 2 | |||||||||||
| m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | |
| Ag | 71 ± 8 | 96 ± 2 | 99 ± 5 | 75 ± 6 | 100 ± 1 | 39 ± 9 | 34 ± 24 | 94 ± 2 | 47 ± 8 | 70 ± 3 | 55 ± 3 | 98 ± 1 |
| As | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 37 ± 11 | <10 | <10 | 79 ± 2 | <10 |
| Ba | 18 ± 12 | 56 ± 6 | 92 ± 1 | 88 ± 2 | 71 ± 5 | <10 | 39 ± 17 | 80 ± 2 | <10 | 14 ± 4 | 20 ± 4 | 90 ± 3 |
| Cd | 17 ± 11 | 64 ± 4 | 92 ± 1 | 91 ± 1 | 66 ± 4 | <10 | 22 ± 5 | 88 ± 2 | <10 | 15 ± 5 | 14 ± 4 | 92 ± 2 |
| Ce | 24 ± 3 | 68 ± 1 | 100 ± 1 | 90 ± 1 | 38 ± 2 | 41 ± 6 | 59 ± 7 | 77 ± 1 | 15 ± 6 | 72 ± 2 | 42 ± 3 | 95 ± 1 |
| Co | <10 | 43 ± 6 | 43 ± 1 | 63 ± 5 | 32 ± 5 | <10 | 14 ± 2 | 46 ± 5 | <10 | <10 | <10 | 75 ± 7 |
| Cr | <10 | 15 ± 7 | 20 ± 2 | 28 ± 3 | 47 ± 3 | <10 | 34 ± 13 | 12 ± 2 | 14 ± 9 | <10 | 43 ± 10 | 66 ± 1 |
| Cu | 51 ± 8 | 75 ± 3 | 75 ± 1 | <10 | 62 ± 3 | 48 ± 6 | 49 ± 21 | 58 ± 1 | 43 ± 6 | 13 ± 1 | 60 ± 3 | 37 ± 1 |
| Fe | 42 ± 20 | <10 | <10 | 72 ± 11 | 38 ± 8 | 30 ± 19 | 13 ± 5 | 42 ± 2 | 11 ± 15 | 53 ± 13 | 40 ± 19 | 38 ± 1 |
| Ga | 31 ± 12 | <10 | <10 | 37 ± 3 | 29 ± 1 | 43 ± 5 | 40 ± 4 | 93 ± 1 | 41 ± 5 | 54 ± 4 | 36 ± 3 | 64 ± 2 |
| In | 73 ± 2 | 92 ± 1 | 76 ± 3 | 99 ± 1 | 55 ± 1 | 92 ± 2 | 92 ± 2 | 96 ± 1 | 87 ± 3 | 48 ± 1 | 89 ± 2 | 96 ± 1 |
| La | 15 ± 6 | 51 ± 1 | 75 ± 1 | 79 ± 1 | 37 ± 1 | 28 ± 7 | 40 ± 13 | 62 ± 1 | 33 ± 8 | 59 ± 1 | 22 ± 4 | 92 ± 1 |
| Mo | 94 ± 2 | 73 ± 2 | 87 ± 1 | 95 ± 5 | 88 ± 1 | 100 ± 1 | 99 ± 1 | 99 ± 1 | 100 ± 1 | 98 ± 1 | 99 ± 1 | 99 ± 1 |
| Ni | <10 | 49 ± 5 | 87 ± 1 | 82 ± 3 | 36 ± 5 | <10 | <10 | 71 ± 3 | <10 | 10 ± 3 | 10 ± 6 | 90 ± 3 |
| Pb | 77 ± 1 | 93 ± 1 | <10 | 99 ± 1 | 90 ± 1 | 29 ± 5 | 88 ± 1 | 97 ± 1 | 17 ± 3 | 95 ± 5 | 79 ± 1 | 95 ± 1 |
| Sb | 46 ± 5 | 25 ± 5 | 19 ± 5 | 43 ± 1 | 32 ± 8 | 81 ± 12 | 82 ± 11 | 88 ± 14 | 83 ± 12 | 72 ± 15 | 93 ± 14 | 70 ± 13 |
| Sn | 86 ± 13 | 66 ± 26 | 57 ± 27 | 91 ± 10 | 59 ± 14 | 99 ± 1 | 92 ± 8 | 97 ± 1 | 99 ± 1 | 92 ± 1 | 99 ± 1 | 97 ± 9 |
| Th | 96 ± 1 | 96 ± 1 | <10 | 96 ± 2 | 97 ± 1 | 98 ± 1 | 91 ± 5 | 98 ± 1 | 96 ± 1 | 97 ± 1 | 92 ± 1 | 95 ± 4 |
| Ti | 76 ± 6 | 61 ± 1 | 100 ± 1 | 77 ± 3 | 82 ± 1 | 99 ± 1 | 92 ± 4 | 99 ± 1 | 100 ± 1 | 98 ± 1 | 100 ± 1 | 99 ± 1 |
| U | 50 ± 7 | 45 ± 1 | 100 ± 1 | 72 ± 2 | 86 ± 1 | 44 ± 3 | 54 ± 7 | 88 ± 1 | 39 ± 3 | 82 ± 1 | 36 ± 4 | 91 ± 1 |
| V | 31 ± 12 | 13 ± 3 | 77 ± 1 | 64 ± 3 | 14 ± 9 | 15 ± 13 | 15 ± 1 | 75 ± 1 | 56 ± 9 | 26 ± 6 | 60 ± 3 | 85 ± 3 |
| W | 100 ± 5 | 67 ± 10 | 93 ± 14 | 67 ± 4 | 93 ± 3 | 100 ± 1 | 100 ± 2 | 100 ± 1 | 100 ± 1 | 100 ± 2 | 100 ± 1 | 99 ± 4 |
| Zn | 14 ± 12 | 55 ± 2 | 88 ± 2 | 86 ± 2 | 22 ± 5 | <10 | 13 ± 7 | 72 ± 3 | <10 | <10 | <10 | 85 ± 5 |
| Adsorption (%) | pH 5.5 | |||||||||||
| m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | m ± SD | |
| Ag | 94 ± 11 | 99 ± 3 | <10 | 99 ± 14 | 29 ± 1 | 98 ± 13 | 99 ± 34 | 96 ± 7 | 99 ± 11 | 99 ± 6 | 100 ± 4 | 85 ± 2 |
| Ba | 89 ± 12 | 93 ± 7 | <10 | 96 ± 2 | 97 ± 5 | 74 ± 13 | 99 ± 16 | 94 ± 10 | 98 ± 7 | 74 ± 8 | 92 ± 3 | 98 ± 2 |
| Cd | 90 ± 14 | 92 ± 12 | 12 ± 15 | 97 ± 11 | 79 ± 10 | 95 ± 7 | 95 ± 8 | 90 ± 8 | 95 ± 12 | 79 ± 8 | 94 ± 9 | 98 ± 9 |
| Co | 54 ± 10 | 45 ± 9 | <10 | 41 ± 9 | 41 ± 6 | 56 ± 9 | 44 ± 2 | 37 ± 4 | 51 ± 8 | 38 ± 3 | 78 ± 4 | 48 ± 8 |
| Cr | <10 | 15 ± 9 | 16 ± 10 | 23 ± 8 | 23 ± 5 | <10 | 18 ± 11 | 30 ± 2 | <10 | 29 ± 5 | 42 ± 9 | 11 ± 1 |
| Cu | 68 ± 18 | 78 ± 17 | 19 ± 5 | 92 ± 13 | 12 ± 8 | 95 ± 15 | 97 ± 28 | 77 ± 2 | 95 ± 9 | 85 ± 10 | 93 ± 8 | 86 ± 7 |
| In | 80 ± 4 | 72 ± 2 | 74 ± 5 | 86 ± 2 | 72 ± 1 | 66 ± 4 | 93 ± 2 | 98 ± 1 | 61 ± 4 | 52 ± 1 | 75 ± 5 | 96 ± 1 |
| Mo | 81 ± 5 | 80 ± 12 | 58 ± 7 | 94 ± 14 | 21 ± 2 | 93 ± 2 | 99 ± 10 | 95 ± 2 | 94 ± 1 | 97 ± 1 | 100 ± 2 | 74 ± 1 |
| Ni | 72 ± 11 | 85 ± 8 | <10 | 94 ± 7 | 57 ± 8 | 93 ± 9 | 94 ± 12 | 88 ± 3 | 92 ± 11 | 65 ± 2 | 89 ± 6 | 96 ± 6 |
| Sb | 26 ± 4 | 25 ± 6 | <10 | 32 ± 15 | 21 ± 8 | 52 ± 15 | 80 ± 16 | 86 ± 16 | 57 ± 14 | 52 ± 16 | 93 ± 15 | <10 |
| V | 82 ± 9 | 64 ± 3 | 11 ± 7 | 90 ± 14 | 55 ± 7 | 90 ± 12 | 99 ± 3 | 95 ± 11 | 91 ± 10 | 97 ± 9 | 100 ± 5 | 95 ± 5 |
| W | 85 ± 9 | 90 ± 7 | 12 ± 19 | 95 ± 2 | 56 ± 10 | 90 ± 6 | 91 ± 8 | 79 ± 7 | 92 ± 15 | 37 ± 3 | 91 ± 9 | 96 ± 8 |
| Zn | 85 ± 9 | 90 ± 7 | 12 ± 19 | 95 ± 2 | 56 ± 10 | 96 ± 6 | 91 ± 8 | 79 ± 7 | 92 ± 15 | 37 ± 3 | 91 ± 9 | 96 ± 8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massimi, L.; Giuliano, A.; Astolfi, M.L.; Congedo, R.; Masotti, A.; Canepari, S. Efficiency Evaluation of Food Waste Materials for the Removal of Metals and Metalloids from Complex Multi-Element Solutions. Materials 2018, 11, 334. https://doi.org/10.3390/ma11030334
Massimi L, Giuliano A, Astolfi ML, Congedo R, Masotti A, Canepari S. Efficiency Evaluation of Food Waste Materials for the Removal of Metals and Metalloids from Complex Multi-Element Solutions. Materials. 2018; 11(3):334. https://doi.org/10.3390/ma11030334
Chicago/Turabian StyleMassimi, Lorenzo, Antonella Giuliano, Maria Luisa Astolfi, Rossana Congedo, Andrea Masotti, and Silvia Canepari. 2018. "Efficiency Evaluation of Food Waste Materials for the Removal of Metals and Metalloids from Complex Multi-Element Solutions" Materials 11, no. 3: 334. https://doi.org/10.3390/ma11030334






