The Preparation, Characterization, Mechanical and Antibacterial Properties of GO-ZnO Nanocomposites with a Poly(l-lactide)-Modified Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
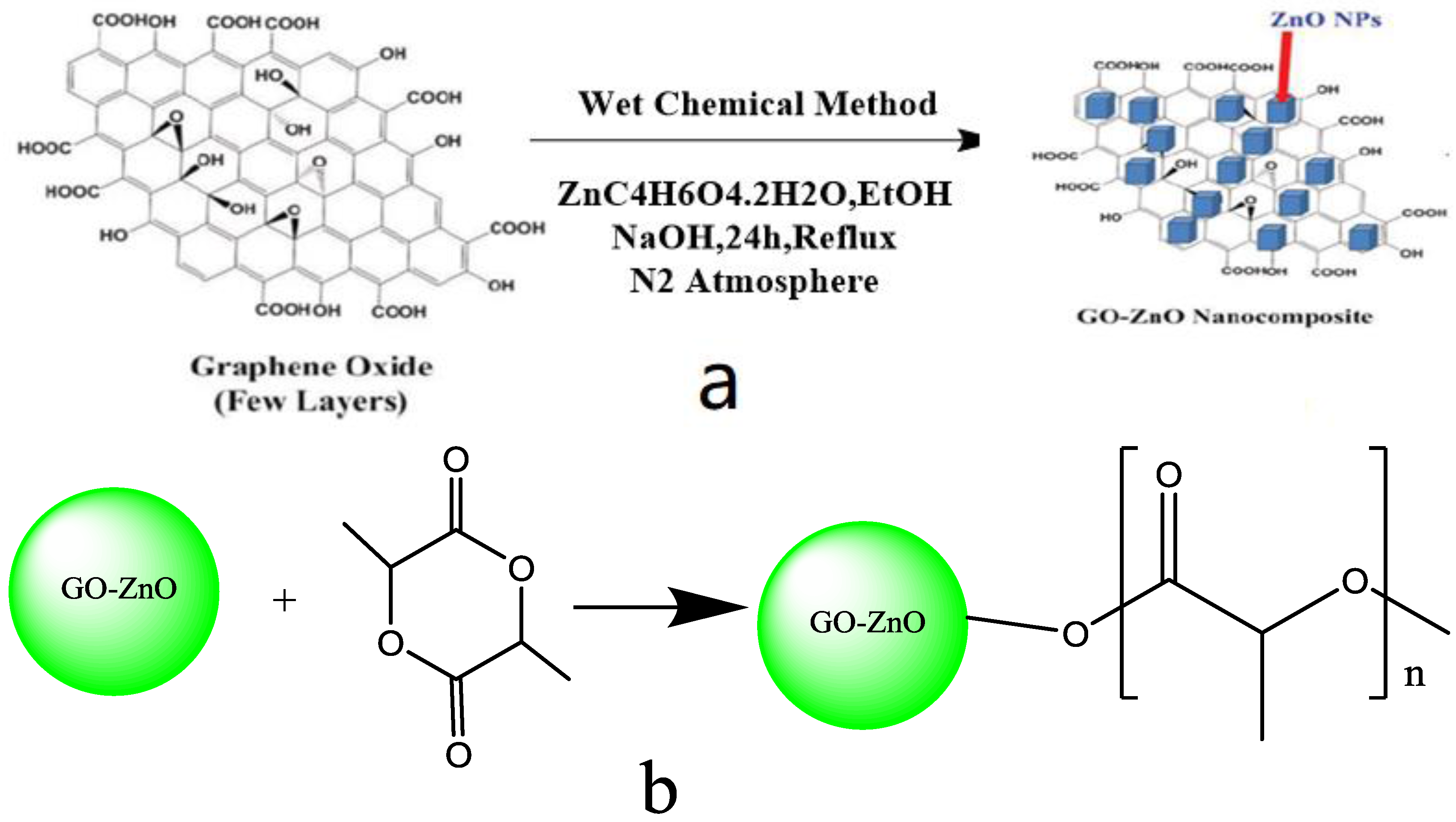
2.1.1. Preparation of Raw Materials
2.1.2. Preparation of GO-ZnO-PLLA
2.1.3. Preparation of the Composite Material GO-ZnO-PLLA/PLLA
2.1.4. Preparation of the GO-ZnO-PLLA/PLLA Composite Film
3. Results and Discussion
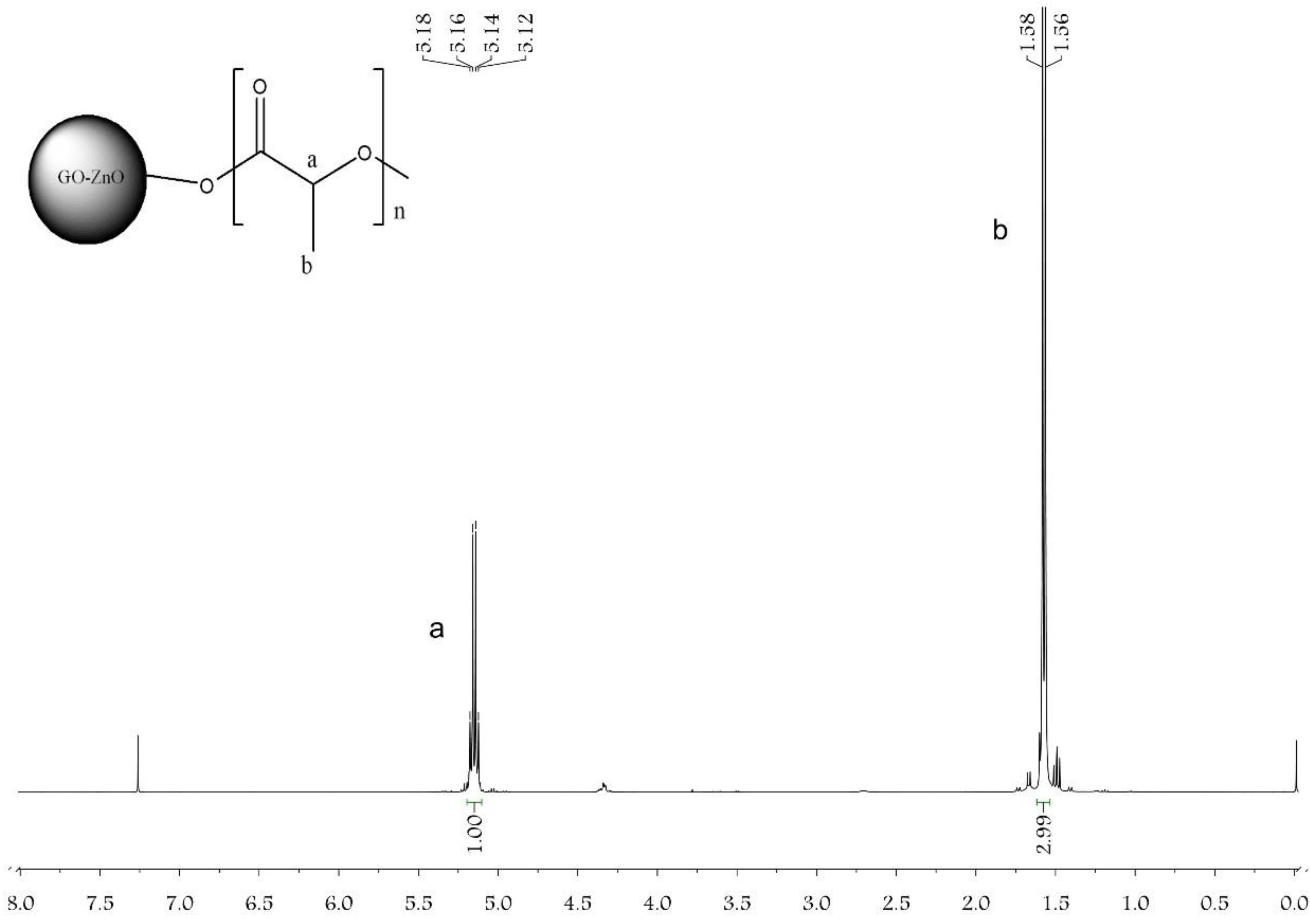
3.1. Characterization of GO-ZnO-PLLA
3.2. GO-ZnO-PLLA Molecular Weight Measurement (Using GPC)
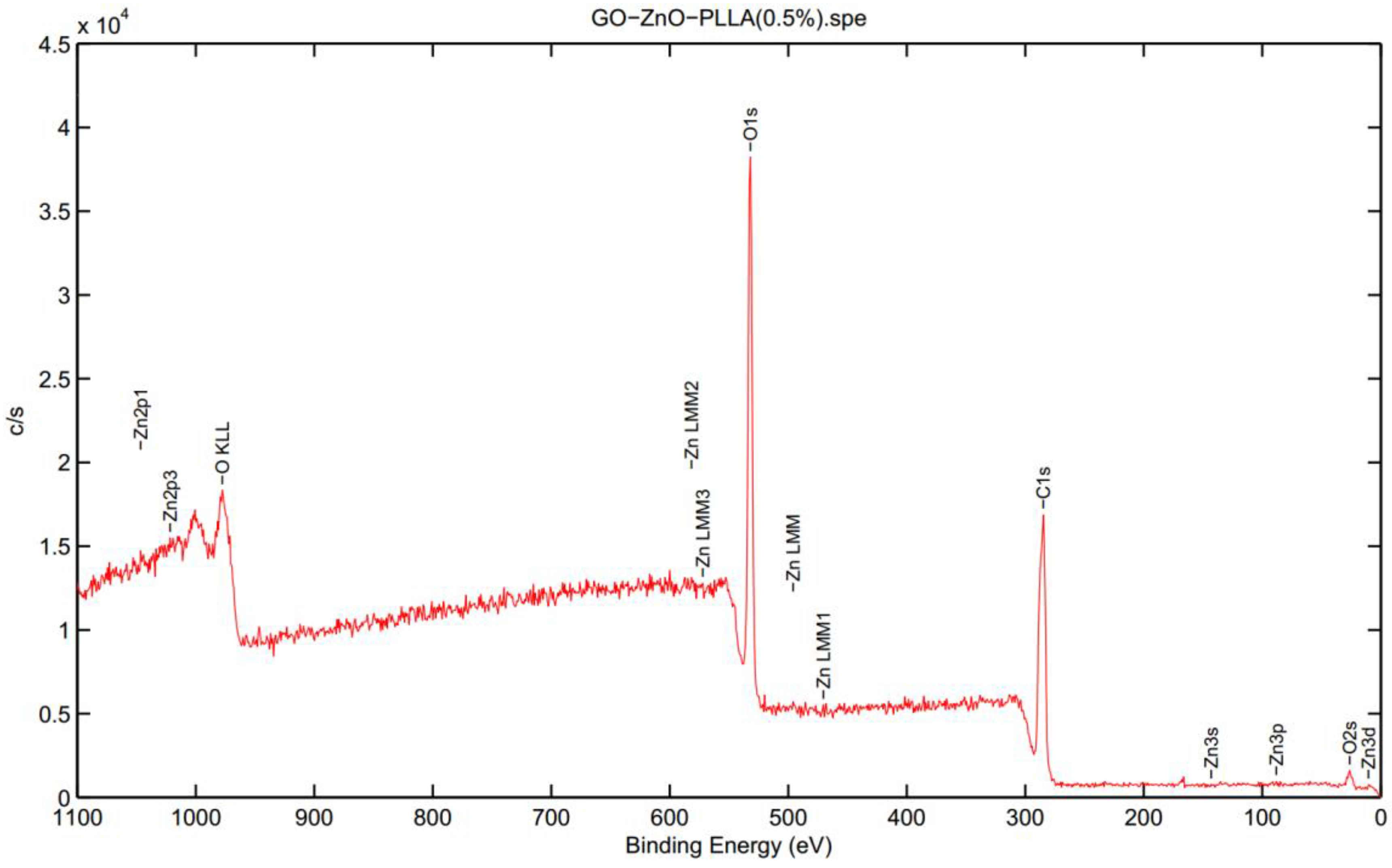
3.3. X-ray Photoelectron Spectroscopy (XPS) Analysis of GO-ZnO-PLLA
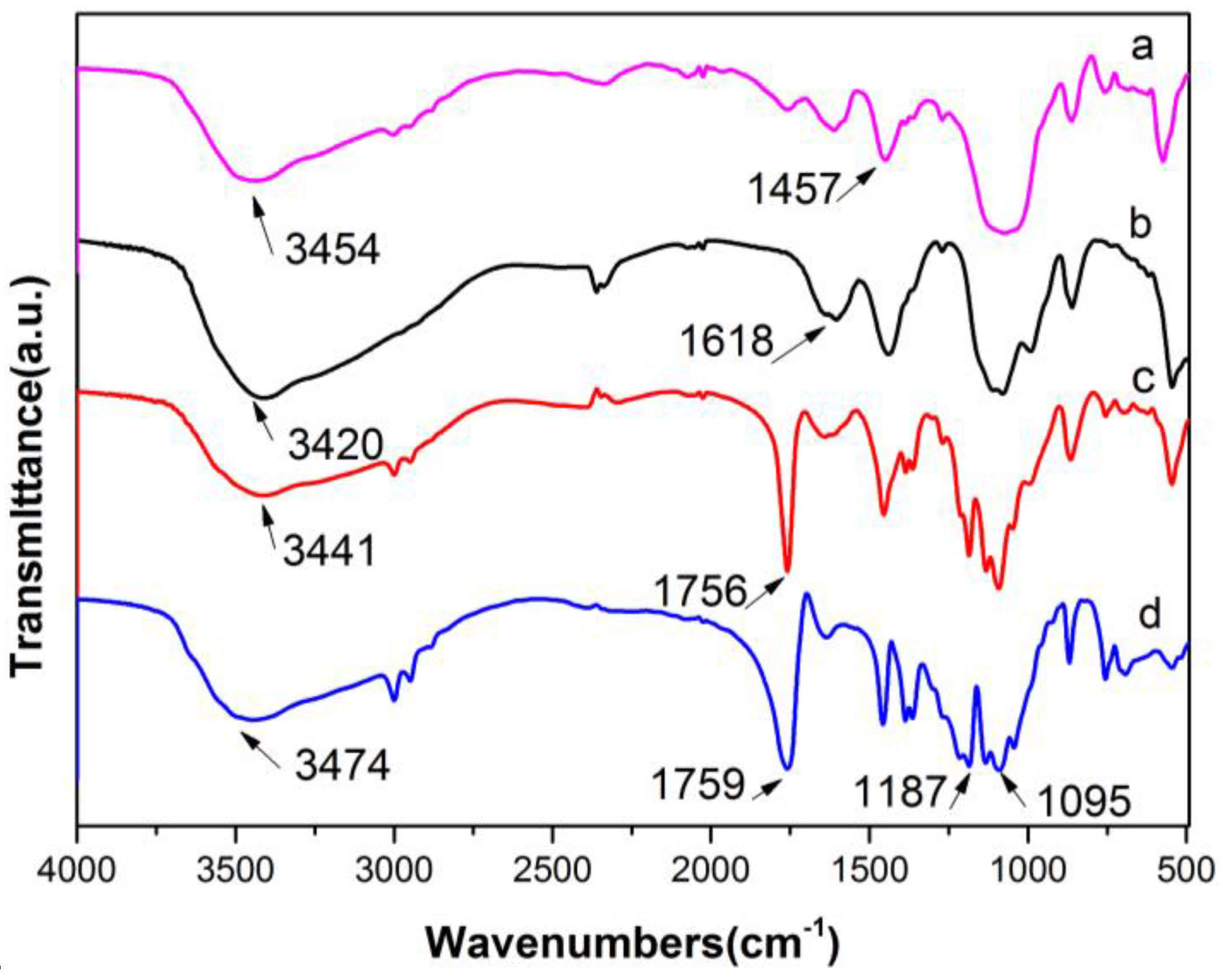
3.4. Infrared (IR) Spectroscopy
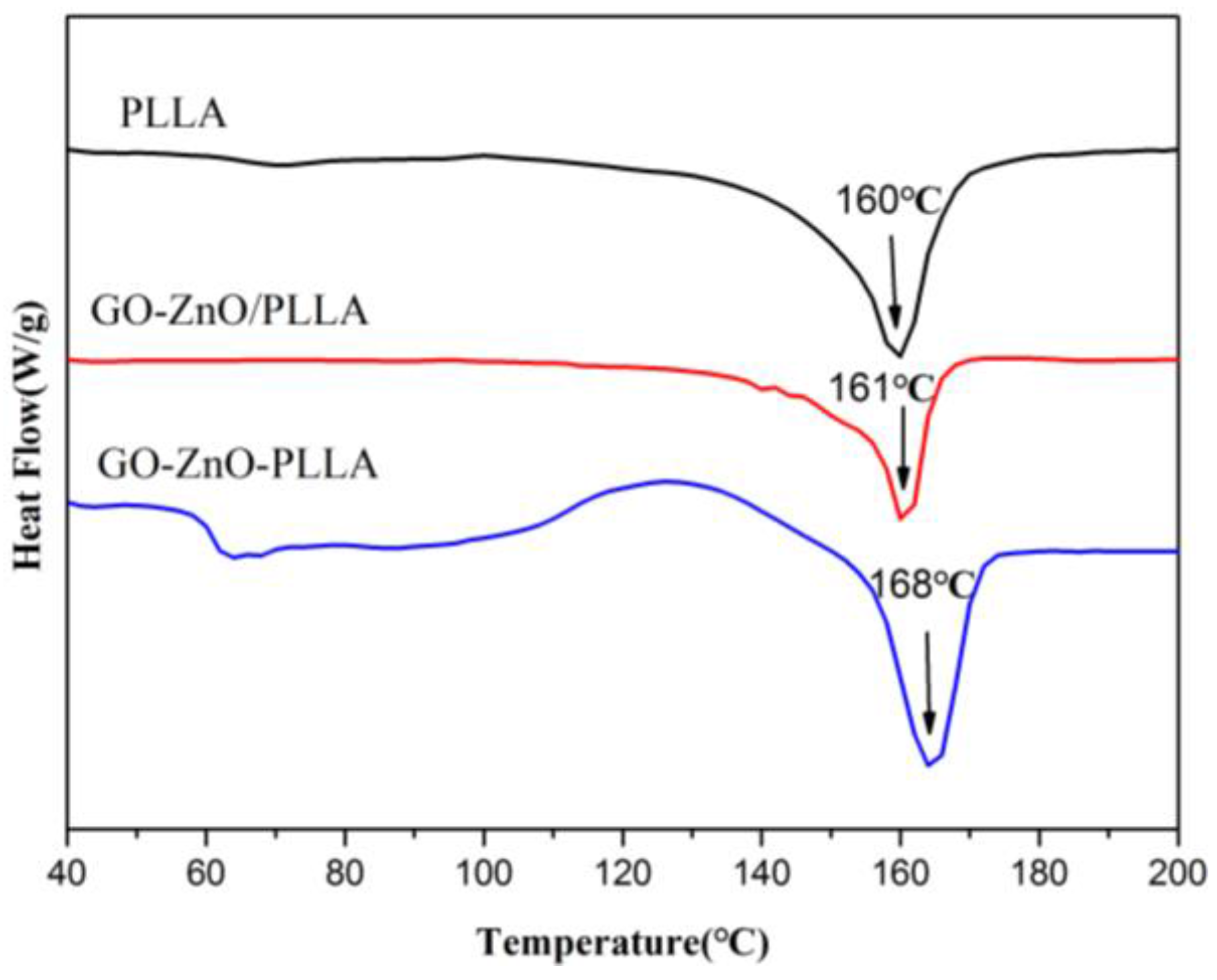
3.5. Differential Scanning Calorimetry (DSC) of PLLA and Its Composites
3.6. Thermogravimetric Analysis (TGA)
3.7. Physical Property Characterization
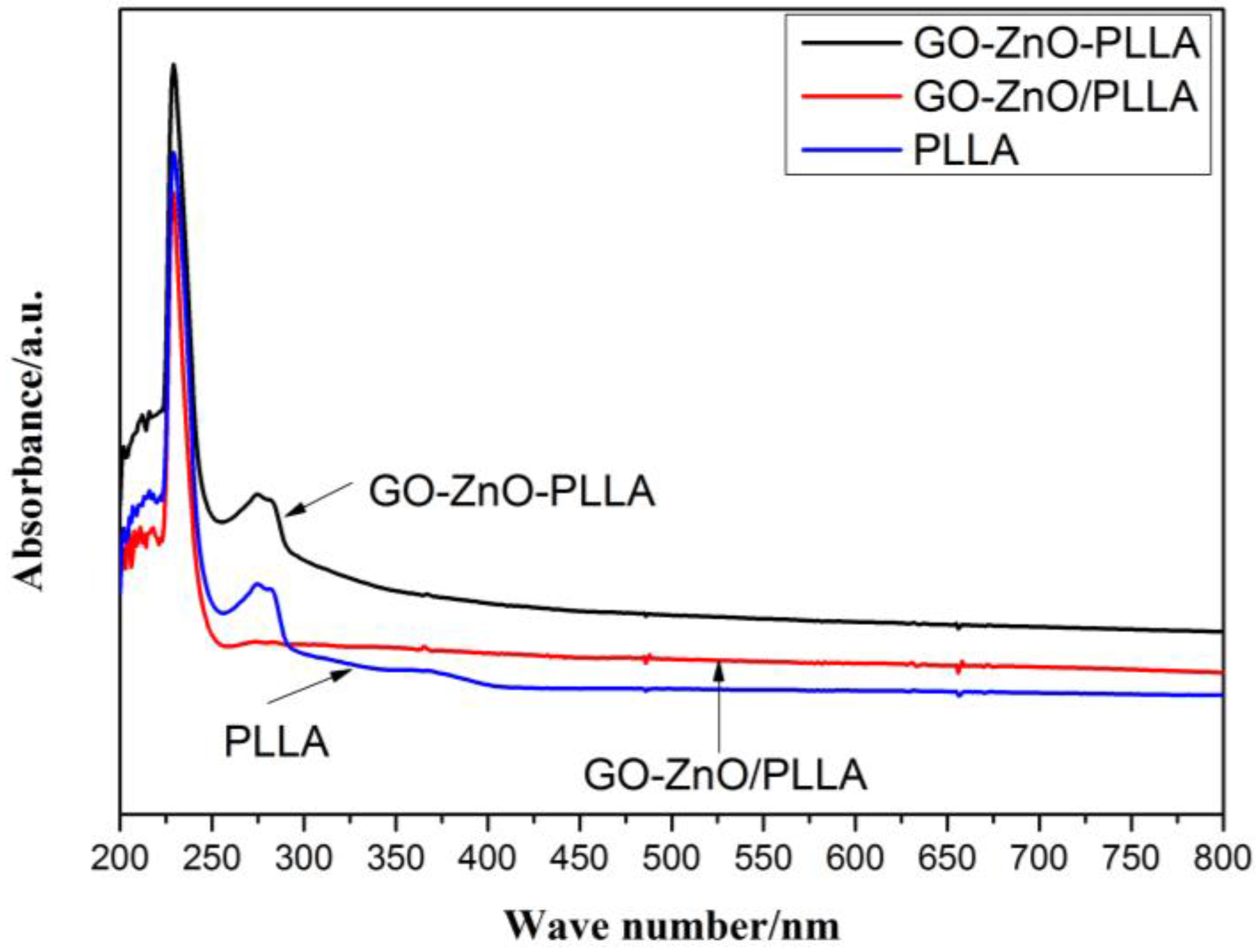
3.8. Dispersions of GO-ZnO–PLLA and GO-ZnO/PLLA in Chloroform
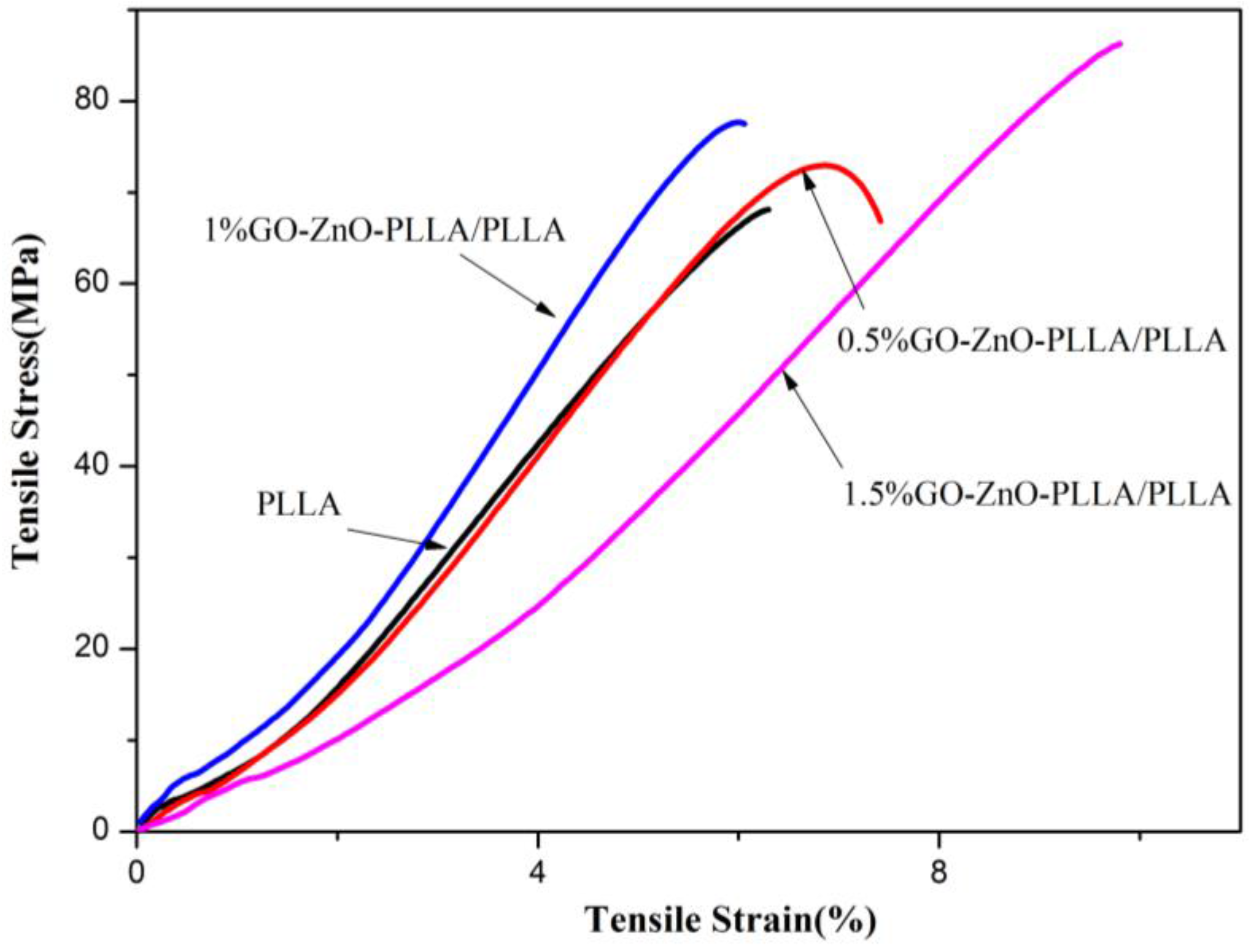
3.9. Testing of Tensile Properties of PLLA and GO-ZnO-PLLA/PLLA Composite Films
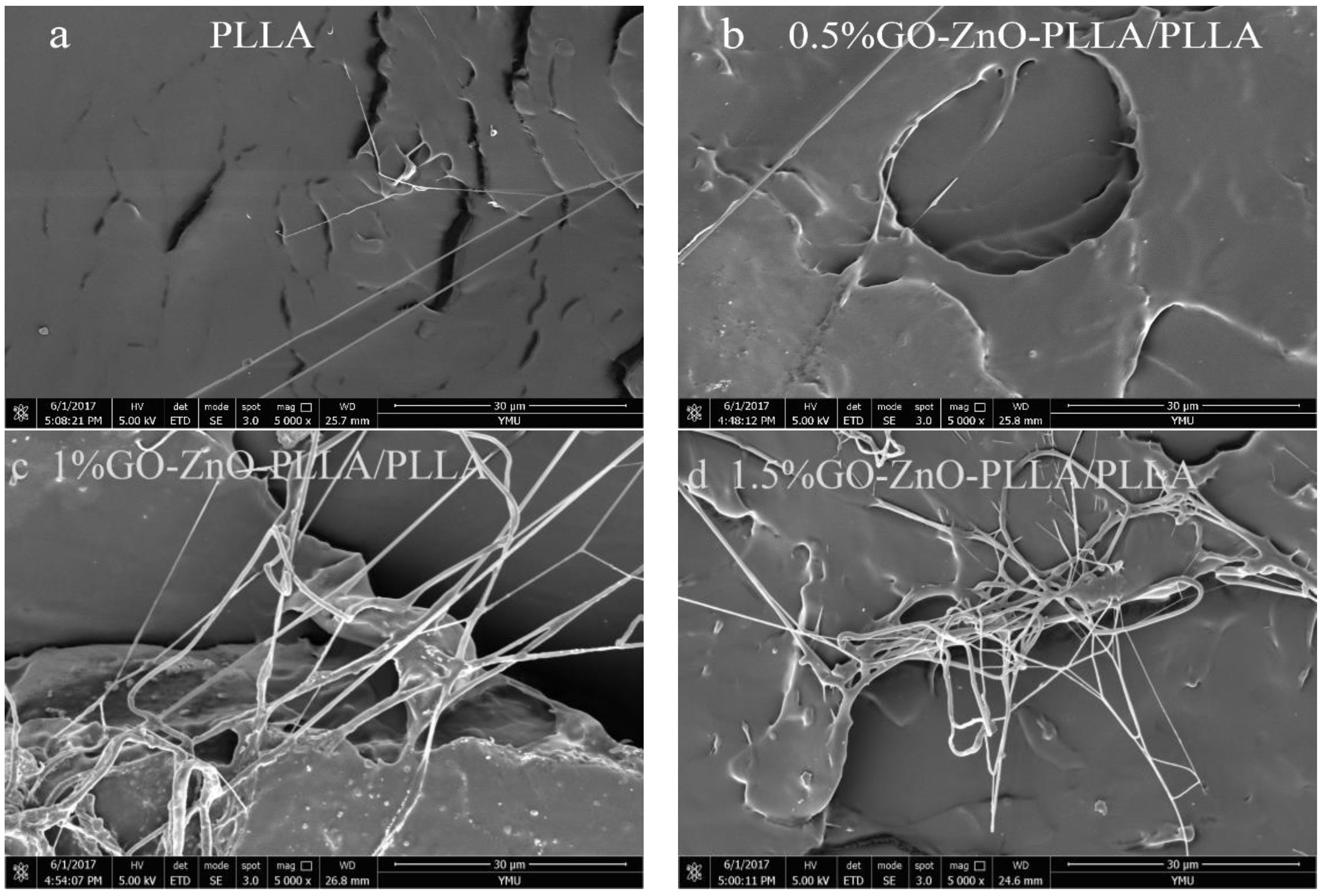
3.10. SEM Analysis of GO-ZnO-PLLA/PLLA
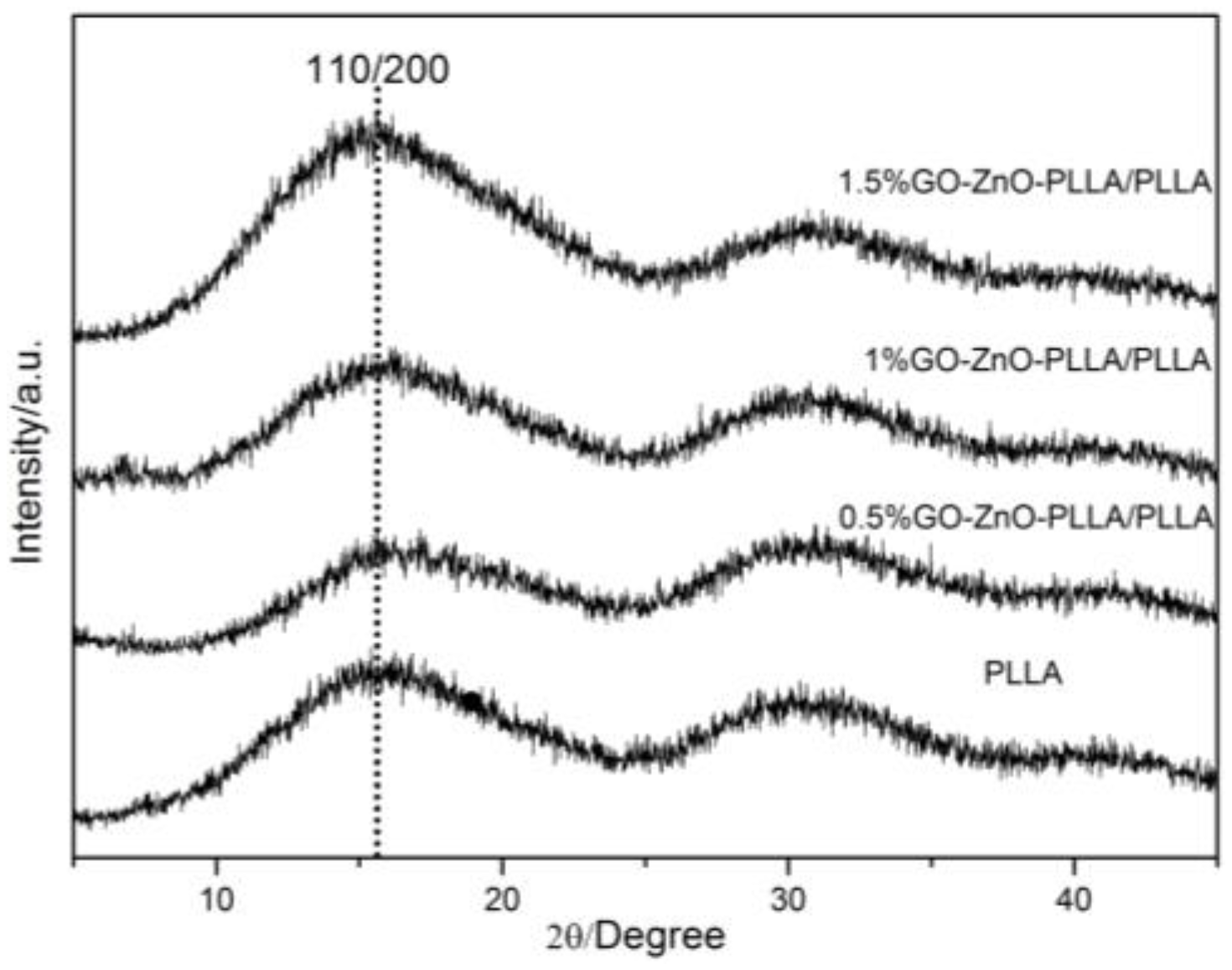
3.11. XRD Analysis of the GO-ZnO-PLLA/PLLA Composite Film
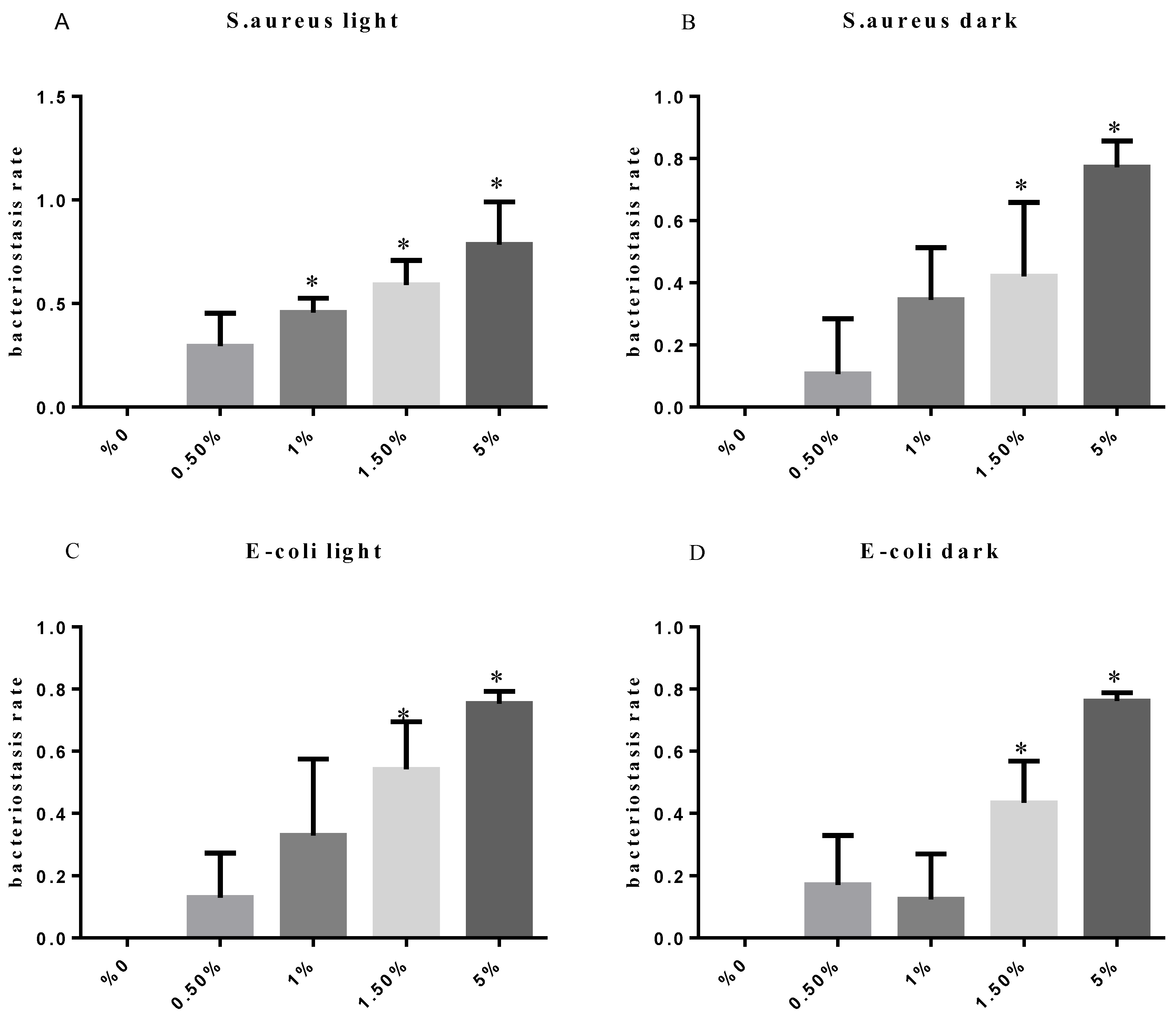
3.12. Test of the Antibacterial Properties of GO-ZnO-PLLA/PLLA Composite Film
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nano composites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar]
- Lagaron, J.M.; Lopez-Rubio, A. Nanotechnology for bioplastics: Opportunities, challenges and strategies. Trends Food Sci. Technol. 2011, 22, 611–617. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R.; Murariu, M.; Dubois, P. PLA/Halloysite nanocomposite films: Water vapor barrier properties and specific key characteristics. Macromol. Mater. Eng. 2014, 299, 104–115. [Google Scholar] [CrossRef]
- Sanchez Reig, C.; Dobon Lopez, A.; Hortal Ramos, M.; Cloquell Ballester, V.A. Nanomaterials: A map for their selection in food packaging applications. Packag. Technol. Sci. 2014, 27, 839–866. [Google Scholar] [CrossRef]
- Guillard, V.; Mauricio-Iglesias, M.; Gontard, N. Effect of novel food processing methods on packaging: Structure, composition, and migration properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 969–988. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Z.; Kumar, P.; Alavi, S.; Sandeep, K.P. Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Doumbia, A.; Bonnaud, L.; Dechief, A.-L.; Paint, Y.; Ferreira, M.; Campagne, C.; Devaux, E.; Dubois, P. High-performance Polylactide/ZnO nanocomposites designed for films and fibers with special end-use properties. Biomacromolecules 2011, 12, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, T.; Zhao, X.; Wang, X.; Zhou, L.; Yang, Y.; Liao, F.; Ju, Y. Poly(lactic acid)/graphene oxide-ZnO nanocomposite films with good mechanical, dynamic mechanical, anti-UV and antibacterial properties. J. Chem. Technol. Biotechnol. 2015, 90, 1677–1684. [Google Scholar] [CrossRef]
- Fan, W.; Zhao, Y.; Zhang, A.; Liu, Y.; Cao, Y.; Chen, J. Effect of a Chain Extender on the Properties of Poly(lactic acid)/Zinc Oxide/Copper Chlorophyll Acid Antibacterial Nanocomposites. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Murariu, M.; Paint, Y.; Murariu, O.; Raquez, J.-M.; Bonnaud, L.; Dubois, P. Current progress in the production of PLA-ZnO nanocomposites: Beneficial effects of chain extender addition on key properties. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Xu, J.-Z.; Chen, T.; Yang, C.-L.; Li, Z.-M.; Mao, Y.-M.; Zeng, B.-Q.; Hsiao, B.S. Isothermal crystallization of Poly(l-lactide) induced by graphene nanosheets and carbon nanotubes: A comparative study. Macromolecules 2010, 43, 5000–5008. [Google Scholar] [CrossRef]
- Murariu, M.; Dechief, A.L.; Bonnaud, L.; Paint, Y.; Gallos, A.; Fontaine, G.; Bourbigot, S.; Dubois, P. The production and properties of polylactide composites filled with expanded graphite. Polym. Degrad. Stab. 2010, 95, 889–900. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, J.; Wu, P. Preparation of organically dispersible graphene nanosheet powders through a lyophilization method and their poly(lactic acid) composites. Carbon 2010, 48, 3834–3839. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, Z. Crystallization behaviors of biodegradable poly(l-lactic acid)/graphene oxide nanocomposites from the amorphous state. Thermochim. Acta 2011, 526, 229–236. [Google Scholar] [CrossRef]
- Xie, M.; Lei, H.; Zhang, Y.; Xu, Y.; Shen, S.; Ge, Y.; Li, H.; Xie, J. Non-covalent modification of graphene oxide nanocomposites with chitosan/dextran and its application in drug delivery. RSC Adv. 2016, 6, 9328–9337. [Google Scholar] [CrossRef]
- Liu, S.; Gordiichuk, P.; Wu, Z.-S.; Liu, Z.; Wei, W.; Wagner, M.; Mohamed-Noriega, N.; Wu, D.; Mai, Y.; Herrmann, A.; et al. Patterning two-dimensional free-standing surfaces with mesoporous conducting polymers. Nat. Commun. 2015, 6, 8817. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, J.; Dong, R.; Gordiichuk, P.; Zhang, T.; Zhuang, X.; Mai, Y.; Liu, F.; Herrmann, A.; Feng, X. Two-dimensional mesoscale-ordered conducting polymers. Angew. Chem.-Int. Ed. 2016, 55, 12516–12521. [Google Scholar] [CrossRef] [PubMed]
- Posa, V.R.; Annavaram, V.; Koduru, J.R.; Ammireddy, V.R.; Somala, A.R. Graphene-ZnO nanocomposite for highly efficient photocatalytic degradation of methyl orange dye under solar light irradiation. Korean J. Chem. Eng. 2016, 33, 456–464. [Google Scholar] [CrossRef]
- Yuan, M.L.; Li, X.H.; Xiong, C.D.; Deng, X.M. Polymerization of lactides and lactones 5. Ring-opening polymerization of epsilon-caprolactone and dl-lactide by rare earth 2-methylphenyl samarium. Eur. Polym. J. 1999, 35, 2131–2138. [Google Scholar] [CrossRef]
- Deng, X.M.; Yuan, M.L.; Xiong, C.D.; Li, X.H. Polymerization of lactides and lactones. II. Ring-opening polymerization of epsilon-caprolactone and dl-lactide by organoacid rare earth compounds. J. Appl. Polym. Sci. 1999, 71, 1941–1948. [Google Scholar] [CrossRef]
- Yuan, M.L.; Xiong, C.D.; Li, X.H.; Deng, X.M. Polymerization of lactides and lactones. III. Ring-opening polymerization of dl-lactide by the (η3-C3H5)2Sm(μ2-Cl)2(μ3-Cl)2Mg(tmed)(μ2-Cl)Mg(tmed) complex. J. Appl. Polym. Sci. 1999, 73, 2857–2862. [Google Scholar] [CrossRef]
- Deng, X.M.; Yuan, M.L.; Xiong, C.D.; Li, X.H. Polymerization of lactides and lactones. IV. Ring-opening polymerization of epsilon-caprolactone by rare earth phenyl compounds. J. Appl. Polym. Sci. 1999, 73, 1401–1408. [Google Scholar] [CrossRef]
- Deng, X.M.; Yuan, M.L.; Li, X.H.; Xiong, C.D. Polymerization of lactides and lactones VII. Ring-opening polymerization of lactide by rare earth phenyl compounds. Eur. Polym. J. 2000, 36, 1151–1156. [Google Scholar] [CrossRef]
- Yuan, M.L.; Wang, Y.H.; Li, X.H.; Xiong, C.D.; Deng, X.M. Polymerization of lactides and lactones. 10. Synthesis, characterization, and application of amino-terminated poly(ethylene glycol)-co-poly(epsilon-caprolactone) block copolymer. Macromolecules 2000, 33, 1613–1617. [Google Scholar] [CrossRef]
- Hua, L.; Kai, W.; Yang, J.; Inoue, Y. A new poly(l-lactide)-grafted graphite oxide composite: Facile synthesis, electrical properties and crystallization behaviors. Polym. Degrad. Stab. 2010, 95, 2619–2627. [Google Scholar] [CrossRef]
- Song, W.; Zheng, Z.; Tang, W.; Wang, X. A facile approach to covalently functionalized carbon nanotubes with biocompatible polymer. Polymer 2007, 48, 3658–3663. [Google Scholar] [CrossRef]
- Yoon, J.T.; Lee, S.C.; Jeong, Y.G. Effects of grafted chain length on mechanical and electrical properties of nanocomposites containing polylactide-grafted carbon nanotubes. Compos. Sci. Technol. 2010, 70, 776–782. [Google Scholar] [CrossRef]
- Sun, Y.; He, C. Synthesis and Stereocomplex Crystallization of Poly(lactide)-Graphene Oxide Nanocomposites. ACS Macro Lett. 2012, 1, 709–713. [Google Scholar] [CrossRef]
- Goffin, A.-L.; Raquez, J.-M.; Duquesne, E.; Siqueira, G.; Habibi, Y.; Dufresne, A.; Dubois, P. From interfacial ring-opening polymerization to melt processing of cellulose nanowhisker-filled polylactide-based nanocomposites. Biomacromolecules 2011, 12, 2456–2465. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.T.; Jeong, Y.G.; Lee, S.C.; Min, B.G. Influences of poly(lactic acid)-grafted carbon nanotube on thermal, mechanical, and electrical properties of poly(lactic acid). Polym. Adv. Technol. 2009, 20, 631–638. [Google Scholar] [CrossRef]
- Amirian, M.; Chakoli, A.N.; Sui, J.H.; Cai, W. Enhanced mechanical and photoluminescence effect of poly(l-lactide) reinforced with functionalized multiwalled carbon nanotubes. Polym. Bull. 2012, 68, 1747–1763. [Google Scholar] [CrossRef]
- Olalde, B.; Aizpurua, J.M.; Garcia, A.; Bustero, I.; Obieta, I.; Jurado, M.J. Single-walled carbon nanotubes and multiwalled carbon nanotubes functionalized with poly(l-lactic acid): A comparative study. J. Phys. Chem. C 2008, 112, 10663–10667. [Google Scholar] [CrossRef]
- Chen, G.X.; Kim, H.S.; Park, B.H.; Yoon, J.S. Controlled functionalization of multiwalled carbon nanotubes with various molecular-weight poly(l-lactic acid). J. Phys. Chem. B 2005, 109, 22237–22243. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Z.; Li, J.; Li, Y.; Shan, M.; Wang, C.; Wang, Z.; Guo, Q.; Liu, L.; Chen, G.; et al. A facile strategy to prepare functionalized graphene via intercalation, grafting and self-exfoliation of graphite oxide. J. Mater. Chem. 2012, 22, 13460–13463. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lin, S.-H.; Lee, Y.-D. Preparation and characterization of poly(l-lactide)-graphene composites using the in situ ring-opening polymerization of PLLA with graphene as the initiator. J. Mater. Chem. 2012, 22, 10805–10815. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and Facile Preparation of Graphene Oxide and Reduced Graphene Oxide Nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chen, L.; Shan, M.; Tian, X.; Yang, C.; Lv, H.; Qian, X. A facile method to produce graphene oxide-g-poly(l-lactic acid) as an promising reinforcement for PLLA nanocomposites. Chem. Eng. J. 2014, 237, 291–299. [Google Scholar] [CrossRef]
- Cao, A.; Liu, Z.; Chu, S.; Wu, M.; Ye, Z.; Cai, Z.; Chang, Y.; Wang, S.; Gong, Q.; Liu, Y. A facile one-step method to poduce graphene-CdS quantum dot nanocomposites as promising optoelectronic materials. Adv. Mater. 2010, 22, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical Properties of Mono layer Graphene Oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z.; Silverajah, V.S.G. Graphene nanoplatelets as novel reinforcement filler in Poly(lactic acid)/epoxidized palm oil green nanocomposites: Mechanical properties. Int. J. Mol. Sci. 2012, 13, 10920–10934. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zeng, G.; Nie, F.; Xu, X.; Chen, S.; Han, Q.; Wang, X. Decorating graphene oxide with CuO nanoparticles in a water-isopropanol system. Nanoscale 2010, 2, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ren, L.; Zhang, C.; Huang, S.; Liu, T. Facile fabrication of functionalized graphene sheets (FGS)/ZnO nanocomposites with photocatalytic property. ACS Appl. Mater. Interfaces 2011, 3, 2779–2785. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shi, T.; Zhou, H. Hydrothermal preparation of ZnO-reduced graphene oxide hybrid with high performance in photocatalytic degradation. Appl. Surf. Sci. 2012, 258, 6204–6211. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, N.; Yuan, J.; Wang, W.; Tang, Y.; Lu, C.; Zhang, J.; Shen, J. Antibacterial and anticoagulation properties of carboxylated graphene oxide-lanthanum complexes. J. Mater. Chem. 2012, 22, 1673–1678. [Google Scholar] [CrossRef]
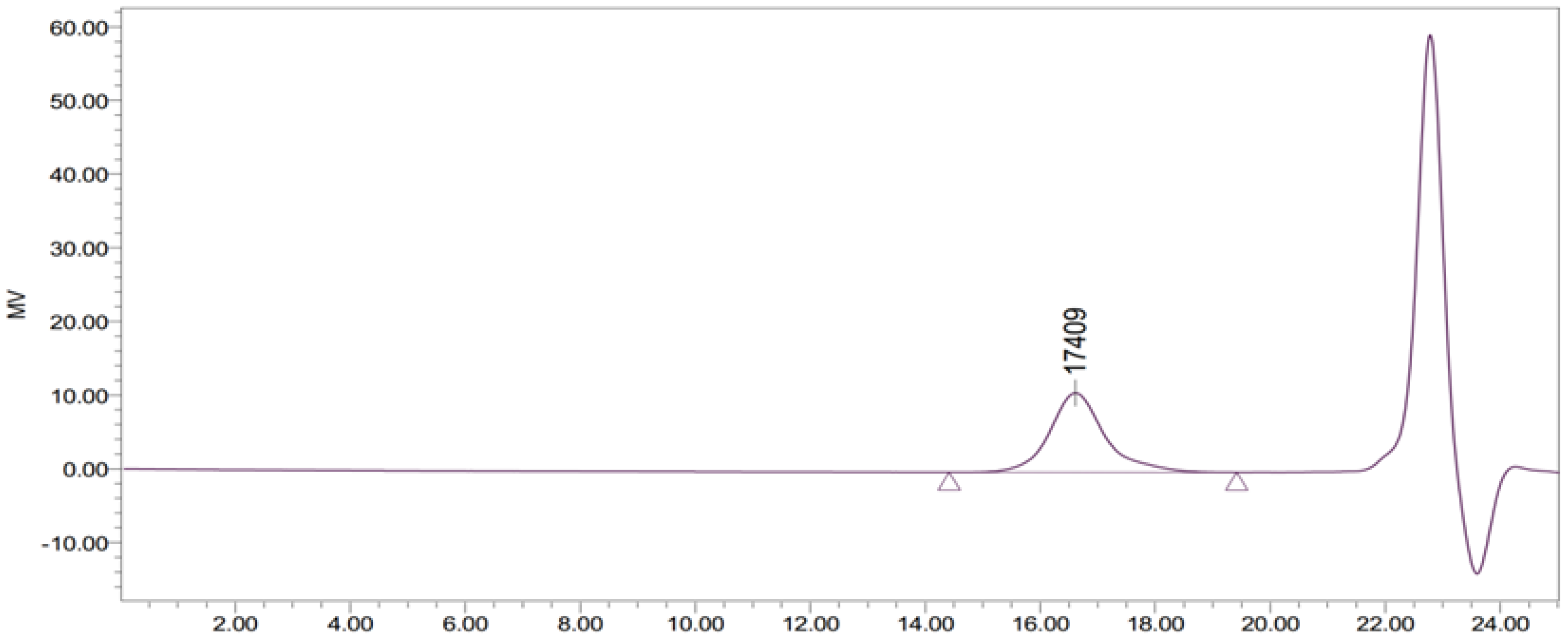



| Mn | Mw | MP | Mz | Mz + 1 | Polydispersity (Mw/Mn) | Mz/Mw |
|---|---|---|---|---|---|---|
| 15,784 | 17,362 | 17,409 | 18,912 | 20,584 | 1.099972 | 1.08925 |
| Sample Name | Elastic Modulus (MPa) | Elongation at Break (%) | Tensile Stress at Break (MPa) | Tensile Strength (MPa) |
|---|---|---|---|---|
| PLLA | 1356.6 | 6.3 | 68.12 | 68.12 |
| (0.5%) GO-ZnO-PLLA/PLLA | 1405.58 | 7.42 | 66.83 | 72.94 |
| (1.0%) GO-ZnO-PLLA/PLLA | 1668.62 | 6.86 | 77.49 | 77.65 |
| (1.5%) GO-ZnO-PLLA/PLLA | 1730.62 | 9.8 | 86.24 | 86.24 |
| Samples | Staphylococcus aureus (S. aureus) | - | Escherichia coli (E. coli) | - |
|---|---|---|---|---|
| - | Colony Photo | Antibacterial Rate R/% | Colony Photo | Antibacterial Rate R/% |
| PLLA |  | - |  | - |
| 0.5% GO-ZnO-PLLA | 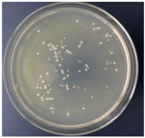 | 29.4 ± 5.3 | 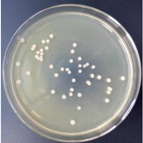 | 12.9 ± 4.7 |
| 1% GO-ZnO-PLLA | 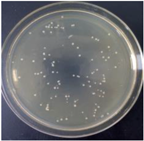 | 45.7 ± 2.3 |  | 32.9 ± 8.1 |
| 1.5% GO-ZnO-PLLA | 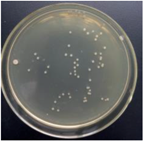 | 58.9 ± 3.9 | 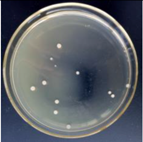 | 54.1 ± 5.1 |
| 5% GO-ZnO-PLLA | 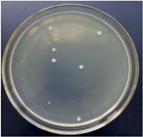 | 78.5 ± 6.8 |  | 75.3 ± 1.3 |
| Sample | Staphylococcus aureus (S. aureus) | - | Escherichia coli (E. coli) | - |
|---|---|---|---|---|
| - | Colony Photo | Antibacterial Rate R/% | Colony Photo | Antibacterial Rate R/% |
| PLLA | 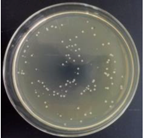 | - | 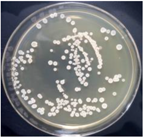 | - |
| 0.5% GO-ZnO-PLLA | 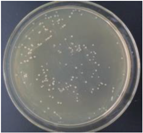 | 10.7 ± 5.9 |  | 17.1 ± 5.2 |
| 1% GO-ZnO-PLLA | 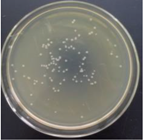 | 34.5 ± 5.5 | 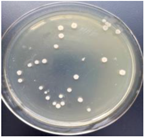 | 12.4 ± 4.8 |
| 1.5% GO-ZnO-PLLA | 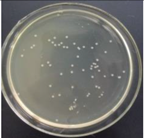 | 42.1 ± 7.9 | 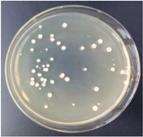 | 43.3 ± 4.5 |
| 5% GO-ZnO-PLLA |  | 77.2 ± 2.8 | 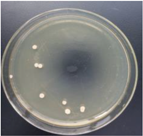 | 76.1 ± 0.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Xiong, C.; Jiang, L.; Li, H.; Yuan, M. The Preparation, Characterization, Mechanical and Antibacterial Properties of GO-ZnO Nanocomposites with a Poly(l-lactide)-Modified Surface. Materials 2018, 11, 323. https://doi.org/10.3390/ma11020323
Yuan M, Xiong C, Jiang L, Li H, Yuan M. The Preparation, Characterization, Mechanical and Antibacterial Properties of GO-ZnO Nanocomposites with a Poly(l-lactide)-Modified Surface. Materials. 2018; 11(2):323. https://doi.org/10.3390/ma11020323
Chicago/Turabian StyleYuan, Mingwei, Chengdong Xiong, Lin Jiang, Hongli Li, and Minglong Yuan. 2018. "The Preparation, Characterization, Mechanical and Antibacterial Properties of GO-ZnO Nanocomposites with a Poly(l-lactide)-Modified Surface" Materials 11, no. 2: 323. https://doi.org/10.3390/ma11020323





