TiO2@PEI-Grafted-MWCNTs Hybrids Nanocomposites Catalysts for CO2 Photoreduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Physico-Chemical and Morphological Characterization of Nanocomposites
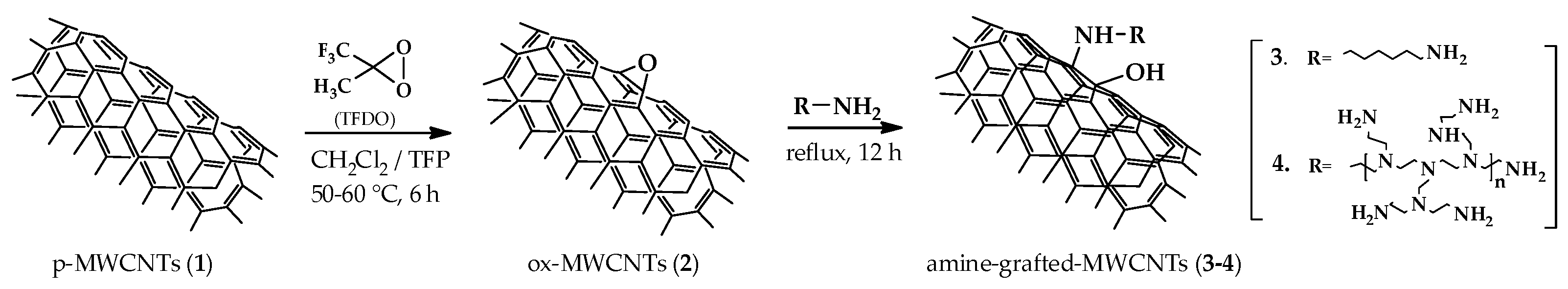
2.3. Preparation of ox-MWCNTs (2) with TFDO
2.4. Epoxide Ring Opening of ox-MWCNTs (2): Preparation of Amine-Grafted-MWCNTs 3 and 4
2.5. Preparation of hybrids nanocomposites photocatalysts 1A, 1C, 4A-C
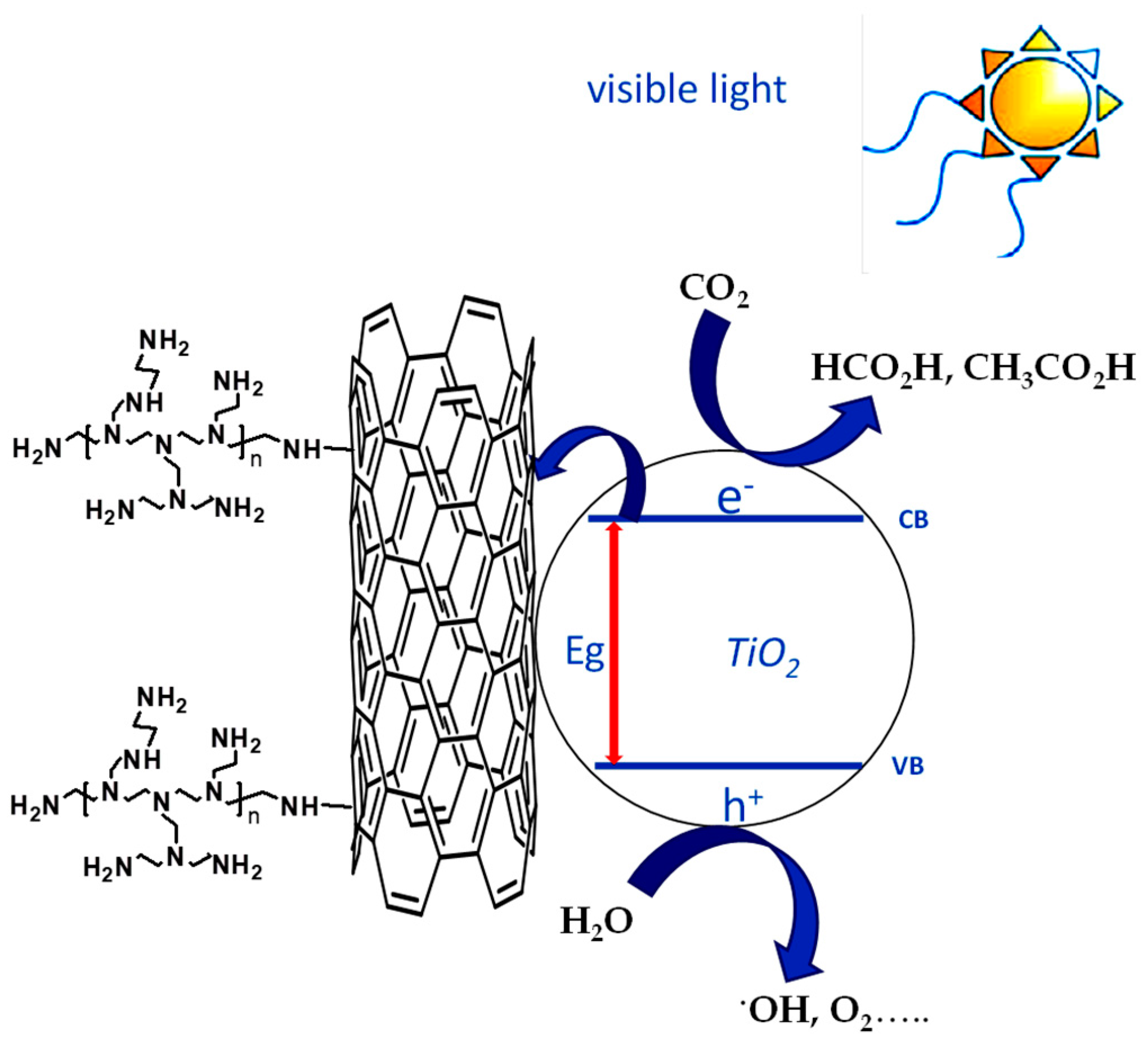
2.6. Photocatalytic Experiments
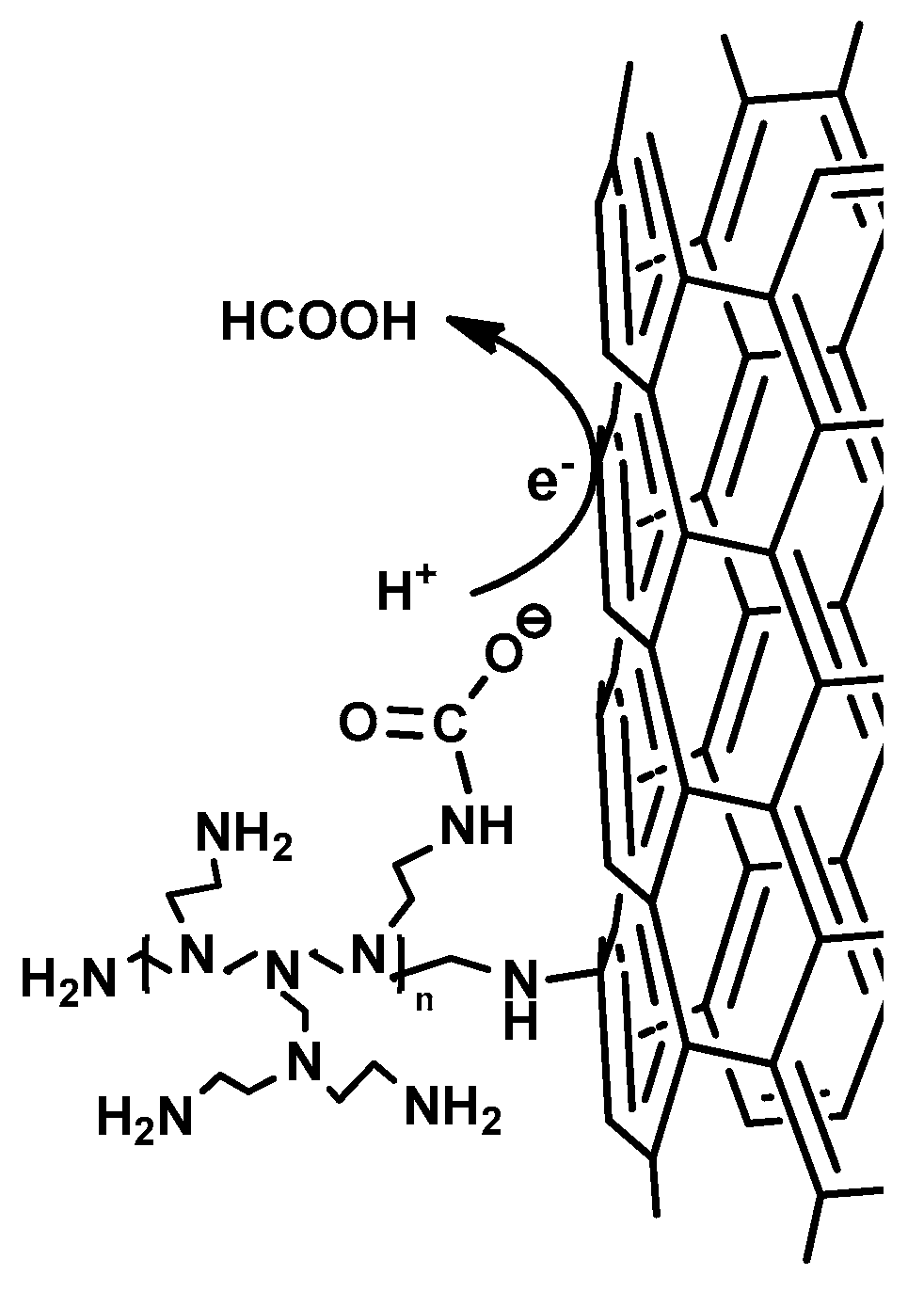
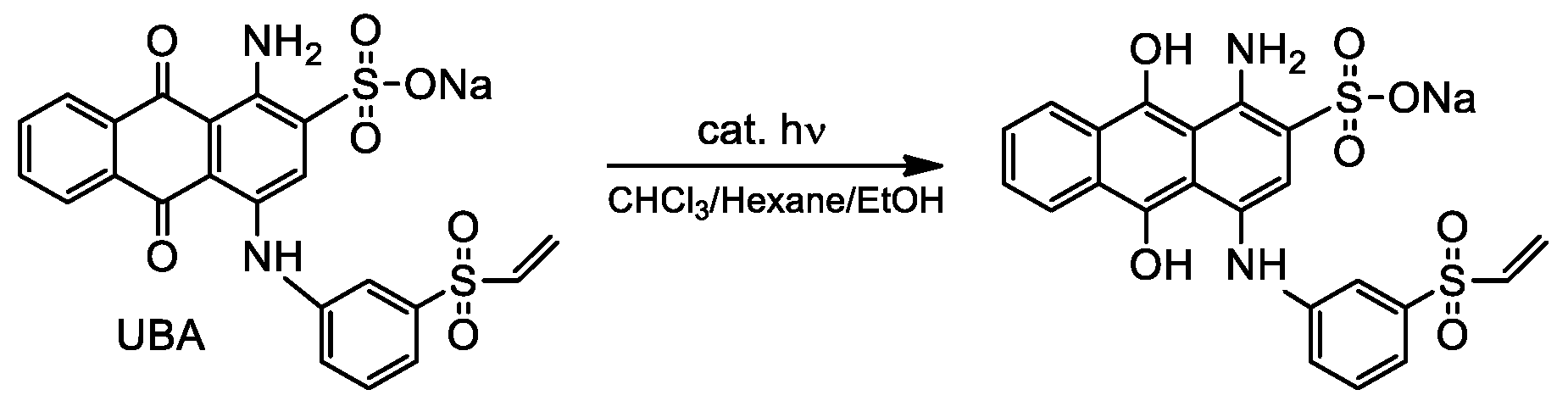
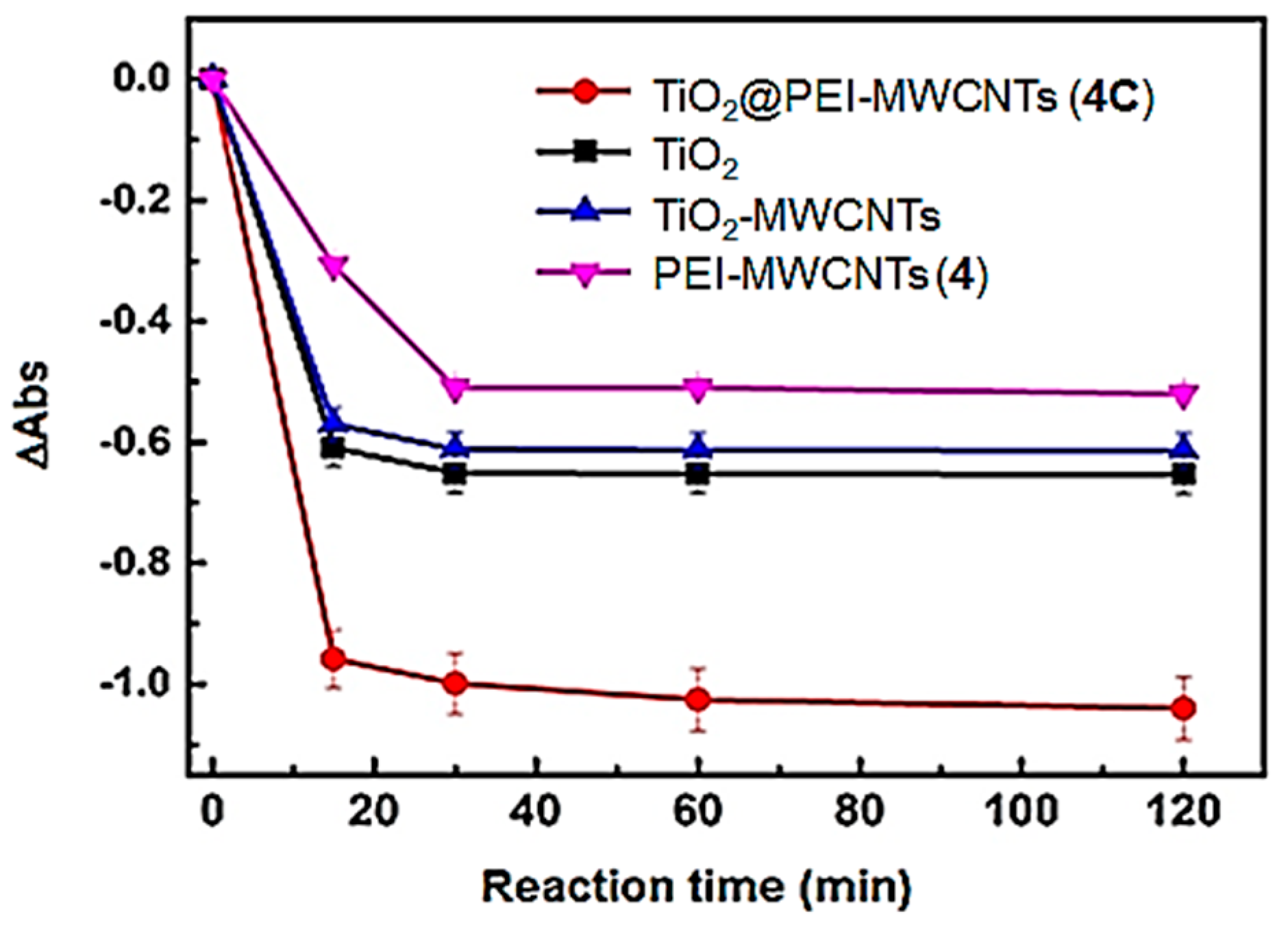
2.7. UBA Reduction Experiments
3. Results and Discussion
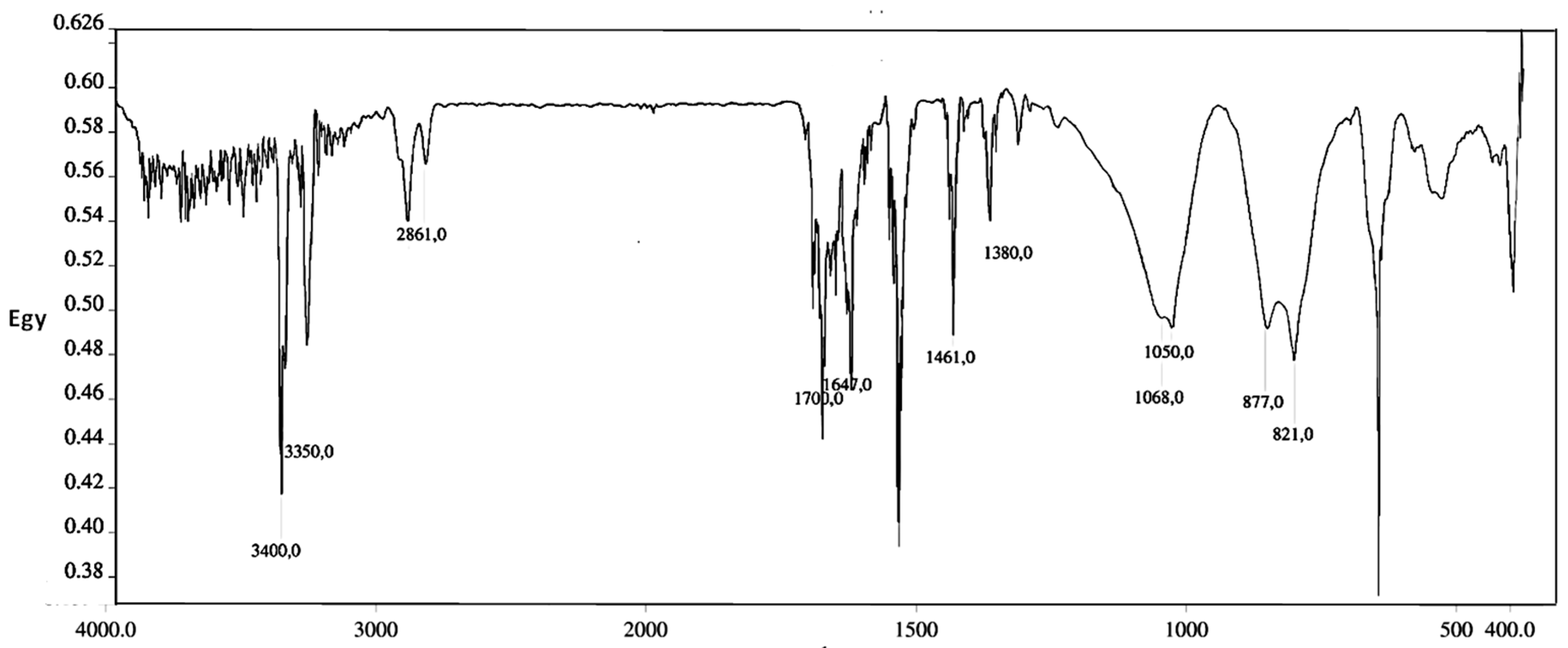
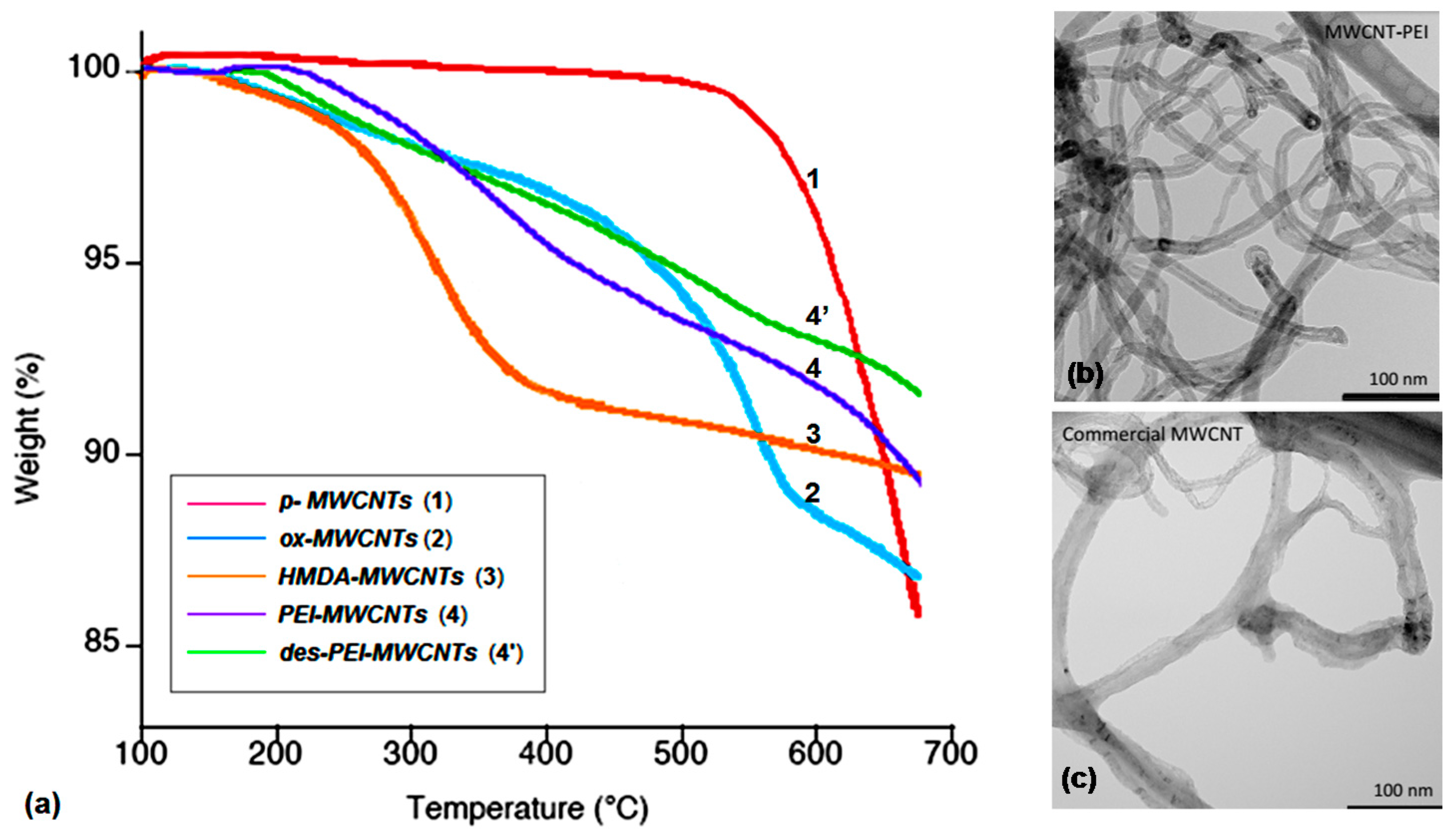
3.1. Synthesis and Characterization of Amine-Grafted-MWCNTs
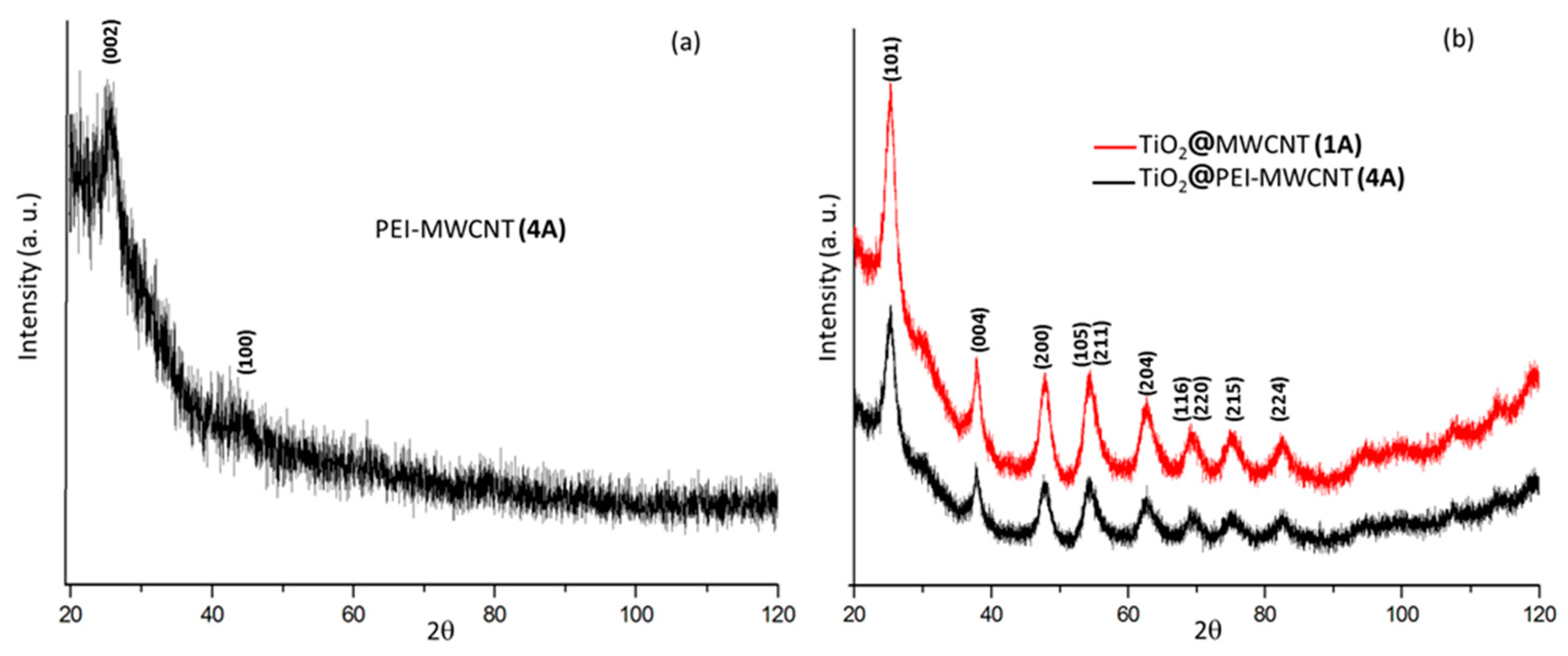
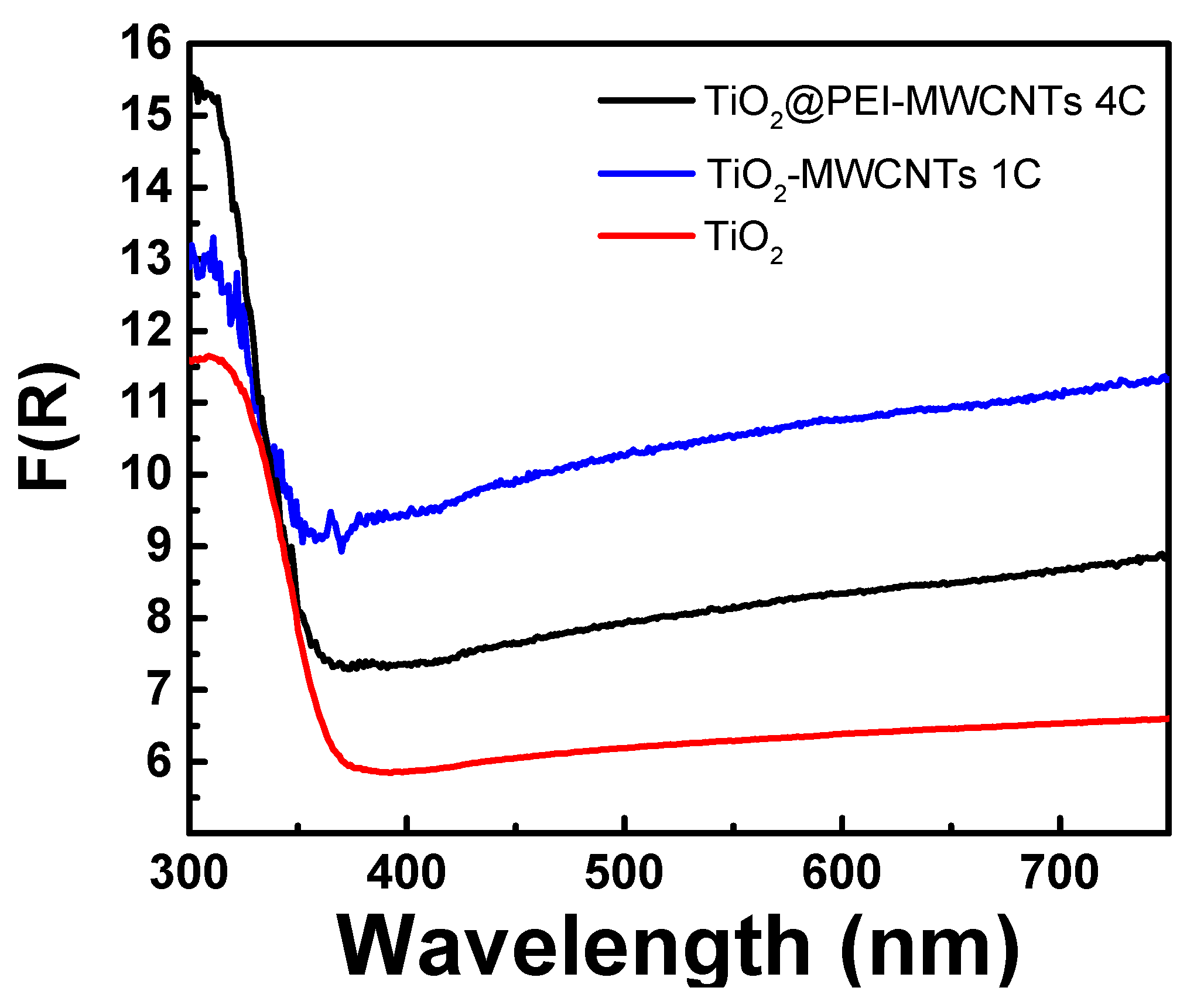
3.2. Preparation and Characterization of Hybrid Nanocomposites Photocatalysts
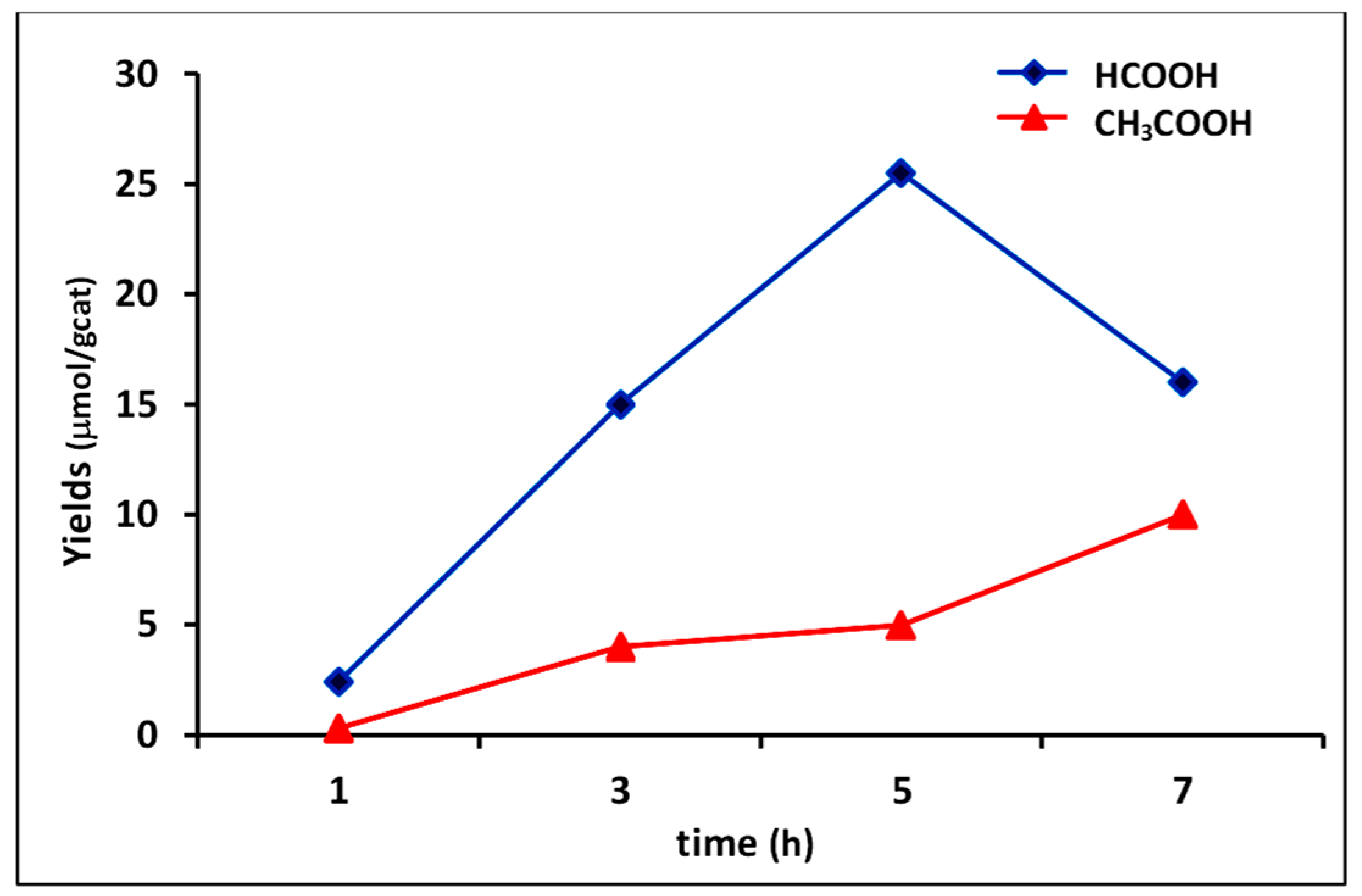
3.3. Phocatalytic Experiments
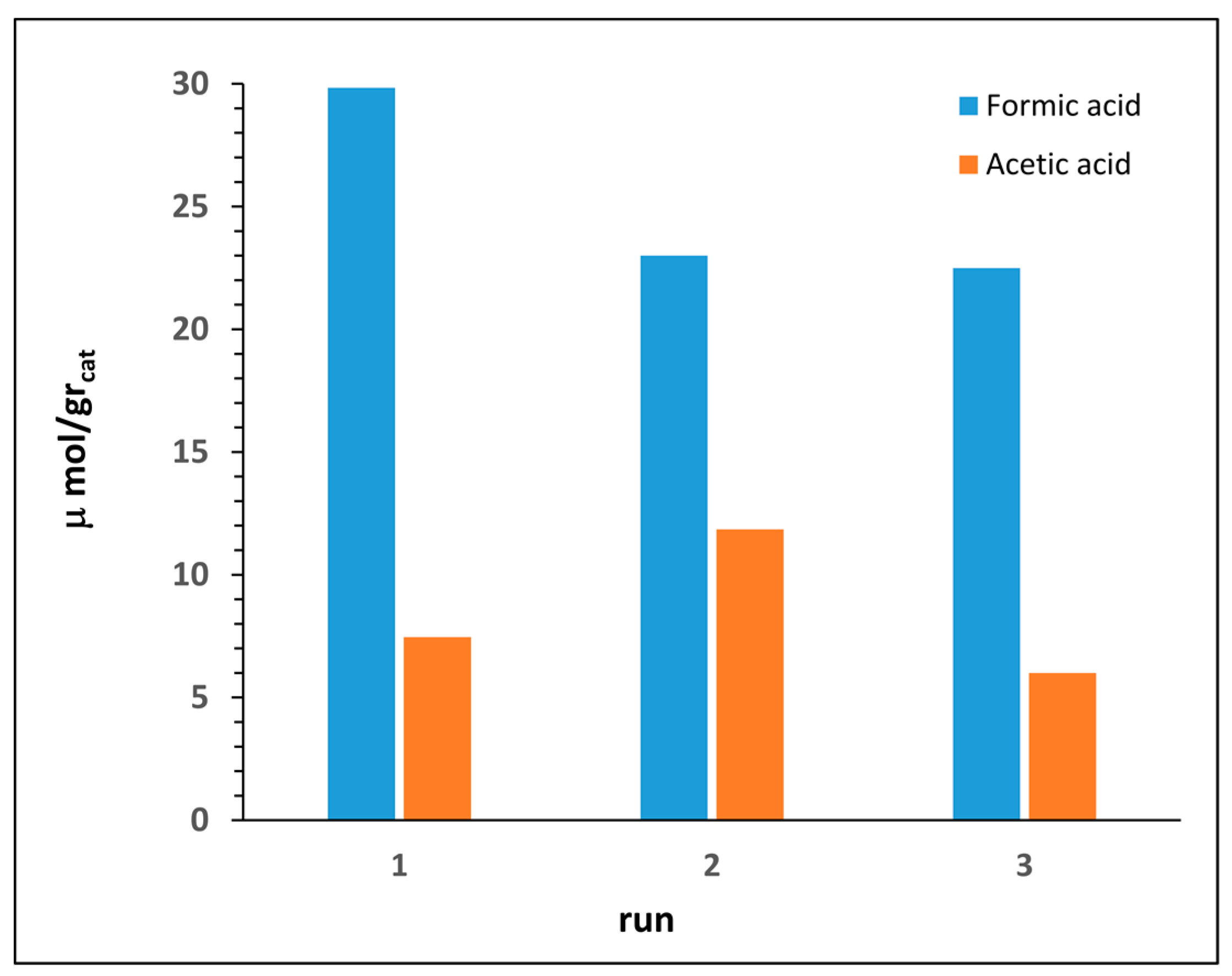
3.4. Catalyst Recycling
3.5. Mechanistic Insights
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, D.; Sakimoto, K.K.; Hong, D.; Yang, P. Artificial Photosynthesis for Sustainable Fuel and Chemical Production. Angew. Chem. Int. Ed. 2015, 54, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Li, K.; Peng, B.; Peng, T. Recent Advances in Heterogeneous Photocatalytic CO2 Conversion to Solar Fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Guan, G.; Kida, T.; Yoshida, A. Reduction of carbon dioxide with water under concentrated sunlight using photocatalyst combined with Fe-based catalyst. Appl. Catal. B 2003, 41, 387–396. [Google Scholar] [CrossRef]
- Wang, W.N.; An, W.J.; Ramalingam, B.; Mukherjee, S.; Niedzwiedzki, D.M.; Gangopadhyay, S.; Biswas, P. Size and structure matter: enhanced CO2 photoreduction efficiency by size-resolved ultrafine Pt nanoparticles on TiO2 single crystals. J. Am. Chem. Soc. 2012, 134, 11276–11281. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.M.; Chai, S.P.; Mohamed, A.R. Modification of MWCNT@ TiO2 core–shell nanocomposites with transition metal oxide dopants for photoreduction of carbon dioxide into methane. Appl. Surf. Sci. 2014, 319, 37–43. [Google Scholar] [CrossRef]
- Gui, M.M.; Wonga, W.M.P.; Chai, S.P.; Mohamed, A.R. One-pot synthesis of Ag-MWCNT@ TiO2 core–shell nanocomposites for photocatalytic reduction of CO2 with water under visible light irradiation. Chem. Eng. J. 2015, 278, 272–278. [Google Scholar] [CrossRef]
- Mele, G.; Annese, C.; D’Accolti, L.; De Riccardis, A.; Fusco, C.; Palmisano, L.; Scarlino, A.; Vasapollo, G. Photoreduction of Carbon Dioxide to Formic Acid in Aqueous Suspension: A Comparison between Phthalocyanine/TiO2 and Porphyrin/TiO2 Catalysed Processes. Molecules 2015, 20, 396–415. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Gui, M.M.; Chai, S.-P.; Mohamed, A.R. Direct growth of carbon nanotubes on Ni/ TiO2 as next generation catalysts for photoreduction of CO2 to methane by water under visible light irradiation. RSC Adv. 2013, 3, 4505–4509. [Google Scholar] [CrossRef]
- Xu, H.; Ouyang, S.; Liu, L.; Wang, D.; Kako, T.; Ye, J. Porous-structured Cu2O/TiO2 nanojunction material toward efficient CO2 photoreduction. Nanotechnology 2014, 25, 165402–165410. [Google Scholar] [CrossRef] [PubMed]
- Suna, Z.; Wanga, H.; Wua, Z.; Wang, L. g-C3N4 based composite photocatalysts for photocatalytic CO2 reduction. Catal. Today 2018, 300, 160–172. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Li, J.; Wang, Y.; Jiang, G.; Zhao, Z.; Liu, B.; Gong, X.; Duan, A.; Liu, Y.; et al. Photocatalytic reduction of CO2 with water vapor on surface La-modified TiO2 nanoparticles with enhanced CH4 selectivity. Appl. Catal. B. 2015, 168–169, 125–131. [Google Scholar] [CrossRef]
- Xia, X.-H.; Jia, Z.-J.; Yu, Y.; Liang, Y.; Wang, Z.; Ma, L.-L. Preparation of multi-walled carbon nanotube supported TiO2 and its photocatalytic activity in the reduction of CO2 with H2O. Carbon 2007, 45, 717–721. [Google Scholar] [CrossRef]
- Tian, L.; Ye, L.; Deng, K.; Zan, L. TiO2/carbon nanotube hybrid nanostructures: Solvothermal synthesis and their visible light photocatalytic activity. J. Solid State Chem. 2011, 184, 1465–1471. [Google Scholar] [CrossRef]
- Gao, B.; Chen, G.Z.; Puma, G.L. Carbon nanotubes/titanium dioxide (CNTs/TiO2) nanocomposites prepared by conventional and novel surfactant wrapping sol–gel methods exhibiting enhanced photocatalytic activity. Appl. Catal. B. 2009, 89, 503–509. [Google Scholar] [CrossRef]
- Cong, Y.; Li, X.; Qin, Y.; Dong, Z.; Yuan, G.; Cui, Z.; Lai, X. Carbon-doped TiO2 coating on multiwalled carbon nanotubes with higher visible light photocatalytic activity. Appl. Catal. B. 2011, 107, 128–134. [Google Scholar] [CrossRef]
- Kongkanand, A.; Kamat, P.V. Electron Storage in Single Wall Carbon Nanotubes. Fermi Level Equilibration in Semiconductor–SWCNT Suspensions. ACS Nano 2007, 1, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Z.-R.; Fu, X.; Xu, Y.-J. TiO2-Graphene Nanocomposites for Gas-Phase Photocatalytic Degradation of Volatile Aromatic Pollutant: Is TiO2-Graphene Truly Different from Other TiO2-Carbon Composite Materials? ACS Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, Q.; Liu, G.; Wang, Y. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures. Chem. Commun. 2016, 52, 35–59. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Ouyang, S.; Kako, T.; Li, P.; Liu, Q.; Wang, T.; Ye, J. Photocatalytic CO2 conversion over alkali modified TiO2 without loading noble metal cocatalyst. Chem. Commun. 2014, 50, 11517–11519. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.J.; Wang, Y.; Zhang, Q.H.; Fan, W.Q.; Deng, W.P.; Wang, Y. Photocatalytic reduction of CO2 with H2O: significant enhancement of the activity of Pt–TiO2 in CH4 formation by addition of MgO. Chem. Commun. 2013, 49, 2451–2453. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An Amine-Functionalized Titanium Metal–Organic Framework Photocatalyst with Visible-Light-Induced Activity for CO2 Reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Cao, S.W.; Yuan, Y.; Gu, Q.; Zhang, Z.; Xue, C. Efficient CO2 Capture and Photoreduction by Amine-Functionalized TiO2. Chem. Eur. J. 2014, 20, 10220–10222. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xie, S.; Zhang, Q.; Tian, Z.; Wang, Y. Carbon dioxide-enhanced photosynthesis of methane and hydrogen from carbon dioxide and water over Pt-promoted polyaniline–TiO2 nanocomposites. Chem. Commun. 2015, 51, 13654–13657. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-C.; Lu, C.; Su, F.; Zeng, W.; Chen, W. Thermodynamics and regeneration studies of CO2 adsorption on multiwalled carbon nanotubes. Chem. Eng. Sci. 2010, 65, 1354–1361. [Google Scholar] [CrossRef]
- Mansoor, A.; Hoseini, V. Development of MWCNT@MIL-101 hybrid composite with enhanced adsorption capacity for carbon dioxide. Chem. Eng. J. 2012, 191, 326–330. [Google Scholar] [CrossRef]
- Lee, M.-S.; Park, S.-J. Silica-coated multi-walled carbon nanotubes impregnated with polyethyleneimine for carbon dioxide capture under the flue gas condition. J. Solid State Chem. 2015, 226, 17–23. [Google Scholar] [CrossRef]
- Dillon, E.P.; Crouse, C.A.; Barron, A.R. Synthesis, Characterization, and Carbon Dioxide Adsorption of Covalently Attached Polyethyleneimine-Functionalized Single-Wall Carbon Nanotubes. ACS Nano 2007, 2, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shi, J.; Wang, Q.; Tao, M.; He, Y.; Shi, Y. Carbon Dioxide Capture with Polyethylenimine-Functionalized Industrial-Grade Multiwalled Carbon Nanotubes. Ind. Eng. Chem. Res. 2014, 53, 17468–17475. [Google Scholar] [CrossRef]
- Yang, B.; Hu, H.; Yu, Q.; Zhang, X.; Lia, Z.; Lei, L. Pretreated multiwalled carbon nanotube adsorbents with amine-grafting for removal of carbon dioxide in confined spaces. RSC Adv. 2014, 4, 56224–56234. [Google Scholar] [CrossRef]
- Khalili, S.; Ghoreyshi, A.A.; Jahanshahi, M.; Pirzadeh, K. Enhancement of Carbon Dioxide Capture by Amine-Functionalized Multi-Walled Carbon Nanotube. Clean Soil Air Water 2013, 41, 939–948. [Google Scholar] [CrossRef]
- Casiello, M.; Iannone, F.; Cotugno, P.; Monopoli, M.; Cioffi, N.; Ciminale, F.; Trzeciak, A.M.; Nacci, A. Copper(II)-catalysed oxidative carbonylation of aminols and amines in water: A direct access to oxazolidinones, ureas and carbamates. J. Mol. Cat. A. Chem. 2015, 407, 8–14. [Google Scholar] [CrossRef]
- Iannone, F.; Casiello, M.; Monopoli, A.; Cotugno, P.; Sportelli, M.C.; Picca, R.A.; Cioffi, N.; Dell’Anna, M.M.; Nacci, A. Ionic liquids/ZnO nanoparticles as recyclable catalyst for polycarbonate depolymerization. J. Mol. Cat. A Chem. 2017, 426, 107–116. [Google Scholar] [CrossRef]
- Annese, C.; Abbrescia, D.I.; Catucci, L.; D’Accolti, L.; Denora, N.; Fanizza, I.; Fusco, C.; La Piana, G. Site-dependent biological activity of valinomycin analogs bearing derivatizable hydroxyl sites. J. Pep. Sci. 2013, 19, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, A.; Stella, V.J. Effect of molecular structure on the relative hydrogen peroxide scavenging ability of some α-keto carboxylic acids. J. Pharm Sci. 2016, 105, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Dentuto, P.L.; Catucci, L.; Cosma, P.; Fini, P.; Agostiano, A.; D’Accolti, L.; Trevithick-Sutton, C.C.; Foote, C.S. Effect of cyclodextrins on the physicochemical properties of chlorophyll a in aqueous solution. J. Phys. Chem. B 2005, 109, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Annese, C.; D’Accolti, L.; Filardi, R.; Tommasi, I.; Fusco, C. Oxidative cleavage of lactams in water using dioxiranes: an expedient and environmentally-safe route to ω-nitro acids. Tet. Lett. 2013, 54, 515–517. [Google Scholar] [CrossRef]
- D’Accolti, L.; Annese, C.; De Riccardis, A.; De Giglio, E.; Cafagna, D.; Fanelli, F.; Fusco, C. Dioxirane-Mediated Heterogeneous Epoxidations with Potassium Caroate: A Solid Catalyst Bearing Anchored Ketone Moieties. Eur. J. Org. Chem. 2012, 24, 4616–4621. [Google Scholar] [CrossRef]
- Annese, C.; D’Accolti, L.; Fusco, C.; Curci, R. Selective Hydroxylation of Methane by Dioxiranes under Mild Conditions. Org. Lett. 2011, 13, 2142–2144. [Google Scholar] [CrossRef] [PubMed]
- Annese, C.; D’Accolti, L.; Armuzza, V.; Da Ros, T.; Fusco, C. Epoxidation of multi-walled carbon nanotubes by organocatalytic oxidation. Eur. J. Org. Chem. 2015, 3063–3068. [Google Scholar] [CrossRef]
- Annese, C.; D’Accolti, L.; Giambastiani, G.; Mangone, A.; Milella, A.; Tuci, G.; Fusco, C. Tunable epoxidation of single-walled carbon nanotubes by isolated methyl(trifluoromethyl)dioxirane. Eur. J. Org. Chem. 2014, 8, 1666–1671. [Google Scholar] [CrossRef]
- Mele, G.; Annese, C.; De Riccardis, A.; Fusco, C.; Palmisano, L.; Vasapollo, G.; D’Accolti, L. Turning lipophilic phthalocyanines/TiO2 composites into efficient photocatalysts for the conversion of CO2 into formic acid under UV–Vis light irradiation. Appl. Cat. A 2014, 481, 169–172. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Fanizza, E.; Comparelli, R.; Curri, M.L.; Agostiano, A.; Laub, D.L. Role of Metal Nanoparticles in TiO2/Ag Nanocomposite-Based Microheterogeneous Photocatalysis. J. Phys. Chem. B 2004, 108, 9623–9630. [Google Scholar] [CrossRef]
- George, C.; Dorfs, D.; Bertoni, G.; Falqui, A.; Genovese, A.; Pellegrino, T.; Roig, A.; Quarta, A.; Comparelli, R.; Curri, M.L.; Cingolani, R.; Manna, L. A Cast-Mold Approach to Iron Oxide and Pt/Iron Oxide Nanocontainers and Nanoparticles with a Reactive Concave Surface. J. Am. Chem. Soc. 2011, 133, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Aviles, F.; Cauich-Rodrıguez, J.V.; Moo-Tah, L.A.; May-Pat, A.; Vargas-Coronado, R. Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon 2009, 47, 2970–2975. [Google Scholar] [CrossRef]
- Shen, X.; Du, H.; Mullins, R.H.; Kommalapati, R.R. Polyethylenimine Applications in Carbon Dioxide Capture and Separation: From Theoretical Study to Experimental Work. Energy Technol. 2017, 5, 822–833. [Google Scholar] [CrossRef]
- Miranda, S.M.; Romanosc, G.E.; Likodimosc, V.; Marquesa, R.R.N.; Favvasc, E.P.; Katsarosc, F.K.; Stefanopoulosc, K.L.; Vilarb, V.J.P.; Faria, J.L.; Falarasc, P.; et al. Pore structure, interface properties and photocatalytic efficiency ofhydration/dehydration derived TiO2/CNT composites. Appl. Catal. B Environ. 2014, 147, 65–81. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Lee, J.Y.; Bajaj, H.C.; Jo, W.-K.; Tayade, R.J. Synthesis of multiwall carbon nanotubes/TiO2 nanotube composites with enhanced photocatalytic decomposition efficiency. Catal. Today 2017, 282, 13–23. [Google Scholar] [CrossRef]
- Gui, M.M.; Chai, S.-P.; Xub, B.-Q.; Mohamed, A.R. Visible-light-driven MWCNT@TiO2 core–shell nanocomposites and the roles of MWCNTs on the surface chemistry, optical properties and reactivity in CO2 photoreduction. RSC Adv. 2014, 4, 24007–24013. [Google Scholar] [CrossRef]
- Chen, Y.; Bian, J.; Qi, L.; Liu, E.; Fan, J. Efficient Degradation of Methylene Blue over Two-Dimensional Au/TiO2 Nanosheet Films with Overlapped Light Harvesting Nanostructures. J. Nanomater. 2015, 16, 254. [Google Scholar] [CrossRef]
- Karamian, E.; Sharifnia, S. On the general mechanism of photocatalytic reduction of CO2. J. CO2 Util. 2016, 16, 194–203. [Google Scholar] [CrossRef]
- Tumuluri, U.; Isenberg, M.; Tan, C.-S.; Chuang, S.S.C. In situ infrared study of the effect of amine density on the nature of adsorbed CO2 on amine-functionalized solid sorbents. Langmuir 2014, 30, 7405–7413. [Google Scholar] [CrossRef] [PubMed]
- Wilfong, W.C.; Srikanth, C.S.; Chuang, S.S.C. In situ ATR and DRIFTS studies of the nature of adsorbed CO2 on tetraethylenepentamine films. ACS Appl. Mater. Interfaces 2014, 6, 13617–13626. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Wang, L.-Y.; Zhang, Q.; Zheng, S.-J.; Li, X.-J.; Huang, C. Correlation between photoreactivity and photophysics of sulfated TiO2 photocatalyst. Mater. Chem. Phys. 2005, 92, 470–474. [Google Scholar] [CrossRef]
- Nazimek, B.; Czech, B. Artificial photosynthesis-CO2 towards methanol. IOP Conf. Ser. Mater. Sci. Eng. 2011, 19, 012010. [Google Scholar] [CrossRef]
- Srinivas, B.; Shubhamangala, B.; Lalitha, K.; Reddy, P.A.K.; Kumari, V.D.; Subrahmanyam, M.; De, B.R. Photocatalytic Reduction of CO2 over Cu-TiO2/Molecular Sieve 5A Composite. Photochem. Photobiol. 2011, 87, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Ishitani, O.; Inoue, C.; Suzuki, Y.; Ibusuki, T.J. Photocatalytic reduction of carbon dioxide to methane and acetic acid by an aqueous suspension of metal-deposited TiO2. Photochem. Photobiol. A Chem. 1993, 72, 269–271. [Google Scholar] [CrossRef]
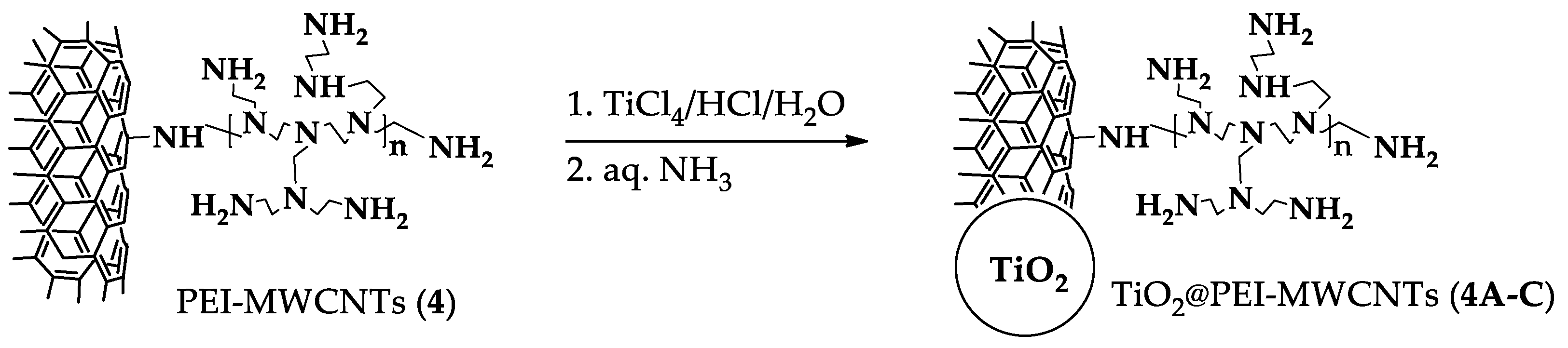



| Nanocomposite | MWCNTs | Amount of MWCNTs (mg) | TiCl4/HCl Mother Solution (mL) | Weight of Nanocomposite (g) | % (w/w) of PEI-MWCNTs in the Hybrid a |
|---|---|---|---|---|---|
| TiO2 (blank) | – | – | 250 | – | – |
| TiO2@MWCNTs (1A) | p-MWCNTs (1) | 20 | 250 | 1.0056 | 2.0% |
| TiO2@MWCNTs (1C) | p-MWCNTs (1) | 500 | 500 | 1.6651 | 30% |
| TiO2@PEI-MWCNTs (4A) | PEI-MWCNTs (4) | 20 | 250 | 1.1152 | 1.8% |
| TiO2@PEI-MWCNTs (4B) | PEI-MWCNTs (4) | 200 | 500 | 1.9508 | 10.3% |
| TiO2@PEI-MWCNTs (4C) | PEI-MWCNTs (4) | 500 | 500 | 1.6197 | 30.9% |
| Amine-Grafted MWCNTs | N-Content (%) by elem. Analysis | Adsorbed CO2 at 25 °C (mL·g−1) a | Desorbed CO2 at 80 °C (mL·g−1) b |
|---|---|---|---|
| p-MWCNTs 1 | – | 11 | 11 |
| HMDA-MWCNTs 3 | 1.01 | 24 | 24 |
| PEI-MWCNTs 4 | 2.63 | 35 | 35 |
| Hybrid a | % (w/w) of PEI-MWCNTs in the Hybrid | BET Surface Area (m2/g) |
|---|---|---|
| 1A (control) | 2.0% b | 176.7 |
| 1C (control) | 30% b | 299.2 |
| 4A | 1.8% | 161.8 |
| 4B | 10.3% | 327.3 |
| 4C | 30.9% | 304.2 |
| Surface Atomic Concentrations (%) | ||||
|---|---|---|---|---|
| Sample | C | O | N | Ti |
| PEI-MWCNTs (4) | 85.8 ± 0.5 | 6.8 ± 0.2 | 7.4 ± 0.2 | – |
| TiO2@PEI-MWCNTs (4C) | 55.2 ± 0.6 | 30.6 ± 0.6 | 3.7 ± 0.3 | 10.5 ± 0.3 |
| TiO2@PEI-MWCNTs (4C) * | 59.8 ± 0.6 | 27.6 ± 0.5 | 3.3 ± 0.2 | 9.3 ± 0.3 |
| Run | Cat. | % (w/w) of PEI-MWCNTs in Hybrid | Cat. Loading (mg/mL) a | Light Source b | Photocatalytic Products (μmol·g−1 Cat.) c | ||
|---|---|---|---|---|---|---|---|
| HCOOH | CH3CO2H | Total Carbonaceous | |||||
| 1 | PEI-MWCNTs (4) | – | 7.5 | H/S | 0.21 | 0.01 | 0.22 |
| 2 | TiO2 | – | 7.5 | H/S | 1.73 | 0.01 | 1.74 |
| 3 | 1A | 2.0% d | 7.5 | H/S | 2.67 | 0.38 | 3.05 |
| 4 | 1C | 30.0% d | 7.5 | H/S | 5.80 | 2.73 | 8.53 |
| 5 | 4A | 1.8% | 7.5 | H/S | 4.05 | 4.30 | 8.35 |
| 6 | 4B | 10.3% | 7.5 | H/S | 13.01 | 8.49 | 21.50 |
| 7 | 4C | 30.9% | 7.5 | H/S | 29.84 | 7.46 | 37.30 |
| 8 | 4C | 30.9% | 7.5 | H | 25.50 | 4.98 | 30.48 |
| 9 | 4C | 30.9% | 7.5 | S | 3.99 | 23.00 | 26.99 |
| 10 | 4C e | 30.9% | 7.5 | H/S | 0.086 | 0.012 | 0.096 |
| 11 | 1A | 2.0% | 25 | H/S | 0.76 | 0.15 | 0.91 |
| 12 | 4A | 1.8% | 25 | H/S | 0.51 | 0.48 | 0.99 |
| 13 | 4B | 10.3% | 25 | H/S | 1.11 | 2.86 | 3.97 |
| 14 | 4C | 30.9% | 25 | H/S | 16.50 | 2.44 | 18.94 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusco, C.; Casiello, M.; Catucci, L.; Comparelli, R.; Cotugno, P.; Falcicchio, A.; Fracassi, F.; Margiotta, V.; Moliterni, A.; Petronella, F.; et al. TiO2@PEI-Grafted-MWCNTs Hybrids Nanocomposites Catalysts for CO2 Photoreduction. Materials 2018, 11, 307. https://doi.org/10.3390/ma11020307
Fusco C, Casiello M, Catucci L, Comparelli R, Cotugno P, Falcicchio A, Fracassi F, Margiotta V, Moliterni A, Petronella F, et al. TiO2@PEI-Grafted-MWCNTs Hybrids Nanocomposites Catalysts for CO2 Photoreduction. Materials. 2018; 11(2):307. https://doi.org/10.3390/ma11020307
Chicago/Turabian StyleFusco, Caterina, Michele Casiello, Lucia Catucci, Roberto Comparelli, Pietro Cotugno, Aurelia Falcicchio, Francesco Fracassi, Valerio Margiotta, Anna Moliterni, Francesca Petronella, and et al. 2018. "TiO2@PEI-Grafted-MWCNTs Hybrids Nanocomposites Catalysts for CO2 Photoreduction" Materials 11, no. 2: 307. https://doi.org/10.3390/ma11020307







