Scale-Up of the Electrodeposition of ZnO/Eosin Y Hybrid Thin Films for the Fabrication of Flexible Dye-Sensitized Solar Cell Modules
Abstract
:1. Introduction
2. Experimental Methods
2.1. Electrodeposition
2.1.1. General Procedures
2.1.2. Fabrication of Small-Scaled ZnO Films
2.1.3. Up-Scaled Fabrication of ZnO Films
2.2. Characterization of ZnO Thin Films
2.3. Device Fabrication
2.3.1. Fabrication of Small-Scaled Flexible DSSCs
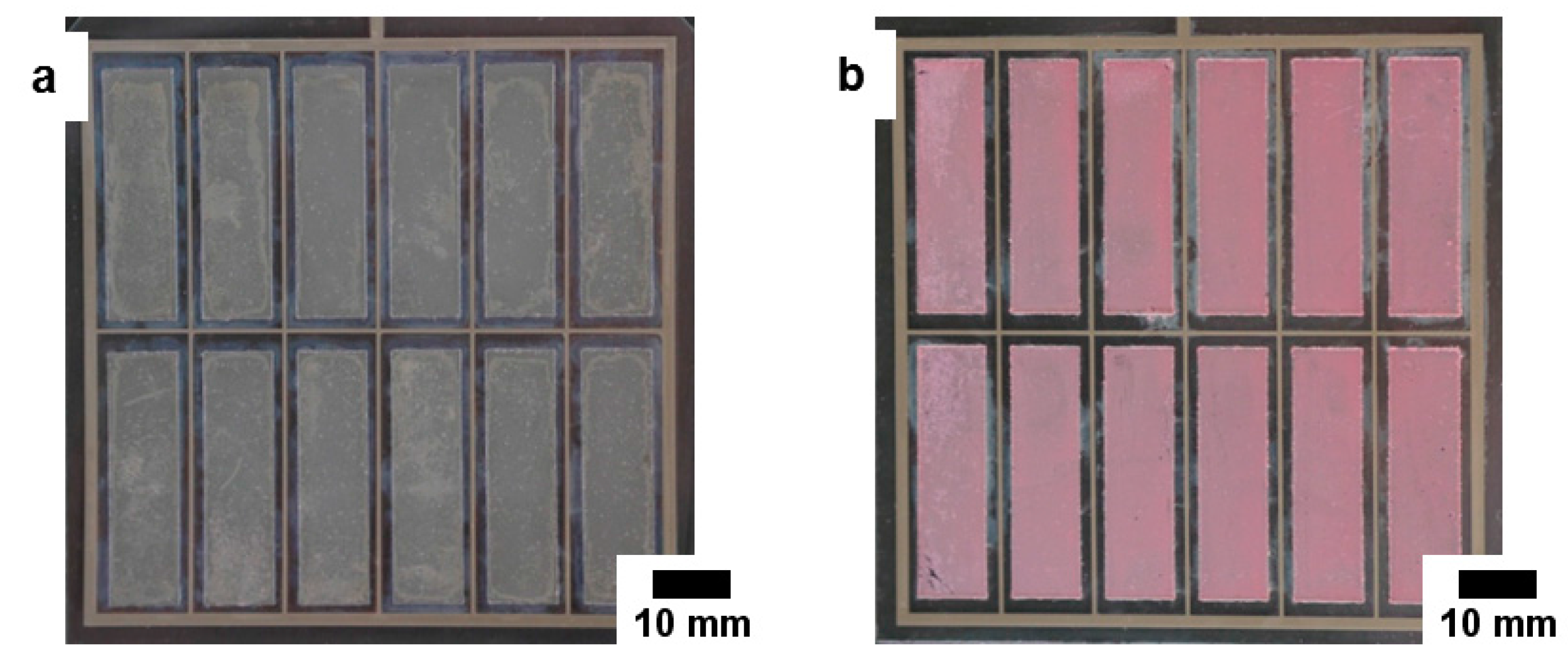
2.3.2. Fabrication of Up-Scaled Flexible DSSC Modules
2.4. Photovoltaic Characterization
2.4.1. I-V Characteristics
2.4.2. Electrochemical Impedance Spectroscopy (EIS)
3. Results and Discussion
3.1. Electrodeposition of ZnO Films
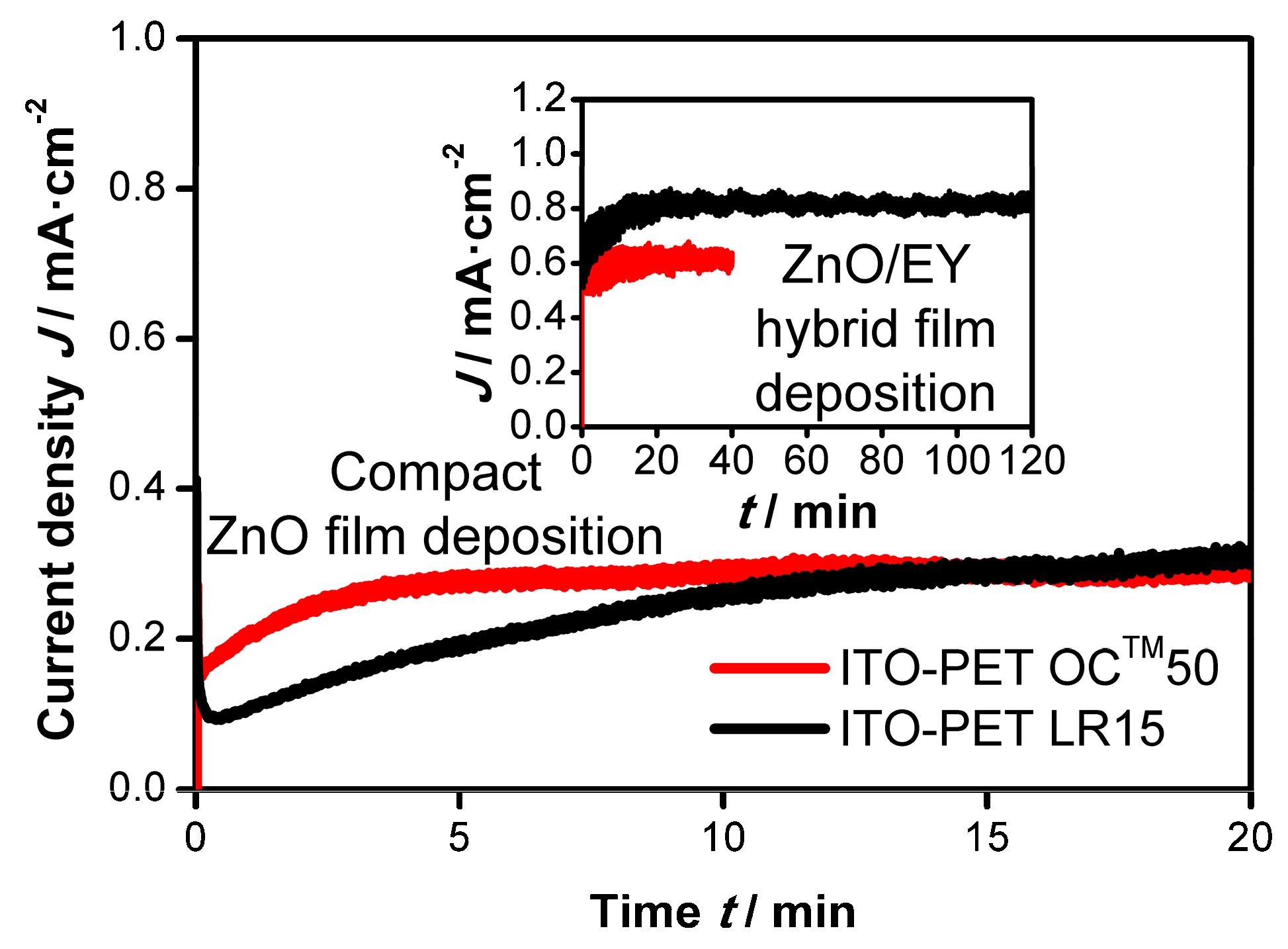
3.1.1. Adaption of the ZnO Electrodeposition Process to the Miniplant Setup
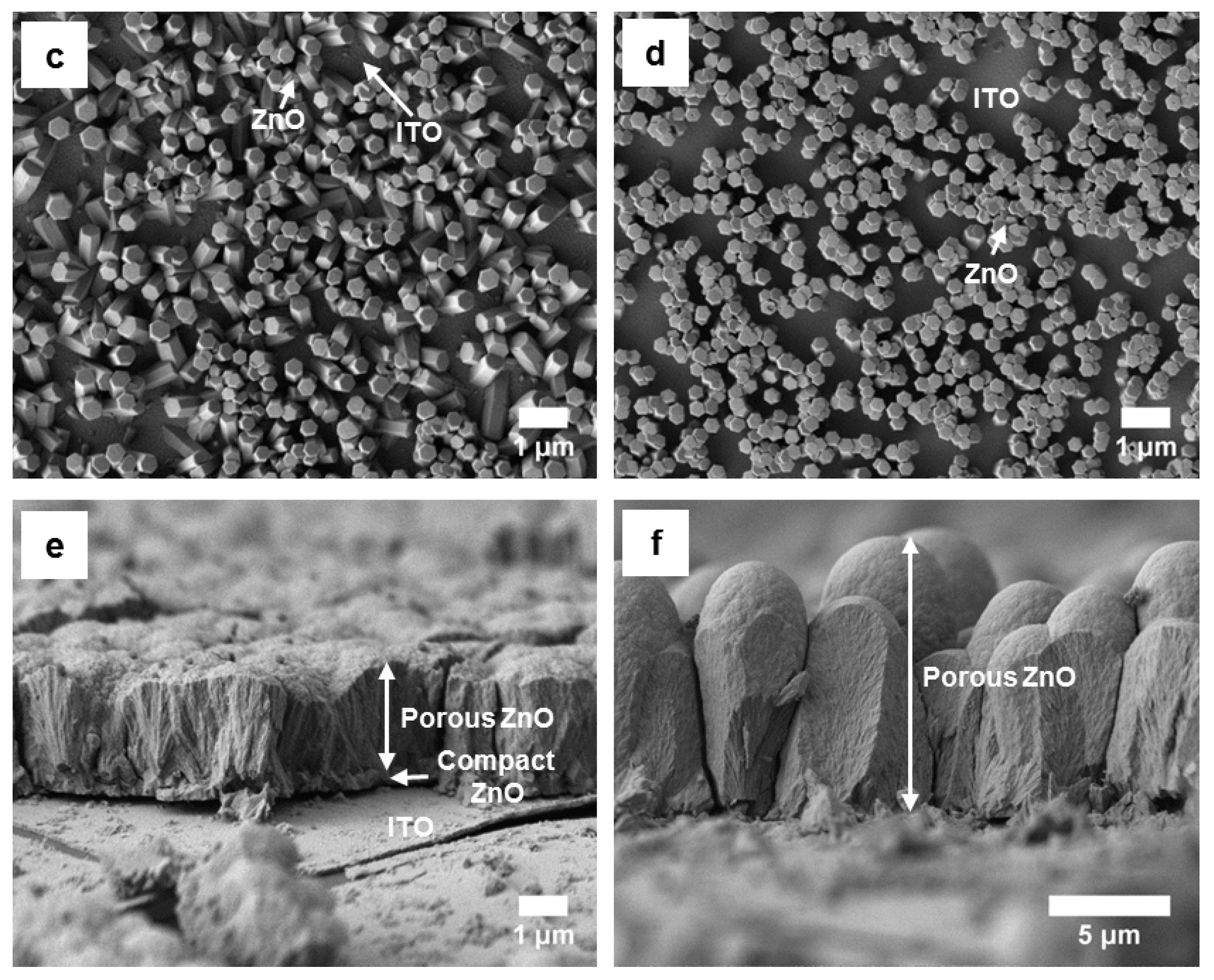
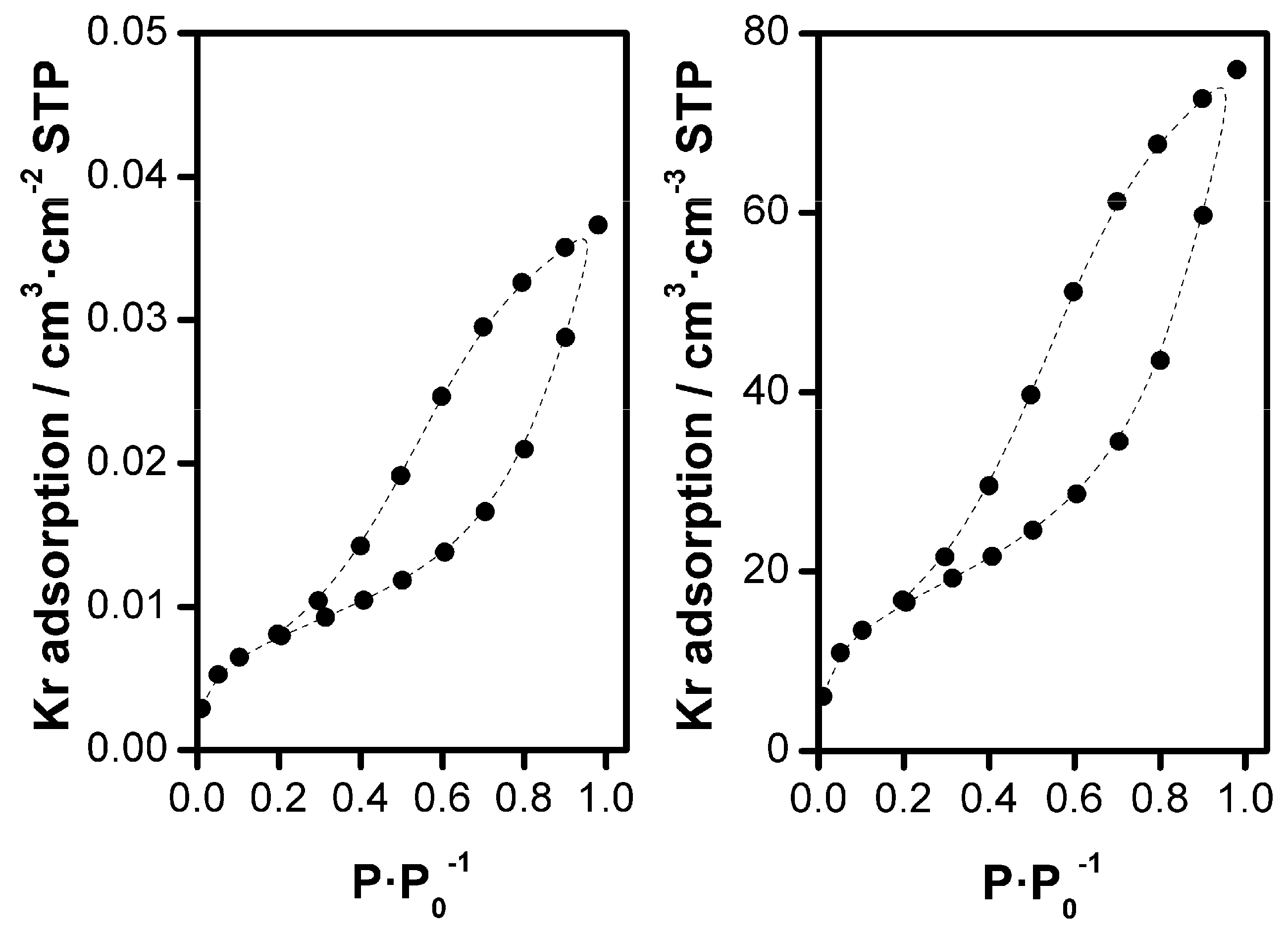
3.1.2. Electrodeposition of ZnO Films on FTO-Glass Substrates
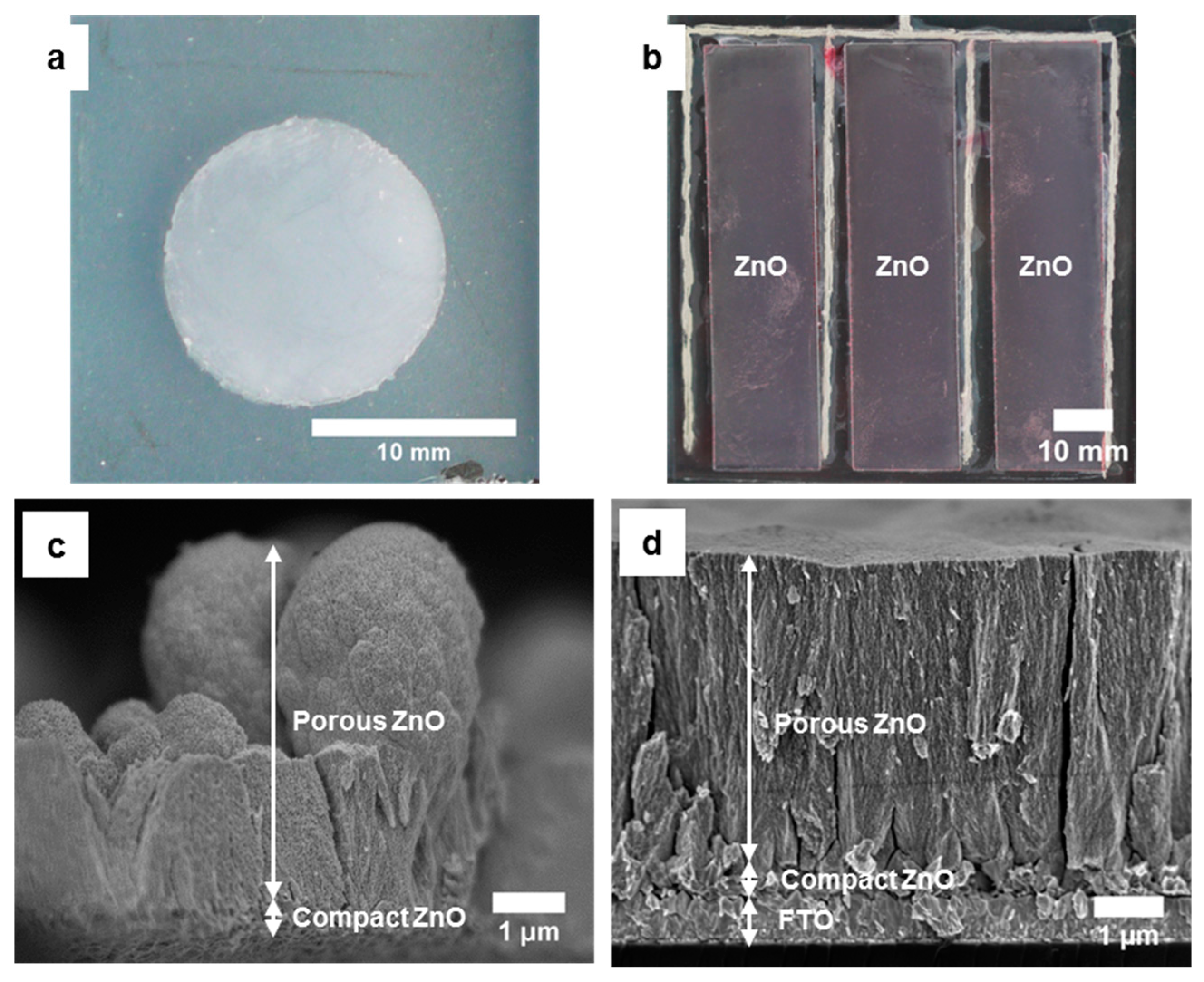
3.1.3. Electrodeposition of ZnO Films on ITO-PET Foils
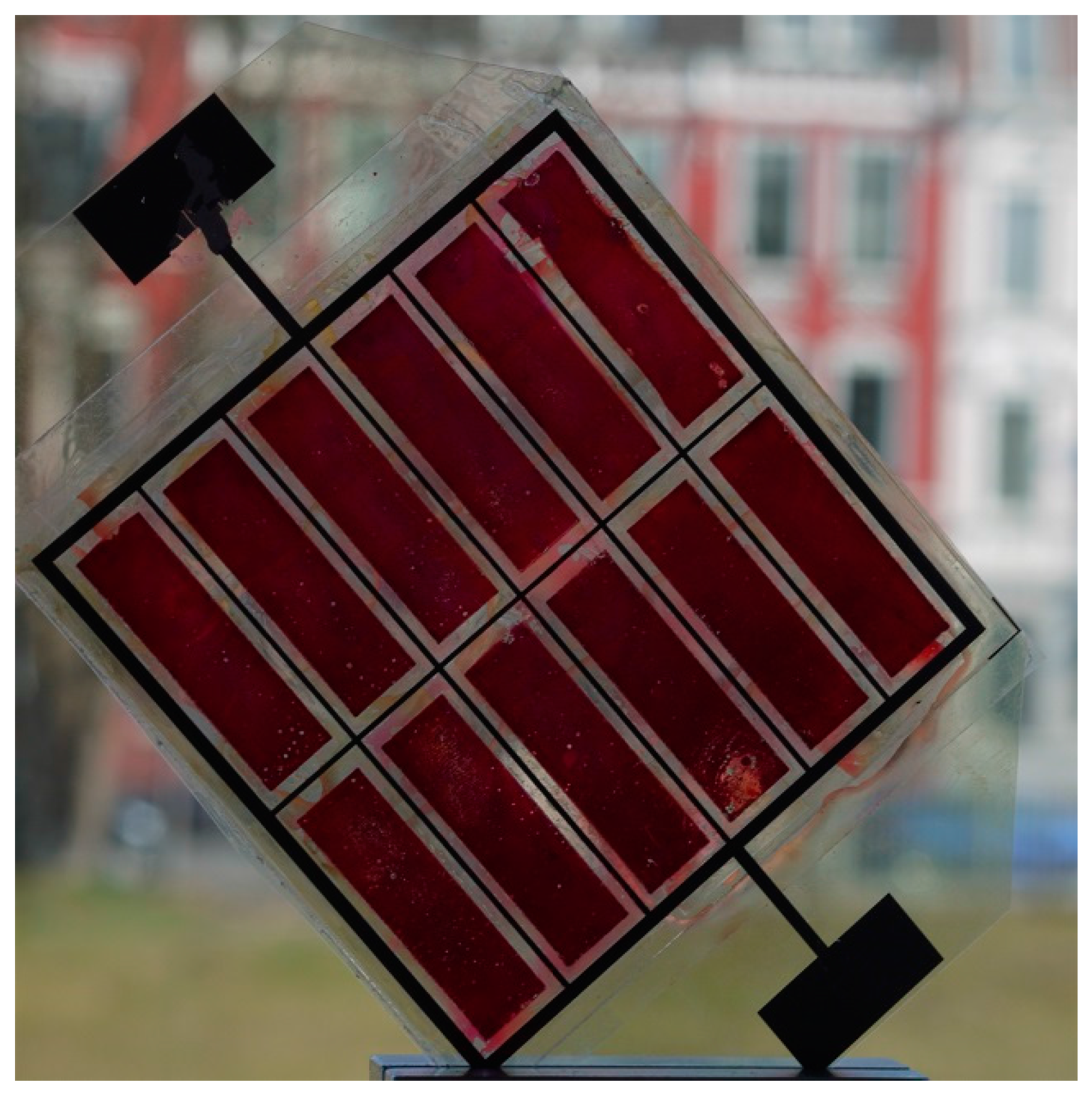
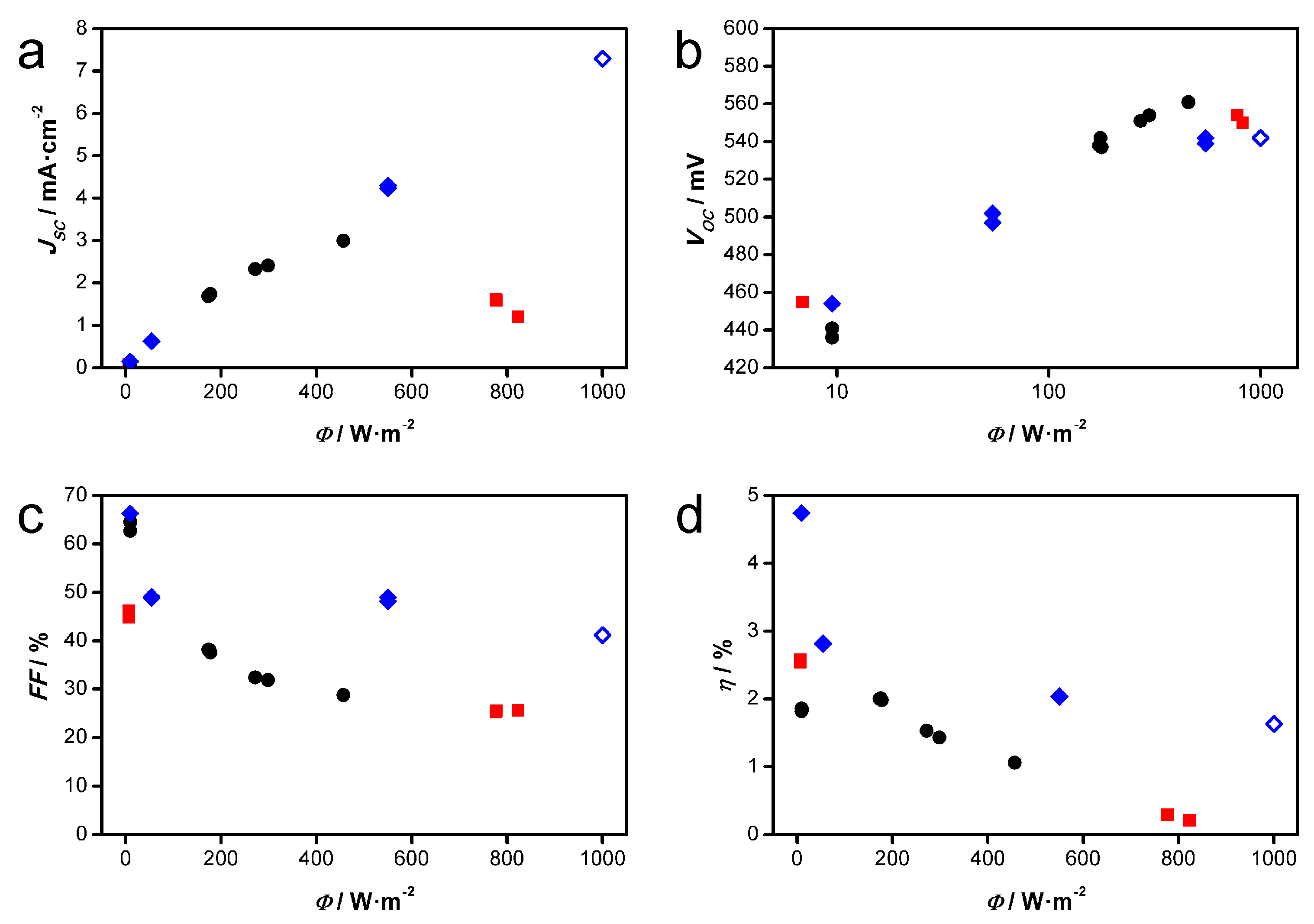
3.2. Flexible DSSC Modules
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schubert, M.B.; Merz, R. Flexible solar cells and modules. Philos. Mag. 2009, 89, 2623–2644. [Google Scholar] [CrossRef]
- Lu, H.; Zhai, X.; Liu, W.; Zhang, M.; Guo, M. Electrodeposition of hierarchical ZnO nanorod arrays on flexible stainless steel mesh for dye-sensitized solar cell. Thin Solid Films 2015, 586, 46–53. [Google Scholar] [CrossRef]
- Baker, J.A.; Worsley, C.; Lee, H.K.H.; Clark, R.N.; Tsoi, W.C.; Williams, G.; Worsley, D.A.; Gethin, D.T.; Watson, T.M. Development of Graphene Nano-Platelet Ink for High Voltage Flexible Dye Sensitized Solar Cells with Cobalt Complex Electrolytes. Adv. Eng. Mater. 2017, 19, 1600652. [Google Scholar] [CrossRef]
- Hashmi, G.; Miettunen, K.; Peltola, T.; Halme, J.; Asghar, I.; Aitola, K.; Toivola, M.; Lund, P.D. Review of materials and manufacturing options for large area flexible dye solar cells. Renew. Sustain. Energy Rev. 2011, 15, 3717–3732. [Google Scholar] [CrossRef]
- Casaluci, S.; Gemmi, M.; Pellegrini, V.; Di Carlo, A.; Bonaccorso, F. Graphene-based large area dye-sensitized solar cell modules. Nanoscale 2016, 8, 5368–5378. [Google Scholar] [CrossRef] [PubMed]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Graetzel, M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)–Based Redox Electrolyte Exceed 12 Percent Efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Hishikawa, Y.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Ho-Baillie, A.W.Y. Solar cell efficiency tables (version 51). Prog. Photovolt. Res. Appl. 2018, 26, 3–12. [Google Scholar] [CrossRef]
- Jackson, P.; Hariskos, D.; Wuerz, R.; Kiowski, O.; Bauer, A.; Friedlmeier, T.M.; Powalla, M. Properties of Cu(In,Ga)Se2 solar cells with new record efficiencies up to 21.7%. Phys. Status Solidi (RRL) 2015, 9, 28–31. [Google Scholar] [CrossRef]
- Park, J.H.; Jun, Y.; Yun, H.-G.; Lee, S.-Y.; Kang, M.G. Fabrication of an Efficient Dye-Sensitized Solar Cell with Stainless Steel Substrate. J. Electrochem. Soc. 2008, 155, F145–F149. [Google Scholar] [CrossRef]
- Fang, X.; Ma, T.; Guan, G.; Akiyama, M.; Kida, T.; Abe, E. Effect of the thickness of the Pt film coated on a counter electrode on the performance of a dye-sensitized solar cell. J. Electroanal. Chem. 2004, 570, 257–263. [Google Scholar] [CrossRef]
- Miettunen, K.; Ruan, X.; Saukkonen, T.; Halme, J.; Toivola, M.; Guangsheng, H.; Lund, P.D. Stability of Dye Solar Cells with Photoelectrode on Metal Substrates. J. Electrochem. Soc. 2010, 157, B814–B819. [Google Scholar] [CrossRef]
- Chang, H.; Chen, T.L.; Huang, K.D.; Chien, S.H.; Hung, K.C. Fabrication of highly efficient flexible dye-sensitized solar cells. J. Alloys Compd. 2010, 504, S435–S438. [Google Scholar] [CrossRef]
- Watson, T.; Mabbett, I.; Wang, H.; Peter, L.M.; Worsley, D. Ultrafast near infrared sintering of TiO2 layers on metal substrates for dye-sensitized solar cells. Prog. Photovolt. Res. Appl. 2011, 19, 482–486. [Google Scholar] [CrossRef] [Green Version]
- Miyasaka, T.; Kijitori, Y. Low-Temperature Fabrication of Dye-Sensitized Plastic Electrodes by Electrophoretic Preparation of Mesoporous TiO2 Layers. J. Electrochem. Soc. 2004, 151, A1767–A1773. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Tobe, N.; Matsumoto, D.; Nagai, T.; Arakawa, H. Highly efficient plastic-substrate dye-sensitized solar cells with validated conversion efficiency of 7.6%. Sol. Energy Mater. Sol. Cells 2010, 94, 812–816. [Google Scholar] [CrossRef]
- Yang, L.; Wu, L.; Wu, M.; Xin, G.; Lin, H.; Ma, T. High-efficiency flexible dye-sensitized solar cells fabricated by a novel friction-transfer technique. Electrochem. Commun. 2010, 12, 1000–1003. [Google Scholar] [CrossRef]
- Park, N.G.; Kim, K.M.; Kang, M.G.; Ryu, K.S.; Chang, S.H.; Shin, Y.-J. Chemical Sintering of Nanoparticles: A Methodology for Low-Temperature Fabrication of Dye-Sensitized TiO2 Films. Adv. Mater. 2005, 17, 2349–2353. [Google Scholar] [CrossRef]
- Li, X.; Lin, H.; Li, J.; Wang, N.; Lin, C.; Zhang, L. Chemical sintering of graded TiO2 film at low-temperature for flexible dye-sensitized solar cells. J. Photochem. Photobiol. A 2008, 195, 247–253. [Google Scholar] [CrossRef]
- Chiu, W.-H.; Lee, K.-M.; Hsieh, W.-F. High efficiency flexible dye-sensitized solar cells by multiple electrophoretic depositions. J. Power Sources 2011, 196, 3683–3687. [Google Scholar] [CrossRef]
- Qin, F.; Meng, W.; Fan, J.C.; Ge, C.; Luo, B.W.; Ge, R.; Hu, L.; Jiang, F.Y.; Liu, T.F.; Jiang, Y.Y.; et al. Enhanced Thermochemical Stability of CH3NH3PbI3 Perovskite Films on Zinc Oxides via New Precursors and Surface Engineering. ACS Appl. Mater. Interfaces 2017, 9, 26045–26051. [Google Scholar] [CrossRef] [PubMed]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Abd-Ellah, M.; Moghimi, N.; Zhang, L.; Thomas, J.P.; McGillivray, D.; Srivastava, S.; Leung, K.T. Plasmonic gold nanoparticles for ZnO-nanotube photoanodes in dye-sensitized solar cell application. Nanoscale 2016, 8, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Oekermann, T.; Yoshida, T.; Minoura, H.; Wijayantha, K.G.U.; Peter, L.M. Electron Transport and Back Reaction in Electrochemically Self-Assembled Nanoporous ZnO/Dye Hybrid Films. J. Phys. Chem. B 2004, 108, 8364–8370. [Google Scholar] [CrossRef]
- Yoshida, T.; Zhang, J.; Komatsu, D.; Sawatani, S.; Minoura, H.; Pauporté, T.; Lincot, D.; Oekermann, T.; Schlettwein, D.; Tada, H.; et al. Electrodeposition of Inorganic/Organic Hybrid Thin Films. Adv. Funct. Mater. 2009, 19, 17–43. [Google Scholar] [CrossRef]
- Peulon, S.; Lincot, D. Cathodic electrodeposition from aqueous solution of dense or open-structured zinc oxide films. Adv. Mater. 1996, 8, 166–170. [Google Scholar] [CrossRef]
- Peulon, S.; Lincot, D. Mechanistic Study of Cathodic Electrodeposition of Zinc Oxide and Zinc Hydroxychloride Films from Oxygenated Aqueous Zinc Chloride Solutions. J. Electrochem. Soc. 1998, 145, 864–874. [Google Scholar] [CrossRef]
- Pauporté, T.; Lincot, D. Electrodeposition of semiconductors for optoelectronic devices: Results on zinc oxide. Electrochim. Acta 2000, 45, 3345–3353. [Google Scholar] [CrossRef]
- Pérez-Hernández, G.; Vega-Poot, A.; Pérez-Juárez, I.; Camacho, J.M.; Arés, O.; Rejón, V.; Peña, J.L.; Oskam, G. Effect of a compact ZnO interlayer on the performance of ZnO-based dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2012, 100, 21–26. [Google Scholar] [CrossRef]
- Pauporté, T.; Lincot, D. Heteroepitaxial electrodeposition of zinc oxide films on gallium nitride. Appl. Phys. Lett. 1999, 75, 3817–3819. [Google Scholar] [CrossRef]
- Yoshida, T.; Pauporté, T.; Lincot, D.; Oekermann, T.; Minoura, H. Cathodic Electrodeposition of ZnO/Eosin Y Hybrid Thin Films from Oxygen-Saturated Aqueous Solution of ZnCl2 and Eosin Y. J. Electrochem. Soc. 2003, 150, C608–C615. [Google Scholar] [CrossRef]
- Canava, B.; Lincot, D. Nucleation effects on structural and optical properties of electrodeposited zinc oxide on tin oxide. J. Appl. Electrochem. 2000, 30, 711–716. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.; Yoshida, T. Spectroelectrochemical studies on redox reactions of eosin Y and its polymerization with Zn2+ ions. J. Electroanal. Chem. 2011, 662, 384–395. [Google Scholar] [CrossRef]
- Goux, A.; Pauporté, T.; Yoshida, T.; Lincot, D. Mechanistic Study of the Electrodeposition of Nanoporous Self-Assembled ZnO/Eosin Y Hybrid Thin Films: Effect of Eosin Concentration. Langmuir 2006, 22, 10545–10553. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Iwaya, M.; Ando, H.; Oekermann, T.; Nonomura, K.; Schlettwein, D.; Woehrle, D.; Minoura, H. Improved photoelectrochemical performance of electrodeposited ZnO/EosinY hybrid thin films by dye re-adsorption. Chem. Commun. 2004, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Rathouský, J.; Loewenstein, T.; Nonomura, K.; Yoshida, T.; Wark, M.; Schlettwein, D. Electrochemically self-assembled mesoporous dye-modified zinc oxide thin films. Stud. Surf. Sci. Catal. 2005, 156, 315–320. [Google Scholar]
- Pauporté, T.; Yoshida, T.; Cortès, R.; Froment, M.; Lincot, D. Electrochemical Growth of Epitaxial Eosin/ZnO Hybrid Films. J. Phys. Chem. B 2003, 107, 10077–10082. [Google Scholar] [CrossRef]
- Guerin, V.-M.; Magne, C.; Pauporté, T.; Le Bahers, T.; Rathouský, J. Electrodeposited Nanoporous versus Nanoparticulate ZnO Films of Similar Roughness for Dye-Sensitized Solar Cell Applications. ACS Appl. Mater. Interfaces 2010, 2, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Rathouský, J.; Kalousek, V.; Yarovyi, V.; Wark, M.; Jirkovský, J. A low-cost procedure for the preparation of mesoporous layers of TiO2 efficient in the environmental clean-up. J. Photochem. Photobiol. A Chem. 2010, 216, 126–132. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Huang, X.-M.; Gao, K.-Y.; Yang, Y.-Y.; Luo, Y.-H.; Li, D.-M.; Meng, Q.-B. How to design dye-sensitized solar cell modules. Sol. Energy Mater. Sol. Cells 2011, 95, 2564–2569. [Google Scholar] [CrossRef]
- Michaelis, E.; Woehrle, D.; Rathousky, J.; Wark, M. Electrodeposition of porous zinc oxide electrodes in the presence of sodium laurylsulfate. Thin Solid Films 2006, 497, 163–169. [Google Scholar] [CrossRef]
- Loewenstein, T.; Nonomura, K.; Yoshida, T.; Michaelis, E.; Woehrle, D.; Rathouský, J.; Wark, M.; Schlettwein, D. Efficient Sensitization of Mesoporous Electrodeposited Zinc Oxide by cis-Bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato)-Ruthenium(II). J. Electrochem. Soc. 2006, 153, A699–A704. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Garcia-Belmonte, G.; Mora-Sero, I.; Bisquert, J. Characterization of nanostructured hybrid and organic solar cells by impedance spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 9083–9118. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yanagida, S. Strategy to improve the performance of dye-sensitized solar cells: Interface engineering principle. Sol. Energy 2011, 85, 3143–3159. [Google Scholar] [CrossRef]
- Ramasamy, E.; Lee, W.J.; Lee, D.Y.; Song, J.S. Portable, parallel grid dye-sensitized solar cell module prepared by screen printing. J. Power Sources 2007, 165, 446–449. [Google Scholar] [CrossRef]
- Ito, S.; Nazeeruddin, M.K.; Liska, P.; Comte, P.; Charvet, R.; Péchy, P.; Jirousek, M.; Kay, A.; Zakeeruddin, S.M.; Graetzel, M. Photovoltaic characterization of dye-sensitized solar cells: Effect of device masking on conversion efficiency. Prog. Photovolt. Res. Appl. 2006, 14, 589–601. [Google Scholar] [CrossRef]
- Miyasaka, T.; Kijitori, Y.; Ikegami, M. Plastic Dye-sensitized Photovoltaic Cells and Modules Based on Low-temperature Preparation of Mesoscopic Titania Electrodes. Electrochemistry 2007, 75, 2–12. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bittner, F.; Oekermann, T.; Wark, M. Scale-Up of the Electrodeposition of ZnO/Eosin Y Hybrid Thin Films for the Fabrication of Flexible Dye-Sensitized Solar Cell Modules. Materials 2018, 11, 232. https://doi.org/10.3390/ma11020232
Bittner F, Oekermann T, Wark M. Scale-Up of the Electrodeposition of ZnO/Eosin Y Hybrid Thin Films for the Fabrication of Flexible Dye-Sensitized Solar Cell Modules. Materials. 2018; 11(2):232. https://doi.org/10.3390/ma11020232
Chicago/Turabian StyleBittner, Florian, Torsten Oekermann, and Michael Wark. 2018. "Scale-Up of the Electrodeposition of ZnO/Eosin Y Hybrid Thin Films for the Fabrication of Flexible Dye-Sensitized Solar Cell Modules" Materials 11, no. 2: 232. https://doi.org/10.3390/ma11020232





