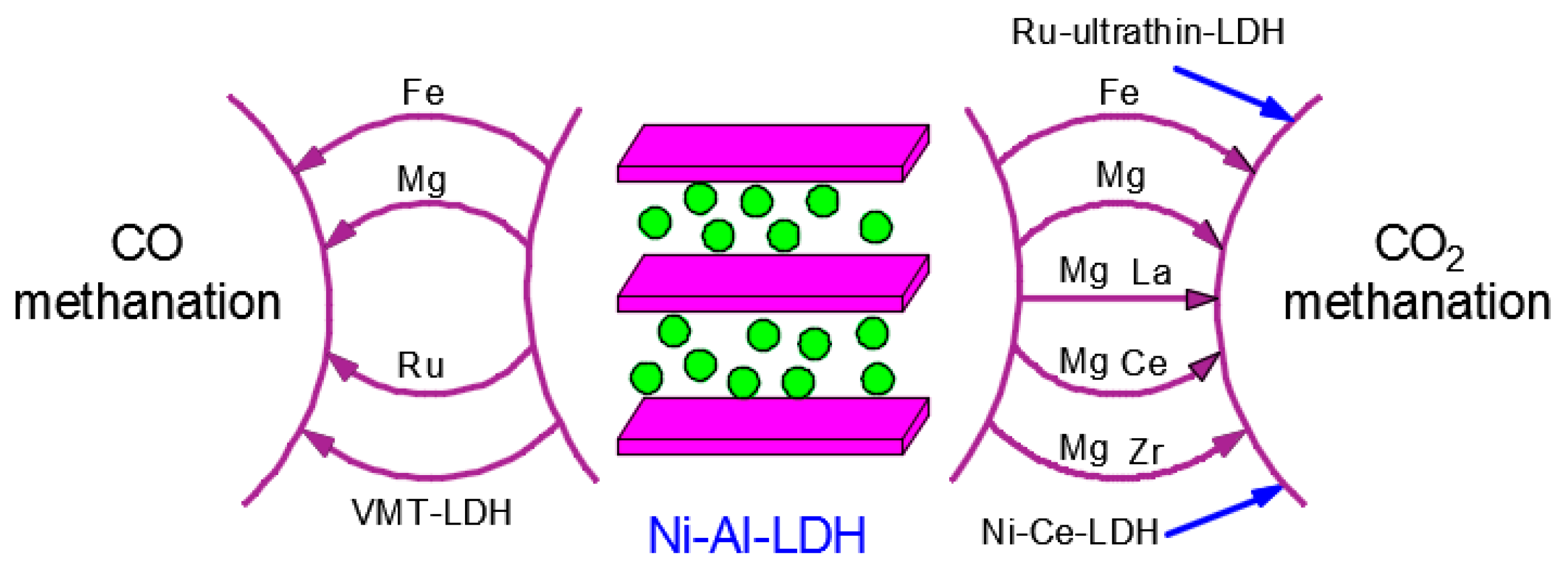
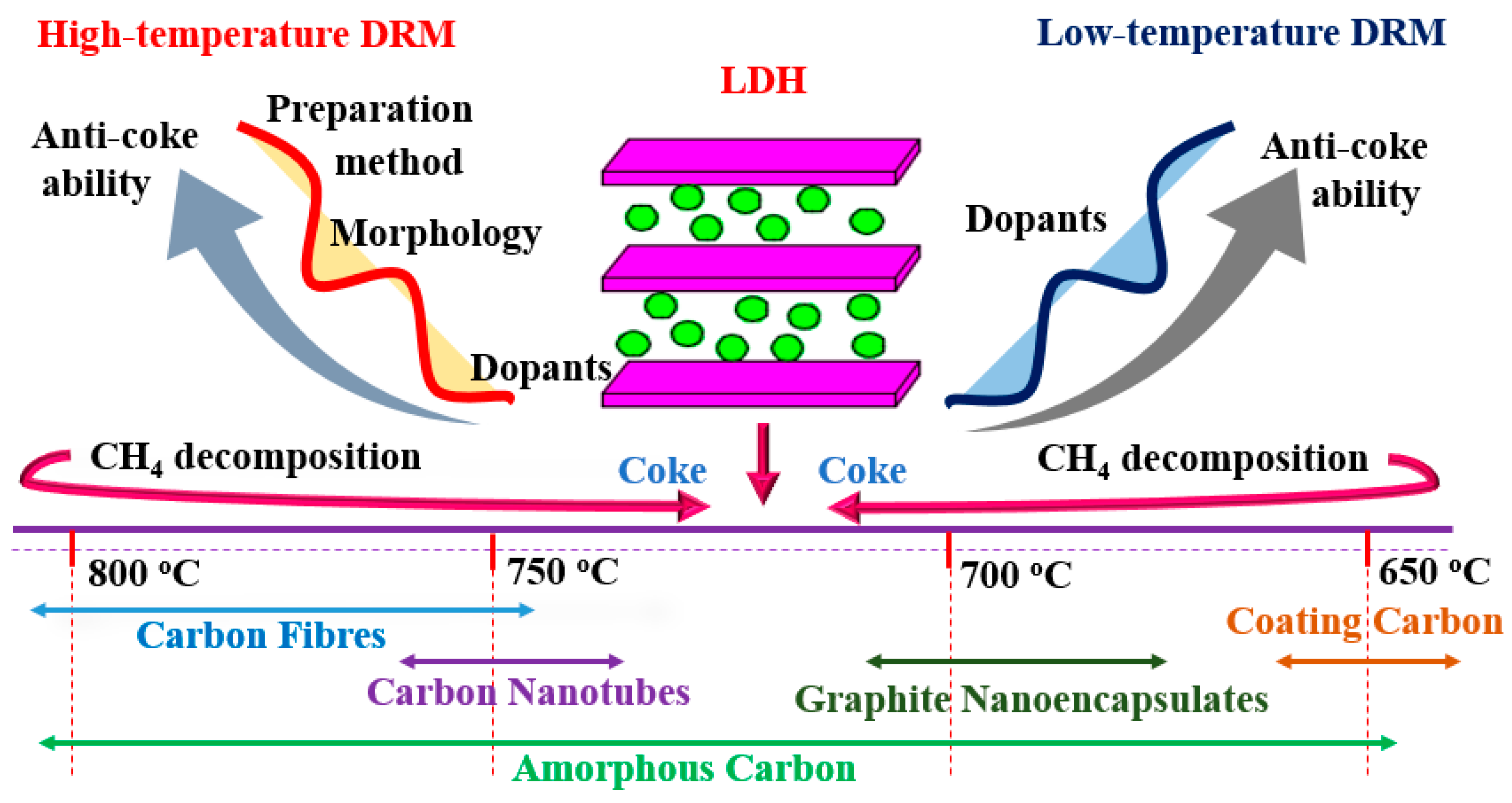
3.1.1. High Temperature Dry Reforming of Methane
Noble metals like Ru reflected excellent catalytic performance and anti-coke deposition ability in the DRM reaction. Supports have great influence on the dispersion of the active component Ru. Ru metal dispersed on different supports followed the order: Ru/Mg
3(Al)O > Ru/MgO > Ru/MgAl
2O
4 > Ru/γ-Al
2O
3. Ru/Mg
3(Al)O catalyst showed higher catalytic performance due to the strong base intensity of support and more surface Ru
0 atoms, which ascribed to Mg(Al)O mixed oxide’s unique properties, including memory effect, low crystallinity and its strong interaction with Ru [
71]. Ru/Mg
3(Al)O catalyst displayed an 84% CH
4 conversion during the 30-h stability test without any deactivation.
Even though noble metal-based catalysts exhibited excellent catalytic performance, industrial applications were limited due to the high cost. Ni was a suitable active catalyst component when taking catalytic performance and cost into consideration. In 1988, Bhattacharyya et al. [
72] investigated the performance of LDH-derived catalysts in CO
2 reforming of methane. Compared with commercial Ni/Al
2O
3 catalysts, the LDH-derived Ni
4Al
2O
7 catalyst showed identical performance at 815 °C and 2.07 MPa. LDH-derived catalysts had superior stability and a coke-resistant ability according to aging studies, and the LDH-derived Mg
5NiAl
2O
9 catalyst exhibited the highest CH
4 conversion of 95.8% under operation conditions at 850 °C, 0.67 MPa, GHSV = 14,400 h
−1 and CO
2/CH = 1.25; while Touahra et al. [
73] discovered that Ni/Al = 2 was optimal for Ni-Al LDH-derived catalysts due to the low Ni
0 crystallite size and high stability of the support (NiAl
2O
4).
Consequently, when Mg was introduced to Ni-Al LDHs, the catalytic performance and anti-coke ability of the catalyst improved. Mette et al. [
74,
75,
76] found that Ni/MgAlOx catalyst has good anti-coke ability, and the catalytic performance revealed no decrease in performance after 19 times of cycling because nickel aluminate overgrowth on the Ni particles blocked all extended metallic Ni sites, which were nucleation centers for carbon formation. As reported by Lopez et al. [
77], the catalytic properties of Ni-Mg-Al catalysts were more affected by the M
II/M
III ratio compared with the Ni/Mg ratio. When M
II/M
III was maintained, the catalyst activity was related to the nickel crystal size and Ni/Mg ratio, while selectivity suffered little from the Ni/Mg ratios. This notwithstanding, when Ni/Mg was constant, the catalyst activity was strongly affected and decreased as the M
II/M
III ratio decreased. Besides, Zhu et al. [
78] showed that NiMgAl catalyst with a Mg/Al ratio of 1 displayed the best activity and stability during the DRM reaction, and it was the formation of LDH precursors and MgNiO
2 that played the key role in stabilization. Interestingly, Li et al. [
79] demonstrated that the performance of Ni/Mg(Al)O catalyst decreased at the beginning due to the MgO film surrounding the Ni particles; however, the catalyst was renewed after MgAl
2O
4 spinel-like phase formation. TheHT-700 catalyst reached approximately 95% CH
4 conversion after 500 h of reaction and maintained for 1500 h. In 2016, Buelens et al. [
80] developed a “super-dry” CH
4 reforming through Le Chatelier’s principle reaction (
Figure 5): Ni/MgAl
2O
4 was used as the catalyst during the “super-dry” CH
4 reforming. Fe
2O
3/MgAl
2O
4 was used as the solid oxygen carrier, which oxidized CH
4 into CO
2 and H
2O. Meanwhile, the Fe
2O
3 reduced to Fe; CaO/Al
2O
3 was used as the CO
2 sorbent, which formed CaCO
3 and then decomposed into CaO and CO
2, and CO
2 reduced to CO by Fe through a redox reaction. “Super-dry” CH
4 reforming also resulted in a very low exergy destruction per mole CO
2 converted; the exergy destruction for CO
2 conversion was up to 25–50% lower as compared with that of conventional DRM. “Super-dry” CH
4 reforming can result in higher CO production and showed both practical and economic benefits compared with conventional dry reforming.
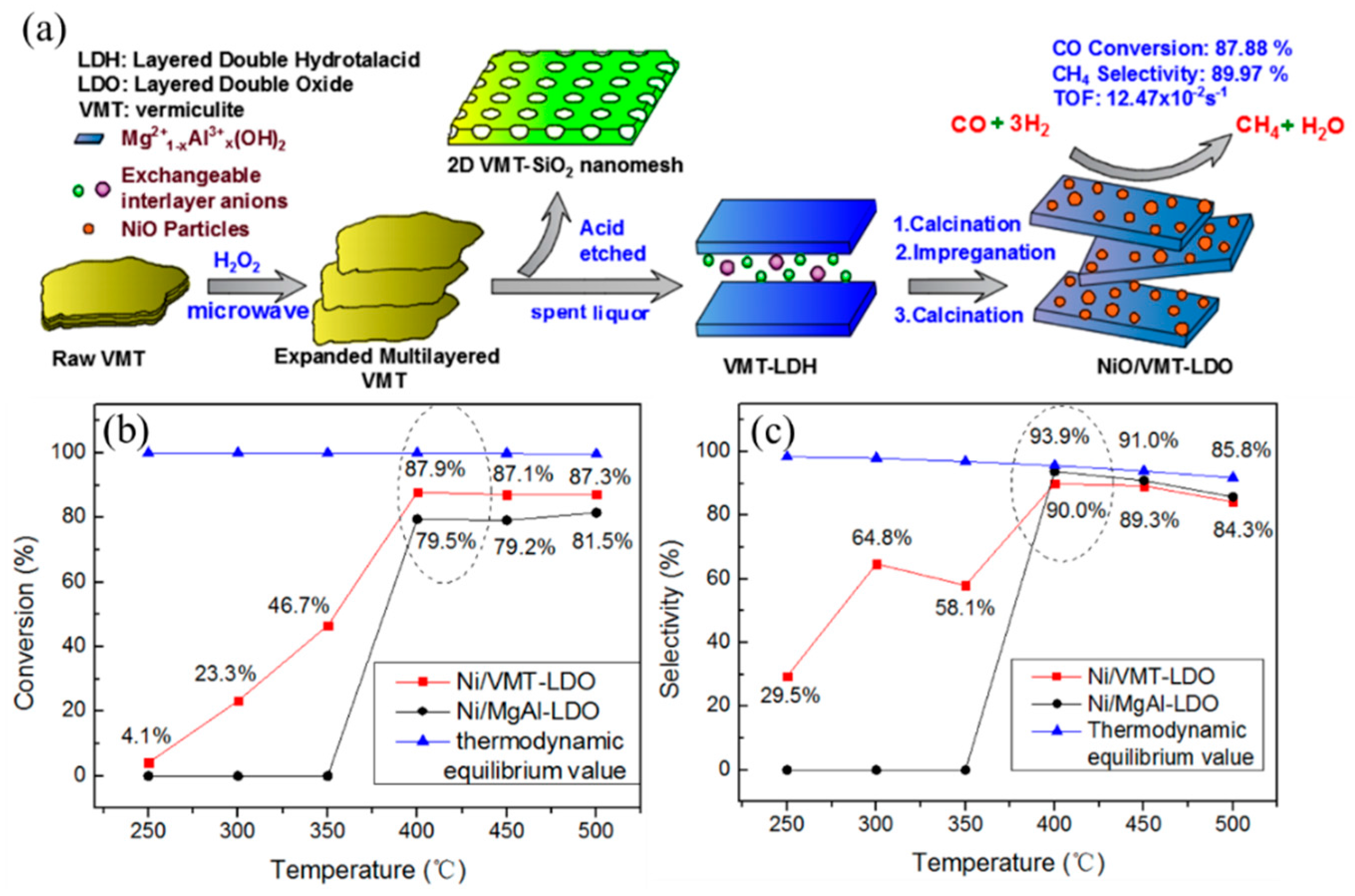
Besides the classic co-precipitation method, other methods also have been employed to prepare high efficiency Ni/Mg/Al LDH-derived catalysts. Meanwhile, Shishido et al. [
81] investigated the influence of the preparation method on the catalytic performance of Ni/Mg-Al catalyst. Likewise, the solid phase crystallization (spc) method can promote Ni
2+ replacement of the Mg
2+ site in LDH, resulting in the formation of highly dispersed 7% Ni metal particles, while the Ni dispersion of imp-Ni/Mg-Al was 4.8% (prepared by impregnation method). The catalyst spc-Ni/Mg-Al exhibited a slightly better catalytic performance than imp-Ni/Mg-Al at 800 °C. In addition, Chai et al. [
82] developed a FeCrAl-fiber-structured nanocomposite NiO-MgO-Al
2O
3 catalyst in one step, using a γ-Al
2O
3/water interface-assisted method, as shown in
Figure 6. The as-obtained catalyst exhibited excellent stability due to Ni nanoparticles being uniformly nested in MgO-Al
2O
3 nano-sheet composites after reducing of the catalyst. Difficult carbon deposition was the main cause for catalyst deactivation, and the deactivation rate was significantly decreased as compared with the Ni/Al
2O
3 catalyst. The NiO-MgO-Al
2O
3 catalyst achieved a CH
4 conversion of 91% at 800 °C with a GHSV of 5000 mL g
−1 h
−1, CH
4/CO
2 = 1.0/1.1. In situ growth of LDH on γ-Al
2O
3 is also an effective way to prepare DMR catalysts. The NiMgAl-LDO/γ-Al
2O
3 catalyst has a small Ni nanoparticles size, a strong metal-support interaction and finely-tailored surface basicity. The NiMgAl-LDO/γ-Al
2O
3 catalyst achieved an 80.7% CH
4 conversion at 700 °C and showed outstanding stability during the 48-h test [
83]. Zhang et al. [
84] reduced LDH by atmospheric cold plasma jet, and the as-obtained C-LDHs/γ-Al
2O
3 catalyst can avoid the side-reaction in CO
2-CH
4 reforming, leading to better carbon deposition resistance. C-LDHs/γ-Al
2O
3 catalyst achieved the same CH
4 conversion of 98% as Ni/MgO/γ-Al
2O
3 catalyst at 800 °C, but had higher H
2 selectivity.
Besides, the main challenge for NiMgAl LDH-derived catalysts was carbon deposition, and many efforts have been made to overcome this obstacle, such as utilizing metal adulteration and changing the morphology of the catalyst. Noble metal Rh as a promoter for Ni catalysts can improve the catalyst activity; Rh adulteration increased the amount of reducible Ni and promoted the dispersion of Ni. In addition, the presence of Rh probably led to Ni segregation with time on stream and generated carbon deposition due to Rh having favorable CH
4 decomposition [
85]. Moreover, Zr adulteration to LDH can form periclase-like mixed oxide and rearrangement of Ni particles during the DRM reaction. The HNiZr
3 catalyst exhibited the highest coking resistance properties due to Zr species in the lattice of periclase-like mixed oxide, resulting in small Ni crystallites, which are inactive in direct methane and Boudouard reaction oxides. Yet, Zr adulteration decreased the activity, and the CH
4 conversion of HT-25Ni catalyst was 40% and 23% for HTNi-Zr catalyst [
86]. La as a promoter can improve nickel metal dispersion and increase the total amount of basic sites and surface Ni content [
87]. With 1.1 wt % La adulteration, the catalyst properties vary as the Mg/Al molar ratio changes, and the higher Mg/Al molar ratio enhanced the catalyst stability with fewer carbon deposition, but decreased activity. The best stability was achieved at Mg/Al = 3 during a 120-h test, and the hydro-magnesite phase was formed with a Mg/Al molar ratio of four [
88,
89,
90,
91].
Ce as a promoter has been revealed by Daza et al. [
92,
93,
94], who explored two adulteration methods. Ce promoted by the co-precipitation method at constant pH formed mixed reducible phases of the NiO-MgO (periclase) type and CeO
2 (fluorite), and the as-obtained catalyst exhibited better performance than the un-promoted catalyst. OM
2 (Ce-NiMgAl-LDO) catalyst with 14.9% CeO
2 displayed about a 75% CH
4 conversion during the 200-min reaction, and the CO
2 conversion was also about 10% higher than that of OM1 (NiMgAl-LDO) catalyst, which contained no CeO
2. Further study revealed that Ce and Mg had a synergic effect on the CO
2 adsorption capacity of the solids, promoted the basicity of oxides and enhanced their catalytic activity in CO
2 reforming of methane. However, high loads of Ce decreased the superficial area of the solid and favored the formation of free NiO, which had a negative impact on the selectivity and increased the formation of coke. The fact-finding of Djebarri et al. [
95] insinuated that the NiMgCe catalyst was a very stable, and a poorly-reducible mixed oxide phase was formed with Ce adulteration, which inhibited the catalysts’ catalytic performance. Besides, the Ce addition affected the catalytic performance. Ren et al. [
96] unveiled that Ce introduced through the incipient impregnation method showed higher Ce
3+ content and appropriate interactions between Ni and NMA compared with catalyst prepared by the co-precipitation method. Daza et al. [
97] also revealed that with Ce introduced by partial reconstruction, the as-prepared catalyst formed periclase and fluorite mixed phases after the calcination, and the reconstruction took place at the external edges of the oxide granules. Ce presented an improvement in the degree of reduction of Ni, the amount and strength of the basic sites. With Ce loading increased, no obvious considerable differences in the catalytic activity and selectivity were perceived, but the anti-coke ability was improved. The optimal amount of Ce doping was 3 wt %, and OM3 (Ce-NiMgAl-LDO) catalysts with (Ni + Mg)/Al = 3) achieved a 90.3% CH
4 conversion at 700 °C, with the CO
2/CH
4/He ratio of 20/20/60 and weight hourly space velocity (WHSV) = 48 L g
−1 h
−1.
Additionally, the anti-coke ability can also be improved through incorporating carbon in the Ni-based catalyst. In 2017, Jin et al. [
33] used sucrose as the carbon source to incorporate carbon in the Ni-based catalyst, and carbon incorporation formed new mesopores, increased the specific surface area and inhibited Ni particle growth. The as-prepared PC-350-1.8 (pretreated catalyst) catalyst exhibited relatively lower initial conversions of CH
4 and CO
2 than pure Ni-LDO catalyst, but showed excellent anti-coke ability. The R-C-350-1.8-800 catalyst was obtained by removing the upper carbon from PC-350-1.8 catalyst, displaying 10% higher CH
4 conversion and showing slightly lower carbon deposition.
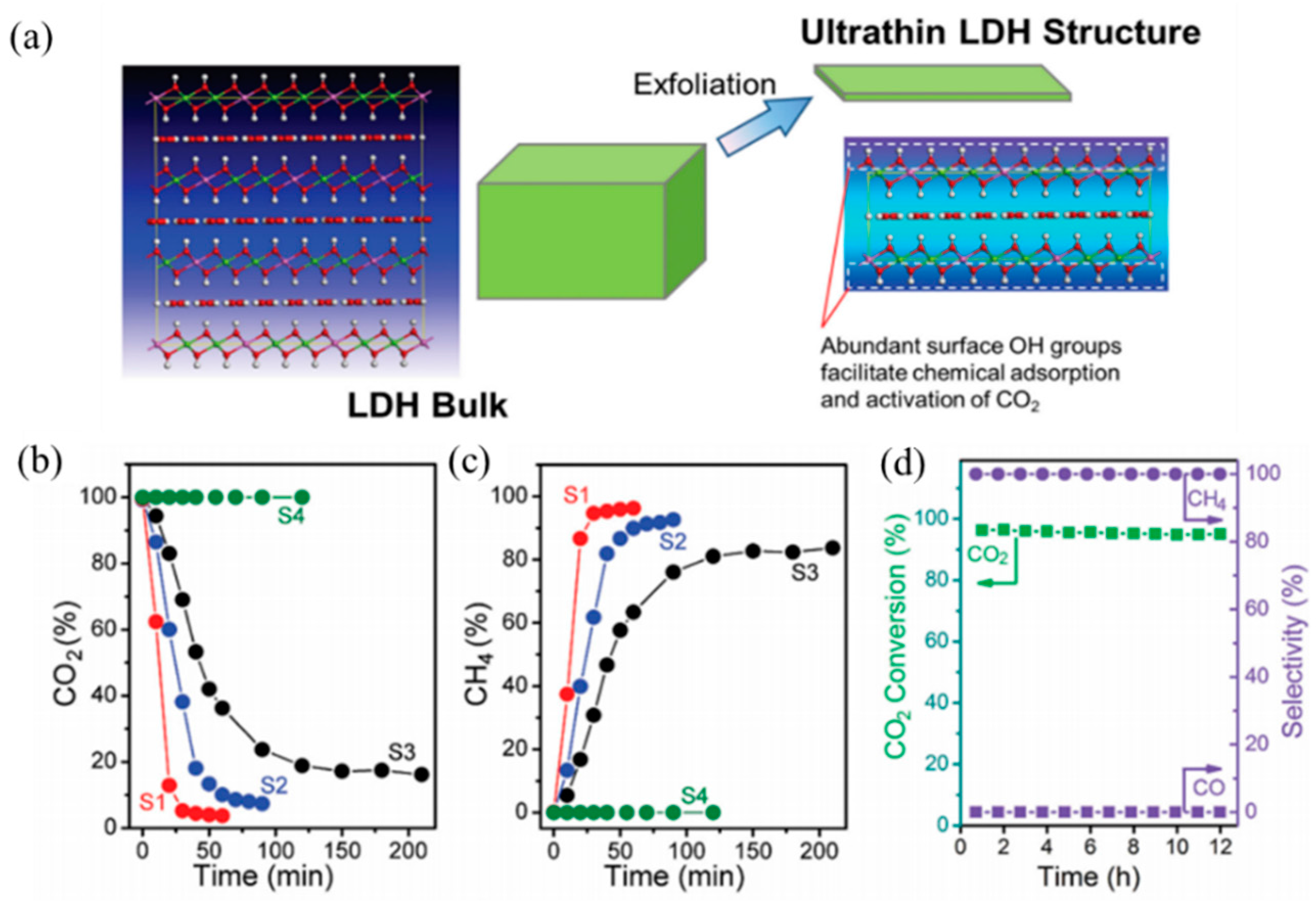
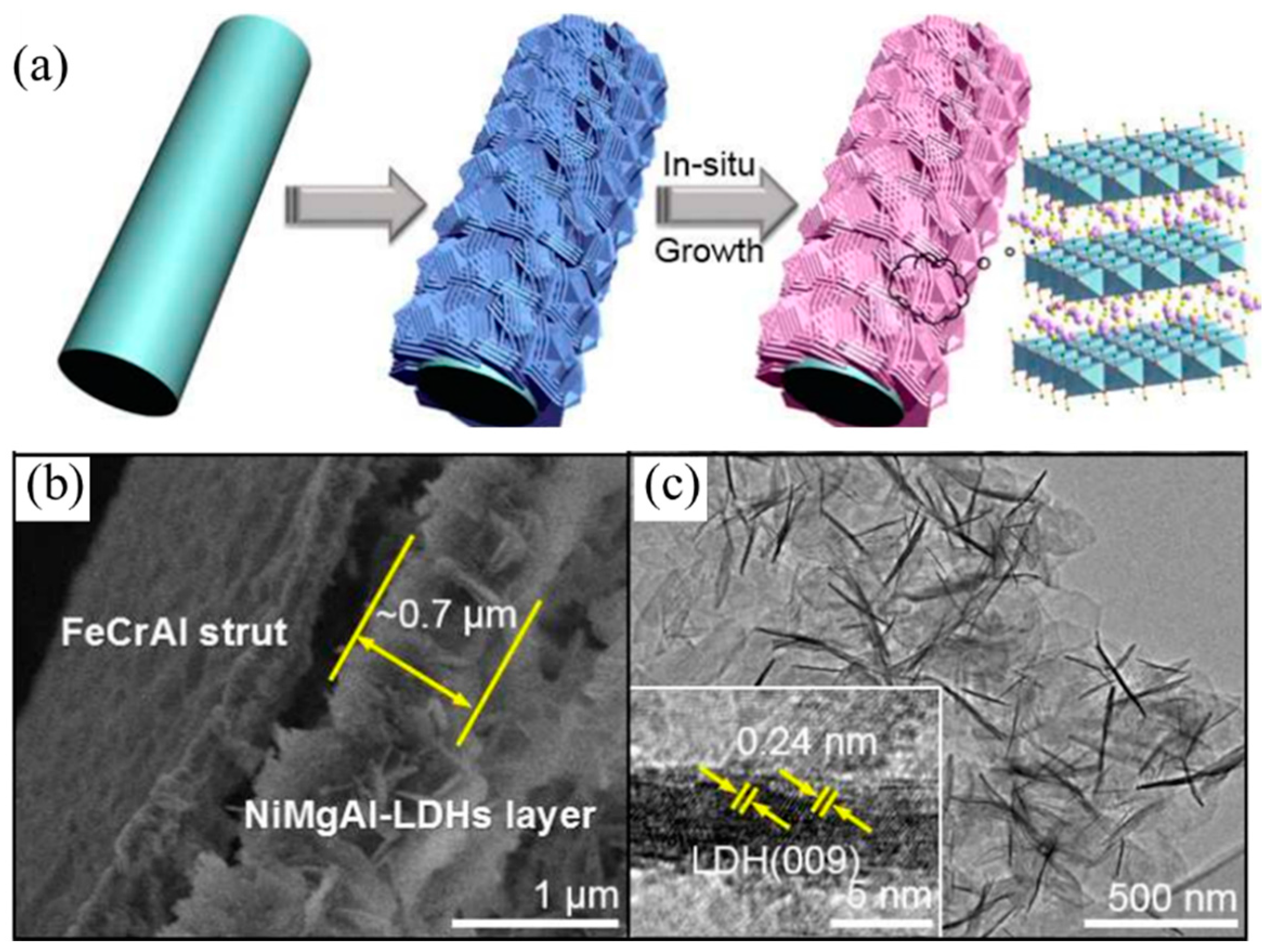
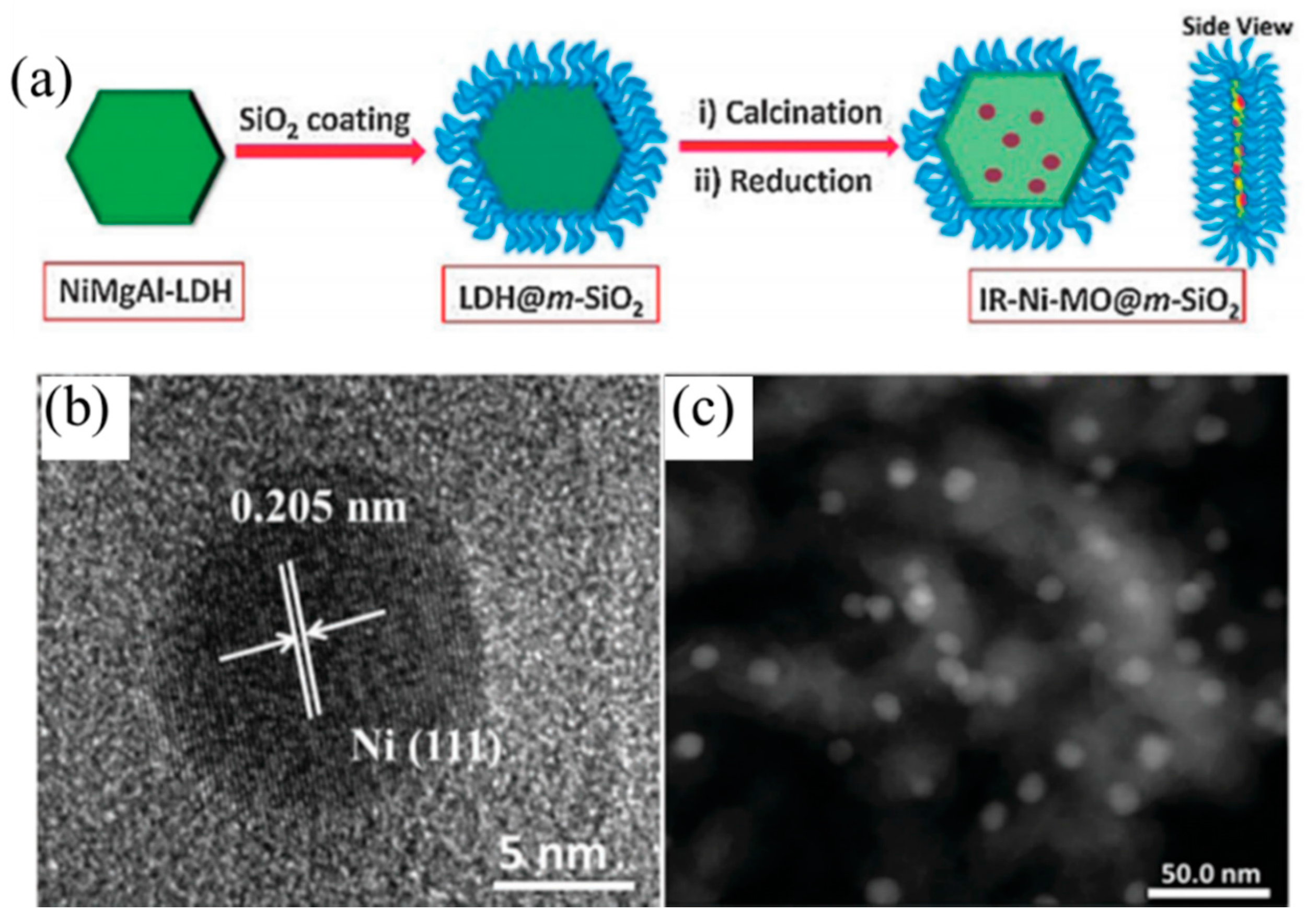
Besides transition elements and rare-earth elements, the advantage of the coke resistance ability changed the morphology of the catalysts and can also promote the anti-carbon deposition ability. Du et al. [
98] synthesized monolithic Ni-Mg-Al LDH catalyst nanosheets via in situ growth on Al wires. The as-prepare catalyst showed a hierarchical porous structure, and the oxide nanosheets were arranged as a dense film on the aluminum substrate. Monolithic catalysts showed strong metal-support interactions and strong basic sites compared with traditional Ni-MgO-Al
2O
3 catalysts. The monolithic catalysts have also displayed excellent sintering resistance and anti-carbon deposition ability, and coke deposition on the monolithic catalysts was one third that on the traditional catalysts. Du et al. [
99] also developed a modular catalyst by combining the Ni-MgO-Al
2O
3 mixed oxide nanoplates with the mesoporous SiO
2 coating, the as-prepared NiMgAl-LDH@m-SiO
2 catalyst core shell structure (
Figure 7) and the dual confinement effects: The first confinement was MgO, which can promote embedded Ni NP dispersion and enhance the chemisorption ability of CO
2, as well as restrain the carbon deposition and Ni NP aggregation. The second confinement resulted from the mesoporous SiO
2 shell, which exhibited another “confinement effect”. These two confinement effects can reinforce each other, which enabled the modular catalysts to show excellent anti-coke and sintering resistance ability. IR-NiMgAl-LDH@m-SiO
2 achieved a 90% CH
4 conversion at 800 °C, much higher than that of the IM-NiMgAl-LDH@m-SiO
2 (~55%) catalyst obtained by the impregnation method. Gonzáleza et al. [
100] made a Ni-Mg-Al nano-spheroid oxide catalyst through the sol-gel method, and this nano-spheroid oxide catalyst with 15 wt % Ni achieved a 95% CH
4 conversion at 800 °C and showed excellent long-term stability. Amorphous carbon formed on the nano-spheroid catalyst surface during the reaction, which seemed not to be detrimental to this reaction. Encapsulation of carbon is the main culprit in the nickel-containing catalyst’s deactivation, because the nickel crystallites are encapsulated by the carbon. However, for this nano-spheroid catalyst, the majority of the carbon was amorphous, and a few seeds of encapsulated carbon benefited from the good conformation of the active sites formed by the nano-crystalline structure of the mixed oxides Mg(Al,Ni)O. In this regard, combining Mg-Al mixed oxides with SBA-15 is also an effective way to improve the anti-coke ability. Zuo et al. [
101] found that the catalyst whose metal oxides were calcined two times showed excellent anti-carbon deposition and catalytic stability, due to the strong metal-support interaction and the channel local effect of SBA-15. The SH-550 (SBA-15 added to the hydrotalcite suspension during the preparation) and HS-550 (hydrotalcite added to the SBA-15 suspension during the preparation)catalysts, for which the metal oxides have been calcined two times, achieved almost an 85% CH
4 conversion and maintained excellent stability at 800 °C. For the HS-550 catalyst, the Ni
0 particles were isolated from one another, so that the clustering of Ni, which is necessary for coke formation, was prevented. In addition, the HS-550 catalyst contained two nickel species: Ni
0 and NiO; the coexistence of these two species favored the high catalyst activity reported by Damyanova et al. [
102].
Furthermore, Co was also an active component of DRM reforming, and Co containing LDH has been used as the DRM catalyst. Liu et al. [
103] discovered that the catalytic activity, stability and coke resistance of Co/MgAl increased with the increase of Co loading, and the 12% Co/Mg
3Al catalyst showed much higher catalytic stability and much less coke deposition at 600 °C as compared to the 12% Ni/Mg
3Al catalyst, which may be attributed to the higher affinity of Co for oxygen species; in addition, Co has a higher interaction with support. The results suggested that Co has the potential compared to Ni to be an active catalyst in the CH
4-CO
2 reforming reaction. In Gennequin et al.’s study [
104], the Co
2Mg
4Al
2HT500 catalyst exhibited better stability as compared with the Co
4Mg
2Al
2HT500 catalyst, though the Co
2Mg
4Al
2HT500 catalyst has a lower initial activity. The Co
6Al
2HT500 catalyst showed comparable catalytic performance to the Co
2Mg
4Al
2HT500 catalyst during the stability test, while Co
6Al
2HT500 showed a larger amount of deposited carbon. After the reaction, three catalysts exhibited a weight decrease corresponding to carbon oxidation following the order: Co
6Al
2HT500 (−52%) > Co
4Mg
2Al
2HT500 (−23%) > Co
2Mg
4Al
2HT500 (−5.5%). Mg oxides could inhibit coke deposition via adsorbed CO
2 species on the basic site and afterwards react with the deposited carbon by the reverse Boudouard reaction. The Co
2Mg
4Al
2HT500 catalyst has a higher Mg content, thus showing excellent anti-coke deposition stability. It could be concluded that for the reaction of the dry reforming of methane, the catalytic performances of CoxMgyAl
2HT500 solids have a great relation to Co and Mg content. Gennequin et al. [
105] used the “memory effect” of LDHs to impregnate the 1 wt % Ru into CoxMgyAl
2, and the as-obtained catalyst exhibited better performance than the conventional impregnation method in the temperature region of 450–750 °C. Regarding this, small amounts of Ru can promote the reducibility of cobalt catalysts and enhance the stability of the catalysts via decreased carbon formation; the catalyst obtained by the “memory effect” generated both metallic and basic sites, which are favorable for the dry reforming reaction of methane.
Moreover, Ni-Co bimetallic LDHs were prepared by the in situ synthesized method on the surface of γ-Al
2O
3. The as-obtained catalysts showed a strong interaction between the active component (Ni and Co) and the catalytic support [
106]. However, Tanios et al. [
107] discovered that the Ni and Co synergistic effect greatly improved the catalytic properties and prevented carbon formation. The 1Co-2Ni-LDH catalyst exhibited the best catalytic performance and resulted in the least coke, achieving a 98.3% CH
4 conversion that decreased to 1.7% after 50 h of reaction at 800 °C, much higher than that of Co/γ-Al
2O
3, which resulted in a 56.2% CH
4 conversion and complete deactivation after the 50-h test; while the optimal Co/Ni ratio was one when using aluminum nitrate as the trivalent ion source.
3.1.2. Low Temperature Dry Reforming of Methane
The DRM reaction was always performed at high temperatures to obtain a better performance, whereas LDHs as the catalyst precursors for low temperature dry reforming of methane were studied by Debek et al. [
108]. Ni-Al LDH was firstly used as the catalyst for the dry reforming of methane. The HT-NiAl catalyst reduced at 900 °C exhibited a 48% CH
4 conversion and about a 55% CO
2 conversion, higher than that of the HT-NiAl catalyst reduced at 550 °C, due to the reduction temperature of 550 °C not being sufficient to reduce all of the nickel species to being metallic. Moreover, the side reaction of CH
4 decomposition occurring due to an excess of H
2 was observed, and a ca. 30% conversion of methane was obtained by CH
4 on decomposition catalysts reduced at 550 and 900 °C, which evinced that methane decomposition strongly influences the overall process; in addition, the CH
4 decomposition was not influenced by the temperature of the catalyst’s pre-treatment.
As the active component, the incorporation method and the content of Ni have a great effect on the DRM reaction. Higher values of CH
4 and CO
2 conversions were obtained for the sample (HTNi) prepared by the co-precipitation method with 63.47 wt % Ni content. About a 55% CH
4 conversion was obtained at 550 °C for the HTNi catalyst, and additional catalytic tests were performed on CH
4 decomposition on the sample HTNi at 550 °C with a feed gas of CH
4/Ar = 2/8. However, HTexNi catalyst with 0.78 wt % Ni displayed higher activity per gram of active material, due to the formation of small Ni NPs or aggregates of nickel oxide on the catalyst surface [
109]. Ni particle size increased with Ni content increasing, and dry reforming of methane and direct methane decomposition showed increased methane conversion. Methane decomposition and carbon formation may occur, especially in the presence of catalysts that contain Ni in considerable amounts, and the catalysts with different Ni contents had the same catalytic performance trend as the DRM reaction. Thus, methane decomposition at low temperatures can be controlled by decreasing the Ni particle/crystal size [
110].
Because the side reaction of methane decomposition was inevitable during the DRM reaction, methane decomposition led to carbon deposition and catalyst deactivation. Daza et al. [
97] found that Ce-promoted catalysts had excellent coke resistance ability. Debek et al. investigated the route cause for this phenomenon. Bigger Ni crystallites on the catalysts with the highest Ni content promoted direct CH
4 decomposition and accelerated catalyst deactivation. Ce-promoted Ni-containing LDH suppressed the side reaction of CH
4 decomposition due to its high basicity enhanced CO
2 adsorption and excellent ability to oxidize the already formed carbon deposits, whereas too high a loading of ceria had a negative effect during the overall process due to the formation of free NiO [
111].
Meanwhile, Zr doping can further promote the coke resistance ability of catalyst and substantially restrain the extensive formation of fishbone-type carbon nanofibers. With the adulteration of Zr, the CH
4 conversion decreased, but Zr considerably inhibited methane’s direct decomposition, favored methane reaction with CO
2 (DMR reaction), together with other important parallel reactions, such as the reverse Boudouard reaction. The HT-25Ni catalyst achieved 48% CH
4 conversion and showed 25% CH
4 conversion at 550 °C; even though both CH
4 and CO
2 conversions of the HTNi-CeZr catalyst were low, almost no carbon was deposited on the catalyst surface during 5 h of DMR reaction [
112]. The catalysts with Ce/Zr loading of 0.6 and 0.3 exhibited a high concentration of strong basic sites and, as a consequence, showed higher catalytic activity than the H-ZrCe1.2 catalyst. The obtained results revealed that the guarantee of low carbon deposition was due to the presence of Zr species in the lattice of periclase-like mixed oxides, which besides influencing basicity, also result in the formation of small Ni crystallites, inactive in direct methane decomposition and the Boudouard reaction [
113].
Unlike Ce and Zr, La as a promoter can not only improve the anti-coke ability, but also enhance catalytic performance [
104]. Side reactions such as methane decomposition were promoted at the same time; however, La can form oxycarbonate species and promote gasification of amorphous carbon deposits, resulting in lower carbon formation during the long-duration isothermal experiments performed at 550 °C. La-NiMgAl-LDO catalyst with 2 wt % La showed a 33% CH
4 conversion at 550 °C, slightly higher than that of the un-promoted NiMgAl-LDO catalyst.