A Novel Class of Injectable Bioceramics That Glue Tissues and Biomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PM-CPC Fabrication
2.3. Mechanical Testing, Characterization
2.4. SEM Analysis
2.5. Solid-State NMR
2.6. Statistics
3. Results and Discussion
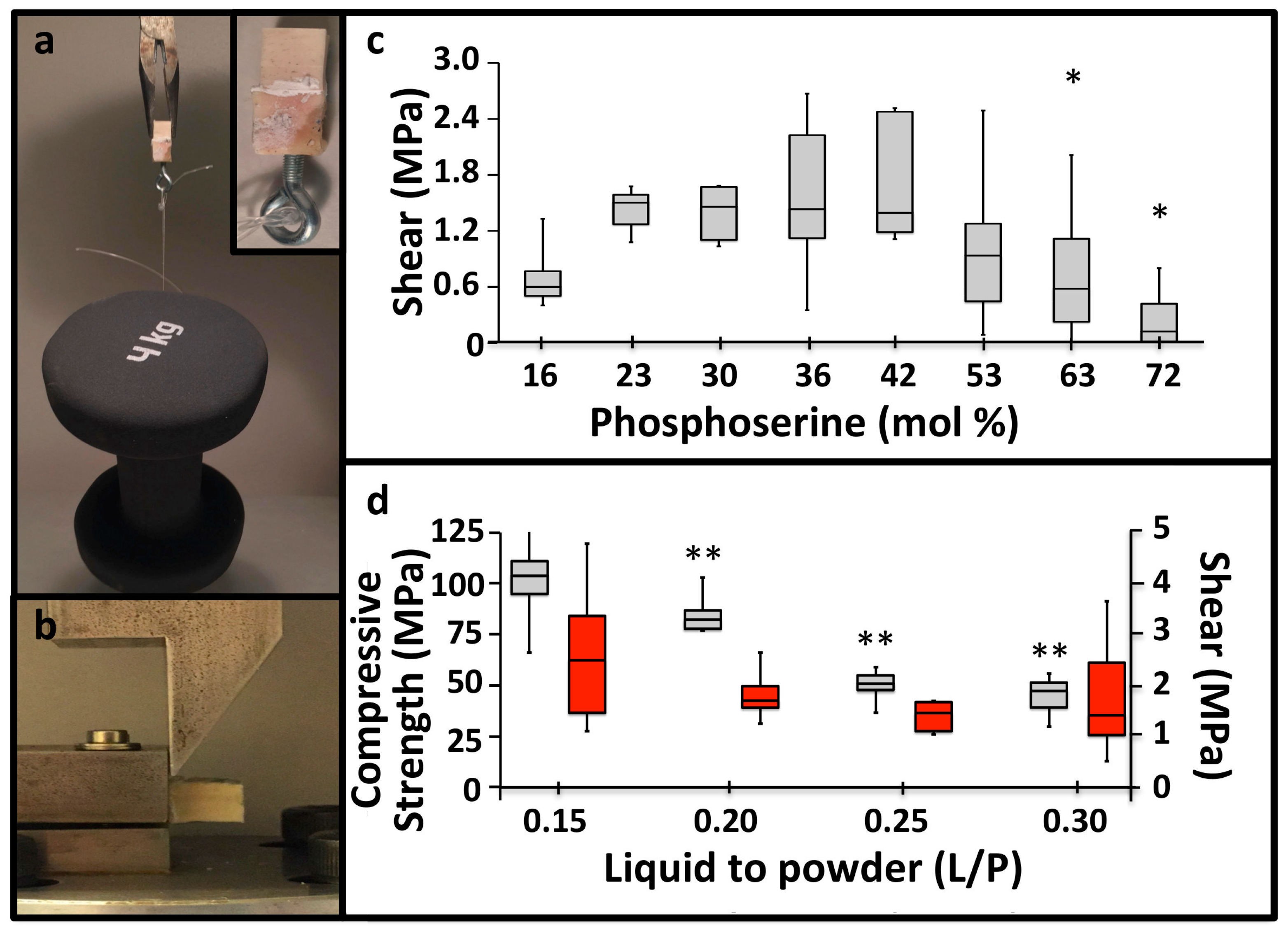
3.1. PM-CPC Setting and Compressive Strength
3.2. PM-CPC Adhesive Strength and Optimal Formulation
3.3. PM-CPC Adhesion to Soft Tissues and Biomaterials
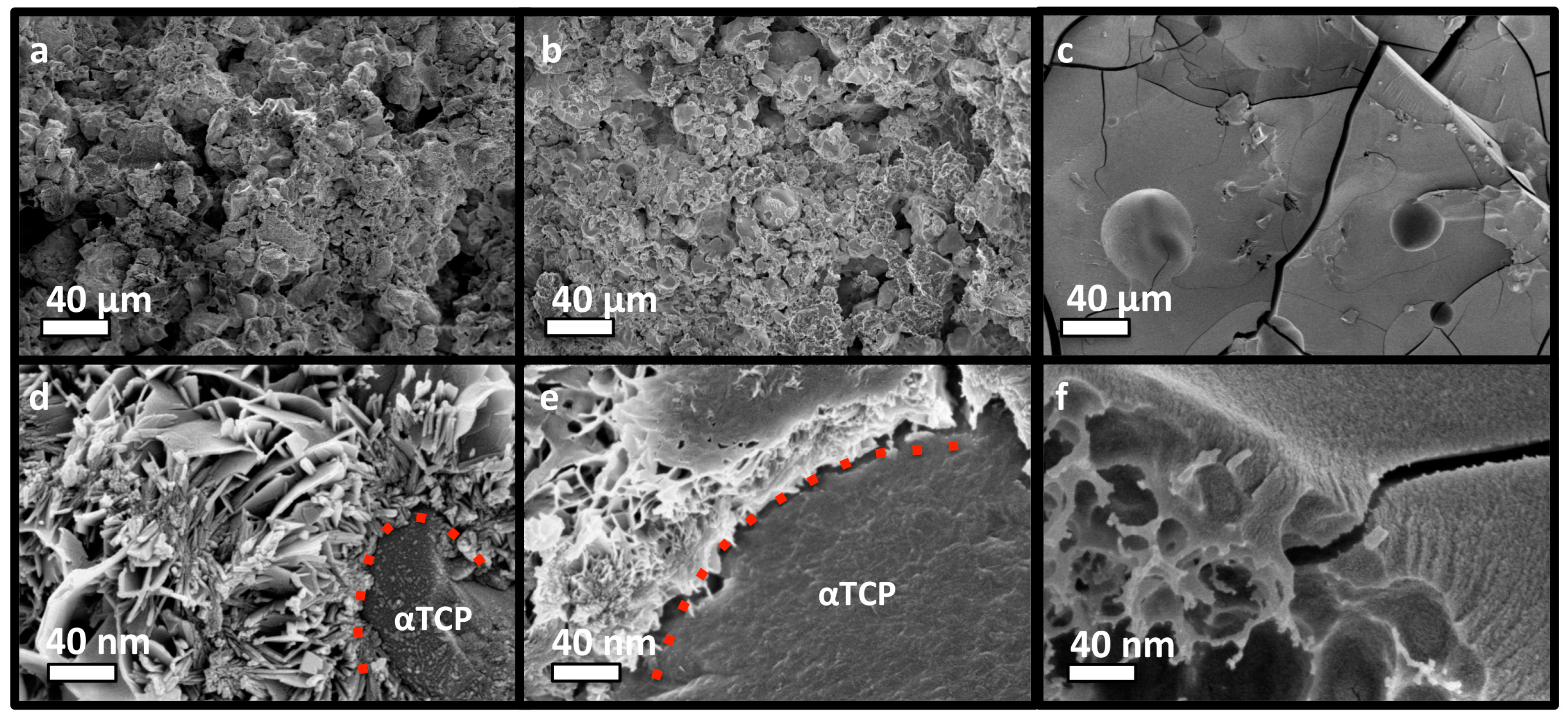
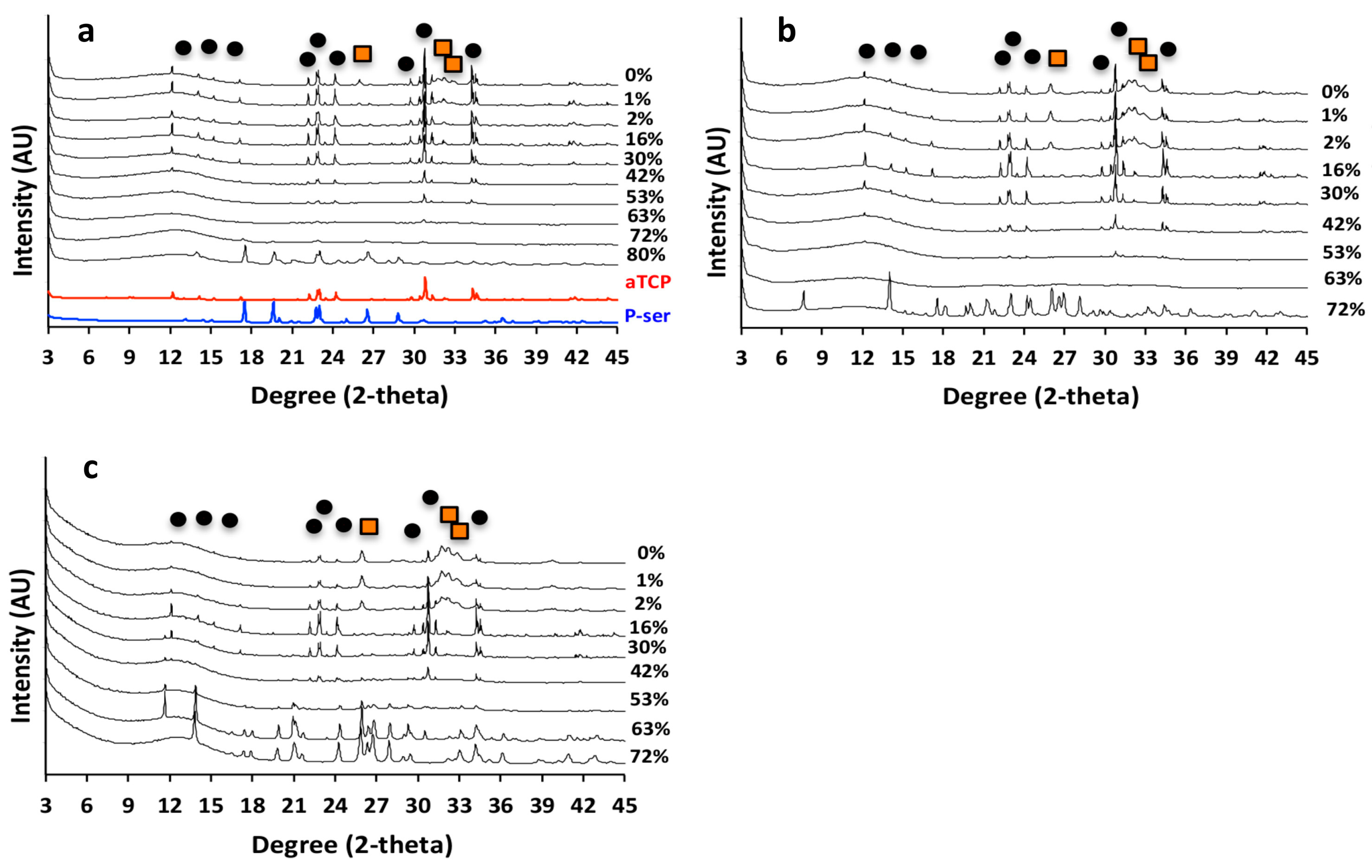
3.4. PM-CPC Physiochemical Analysis of PM-CPC
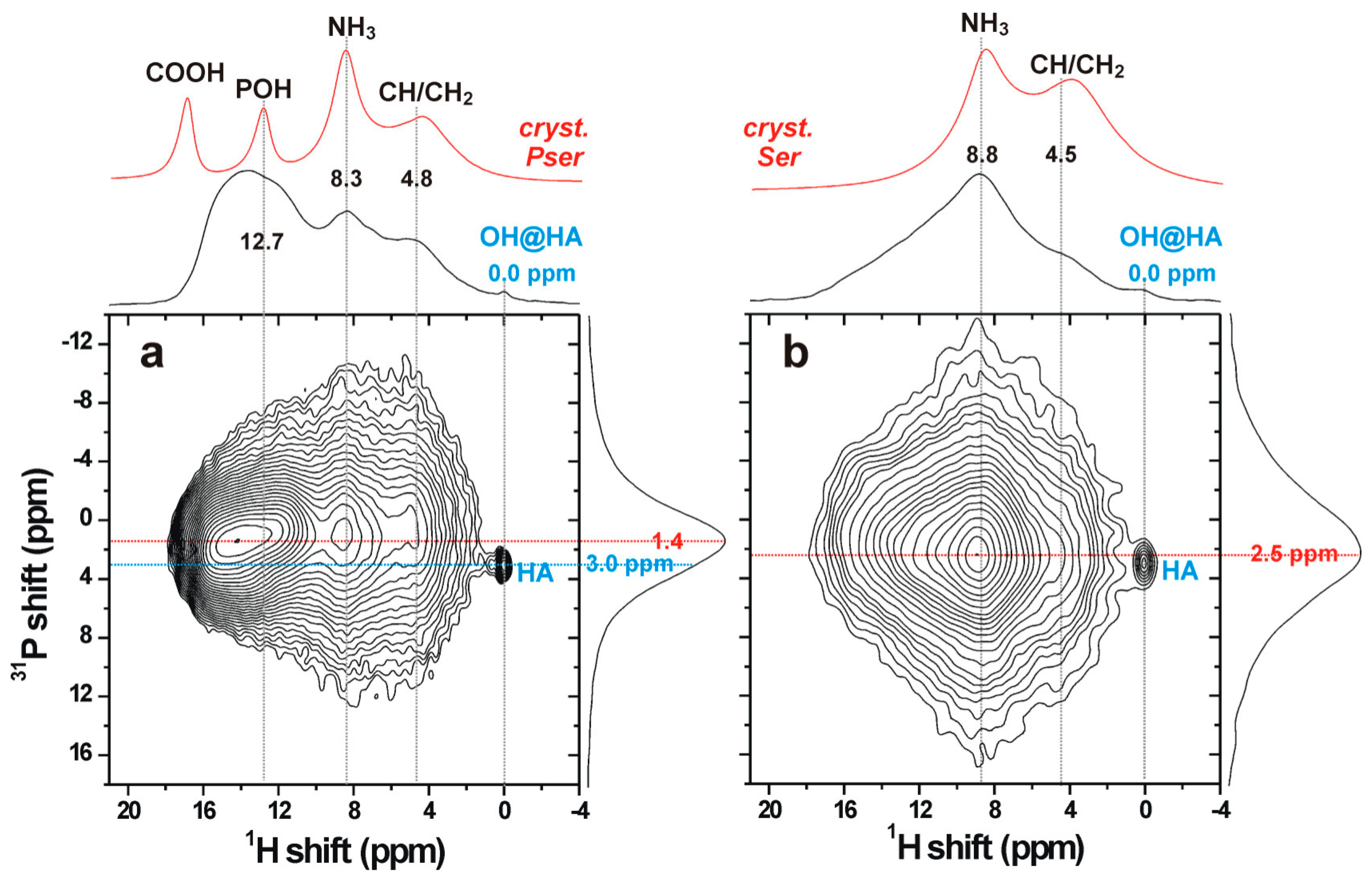
3.5. The Organic/Inorganic Interface
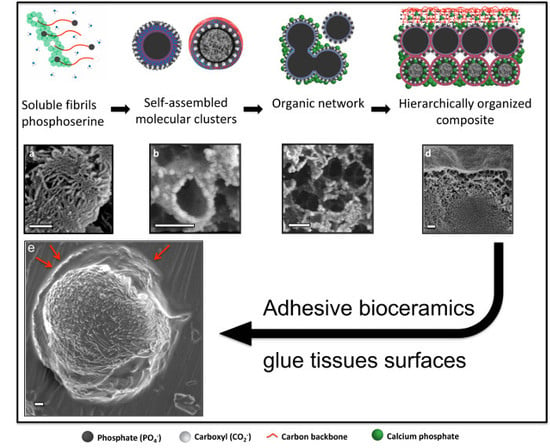
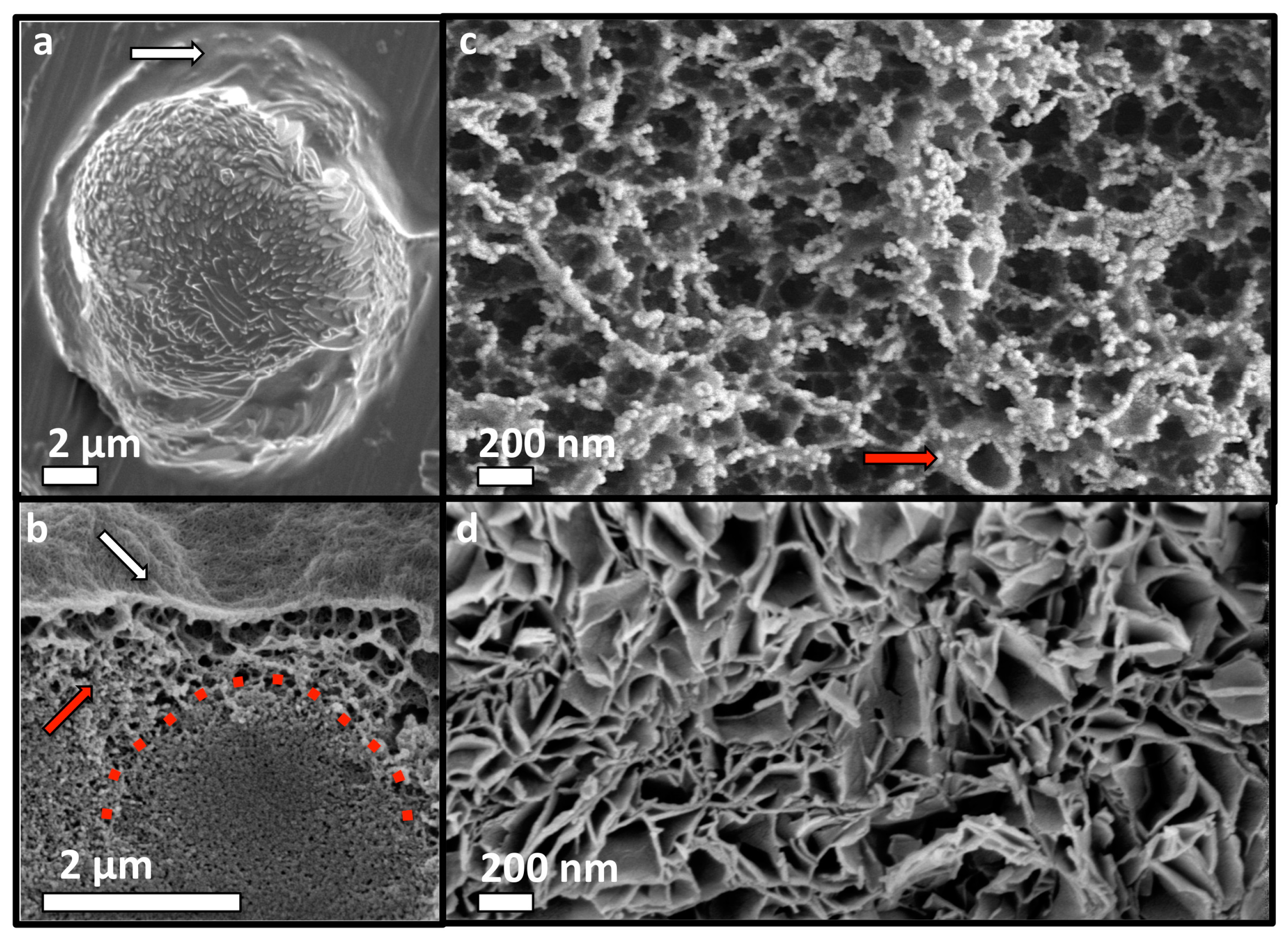
3.6. Adhesive Mechanism and Macrostructure of PM-CPC
3.7. Hierarchical Organization and Templating of PM-CPC by Phosphoserine
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Thurner, P.J.; Lam, S.; Weaver, J.C.; Morse, D.E.; Hansma, P.K. Localization of Phosphorylated Serine, Osteopontin, and Bone Sialoprotein on Bone Fracture Surfaces. J. Adhes. 2009, 85, 526–545. [Google Scholar] [CrossRef]
- Taylor, M.G.; Simkiss, K.; Simmons, J.; Wu, L.N.; Wuthier, R.E. Structural studies of a phosphatidyl serine-amorphous calcium phosphate complex. Cell. Mol. Life Sci. CMLS 1998, 54, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Fantner, G.E.; Adams, J.; Turner, P.; Thurner, P.J.; Fisher, L.W.; Hansma, P.K. Nanoscale ion mediated networks in bone: Osteopontin can repeatedly dissipate large amounts of energy. Nano Lett. 2007, 7, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- De Yoreo, J.J.; Gilbert, P.U.; Sommerdijk, N.A.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.; Lode, A.; Bernhardt, A.; Reinstorf, A.; Nies, B.; Gelinsky, M. Modifications of a calcium phosphate cement with biomolecules—Influence on nanostructure, material, and biological properties. J. Biomed. Mater. Res. A 2010, 95, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Reinstorf, A.; Hempel, U.; Olgemöller, F.; Domaschke, H.; Schneiders, W.; Mai, R.; Stadlinger, B.; Rösen-Wolff, A.; Rammelt, S.; Gelinsky, M.; et al. O-phospho-L-serine modified calcium phosphate cements—Material properties, in vitro and in vivo investigations. Materialwiss. Werkstofftech. 2006, 37, 491–503. [Google Scholar] [CrossRef]
- Pujari-Palmer, M.; Pujari-Palmer, S.; Lu, X.; Lind, T.; Melhus, H.; Engstrand, T.; Karlsson-Ott, M.; Engqvist, H. Pyrophosphate Stimulates Differentiation, Matrix Gene Expression and Alkaline Phosphatase Activity in Osteoblasts. PLOS ONE 2016, 11, e0163530. [Google Scholar] [CrossRef]
- Mai, R.; Lux, R.; Proff, P.; Lauer, G.; Pradel, W.; Leonhardt, H.; Reinstorf, A.; Gelinsky, M.; Jung, R.; Eckelt, U.; et al. O-phospho-L-serine: A modulator of bone healing in calcium-phosphate cements. Biomed. Tech. 2008, 53, 229–233. [Google Scholar] [CrossRef]
- Kirillova, A.; Kelly, C.; Windheim, N.; Gall, K. Bioinspired Mineral–Organic Bioresorbable Bone Adhesive. Adv. Healthc. Mater. 2018, 7, 1800467. [Google Scholar] [CrossRef]
- Grover, L.M.; Gbureck, U.; Farrar, D.; Barralet, J.E. Adhesion of a Novel Calcium Phosphate Cement to Cortical Bone and Several Common Biomaterials. Key Eng. Mater. 2006, 309–311, 849–852. [Google Scholar] [CrossRef]
- Engstrand, T.; Kihlström, L.; Lundgren, K.; Trobos, M.; Engqvist, H.; Thomsen, P. Bioceramic Implant Induces Bone Healing of Cranial Defects. Plast. Reconstr. Surg. Glob. Open 2015, 3, e491. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, J.; Persson, C.; Engqvist, H. The effect of composition on mechanical properties of brushite cements. J. Mech. Behav. Biomed. Mater. 2014, 29, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Merkle, H.P.; Landuyt, P.V.; Trophardy, G.; Lemaitre, J. Effect of several additives and their admixtures on the physico-chemical properties of a calcium phosphate cement. J. Mater. Sci. Mater. Med. 2000, 11, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Walsh, F.; Gludovatz, B.; Delattre, B.; Huang, C.; Chen, Y.; Tomsia, A.P.; Ritchie, R.O. Bioinspired Hydroxyapatite/Poly(methyl methacrylate) Composite with a Nacre-Mimetic Architecture by a Bidirectional Freezing Method. Adv. Mater. 2015, 28, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Giocondi, J.L.; El-Dasher, B.S.; Nancollas, G.H.; Orme, C.A. Molecular mechanisms of crystallization impacting calcium phosphate cements. Phil. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1937–1961. [Google Scholar] [CrossRef] [Green Version]
- Chatzipanagis, K.; Iafisco, M.; Roncal-Herrero, T.; Bilton, M.; Tampieri, A.; Kröger, R.; Delgado-López, J.M. Crystallization of citrate-stabilized amorphous calcium phosphate to nanocrystalline apatite: A surface-mediated transformation. Cryst. Eng. Comm. 2016, 18, 3170–3173. [Google Scholar] [CrossRef]
- Barradas, A.M.; Yuan, H.; van Blitterswijk, C.A.; Habibovic, P. Osteoinductive biomaterials: Current knowledge of properties, experimental models and biological mechanisms. Eur. Cell. Mater. 2011, 21, 407–429, discussion 429. [Google Scholar] [CrossRef]
- Luo, J.; Ajaxon, I.; Ginebra, M.P.; Engqvist, H.; Persson, C. Compressive, diametral tensile and biaxial flexural strength of cutting-edge calcium phosphate cements. J. Mech. Behav. Biomed. Mater. 2016, 60, 617–627. [Google Scholar] [CrossRef]
- Farrar, D.F. Bone adhesives for trauma surgery: A review of challenges and developments. Int. J. Adhes. Adhes. 2012, 33, 89–97. [Google Scholar] [CrossRef]
- Wang, C.S.; Stewart, R.J. Localization of the bioadhesive precursors of the sandcastle worm, Phragmatopoma californica (Fewkes). J. Exp. Biol. 2012, 215, 351. [Google Scholar] [CrossRef]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Gu, W.; Pan, H.; Jiang, S.; Tang, R. Stabilizing amorphous calcium phosphate phase by citrate adsorption. Cryst. Eng. Comm. 2014, 16, 1864–1867. [Google Scholar] [CrossRef]
- Meikle, S.T.; Bianchi, G.; Olivier, G.; Santin, M. Osteoconductive phosphoserine-modified poly(ε-lysine) dendrons: Synthesis, titanium oxide surface functionalization and response of osteoblast-like cell lines. J. R. Soc. Interface 2013, 10, 20120765. [Google Scholar] [CrossRef] [PubMed]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Trebosc, J.; Hu, B.; Amoureux, J.P.; Gan, Z. Through-space R3-HETCOR experiments between spin-1/2 and half-integer quadrupolar nuclei in solid-state NMR. J. Magn. Reson. 2007, 186, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, A.; Kentgens, A.P.M. Sensitivity Enhancement and Heteronuclear Distance Measurements in Biological 17O Solid-State NMR. J. Phys. Chem. B 2006, 110, 16089–16101. [Google Scholar] [CrossRef] [PubMed]
- Marion, D.; Ikura, M.; Tschudin, R.; Bax, A. Rapid recording of 2D NMR spectra without phase cycling. Application to the study of hydrogen exchange in proteins. J. Magn. Reson. 1989, 85, 393–399. [Google Scholar] [CrossRef]
- Shao, H.; Bachus, K.N.; Stewart, R.J. A water-borne adhesive modeled after the sandcastle glue of P. californica. Macromol. Biosci. 2009, 9, 464–471. [Google Scholar] [CrossRef]
- Nyarko, A.; Barton, H.; Dhinojwala, A. Scaling down for a broader understanding of underwater adhesives—A case for the Caulobacter crescentus holdfast. Soft Matter 2016, 12, 9132–9141. [Google Scholar] [CrossRef]
- Hoffmann, B.; Volkmer, E.; Kokott, A.; Augat, P.; Ohnmacht, M.; Sedlmayr, N.; Schieker, M.; Claes, L.; Mutschler, W.; Ziegler, G. Characterisation of a new bioadhesive system based on polysaccharides with the potential to be used as bone glue. J. Mater. Sci. Mater. Med. 2009, 20, 2001–2009. [Google Scholar] [CrossRef]
- Jäger, C.; Welzel, T.; Meyer-Zaika, W.; Epple, M. A solid-state NMR investigation of the structure of nanocrystalline hydroxyapatite. Magn. Reson. Chem. 2006, 44, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Von Euw, S.; Fernandes, F.M.; Cassaignon, S.; Selmane, M.; Laurent, G.; Pehau-Arnaudet, G.; Coelho, C.; Bonhomme-Coury, L.; Giraud-Guille, M.-M.; et al. Water-mediated structuring of bone apatite. Nat. Mater. 2013, 12, 1144. [Google Scholar] [CrossRef]
- Xie, R.; Feng, Z.; Li, S.; Xu, B. EDTA-Assisted Self-Assembly of Fluoride-Substituted Hydroxyapatite Coating on Enamel Substrate. Cryst. Growth Des. 2011, 11, 5206–5214. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Carloni, J.D.; Demarchi, B.; Sparks, D.; Reid, D.G.; Kunitake, M.E.; Tang, C.C.; Duer, M.J.; Freeman, C.L.; Pokroy, B.; et al. Tuning hardness in calcite by incorporation of amino acids. Nat. Mater. 2016, 15, 903. [Google Scholar] [CrossRef]
- Jawor-Baczynska, A.; Moore, B.D.; Lee, H.S.; McCormick, A.V.; Sefcik, J. Population and size distribution of solute-rich mesospecies within mesostructured aqueous amino acid solutions. Faraday Discuss. 2013, 167, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Hagmeyer, D.; Ruesing, J.; Fenske, T.; Klein, H.-W.; Schmuck, C.; Schrader, W.; da Piedade, M.E.M.; Epple, M. Direct experimental observation of the aggregation of α-amino acids into 100–200 nm clusters in aqueous solution. RSC Adv. 2012, 2, 4690–4696. [Google Scholar] [CrossRef]
- Hagmeyer, D.; Ganesan, K.; Ruesing, J.; Schunk, D.; Mayer, C.; Dey, A.; Sommerdijk, N.A.J.M.; Epple, M. Self-assembly of calcium phosphate nanoparticles into hollow spheres induced by dissolved amino acids. J. Mater. Chem. 2011, 21, 9219–9223. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, M.; Bewick, S.; Suo, Z. Nanoparticles to Increase Adhesive Properties of Biologically Secreted Materials for Surface Affixing. J. Biomed. Nanotechnol. 2009, 5, 294–299. [Google Scholar] [CrossRef]
- Meddahi-Pellé , A.; Legrand, A.; Marcellan , A.; Louedec, L.; Letourneur, D.; Leibler, L. Organ Repair, Hemostasis, and In Vivo Bonding of Medical Devices by Aqueous Solutions of Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 6369–6373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Peng, Y.; Yang, C.; Wang, M. Silica Nanoparticles as Adhesives for Biological Tissues? Re-Examining the Effect of Particles Size, Particle Shape, and the Unexpected Role of Base. Part. Part. Syst. Charact. 2017, 34, 1700286. [Google Scholar] [CrossRef]
- Abbas, Z.; Labbez, C.; Nordholm, S.; Ahlberg, E. Size-Dependent Surface Charging of Nanoparticles. J. Phys. Chem. C 2008, 112, 5715–5723. [Google Scholar] [CrossRef]
- Good, R.J.; Hawa, A.K. Acid/Base Components in the Molecular Theory of Adhesion. J. Adhes. 1997, 63, 5–13. [Google Scholar] [CrossRef]
- Willett, R.L.; Baldwin, K.W.; West, K.W.; Pfeiffer, L.N. Differential adhesion of amino acids to inorganic surfaces. Proc. Natl. Acad. Sci. USA 2005, 102, 7817. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Cervino, G.; Herford, A.S.; Famà, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial Bone Reconstruction Using both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar. Drugs 2018, 13, 27. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujari-Palmer, M.; Guo, H.; Wenner, D.; Autefage, H.; Spicer, C.D.; Stevens, M.M.; Omar, O.; Thomsen, P.; Edén, M.; Insley, G.; et al. A Novel Class of Injectable Bioceramics That Glue Tissues and Biomaterials. Materials 2018, 11, 2492. https://doi.org/10.3390/ma11122492
Pujari-Palmer M, Guo H, Wenner D, Autefage H, Spicer CD, Stevens MM, Omar O, Thomsen P, Edén M, Insley G, et al. A Novel Class of Injectable Bioceramics That Glue Tissues and Biomaterials. Materials. 2018; 11(12):2492. https://doi.org/10.3390/ma11122492
Chicago/Turabian StylePujari-Palmer, Michael, Hua Guo, David Wenner, Hélène Autefage, Christopher D. Spicer, Molly M. Stevens, Omar Omar, Peter Thomsen, Mattias Edén, Gerard Insley, and et al. 2018. "A Novel Class of Injectable Bioceramics That Glue Tissues and Biomaterials" Materials 11, no. 12: 2492. https://doi.org/10.3390/ma11122492