Development of High-Performance Enamel Coating on Grey Iron by Low-Temperature Sintering
Abstract
:1. Introduction
2. Experimental Procedure
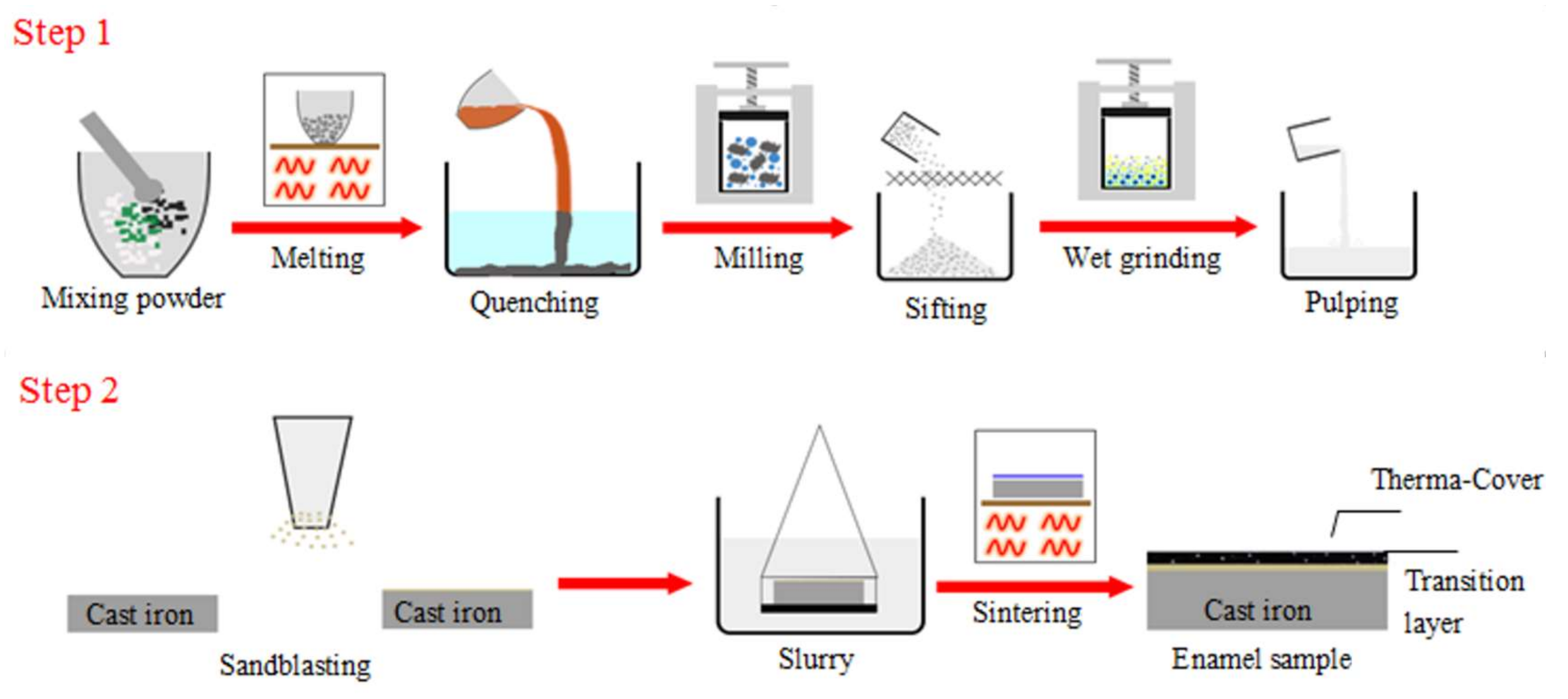
2.1. Coating Formation Processing
2.2. Microstructure Observation
2.3. Mechanical Property Testing
2.4. Wear-Resisting Property Testing
3. Results and Discussion
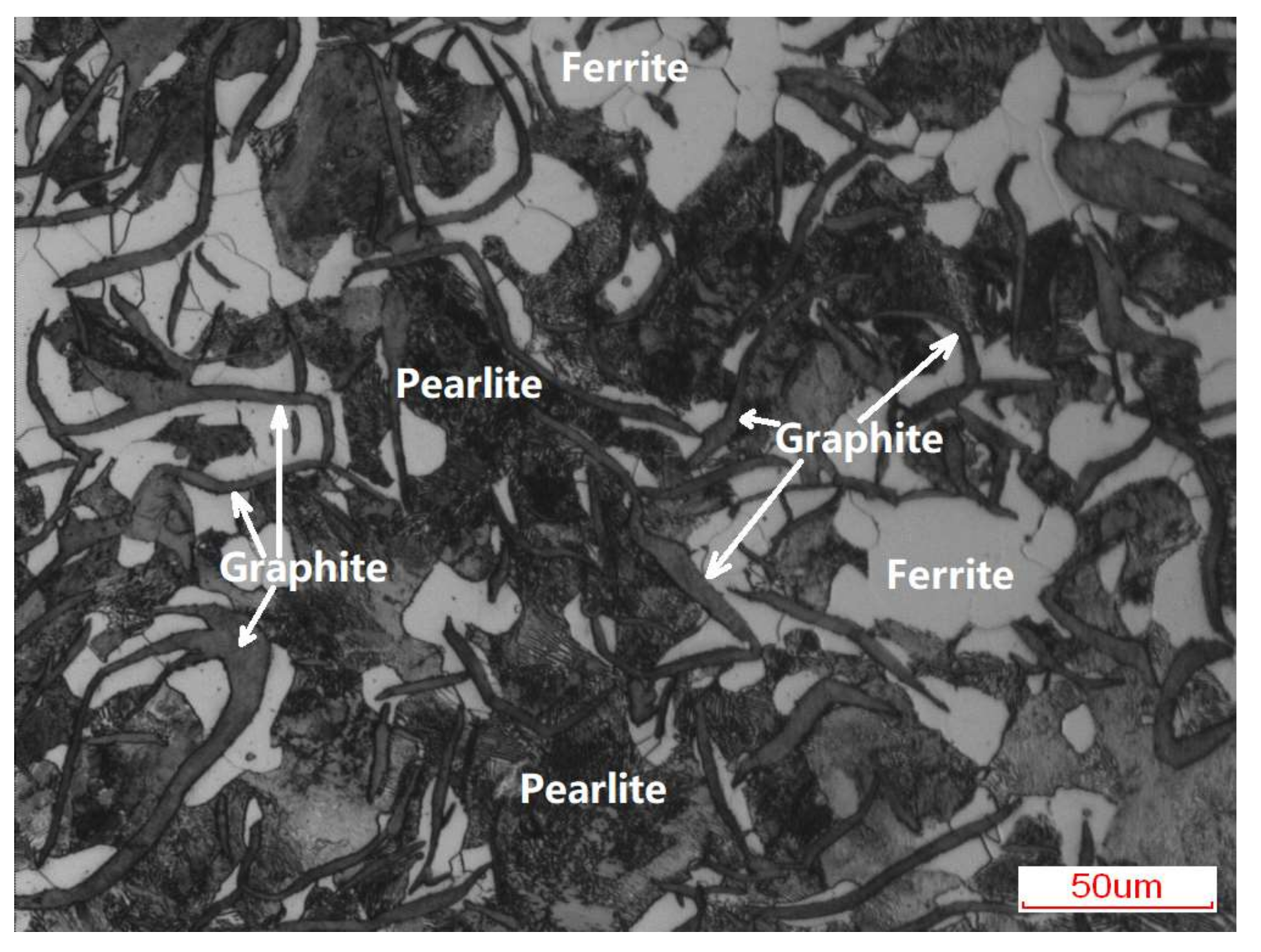
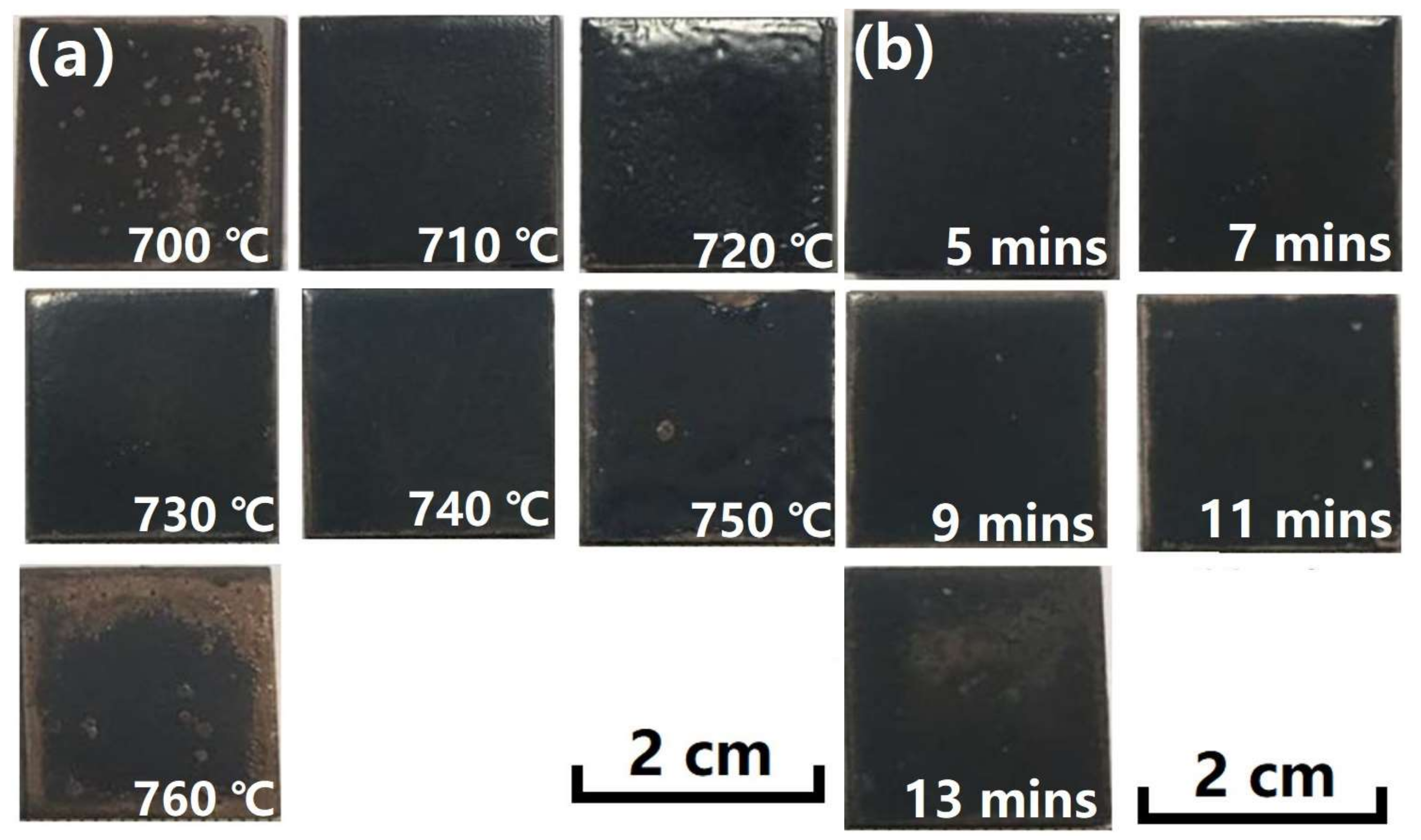
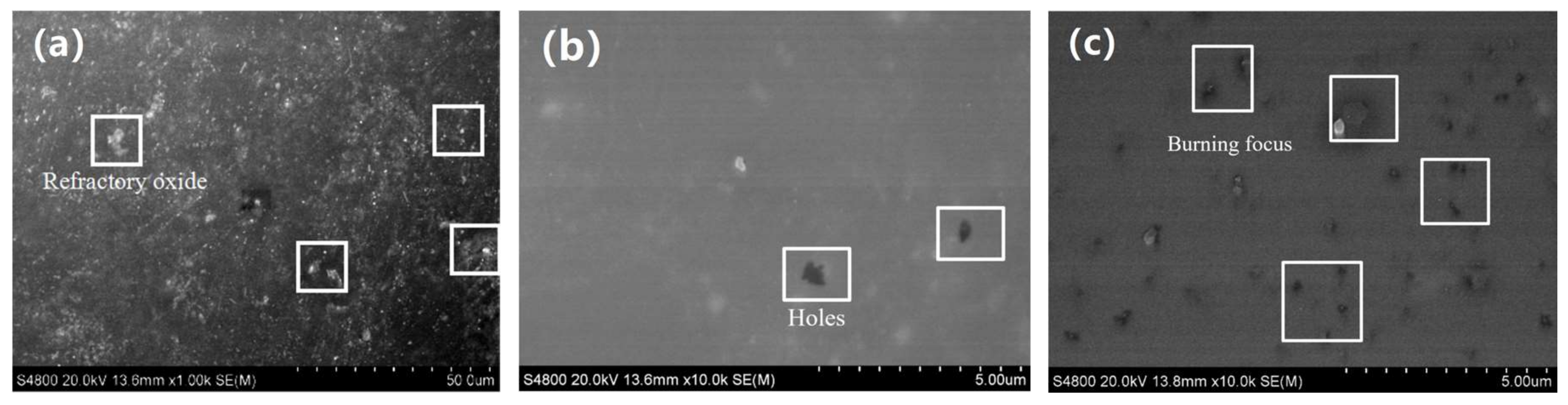
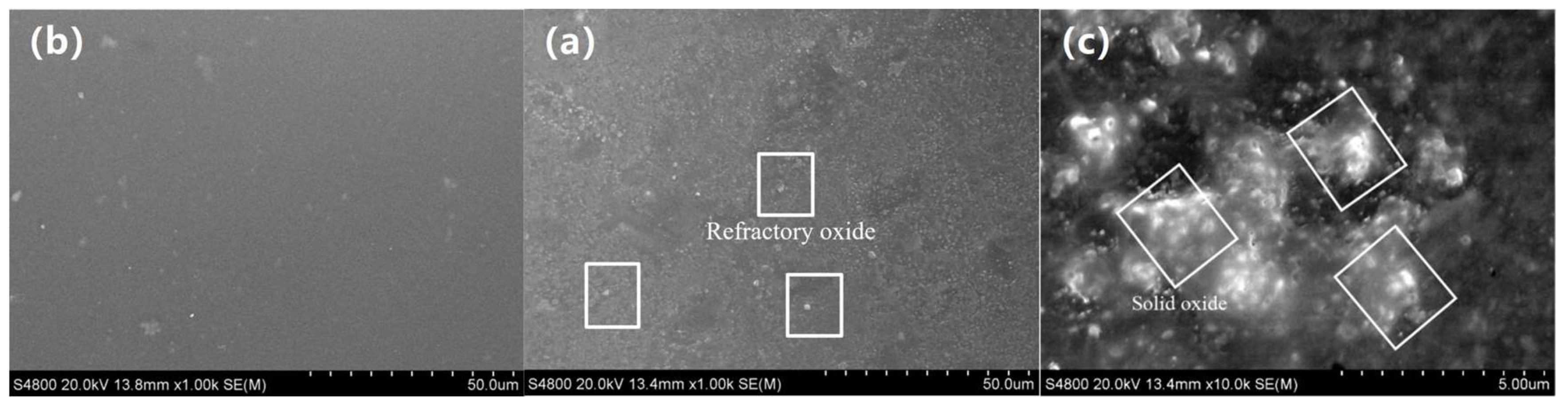
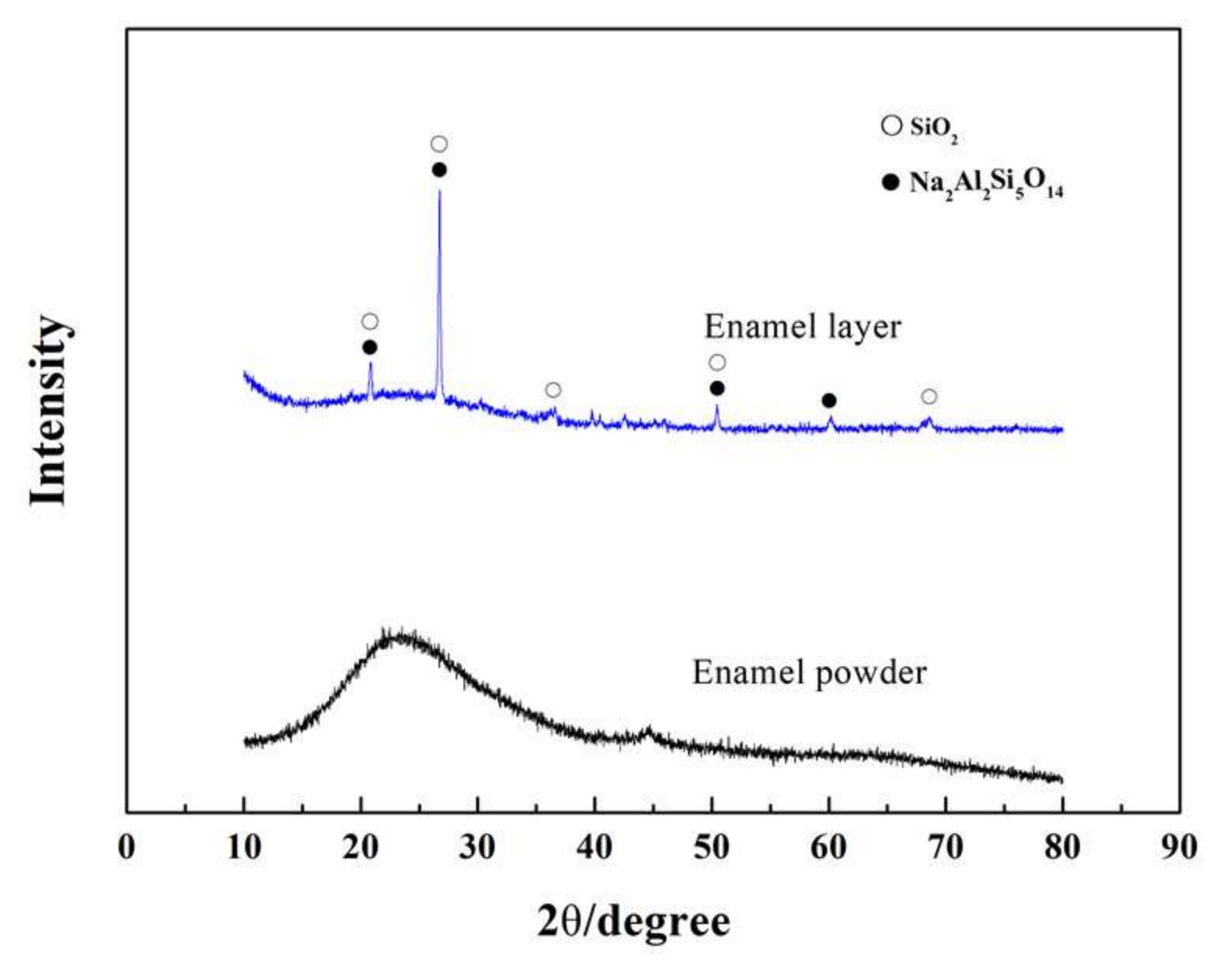
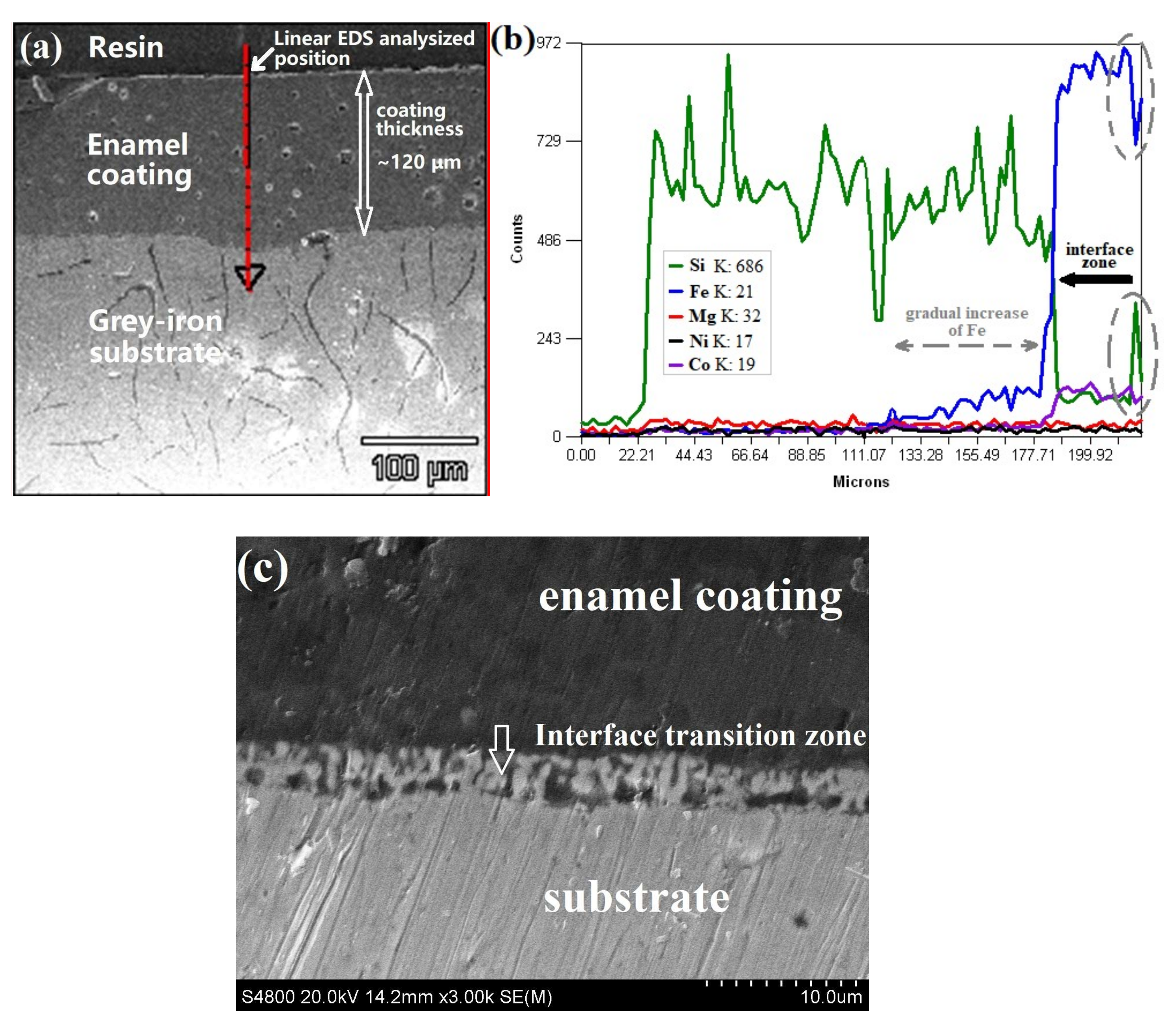
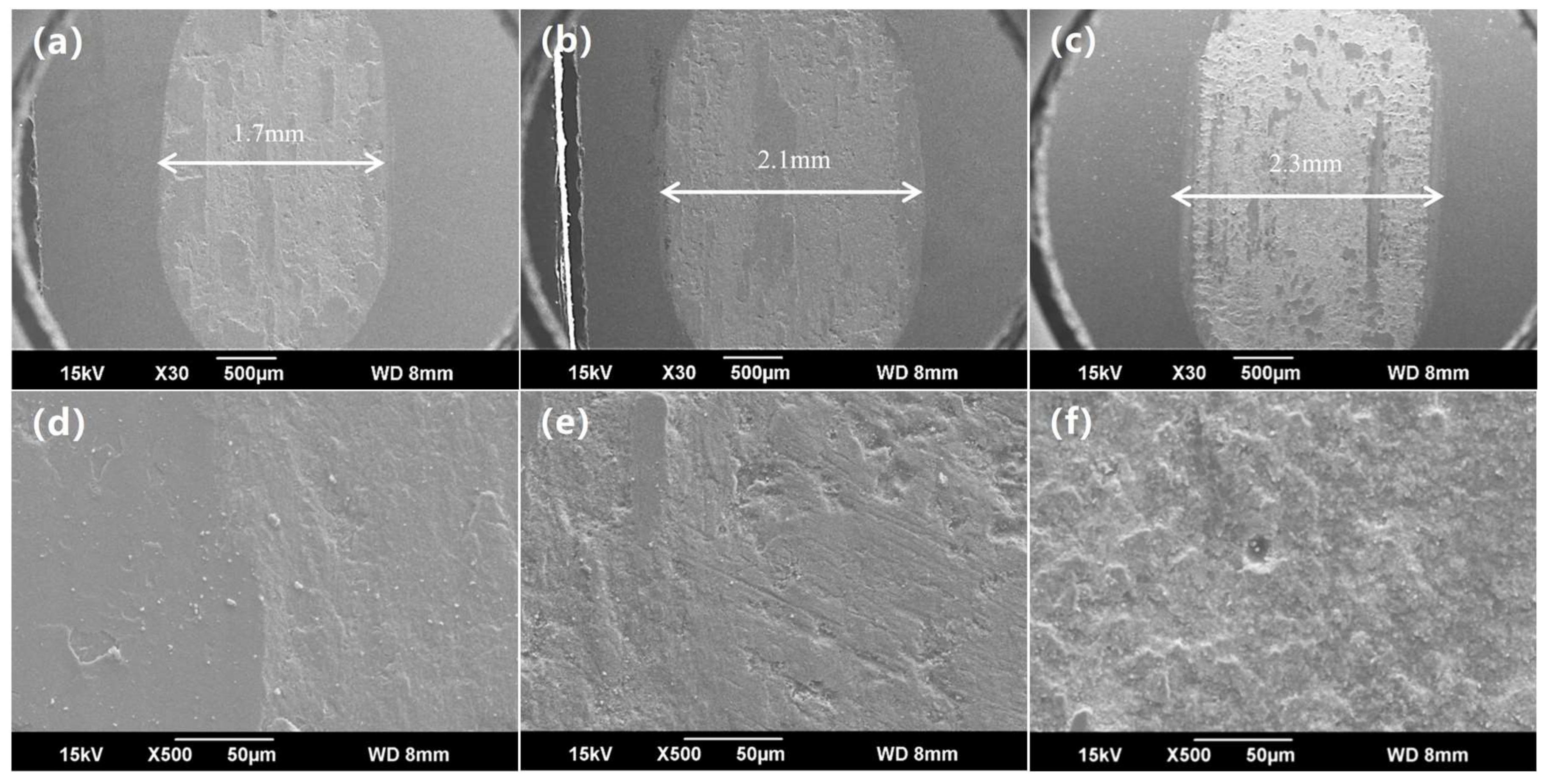
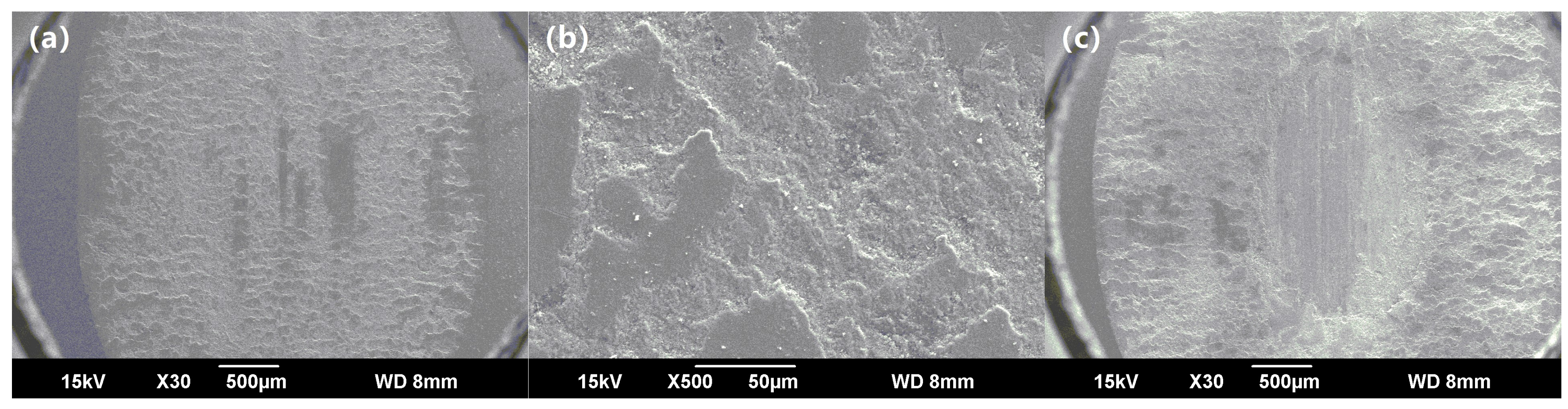
3.1. Macro-Morphologies and Microstructure Characteristic of the Enamel Coating
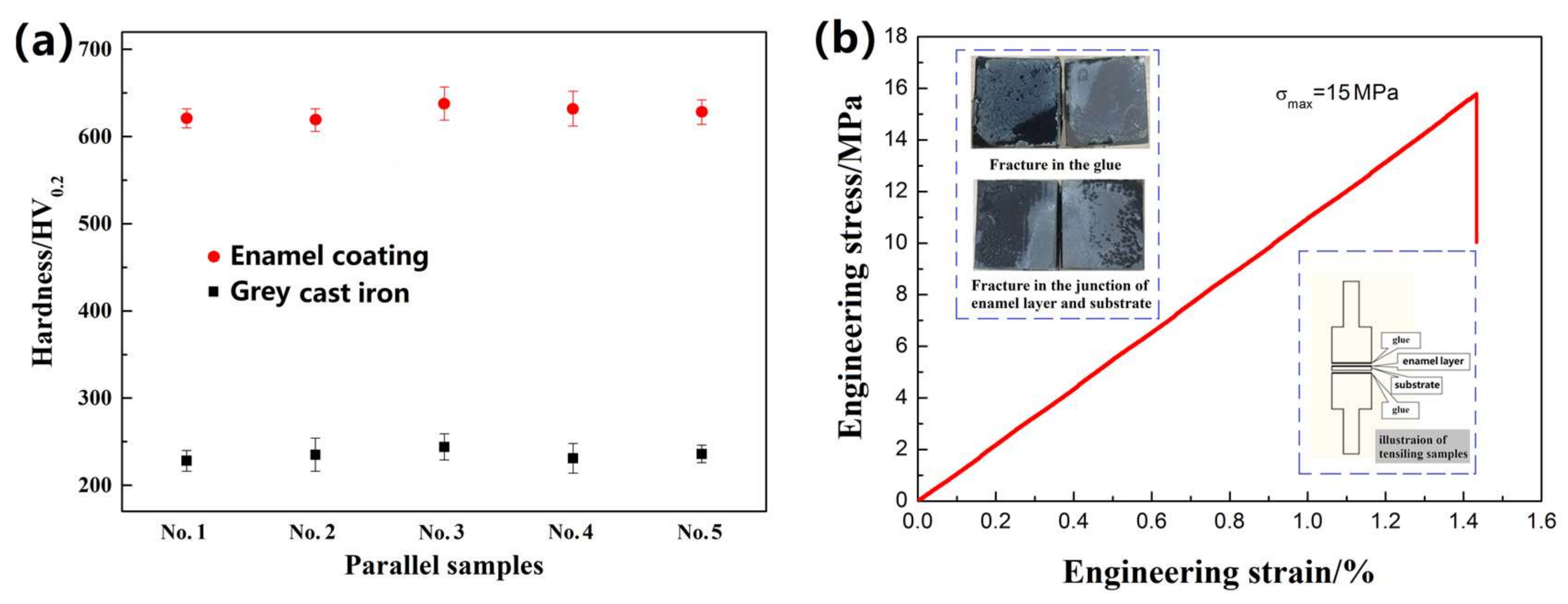
3.2. Mechanical Properties of the Enamel Coating
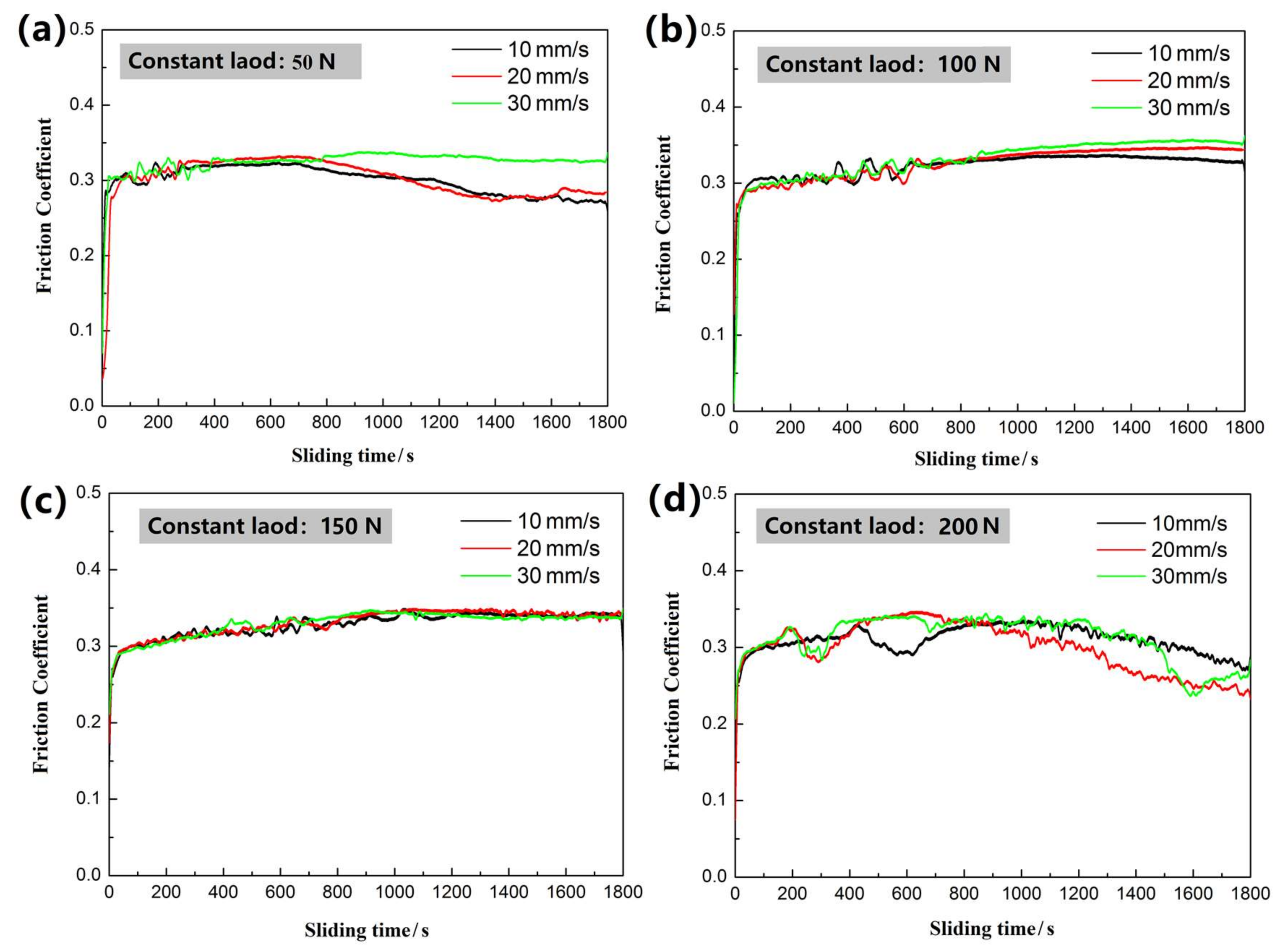
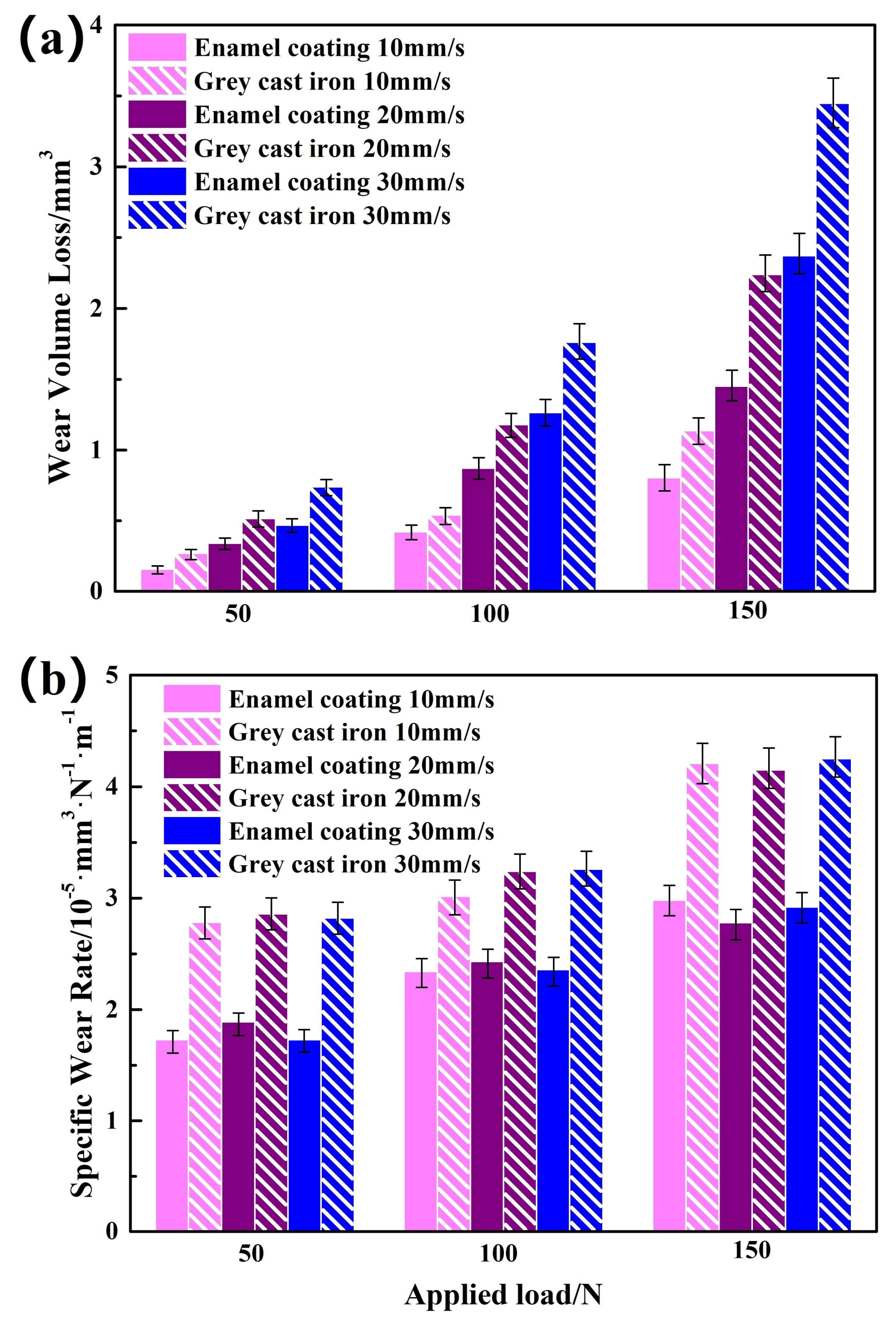
3.3. Wear-Resisting Property of the Enamel Coating
4. Conclusions
- The traditional SiO2–Al2O3–B2O3 composited prescription for an enamel coating was modified to sinter a complete and compact enamel coating at a relatively lower temperature. In the optimized prescription, some quantity of SiO2 was used instead of B2O3, and some of the alkali metals were used instead of Li2O. The optimized sintering process of the enamel coating was 730 °C at seven minutes. This coating was composed of both an amorphous phase and a Na2Al2Si5O14 crystalline phase.
- The enamel coating sintered by the optimized parameter had a thickness of 150 µm, which uniformly and completely covered the metal substrate with a good gloss. The micro-hardness of the enamel coating was approximately 630 HV0.2, which was approximately 2.7 times of the metal substrate. The enamel coating and the grey cast iron substrate had a metallurgical bond, which endowed the sufficient coating bonding stress of approximately 15 MPa.
- The wear mechanism of the enamel coating was a typical brittle exfoliation under the studied friction situation. The wear volume loss and the specific wear rate of the enamel coating were obviously lower than that of the grey cast iron at the different applied load and sliding speed.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Padhy, M.K.; Saini, R.P. A review on silt erosion in hydro turbines. Renew. Sustain. Energy Rev. 2008, 12, 1974–1987. [Google Scholar] [CrossRef]
- Fan, L.; Tang, F.; Reis, S.T.; Chen, G.; Koenigstein, M.L. Corrosion resistance of transmission pipeline steel coated with five types of enamels. Acta Metall. Sin. Engl. Lett. 2017, 30, 390–398. [Google Scholar] [CrossRef]
- Ion, M.; Ilare, B.; Marius, P.; Corneliu, C. Ultrasonic cavitation erosion of nodular cast iron with ferrite-pearlite microstructure. Ultrason. Somochem. 2015, 23, 385–390. [Google Scholar]
- Zhou, Y.X.; Lu, Z.L.; Zhan, M. An investigation of the erosion-corrosion characteristics of ductile cast iron. Mater. Des. 2007, 28, 260–265. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Ouyang, C.; Qiao, Y.X.; Zhou, X.W. Wear Characteristic of Stellite 6 Alloy Hardfacing Layer by Plasma Arc Surfacing Processes. Scanning 2017, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Conde, A.; Damborenea, J.J.D. Electrochemical impedance spectroscopy for studying the degradation of enamel coatings. Corros. Sci. 2002, 44, 1555–1567. [Google Scholar] [CrossRef] [Green Version]
- Ilona, P.; Kaspars, M.; Gundars, M.; Laimons, B.; Margarita, K. Hard TiO2-SiO2 sol–gel coatings for enamel against chemical corrosion. Surf. Coat. Technol. 2014, 258, 206–210. [Google Scholar]
- Scrinzi, E.; Rossi, S. The aesthetic and functional properties of enamel coatings on steel. Mater. Des. 2010, 31, 4138–4146. [Google Scholar] [CrossRef]
- Barbour, M.E.; Rees, J.S. The laboratory assessment of enamel erosion: A review. J. Dent. 2004, 32, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Noui-Mehidi, M.N.; Graham, L.J.W.; Wu, J.; Nguyen, B.V.; Smith, S. Study of erosion behaviour of paint layers for multilayer paint technique applications in slurry erosion. Wear 2008, 264, 737–743. [Google Scholar] [CrossRef]
- Fan, L.; Reis, S.T.; Chen, G.D.; Koenigstein, M.L. Corrosion Resistance of Pipeline Steel with Damaged Enamel Coating and Cathodic Protection. Coatings 2018, 8, 185. [Google Scholar] [CrossRef]
- Lazutkina, O.R.; Kostenko, M.G.; Komarova, S.A.; Kazak, A.K. Highly reliable energy-efficient glass coatings for pipes transporting energy carriers, liquids, and gases. Glass Ceram. 2007, 64, 93–95. [Google Scholar] [CrossRef]
- Fan, L.; Tang, F.; Reis, S.T.; Chen, G.; Koenigstein, M.L. Corrosion resistances of steel pipes internally coated with enamel. Corrosion 2017, 73, 1335–1345. [Google Scholar] [CrossRef]
- Tao, J.H.; Hu, S.B.; Kong, J.H.; Zhang, Y.W. Effect of process temperature on the microstructures and properties of enamels coated on Ti-bearing substrate RT360. Ceram. Int. 2017, 43, 6276–6285. [Google Scholar] [CrossRef]
- Rossi, S.; Parziani, N.; Zanella, C. Abrasion resistance of vitreous enamel coatings in function of frit composition and particles presence. Wear 2015, 332–333, 702–709. [Google Scholar] [CrossRef]
- Wang, D.Q. Effect of crystallization on the property of hard enamel coating on steel substrate. Appl. Surf. Sci. 2009, 255, 4640–4645. [Google Scholar] [CrossRef]
- Samiee, L.; Sarpoolaky, H.; Mirhabibi, A. Microstructure and adherence of cobalt containing and cobalt free enamels to low carbon steel. Mater. Sci. Eng. A 2007, 458, 88–95. [Google Scholar] [CrossRef]
- ASTM D4541-09(2009). Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers; ASTM Standards: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Hiroshi, M.; Alan, H. Wear equation for adhesive wear established through elementary process of wear. Wear 2013, 308, 186–192. [Google Scholar]
- Siligardi, C.; Wu, J.P.; Boccaccini, A.R. Sintering and crystallisation of vanadium doped CaO-ZrO2-SiO2 glass-ceramics. Mater. Lett. 2006, 60, 1607–1612. [Google Scholar] [CrossRef]
- Ahmed, B.; Assia, M.; Domingos, D.S.M.; Emmanuel, V.; Yacine, S.; Patrick, E. Study of the firing type on the microstructure and color aspect of ceramic enamels. J. Alloy Compd. 2018, 735, 2479–2485. [Google Scholar]
- Ehab, Z.A.; Ilnaz, H.; Syozi, N.; Yasushi, S.; Turki, A.B.; Junji, T.; Alireza, S. Effects of coating materials on nanoindentation hardness of enamel and adjacent areas. Dent. Mater. 2016, 32, 807–816. [Google Scholar]
- Rossi, S.; Scrinzi, E. Evaluation of the abrasion resistance of enamel coatings. Chem. Eng. Process. 2013, 68, 74–80. [Google Scholar] [CrossRef]
- Rossi, S.; Zanella, C.; Sommerhuber, R. Influence of mill additives on vitreous enamel properties. Mater. Des. 2014, 55, 880–887. [Google Scholar] [CrossRef]
- Mckellop, H.; Clarke, I.; Markolf, K. Friction and wear properties of polymer, metal, and ceramic prosthetic joint materials evaluated on a multichannel screening device. J. Biomed. Mater. Res. A 1981, 15, 619–653. [Google Scholar] [CrossRef] [PubMed]
- Molla, A.R.; Kumar, B.V.M. Basu, B. Friction and wear mechanisms of K2O-B2O3-Al2O3-SiO2-MgO-F glass-ceramics. J. Eur. Ceram. Soc. 2009, 29, 2481–2489. [Google Scholar] [CrossRef]
- Bai, X.Q.; Yu, A.B.; Jia, D.W.; Chang, X.J. Wear Experiments of Fluorophlogopite Glass Ceramics against Cemented Carbide Materials. J. Rare Earth 2007, 25, 334–336. [Google Scholar]
- Tozetti, K.D.; Albertin, E.; Scandian, C. Abrasive size and load effects on the wear of a 19.9% chromium and 2.9% carbon cast iron. Wear 2017, 376–377, 46–53. [Google Scholar] [CrossRef]
- Telliskivi, T. Simulation of wear in a rolling–sliding contact by a semi-Winkler model and the Archard’s wear law. Wear 2004, 256, 817–831. [Google Scholar] [CrossRef]
- Ruiz-Andres, M.; Conde, A.; de Damborenea, J.; Garcia, I. Friction and wear behaviour of dual phase steels in discontinuous sliding contact conditions as a function of sliding speed and contact frequency. Tribol. Int. 2015, 90, 32–42. [Google Scholar] [CrossRef] [Green Version]
| Component | SiO2 | B2O3 | Al2O3 | Na2O | Li2O | ZnO | MgO | Na2SiF6 | CaF2 | NiO | CoO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt/% | 52 | 14 | 3.5 | 6 | 12 | 4.4 | 0.5 | 3 | 2 | 1.6 | 1 |
| Component | Enamel Powder | Ball Clay | Sb2O3 | Borax | Ethyl Alcohol |
|---|---|---|---|---|---|
| Relative Mass Ratio | 100 | 6 | 0.2 | 0.2 | 70 |
| Testing Parameters | Value |
|---|---|
| Friction time/min | 30 |
| Sliding stroke /mm | 1 |
| Acceleration /mm·s−1 | 0.1 |
| Applied load /N | 50, 100, 150, 200 |
| Sliding speed/mm·s−1 | 10, 20, 30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Tang, R.; Yang, F.; Qiao, Y.; Sun, J.; Jiang, J.; Ma, A. Development of High-Performance Enamel Coating on Grey Iron by Low-Temperature Sintering. Materials 2018, 11, 2183. https://doi.org/10.3390/ma11112183
Song D, Tang R, Yang F, Qiao Y, Sun J, Jiang J, Ma A. Development of High-Performance Enamel Coating on Grey Iron by Low-Temperature Sintering. Materials. 2018; 11(11):2183. https://doi.org/10.3390/ma11112183
Chicago/Turabian StyleSong, Dan, Ren Tang, Falin Yang, Yanxin Qiao, Jiapeng Sun, Jinghua Jiang, and Aibin Ma. 2018. "Development of High-Performance Enamel Coating on Grey Iron by Low-Temperature Sintering" Materials 11, no. 11: 2183. https://doi.org/10.3390/ma11112183





