Effects of Pr and Yb Dual Doping on the Thermoelectric Properties of CaMnO3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Synthesis
2.2. Characterization
2.3. Thermoelectric Properties
2.4. Computational Method
3. Results
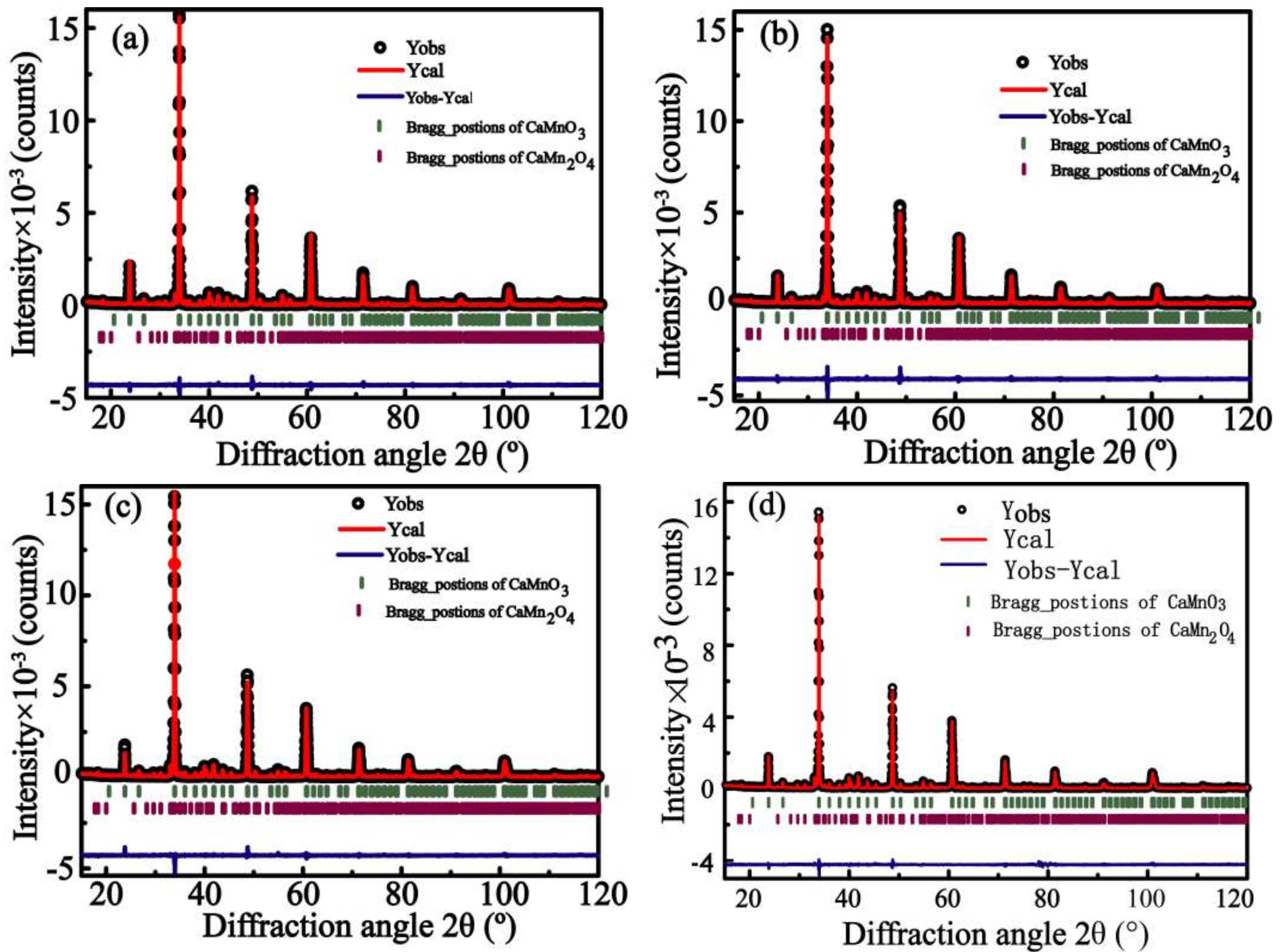
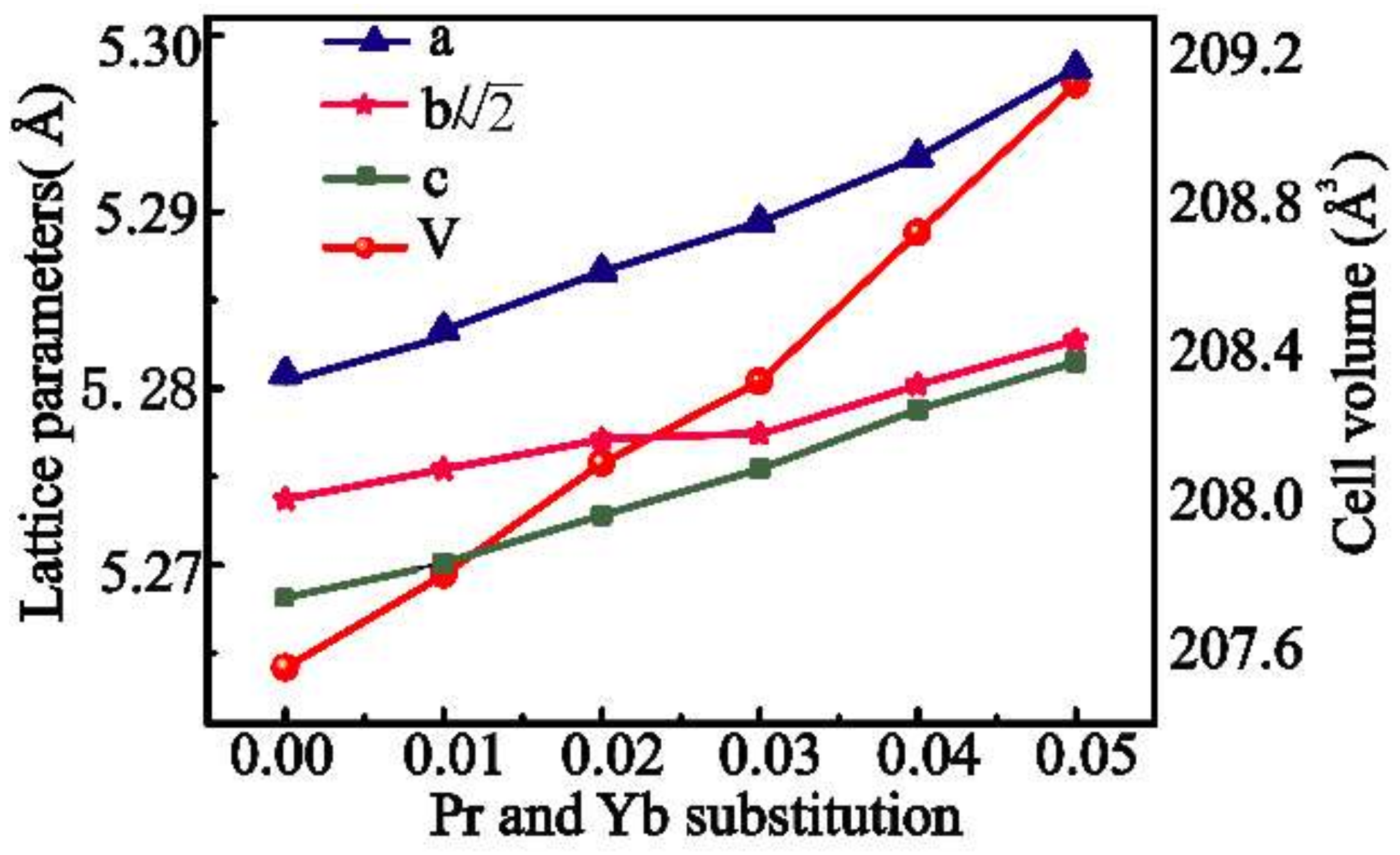
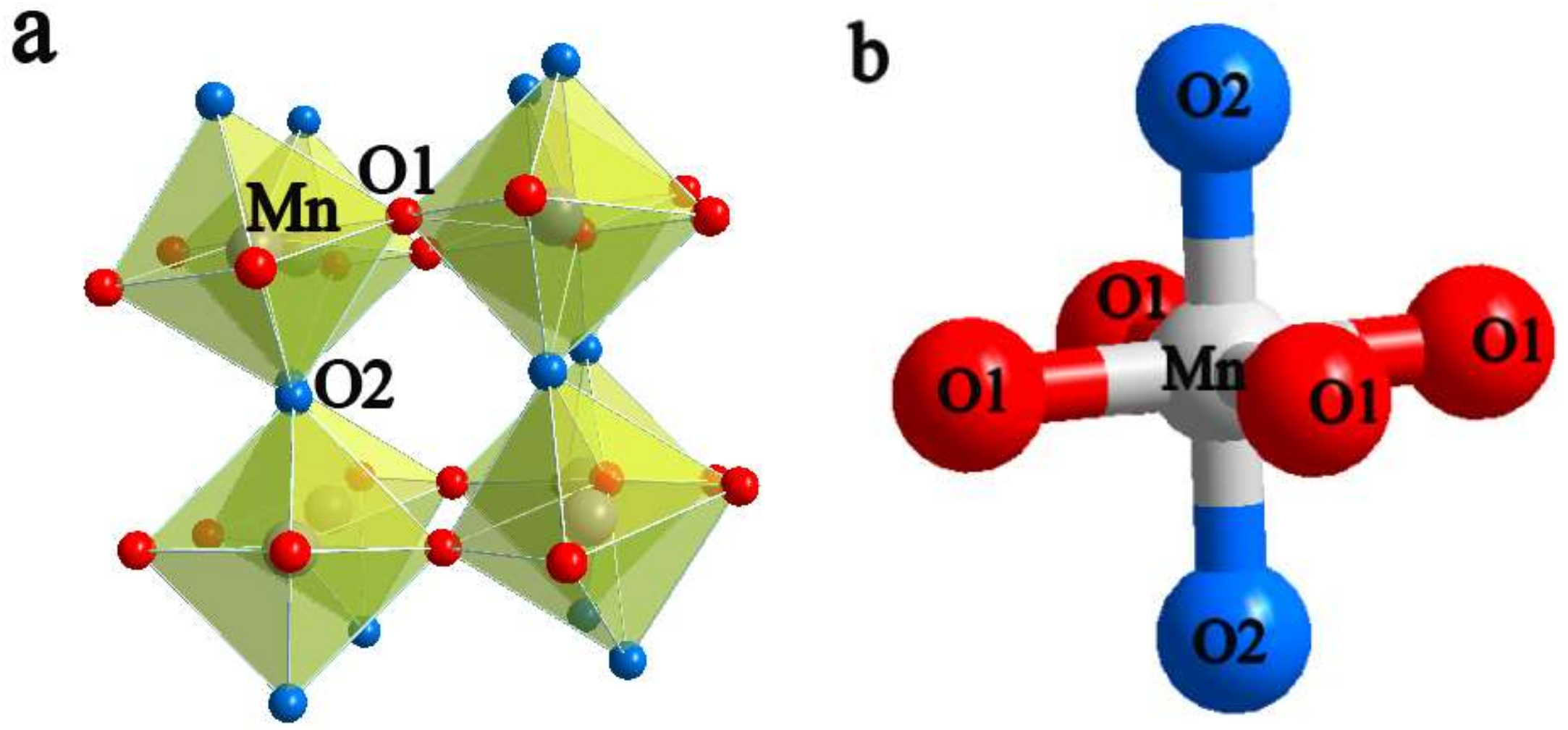
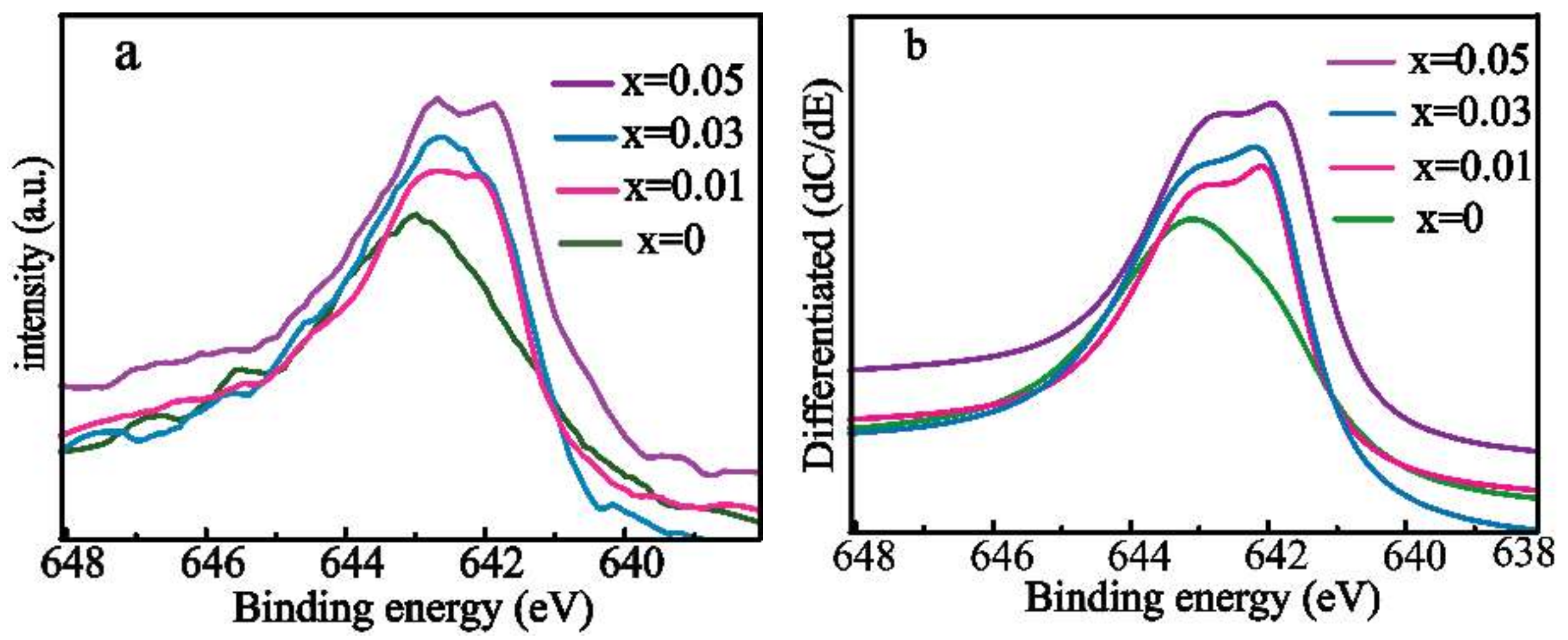
3.1. Characterization of Structure and Composition
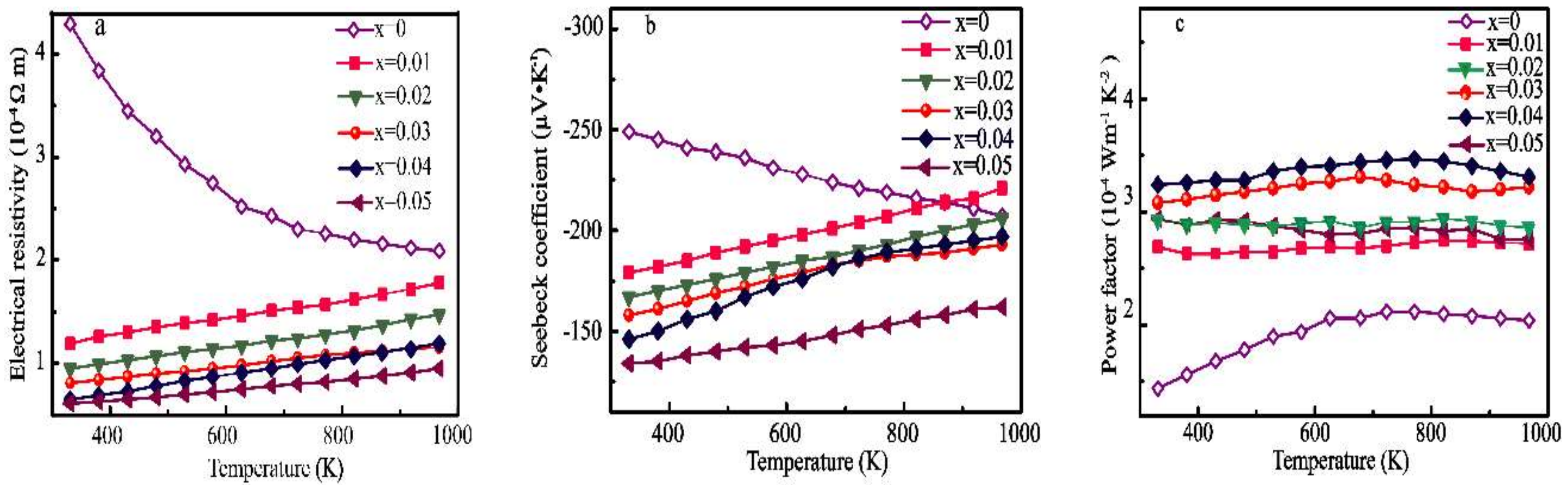
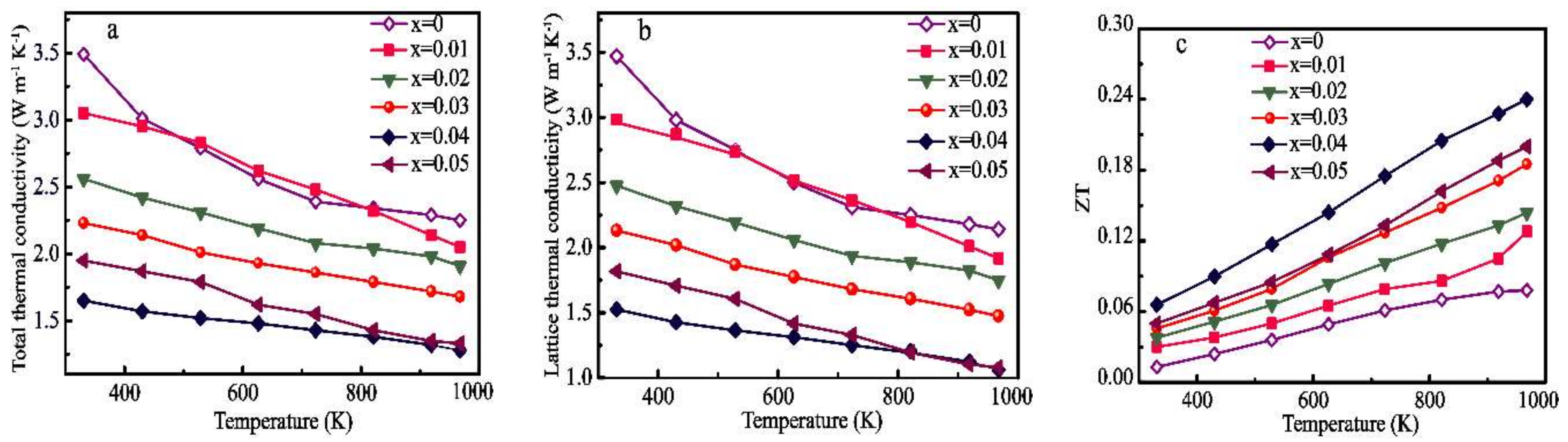
3.2. Thermoelectric Properties
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bilen, K.; Ozyurt, O.; Bakırcı, K.; Karslı, S.; Erdogan, S.; Yılmaz, M.; Comaklı, O. Energy production, consumption, and environmental pollution for sustainable development: A case study in Turkey. Renew. Sustain. Energy Rev. 2008, 12, 1529–1561. [Google Scholar] [CrossRef]
- Shu, G.Q.; Liang, Y.C.; Wei, H.Q.; Tian, H.; Zhao, J.; Liu, L.N. A review of waste heat recovery on two-stroke IC engine aboard ships. Renew. Sustain. Energy Rev. 2013, 19, 385–401. [Google Scholar] [CrossRef]
- He, W.; Zhang, G.; Zhang, X.X.; Ji, J.; Li, G.Q.; Zhao, X.D. Recent development and application of thermoelectric generator and cooler. Appl. Energy 2015, 143, 1–25. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of Thermoelectric Efficiency in PbTe by Distortion of the Electronic Density of States. Science 2008, 321, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.D.; Lo, S.H.; Zhang, Y.S.; Sun, H.; Tan, G.G.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; He, J.; Blum, I.D.; Chun-IWu; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414–418. [Google Scholar]
- He, Y.; Lu, P.; Shi, X.; Xu, F.F.; Zhang, T.S.; Snyder, G.J.; Uher, C.; Chen, L.D. Ultrahigh Thermoelectric Performance in Mosaic Crystals. Adv. Mater. 2015, 27, 3639–3644. [Google Scholar] [CrossRef] [PubMed]
- Appel, O.; Schwall, M.; Kohne, M.; Balke, B.; Gelbstein, Y. Effects of Microstructural Evolution on the Thermoelectric Properties of Spark-Plasma-Sintered Ti0.3Zr0.35Hf0.35NiSn Half-Heusler Compound. J. Electron. Mater. 2013, 42, 1340–1345. [Google Scholar] [CrossRef]
- Vizel, R.; Bargig, T.; Beeri, O.; Gelbstein, Y. Bonding of Bi2Te3-Based Thermoelectric Legs to Metallic Contacts Using Bi0.82Sb0.18 Alloy. J. Electron. Mater. 2016, 45, 1296–1300. [Google Scholar] [CrossRef]
- Zhang, F.P.; Lu, Q.M.; Zhang, X.; Zhang, J.X. Electrical transport properties of CaMnO3 thermoelectric compound: A theoretical study. J. Phys. Chem. Solids 2013, 74, 1859–1864. [Google Scholar] [CrossRef]
- Ohtaki, M.; Koga, H.; Tokunaga, T.; Eguchi, K.; Arai, H. Electrical Transport Properties and High-Temperature Thermoelectric Performance of (Ca0.9M0.1)MnO3 (M = Y, La, Ce, Sm, In, Sn, Sb, Pb, Bi). J. Solid State Chem. 1995, 120, 105–111. [Google Scholar] [CrossRef]
- Flahaut, D.; Mihara, T.; Funahashi, R.; Nabeshima, N.; Lee, K. Thermoelectrical properties of A-site substituted Ca1−xRexMnO3 system. J. Appl. Phys. 2006, 100, 084911-1–084911-4. [Google Scholar] [CrossRef]
- Wang, Y.; Sui, Y.; Fan, H.G.; Wang, X.J.; Su, Y.T.; Su, W.H.; Liu, X.Y. High Temperature Thermoelectric Response of Electron-Doped CaMnO3. Chem. Mater. 2009, 21, 4653–4660. [Google Scholar] [CrossRef]
- Torres, A.S.M.; Madre, M.; Diez, J. Effect of synthesis process on the densification, microstructure, and electrical properties of Ca0.9Yb0.1MnO3 ceramics. Int. J. Appl. Ceram. Technol. 2017, 14, 1190–1196. [Google Scholar]
- Zhang, F.P.; Niu, B.C.; Zhang, K.S.; Zhang, X.; Lu, Q.M.; Zhang, J.X. Effects of praseodymium doping on thermoelectric transport properties of CaMnO3 compound system. J. Rare Earths 2013, 37, 885–890. [Google Scholar] [CrossRef]
- Cong, B.T.; Tsuji, T.; Thao, P.X.; Thanh, P.Q.; Yamamura, Y. High-temperature thermoelectric properties of Ca1−xPrxMnO3−δ (0 ≤ x < 1). Physica B 2004, 352, 352–357. [Google Scholar]
- Soteloa, A.; Depriesterb, M.; Torresa, M.A.; Sahraouib, A.H.; Madrea, M.A.; Dieza, J.C. Effect of simultaneous K, and Yb substitution for Ca on the microstructural and thermoelectric characteristics of CaMnO3 ceramics. Ceram. Int. 2017, 44, 12697–12701. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Su, W.B.; Liu, J.; Zhou, Y.C.; Li, J.; Zhang, X.; Du, Y.; Wang, C.L. Effects of Dy and Yb co-doping on thermoelectric properties of CaMnO3 ceramics. Ceram. Int. 2015, 41, 1535–1543. [Google Scholar] [CrossRef]
- Wang, Y.; Sui, Y.; Wang, X.; Su, W. Enhancement of thermoelectric efficiency in (Ca,Dy)MnO3–(Ca,Yb)MnO3 solid solutions. Appl. Phys. Lett. 2010, 97, 4. [Google Scholar] [CrossRef]
- Kosuga, A.; Isse, Y.; Wang, Y.; Koumoto, K.; Funahashi, R. High-temperature thermoelectric properties of Ca 0.9−xSrxYb0.1 MnO3. J. Appl. Phys. 2009, 105, 093717-1–093717-6. [Google Scholar] [CrossRef]
- Kim, C.M.; Seo, J.W.; Cha, J.S.; Park, K. Electrical transport properties of Ca0.9La0.1−xBixMnO3−δ (0 ≤ x ≤ 0.1) thermoelectric materials. Int. J. Hydrogen Energy 2015, 40, 15556–155568. [Google Scholar] [CrossRef]
- Wang, H.C.; Wang, C.L. Synthesis of Dy doped Yb0.1Ca0.9MnO3 ceramics with a high relative density and their thermoelectric properties. Mater. Res. Bull. 2012, 47, 2252–2256. [Google Scholar] [CrossRef]
- Löhnert, R.; Stelter, M.; Töpfer, J. Evaluation of soft chemistry methods to synthesize Gd-doped CaMnO3−δ with improved thermoelectric properties. Mater. Sci. Eng. B 2017, 223, 185–193. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J.; Fullprof, K. Version 4.6 c–Mar 2002. Physica B 1993, 192, 55–69. [Google Scholar]
- Taguchi, H.; Sonoda, M.; Nagao, M. Relationship between Angles for Mn–O–Mn and Electrical Properties of Orthorhombic Perovskite-Type (Ca1−xSrx)MnO3. J. Solid State Chem. 1998, 137, 82–86. [Google Scholar] [CrossRef]
- Giesber, H.G.; Pennington, W.T.; Kolis, J.W. Redetermination of CaMn2O4. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2001, C57, 329–330. [Google Scholar] [CrossRef]
- Li, C.Q.; Chen, Q.L.; Yan, Y.A.; Li, Y.N.; Zhao, Y. High-temperature thermoelectric properties of Ca0.92La0.04RE0.04MnO3 (RE = Sm, Dy and Yb) prepared by coprecipitation. Mater. Res. Express 2018. [Google Scholar] [CrossRef]
- VandeVondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. QUICKSTEP: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Goedecker, S.; Teter, M.; Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 1996, 54, 1703–1709. [Google Scholar] [CrossRef]
- VandeVondele, J.; Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 2007, 127, 114105-1–114105-9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. E 1976, 32, 751–767. [Google Scholar] [CrossRef] [Green Version]
- Zhun, Y.; Wang, C.; Su, W.; Li, J.; Liu, J.; Du, Y.; Mei, L. High-temperature thermoelectric performance of Ca0.96Dy0.02RE0.02MnO3 ceramics (R = Ho, Er, Tm). Ceram. Int. 2014, 40, 15531–15536. [Google Scholar]
- Bocher, L.; Aguirre, M.H.; Logvinovich, D.; Shkabko, A.; Robert, R.; Trottmann, M.; Weidenkaff, A. CaMn1−xNbxO3 (x = 0.08) Perovskite-Type Phases As Promising New High-Temperature n-Type Thermoelectric Materials. Inorg. Chem. 2008, 47, 8077–8085. [Google Scholar] [CrossRef] [PubMed]
- Granado, E.; Moreno, N.O.; Martinho, H.; García, A.; Sanjurjo, J.A.; Rettori, I.T.C.; Neumeier, J.J.; Oseroff, S.B. Dramatic Changes in the Magnetic Coupling Mechanism for La-Doped CaMnO3. Phys. Rev. Lett. 2001, 86, 5385–5388. [Google Scholar] [CrossRef] [PubMed]
- Jahn, H.A.; Teller, E. Stability of Polyatomic Molecules in Degenerate Electronic States I-Orbital Degeneracy. Proc. R. Soc. Lond. Ser. A 1937, 161, 220–235. [Google Scholar] [CrossRef]
- Bocher, L.; Aguirre, M.H.; Robert, R.; Logvinovich, D.; Bakardjieva, S.; Hejtmanek, J.; Weidenkaff, A. High-temperature stability, structure and thermoelectric properties of CaMn1−xNbxO3 phases. Acta Mater. 2009, 57, 5667–5680. [Google Scholar] [CrossRef]
- Wei, Y.G.; Kim, K.-B.; Chen, G. Evolution of the local structure and electrochemical properties of spinel LiNixMn2−xO4 (0 ≤ x ≤ 0.5). Electrochim. Acta 2006, 51, 3365–3373. [Google Scholar] [CrossRef]
- Zhan, B.; Lan, J.L.; Liu, Y.C.; Lin, Y.H.; Shen, Y.; Nan, C.W. High Temperature Thermoelectric Properties of Dy-doped CaMnO3 Ceramics. J. Mater. Sci. Technol. 2014, 30, 821–825. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Wang, J.; Zou, T.; Zhang, S.; Yaer, X.B.; Ding, N.; Liu, C.Y.; Miao, L.; Li, Y.; Wu, Y. High thermoelectric performance of Nb-doped SrTiO3 bulk materials with different doping levels. J. Mater. Chem. C 2015. [Google Scholar] [CrossRef]
- Zhang, F.P.; Zhang, X.; Lu, Q.M.; Zhang, J.X.; Liu, Y.Q.; Fan, R.F.; Zhang, G.Z. Doping induced electronic structure and estimated thermoelectric properties of CaMnO3 system. Phys. B Condens. Matter 2011, 406, 1258–1262. [Google Scholar] [CrossRef]
- Zhang, X.H.; Li, J.C.; Du, Y.L.; Wang, F.N.; Liu, H.Z.; Zhu, Y.H.; Liu, J.; Su, W.B.; Wang, C.L.; Mei, L.M. Thermoelectric properties of A-site substituted Lanthanide Ca0.75R0.25MnO3. J. Alloys Compd. 2015, 634, 1–5. [Google Scholar] [CrossRef]
- Zhang, F.P.; Zhang, X.; Lu, Q.M.; Zhang, J.X.; Liu, Y.Q. Electronic structure and thermal properties of doped CaMnO3 systems. J. Alloys Compd. 2011, 509, 4171–4175. [Google Scholar] [CrossRef]
- Kabir, R.; Zhang, T.; Wang, D.; Donelson, R.; Tian, R.; Tan, T.T.; Li, S. Improvement in the thermoelectric properties of CaMnO3 perovskites by W doping. J. Mater. Sci. 2014, 49, 7522–7528. [Google Scholar] [CrossRef]
- Wang, Y.; Sui, Y.; Su, W. High temperature thermoelectric characteristics of Ca0.9R0.1MnO3 (R = La, Pr, …, Yb). J. Appl. Phys. 2008, 104, 093703–093703-7. [Google Scholar] [CrossRef]
- Wang, Y.; Sui, Y.; Wang, X.; Su, W.; Liu, X.; Fan, H.J. Thermal conductivity of electron-doped CaMnO3 perovskites: Local lattice distortions and optical phonon thermal excitation. Acta Mater. 2010, 58, 6306–6316. [Google Scholar] [CrossRef]


| Ca1−2xPrxYbxMnO3 | x = 0 | x = 0.01 | x = 0.02 | x = 0.03 | x = 0.04 | x = 0.05 |
|---|---|---|---|---|---|---|
| a (Å) | 5.2808(2) | 5.2833(2) | 5.2866(2) | 5.2894(2) | 5.2931(4) | 5.2981(4) |
| b (Å) | 7.4570(3) | 7.4595(4) | 7.4619(4) | 7.4622(3) | 7.4662(6) | 7.4698(6) |
| b/ | 5.2737 | 5.2754 | 5.2771 | 5.2774 | 5.2802 | 5.2827 |
| c (Å) | 5.2682(2) | 5.2701(2) | 5.2728(3) | 5.2754(2) | 5.2788(4) | 5.2815(4) |
| V (Å3) | 207.45 | 207.70 | 208.0 | 208.22 | 208.62 | 209.02 |
| Rw | 11.1 | 11.0 | 10.4 | 12.7 | 11.7 | 13.5 |
| Rp | 7.39 | 7.44 | 6.87 | 8.26 | 7.64 | 8.89 |
| χ2 | 1.74 | 1.78 | 1.68 | 2.63 | 2.32 | 3.22 |
| Mn-O2 × 2 (Å) | 1.8708(2) | 1.8716(2) | 1.8728(2) | 1.8737(4) | 1.8750(4) | 1.8765(4) |
| Mn-O1 × 2 (Å) | 1.9110(2) | 1.9117(1) | 1.9123(2) | 1.9125(2) | 1.9135(2) | 1.9144(2) |
| Mn-O1 × 2 (Å) | 1.9281(2) | 1.9289(2) | 1.9300(2) | 1.9309(4) | 1.9322(4) | 1.9335(4) |
| Mn-O1-Mn (°) | 158.077(1) | 158.078(1) | 158.080(1) | 158.084(1) | 158.085(1) | 158.087(1) |
| Mn-O2-Mn (°) | 154.594(1) | 154.593(1) | 154.589(1) | 154.578(1) | 154.575(1) | 154.573(1) |
| T | 1.000 | 0.9982 | 1.9967 | 1.9950 | 0.9933 | 0.9916 |
| Nominal Composition (x) | Pr Content by ICP | Yb Content by ICP | dt (g/cm3) | da (g/cm3) | Relative Density (%) |
|---|---|---|---|---|---|
| 0 | - | - | 4.58 | 3.62 | 79 |
| 0.01 | 0.0089 | 0.0078 | 4.64 | 4.08 | 85 |
| 0.02 | 0.0179 | 0.0187 | 4.71 | 4.10 | 87 |
| 0.03 | 0.0285 | 0.0282 | 4.78 | 4.30 | 89 |
| 0.04 | 0.0388 | 0.0386 | 4.84 | 4.21 | 90 |
| 0.05 | 0.0487 | 0.0483 | 4.92 | 4.38 | 89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Chen, Q.; Yan, Y. Effects of Pr and Yb Dual Doping on the Thermoelectric Properties of CaMnO3. Materials 2018, 11, 1807. https://doi.org/10.3390/ma11101807
Li C, Chen Q, Yan Y. Effects of Pr and Yb Dual Doping on the Thermoelectric Properties of CaMnO3. Materials. 2018; 11(10):1807. https://doi.org/10.3390/ma11101807
Chicago/Turabian StyleLi, Cuiqin, Qianlin Chen, and Yunan Yan. 2018. "Effects of Pr and Yb Dual Doping on the Thermoelectric Properties of CaMnO3" Materials 11, no. 10: 1807. https://doi.org/10.3390/ma11101807




