3.1. Oxidation Kinetics
Coated inserts were oxidized at 900 °C in air for different times. Oxidation can be fully finished at the temperature of 900 °C according to previous reports [
4,
5]. Macrographs represented in
Figure 3. At the early stage, the compact coating acted as an effective diffusion barrier, blocking the inward diffusion of oxygen and the outward diffusion of the elements inside. After being oxidized for 10 min, the coating was still smooth. Only a few blue-gray oxides could be seen upwardly bulging from the edges and corners, where the defects were concentrated (
Figure 1a). Defects provided a rapid path for oxygen to diffuse into the substrate. Oxidation was initiated from the damaged edges and corners, especially the local loss of the protective coating (
Figure 3). More bulges can be found with further oxidation. When oxidized for 60 min, the color of the outermost layer becomes uneven. After oxidation for 120 min, it could be seen that some bulges came out of the middle of the coating on the flank faces, not only on the edges. The front faces were a little yellow when oxidized for 180 min. However, the untreated samples approximately remained as a square shape and were only partially oxidized for 180 min (
Figure 3a). This was different from the result reporting that the CrAlN coated WC-9% Co insert tip scraps were fully oxidized at 900 °C for 180 min [
12]. This discrepancy can be attributed to the better oxidation resistance of the Al
2O
3 coating to CrAlN. Therefore, different coatings should be given a different treatment.
Figure 3b shows the macrographs of the fragments with different oxidation times. As shown in pictures, when oxidized for 10 min, the shape changed considerably. Swelling was visible at the locations of coating loss where the oxidation reaction was not limited by the coatings. Oxidation continued individually at many of the different damaged locations, so the shape was irregular. When undergoing oxidation for 180 min, the volume was up to more than 200%. The result revealed that the oxidation rate was apparently accelerated when the hardmetal scrap was broken into pieces since more bare WC-Co phases could come into contact with the oxygen directly.
Figure 3c shows the milled powders that were isothermally heated at 900 °C in air for various times. When oxidized for 10 min, many black particles could be seen, and the quantity of the particles decreased with further oxidation. The powders became a completely light grey-green in color after oxidation for 180 min and no black un-oxidized particles were found, indicating that the milled powders were fully oxidized after oxidation treatment for 180 min. Compared to the untreated inserts and fragmented samples, the oxidation rate greatly accelerated after the milling procedure.
To examine the optimal oxidation temperature, thermal analysis was employed with air flow. The TG-DSC results are shown in
Figure 4a. The mass fraction indicates the total weight of the oxidized samples as a percentage compared to the initial weight. It can be seen that the weight changed obviously with increasing temperature after 400 °C and increased sharply around 720 °C. This means that oxidation began at around 400 °C, so the theoretical oxidation temperature for recycling the WC-Co cemented carbide scrap should be 720 °C. As shown in
Figure 4a, the TG curve remained almost flat when the value of the mass fraction reached 118.20%, which as consistent with the theoretical calculation value when fully oxidized, indicating that the weight gain was about 18.20% when fully oxidized [
19].
Figure 4b shows the relationship between the weight ratio
W/W0 and oxidation time.
W is the total weight of the oxidized samples compared and
W0 is the initial weight. According to the result in
Figure 4a, the coated inserts could be rapidly oxidized at 720 °C. However, the curve appeared flat when the untreated hardmetal scraps were oxidized at 720 °C (Curve 1), i.e., the whole insert tip scrap could not be completely oxidized at 720 °C since the coating strongly resisted the oxidation. It has been reported that the oxidation process is controlled by the reaction at the interfaces [
19]. For the untreated samples, the WC-Co surfaces were separated from oxygen by the coating, and as the oxygen could not directly make contact with the inner WC-Co phase, the oxidation rate slowed down for the protective coatings. In order to accelerate the oxidation process, it was necessary to reveal more bare WC-Co phases to oxygen, thus fragmentation was conducted. The weight ratio still increased slowly when at 720 °C (Curve 2), so a higher temperature was required. When the oxidation temperature rose to 900 °C, an obvious weight increment could be observed and the oxidation rate was significantly accelerated (Curve 3). This revealed that fine particles and increasing oxidation temperature led to an increasing oxidation rate. Coated inserts were milled to powders with a size below 0.150 mm. Thus, nearly all of the WC-Co surfaces were exposed to air, just like the uncoated particles. Oxygen could therefore make contact with the WC-Co surfaces directly and reacted with them. Furthermore, the oxygen also could easily diffuse into the WC-Co surfaces near the coating so the weight ratio increased markedly and reached 1.138 when oxidized for 120 min. With further oxidation for 180 min, it reached 1.183, approximate to the max mass value of 1.182 in
Figure 4a. This implies that the powders that had been passed through a 100-mesh sieve were fully oxidized at 900 °C for 180 min. The results demonstrated that the coating slowed down the oxidation rate. Considering the cost and production cycle, the scrap should be milled into powders for rapid processing.
3.2. Phase Transitions
Figure 5 shows the enlarged macrographs of the coated WC-Co hardmetal scraps oxidized at 900 °C in air for 180 min and 300 min. It can be observed from
Figure 5a that the yellow coating and black coating connected to the substrate when oxidized for 180 min. This implies that the TiCN/Al
2O
3/TiN and TiCN/Al
2O
3 coatings were not fully oxidized. With further oxidization for 300 min, red porous oxides were seen clearly in
Figure 5b,c, indicating the phase transition. Bulges were porous and could be easily broken down for the cracking of the hard phases and bond metals during oxidation treatment [
19].
Figure 6 shows the chemical phases of the fragments oxidized for different times.
Figure 6a presents the XRD patterns of the outermost yellow TiN coating. When oxidized for 60 min, the TiO
2 (PDF#21-1276) phase appeared. The result showed that the yellow coating that was oxidized mainly consisted of the TiO
2 phase. TiO
2 was the final oxidation product of the TiN coating as well as TiCN. This can be described by the reactions as [
20].
On the other hand, the black coating was mainly composed of the Al
2O
3 (PDF#46-1212) phase as originally based on the XRD pattern in
Figure 6b. The chemical compound Al
2O
3 is impossible to oxidize due to its good oxidation resistance at 900 °C [
20]. Therefore, the phases were almost the same with different oxidation times.
Figure 6b,c show the XRD results of the substrate and milled powders oxidized for different oxidation times. It was found that the peaks of WC (PDF#51-0939) decreased with further oxidation. After oxidation for 180 min, the present coated WC-Co hardmetal powders were subsequently transformed into a mixture of WO
3 (PDF#20-1324) and CoWO
4 (PDF#15-0867) as well as the exposed substrate. The following oxidation reactions could be proposed [
15,
19].
In accordance with the reported thermodynamic calculations [
12,
14], the standard Gibbs free energy of reactions (Equations (3)–(5)) were all below zero and the reactions took place under standard pressure at 900 °C. This was demonstrated by the XRD result of the substrate surface in
Figure 6a. When the WC-Co hardmetal scrap covered with the TiCN/Al
2O
3/TiN multilayer was fully oxidized, it could be seen as the schemes shown in
Figure 6e,f, and the unbroken oxidized layer could be TiO
2/Al
2O
3/TiO
2. In fact, it was difficult to keep the whole shape as the brittle oxide layers and porous substrates were easily broken down.
After oxidation for 180 min, no un-oxidized WC peaks were detected in the powders (
Figure 6d), but could be found on the surface of the fragments (
Figure 6a,b), i.e., the milled powders passed through a 100-mesh were fully oxidized, but the bulks were not. This contradiction can be explained by the protective coatings. Therefore, the crushing process should be done before oxidation for a shorter process, which is meaningful for reducing energy consumption.
The intensity peaks caused by TiO
2 and Al
2O
3 were not detectable in the oxide powders due to their relatively low content as well as Ta
2O
5 and Nb
2O
5, which are the oxides of Ta and Nb, respectively [
13,
21]. It was speculated that their peaks were hidden by the XRD pattern noise given their weak diffraction signals. The possible chemical reactions and oxidation behavior of TaC, NbC, and TiC were the focus of our study and will be discussed in our future publication.
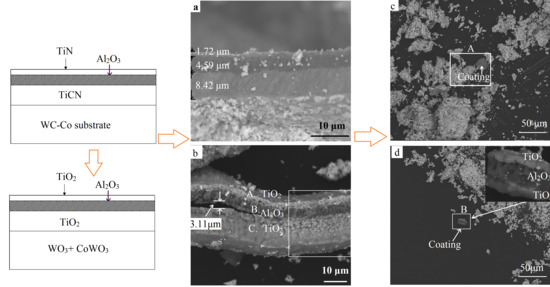
Figure 7 shows the SEM images and the EDS results of the fully oxidized powders. According to the XRD patterns in
Figure 6d, the final oxides were mainly consistent with WO
3 and CoWO
4. This agreed well with the results reported [
4,
5]. As shown in
Figure 7a, a gray fragment could be seen clearly among the white final oxides in the square area A. This fragment was rich in titanium and oxygen, while tungsten and cobalt were concentrated in the white particles. This could be the exfoliation of the TiO
2 layer according to the XRD result in
Figure 6a. Pieces of the multilayer could be found in area B in
Figure 7b where oxygen was focused. Aluminum was concentrated in the middle layer, while titanium was distributed on the inner and outermost layers. Based on the XRD results in
Figure 6, this could be the TiO
2/Al
2O
3/TiO
2 layers. Therefore, the TiO
2 and Al
2O
3 phases remained in the oxide powders. Although the Al
2O
3 layer was thin, it was difficult to fuse or react with the WC and Co. It mainly distributes in the binder Co and decreases the bonding strength of WC/Co [
17]. Future work will focus on the influence of Al
2O
3 coatings on the subsequent reduction and carburization processes as well as the recycled products of the W powders, WC-Co composite powders, and the final recycled hard alloy.
3.3. Evolution of Coatings
To understand the development of the coated insert and the interaction between oxygen, the coating, and substrate during oxidation, the morphologies of the samples were tested, as shown in
Figure 8 and
Figure 9. Before oxidation, many pits could be seen on the surfaces, and were concentrated at the corners and edges. These defects mainly appeared during the repetitive high-speed cutting operation, with even some bare WC-Co surfaces exposed to the local loss of coatings (
Figure 3a). Stress was another main reason for the pits and micro-cracks. When cutting metals or alloys, the temperature in the inserts increased, then stress was generated for the different coefficients of thermal expansion (
Table 2 [
22]).
After oxidation for 60 min, more pits were found on the surface (
Figure 8b). The outermost coating became uneven and porous. Furthermore, many cracks were presented and could be seen clearly in the magnified image on the left. The stress that appeared when isothermally heated at 900 °C was the main reason for more defects. Defects provided fast paths for the element diffusion. Oxygen diffused to the inner TiCN coating and inside the substrate through cracks and pits. Meanwhile, the elements inside were out-diffused. Some white oxide particles were piled up on the surface along the cracks. Some cracks were even healed by the white particles. After oxidation for 300 min, more and deeper cracks could be found. The widest crack exceeded 3 μm (
Figure 8b). As shown in the EDS results (
Figure 8c,d), the elements W was tested in the white particles and implies that W out-diffused to the surface.
Figure 9 shows the cross-section images and EDS results forth fragmented samples. Most coatings remained compact and protective after oxidation for 10 min (
Figure 9a). After a mere 30 min of oxidation at 900 °C, the inner and outermost layers obviously expanded, especially for the inner coating where the thickness increased by 24.58% (
Figure 9b). A number of pores and voids could be seen. Lofaj [
19] suggested the presence of oxides and volatile gas as the main reason for the extensive swelling. As shown in Reactions (1) and (2), the TiN and TiCN layers were transformed to porous TiO
2, accompanying the formation of volatile gas N
2 and CO
2. The volume expansion and growth of defects were caused and promoted by the presence of volatile gas as well as the volatile tungsten oxides [
19,
23]. As a consequence, the oxide particles in the porous inner and outermost layers were weakly bonded with each other, so that they were easily separated.
As the coating became un-protective, more oxygen moved into the inner TiCN coating and WC-Co substrate rapidly through these defects. The adhesion between the coatings became poor. As shown in
Figure 9c, after a holding time of 300 min, the coating was highly cracked. A fissure with a maximum width exceeding 3 μm could be observed lying between the oxide layers. This indicated poor interfacial adhesion, which meant that the coating was easily broken down, resulting in the exfoliation of the coatings, as shown in
Figure 5 and
Figure 9d. Therefore, poor interfacial adhesion and defects are the main reasons for the breakdown of coatings [
24].
Figure 9e shows the oxides when the powder sample was oxidized for 300 min. From the EDS result, there no C element could be found, only W, Co, and O were detected, indicating that the hardmetal was fully oxidized, and the stripped coating can be clearly seen in
Figure 9d. This was in good agreement with the result in
Figure 7. The coatings were distributed unevenly in the final oxides.
As seen in the EDS results of the square area in
Figure 9c, the coating included three distinct layers. The outermost and inner layers were rich in titanium and oxygen. It confirmed the fact that both TiN and TiCN transformed to the TiO
2 phase when the holding time was extended to 300 min. Although part of the area was hidden, Al could be seen to be concentrated in the middle layer, where there was an O element enrichment area. This indicates that the chemical phase Al
2O
3 remained, which was confirmed by the XRD pattern of the coatings. The dense Al
2O
3 coating acted as a barrier layer and the elements could hardly pass through it [
20]. The outward diffusion of tungsten and the inward diffusion of oxygen mainly went through the defects, which are vulnerable, providing the most preferential and fast diffusion paths. Therefore, it can be seen that tungsten was distributed evenly in the outermost layers (
Figure 9c) and on the surface of the samples (
Figure 8c,d). This was confirmed by the WO
3 peaks in the XRD results of the yellow coating in
Figure 6a. However, only a little white WO
3 could be observed, ascribing to a much more rapid in-diffusion of oxygen by gas phase diffusion as compared with the out-diffusion of W by solid state diffusion [
25].