1. Introduction
Since the first scientific on the mechanical exfoliation of graphite into graphene monolayers by scotch-tape, several interesting studies have been published on graphene [
1,
2,
3]. Graphene has become very popular research material during the last few years [
2]. This method (scotch-tape approach) of obtaining graphene is inefficient for large-scale production [
4]; therefore, many efforts have been undertaken to find a better way to get graphene monolayers. Promising large-scale methods of graphene fabricate are bottom-up methods (especially epitaxially growth on SiC crystal, chemical vapour deposition (CVD)), top-down methods, such as chemical, electrochemical, plasma-assisted, and mechanical exfoliation [
5]. These methods mainly differ in the quality and efficiency of the obtained graphenes.
Bottom-up methods allow for the production of wafer graphene, which covers the demand of the electronic industry. The high quality of graphene monolayer shows the high electrical conductivity and transparency of this material. Quality of graphene produced by bottom-up approach shows well-builded one-layer structure without atomic defects [
6]. The method of manufacturing this type of graphene was developed and patented by Institute of Electronic Materials Technology in Warsaw, Poland [
7], and it is process of implementing at the industry scale. The main applications of these graphenes are focused to apply in displays, solar cells [
8], integrated circuits elements (transistors [
9]), and many other electronic devices.
Top-down approach of obtaining graphene is based on graphite as a raw material. In this way, graphite is exfoliated as the result of breaking inter-plane π-π interactions of stacked graphene in graphite crystal. Exfoliation can be carried in a mechanical, chemical, electrochemical, and plasma assisted way. According to top-down production methods, the obtained graphenes can differ in chemical and physical properties. The main differences are in crystal defects, various multi-layer stacked structure, polydispersity of basal plane, and thickness. The monolayer structure of top-down graphene can be folded and sharp-edged. We can also add function groups by various chemical modifications. These modifications have a strong effect on the electrical, mechanical, and optical properties of graphene obtained by top-down ways.
Top-down graphene is promising nanofiller for segmented polyurethane matrix. It can be introduced to matrix via in-situ polymerization, solvent-methods and during extrusion [
10,
11,
12], or injection molding [
8]. The addition of graphene allows for meeting the increasing requirements put in many applications. Besides the improvement of mechanical properties [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26] and thermal stability [
10,
16,
27,
28], the modification of segmented polyurethanes by graphene can improve shape memory effect [
15,
21,
24,
25,
29,
30,
31], gas-barrier properties [
19], and electrical conductivity [
16,
18,
19,
23,
32]. Graphene-polyurethane nanocomposites can be used as a selective membrane in filtration [
33], piezoelectric sensors [
34,
35], actuators [
30,
36], or self-healing materials [
20,
37,
38].
Elastomeric materials can be successfully modified with many nanofillers [
39], especially clays, CNT [
40], or graphene derivatives [
41,
42]. These different nano-modificators could cause high improvement in the properties (at low addition, up to 5%) of the polyurethane (PU) matrix because of the interactions between polyurethane chains and fillers at the nano-scale levels. Also, specific geometry (one-dimensional (1D), two-dimensional (2D), and three-dimensional (3D)) of these nanofillers [
43] influence on the mechanical [
44], thermal [
44], or barrier properties [
19] of polyurethane nanocomposites. Simply unmodified nanofillers have limited use, because of the compatibility issues of the polyurethane chains of hard and soft segments with the nano scale fillers. Therefore, the useful list of nanofillers include some “defected” form of graphene, modified CNT, and organically modified clays. The most important improvement of polyurethane matrix was achieved by using OMMT, CNT, or graphene derivatives, which are widely investigated and presented into
Table 1.
Segmented polyurethanes contain flexible and rigid chains alternately in the polymer back bone. The precursor of soft flexible segments is polymeric macromolecules with hydroxyl-end groups, while rigid segments are obtained because of reaction diisocyanate with low-molecular diol or diamine. Due to the chemical incompatibility between the two types of segments (soft and hard), segmented polyurethanes are phase separated on a nanoscale. This phenomenon leads to the creation hard and soft domains, in which hard domains (HS) are rich in rigid segments and soft domains (SS) are rich in flexible chains. Hard domains are responsible for mechanical strength and hardness, and on the other hand, soft domains provide elasticity [
53]. Furthermore, hard domains act as a physical cross-linking by the so-called reversible cross-links through hydrogen bonding interactions. Therefore, these materials are excellent thermoplastic elastomers (TPUs), which allow them to be processed using thermoplastic machinery such as extrusion and injection molding for techniques. The soft segments provide elastic properties to behave like a rubber at ambient conditions.
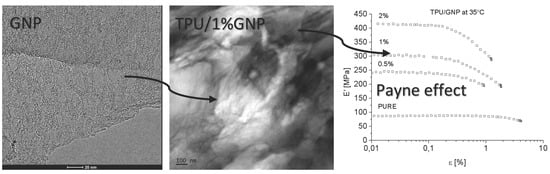
The aim of this study is to explore the influence of graphene-like nano structured carbon fillers, RGO (reduced graphene oxide), and GNP (graphene nanoplatelets) on the morphology and proprieties of segmented polyurethane thermoplastic elastomers. The filler dispersion, filler orientation and filler-filler networking (Payne effect) have been carefully studied and compared. Morphology-property correlation has been established. Techniques such as high-resolution transmission electron microscopy (HRTEM), DSC, TGA, XRD, Fourier Transformation Infrared Spectroscopy (FTIR), and UTM have been employed to characterize the morphology and proprieties of the system. Theoretical models have been used to fit the experimental data.
2. Experimental Section
2.1. Chemicals and Materials
In the following research graphite flakes, Micro 850 (99% at. C, Asbury Carbons, Asbury, NJ, USA) was used as a precursor to obtain Graphite Oxide (GO). Also, potassium permanganate (KMnO4, Stanlab, >99%), hydrogen peroxide (H2O2, 30% aq.), hydrochloric acid (H2SO4, 35–38% aq.), phosphoric acid (H3PO4, 85%), sulfuric acid (H2SO4, 96%), ethanol (C2H5OH, 96%), and diethyl ether ((C2H5)2O), all from POCh S.A., Poland, were used in GO synthesis. Compressed argon (Ar, >99% mol.) was used in thermal reduction of GO to reduced graphene oxide (RGO). Graphene platelets (GNP) were supplied by ACS Materials (Advanced Chemicals Supplier, Medford, OR, USA). Thermoplastic polyester urethane (TPU) 52DE55 (Applicazioni Plastische Industriali S.P.A, Mussolente, Italy) was used as a nanocomposite polymer matrix. N,N-Dimethylmethanamide (DMF) ((HCON(CH3)2, 99%, POCh S.A., Gliwice, Poland) was used as a solvent in composite preparation.
2.2. Preparation of RGO
Reduced graphene oxide (RGO) was obtained in two-step synthesis. On the first step, graphite oxide (GO) was obtained using modified Hummer’s method described in the following article [
54]. On the second step, GO was thermally reduced to RGO at a temperature of 200 °C and normal pressure for 15 min within argon. The efficiency of reduction was 30%.
2.3. Nanocomposites Preparation
Nanocomposites were prepared by the solvent blending method. TPU was dissolved by adding it to DMF at temperature of 80 °C with continuously stirring, the final concentration of TPU/DMF solutions was 25% by weight. Next, the proper amounts of nanofillers was added to prepared solution and was homogenized by high-shear mixer (Omni Macro Homogenizer, 35 mm mixing head diameter) with 2500 rpm for 30 min. Obtained polymer nanocomposite solutions, with good quality of dispersion, were poured onto Petri pans and the DMF was fully evaporated at 70 °C for 72 h. The obtained nanocomposites were compression molded (under a pressure of 1 ton) to thin film with 0.7 mm thickness at the temperature of 195 °C. Press molded films (
Table 2) was used later for characterization.
3. Characterization
3.1. FTIR
Fourier Transformation Infrared Spectroscopy of polyurethane materials has been carried using ThermoElectron Corporation (Waltham, MA, USA) Nicolet 8700 Spectrometer in the Attenuated Total Reflectance (ATR, Gold State II) mode in the range of 500–4000 cm−1. The measurement was carried in standard conditions (normal pressure and at room temperature).
3.2. XRD
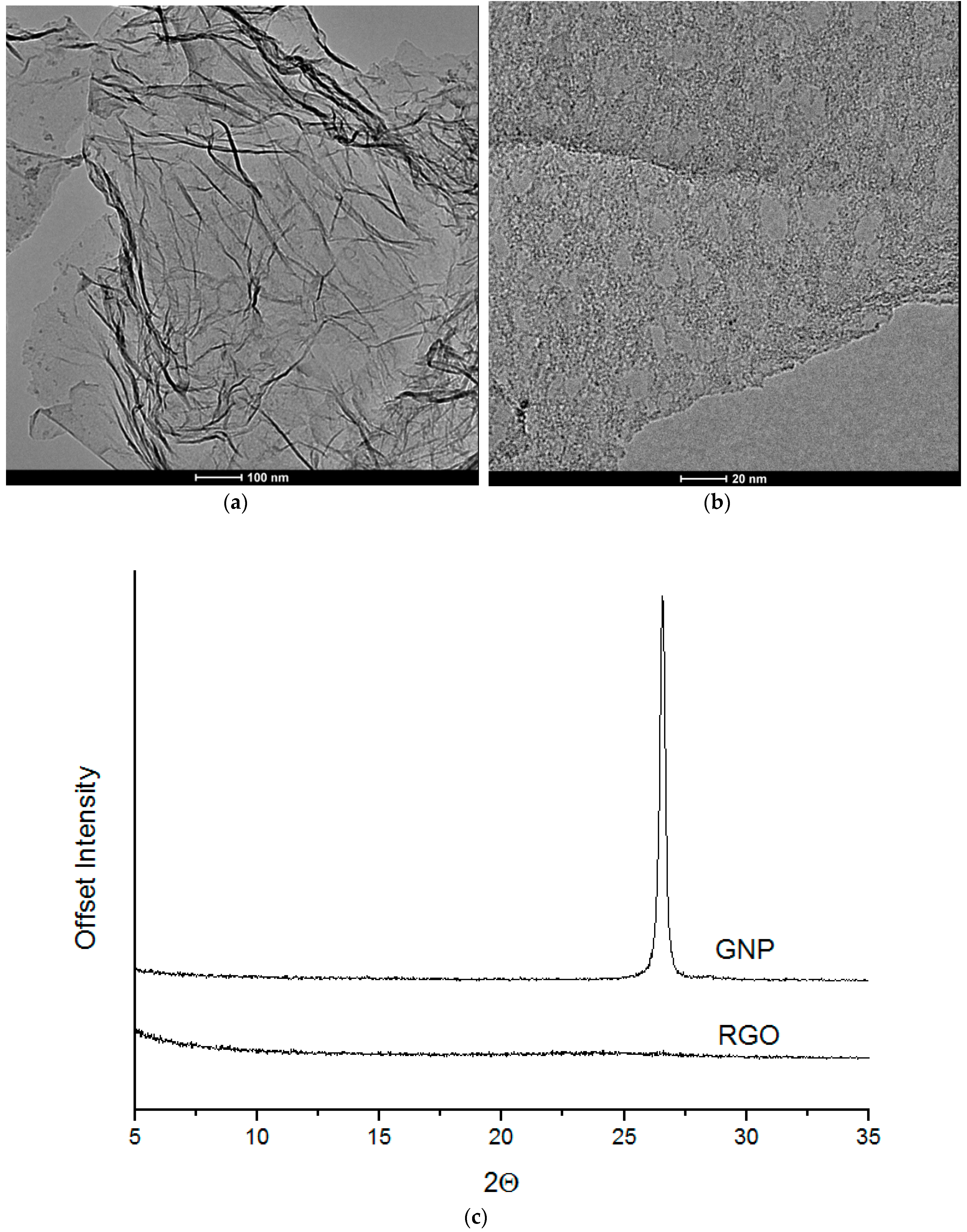
Wide-angle X-ray scattering measurements of nanofillers and polyurethane composites were performed using a Bragg–Brentano X’PERT PHILIPS diffractometer (Malvern Panalytical B. V., Almelo, Netherlands), equipped with a Cu Anode X-ray tube and diffracted beam monochromator (40 kV, 30 mA, λ Cu Kα = 0.1542 nm). TPU samples were scanned in 2θ range from 5° to 35°.
3.3. TEM
Microscopy analysis (for nanofiller GNP and RGO) was performed by means of STEM-EDX technique using Transmission Electron Microscope FEI Europe (Eindhoven, The Netherlands), Tecnai F20 X-Twin coupled with EDX Spectrometer (Tecnai, Hillsboro, Oregon, USA) (samples were cut in cryo-mode using ultramicrotome). HRTEM (for nanocomposite systems) is from JEOL (Tokyo, Japan), model JEM-2100. The samples were sliced with LEICA EM UC7 Ultra Microtome with cryo-mode (EM FC7, LEICA, Wiesral, Germany) using glass knife. The thickness of the samples was 150 nm and the slices were deposited on a 200 mesh Cu Grid.
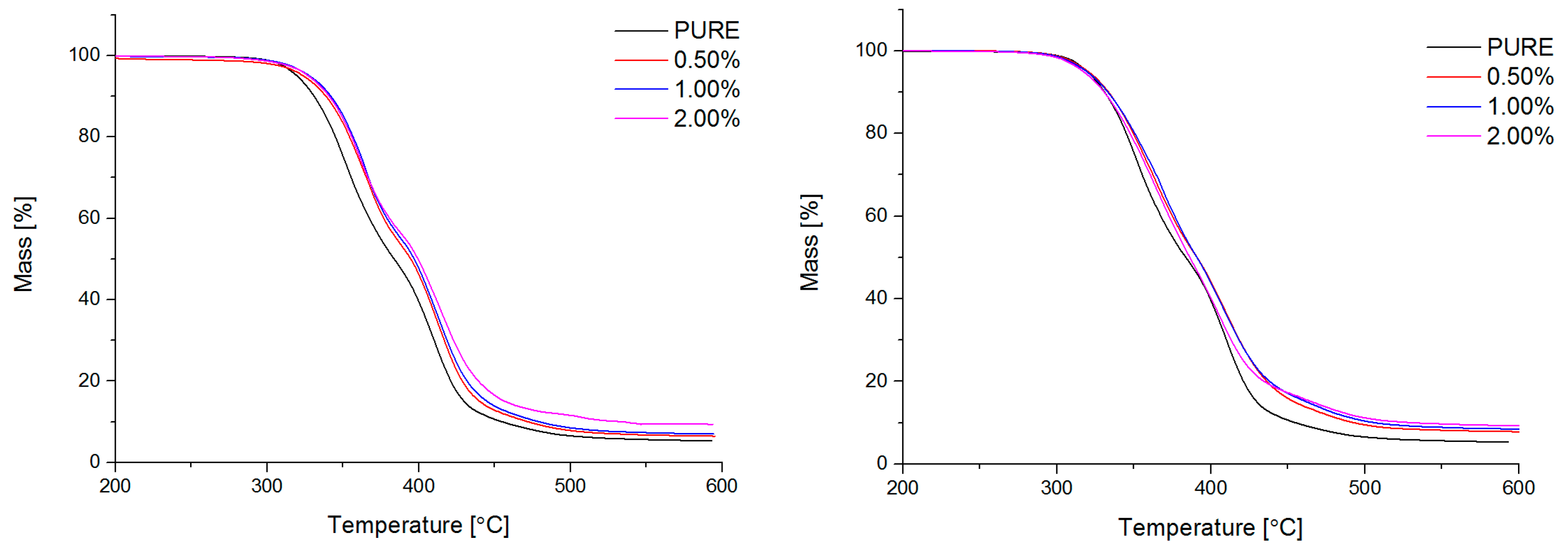
3.4. TG
Thermogravimetry (TG; (International Organization for Standardization) ISO 11358) of obtained materials was conducted by Netzsch TG209F3 TG analyzer (NETZSCH, Selb, Germany). Degradation process has been performed in the temperature range from 35 °C to 700 °C, nitrogen atmosphere with gas flow rate 40 mL/min, and heating rate 20 °C/min.
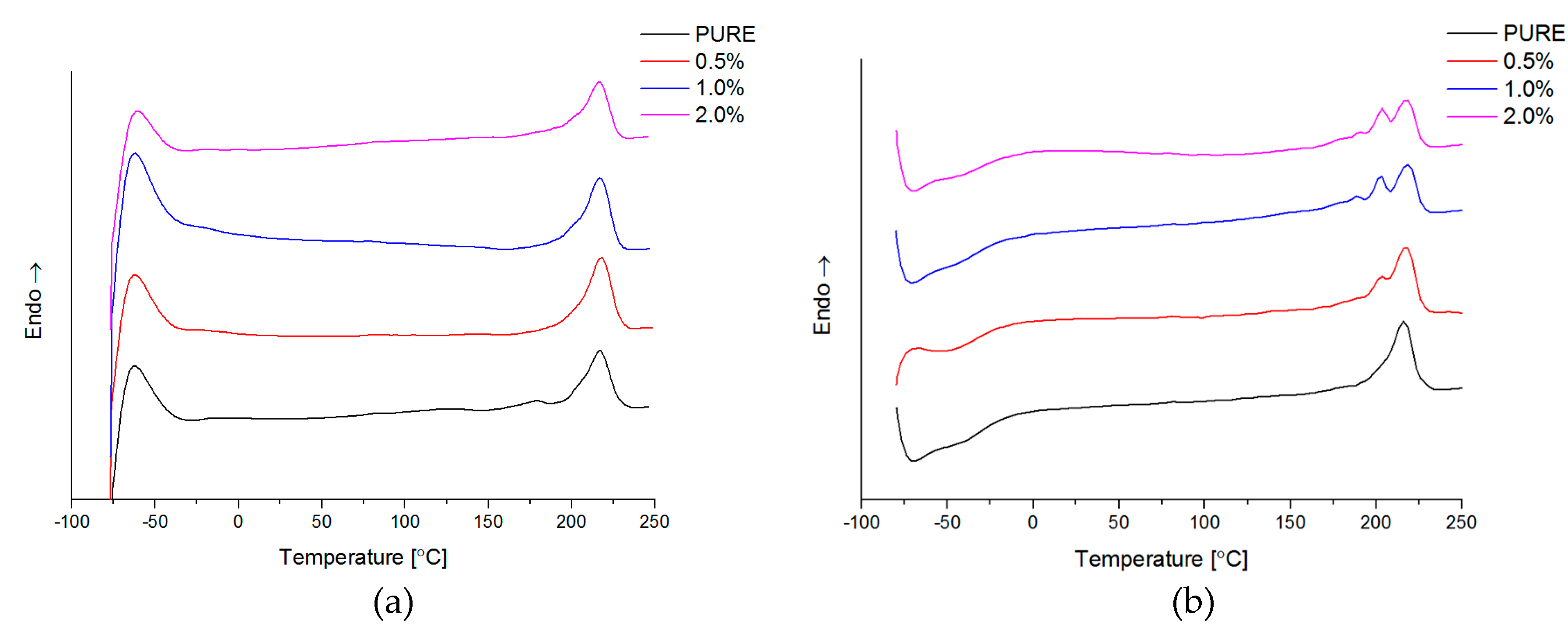
3.5. DSC
Differential Scanning Calorimetry (DSC; ISO 11357-1, 11357-3) measurements have been carried by Netzsch DSC209F1 instrument (NETZSCH, Selb, Germany) in the temperature range from −85 °C to 250 °C. The samples weight was about 10 mg, and analysis was conducted in nitrogen atmosphere with gas flow 40 mL/min. The samples were pre-heated (1st-heating) to eliminate thermal history, then cooling and 2nd-heating at rate 10 °C/min.
3.6. Mechanical Properties (Dynamic and Static Mode)
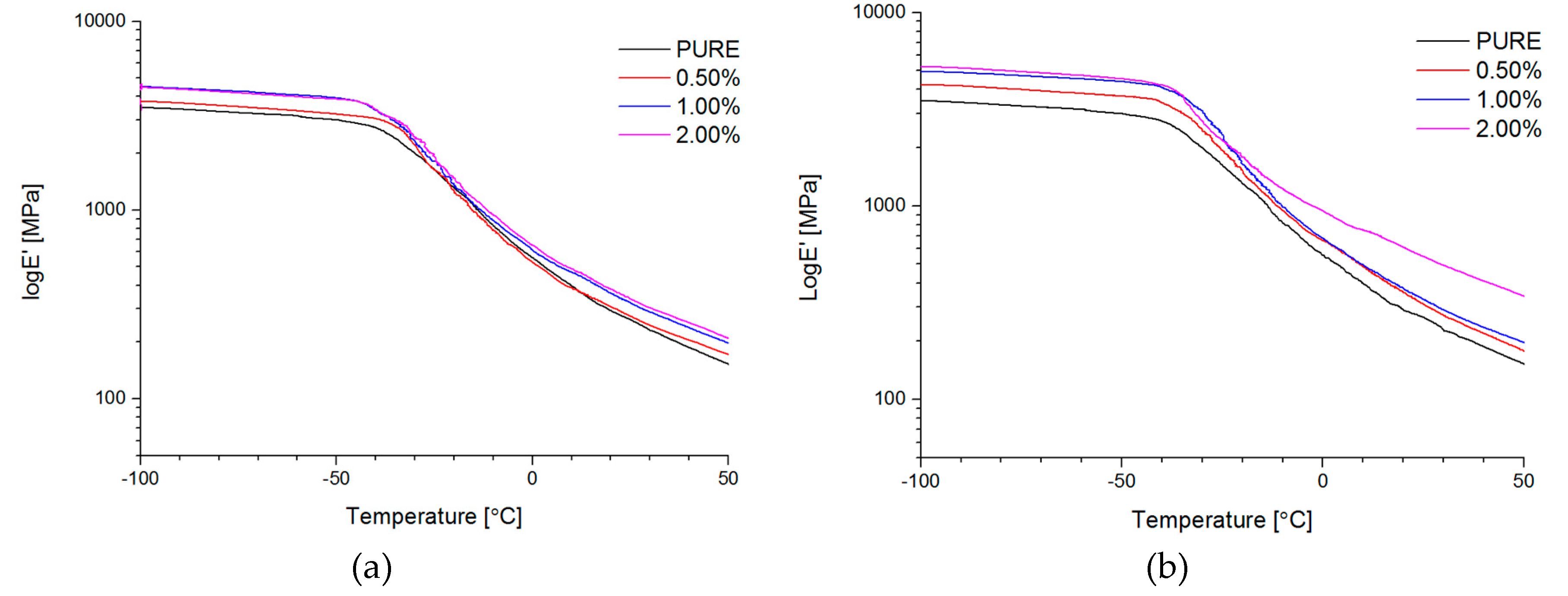
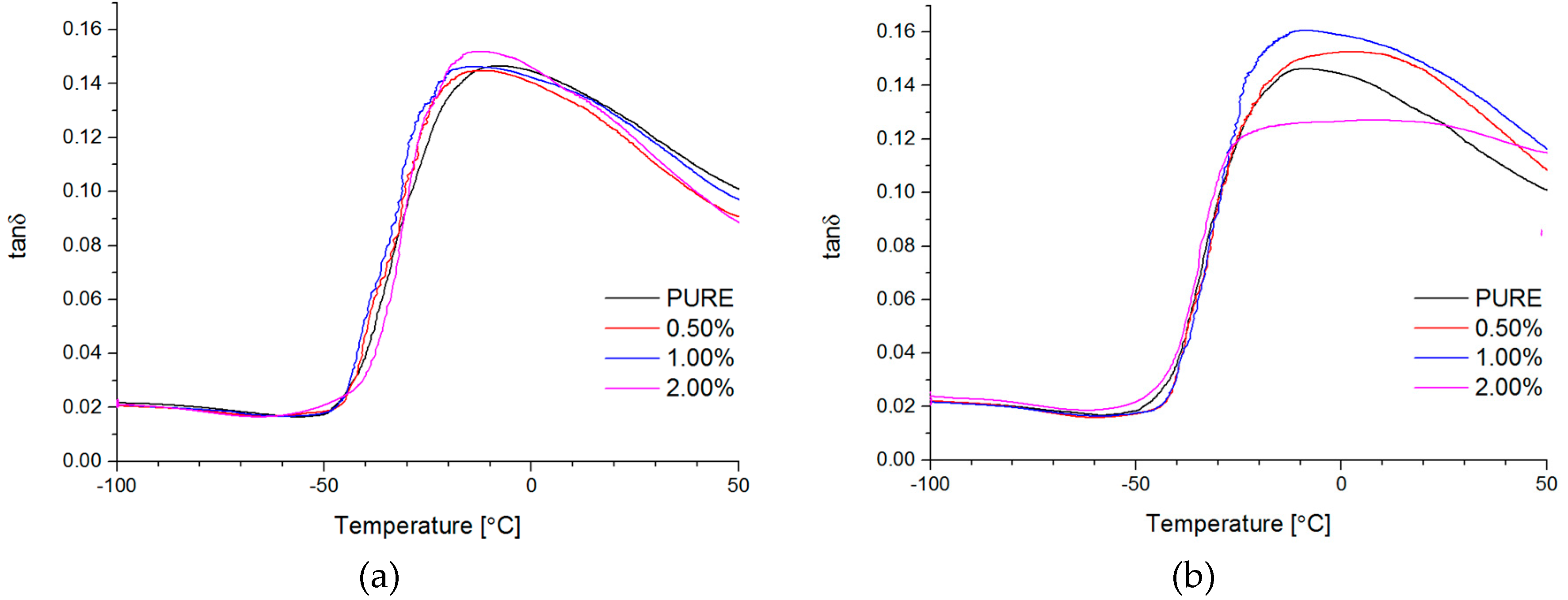
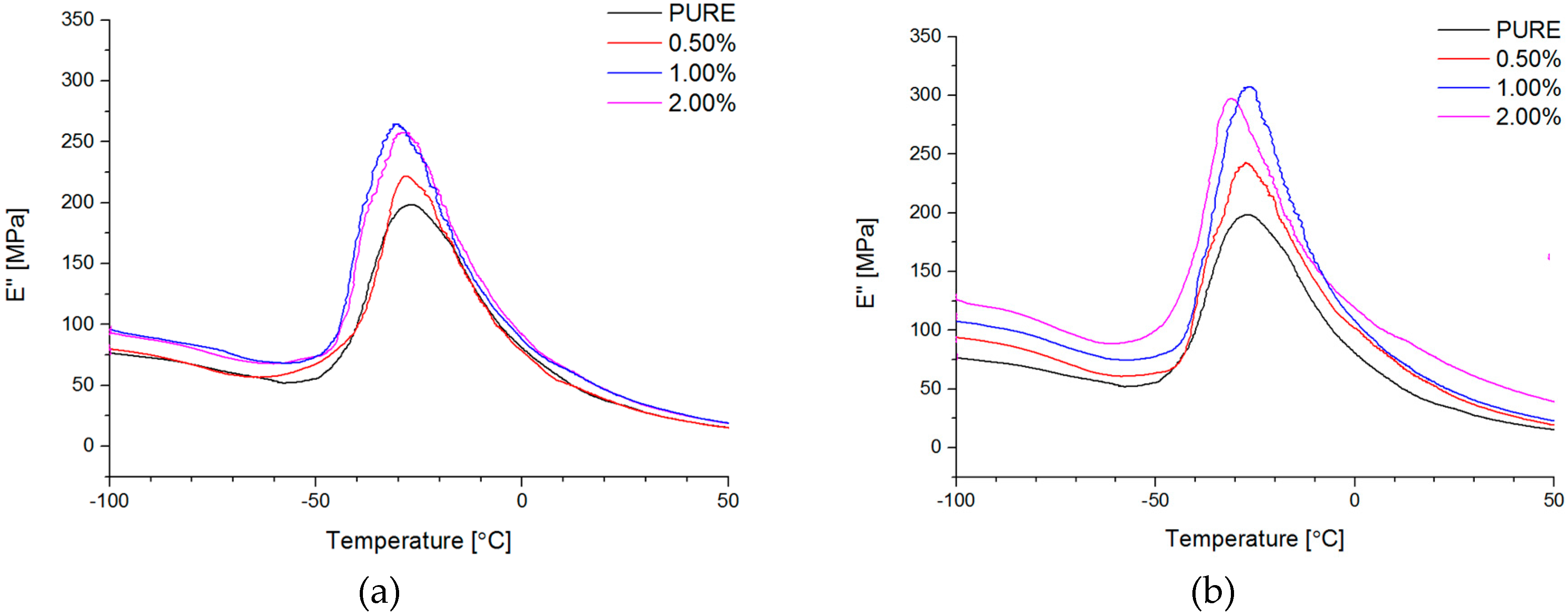
Dynamical Mechanical Analysis (DMA; (American Society for Testing and Materials) ASTM D4065, D7028-07) has been carried by TA Instruments DMA Q800 analyzer (DMA, West Kentucky, PA, USA). Temperature investigation mode has been performed in uniaxial tension mode, in the temperature range −100–100 °C, 1Hz frequency amplitude of deformation 20 µm and heating rate 4 °C/min using liquid nitrogen as cooling medium. Strain sweep analysis for Payne effect investigation has been conducted in three different temperatures (35 °C, 55 °C, and 75 °C), in the same tension mode and frequency of deformation, but in the amplitude range from 0.25 to 170 µm. The dimensions of thin samples were 5.0 × 5.0 × 0.7 mm3. Tensile (in static mode) tests were carried out using universal testing machine (UTM) Zwick/Roell Z020 (Zwick, Kennesaw, GA, USA). All of the tests were performed according to ISO 527-1 standard.
5. Conclusions
Based on this study it was successfully obtained polyurethane nanocomposites containing reduced graphene oxide (RGO) or graphene nanoplatelets (GNP). The addition of RGO or GNP undoubtedly influenced investigated properties of the polyurethane matrix.
The authors showed the interaction behavior of the nanofillers with the polyurethane matrix by using spectroscopic and diffraction investigation. Morphology investigation confirmed good exfoliation nanoparticles into the polyurethane matrix and shows a better compatibly of RGO nanofiller to the matrix, in comparison to the GNP (where higher agglomeration of this nanofiller is observed,
Figure 4d). Phase transition investigation gives information about the effect on melting and crystallization behavior, where GNP nanofiller causes an increased crystallization temperature of the nanocomposite systems. The storage modulus (E′) of nanocomposites containing TPU/GNP materials significantly increased in comparison to the non-modified (PURE TPU) material. The modulus and thermal stability of the TPU/GNP (RGO) nanocomposites at 2 wt % nanofiller loading are higher, especially above glass transition temperature (T
g), respectively, than those of the PURE TPU material. It is seen (
Table 5;
δmax) that the addition of the nanofillers improves the mechanical strength of TPU matrix. Enhanced mechanical properties were observed when add maximum 2 wt % of nanofiller and higher tensile strength properties obtain for RGO nanofiller in comparison to the GNP modificator.
Both nanofillers, GNP and RGO, enhanced the mechanical and thermal properties of the TPU material. Furthermore, it should be highlighted that by using RGO and GNP nanofiller, the Payne effect is visible for obtained nanocomposite systems. It is demonstrated that different Payne effect behavior is visible for commercial graphene nanoplatelets (GNP) and synthesized reduced graphene oxide (RGO) nanofiller. We could observe agglomeration (strong filler/filler interaction) at higher filler loading, which was evidenced by the high Payne effect. When comparing with results in literature [
67] based on TPU matrix and the same amount of filler, nanocomposites with a good balance between thermal and mechanical properties were achieved in this study. The authors believe that these industries based materials could be easily applied in many fields, due to their enhanced properties.