Effect of Lithium Disilicate Reinforced Liner Treatment on Bond and Fracture Strengths of Bilayered Zirconia All-Ceramic Crown
Abstract
:1. Introduction
2. Results
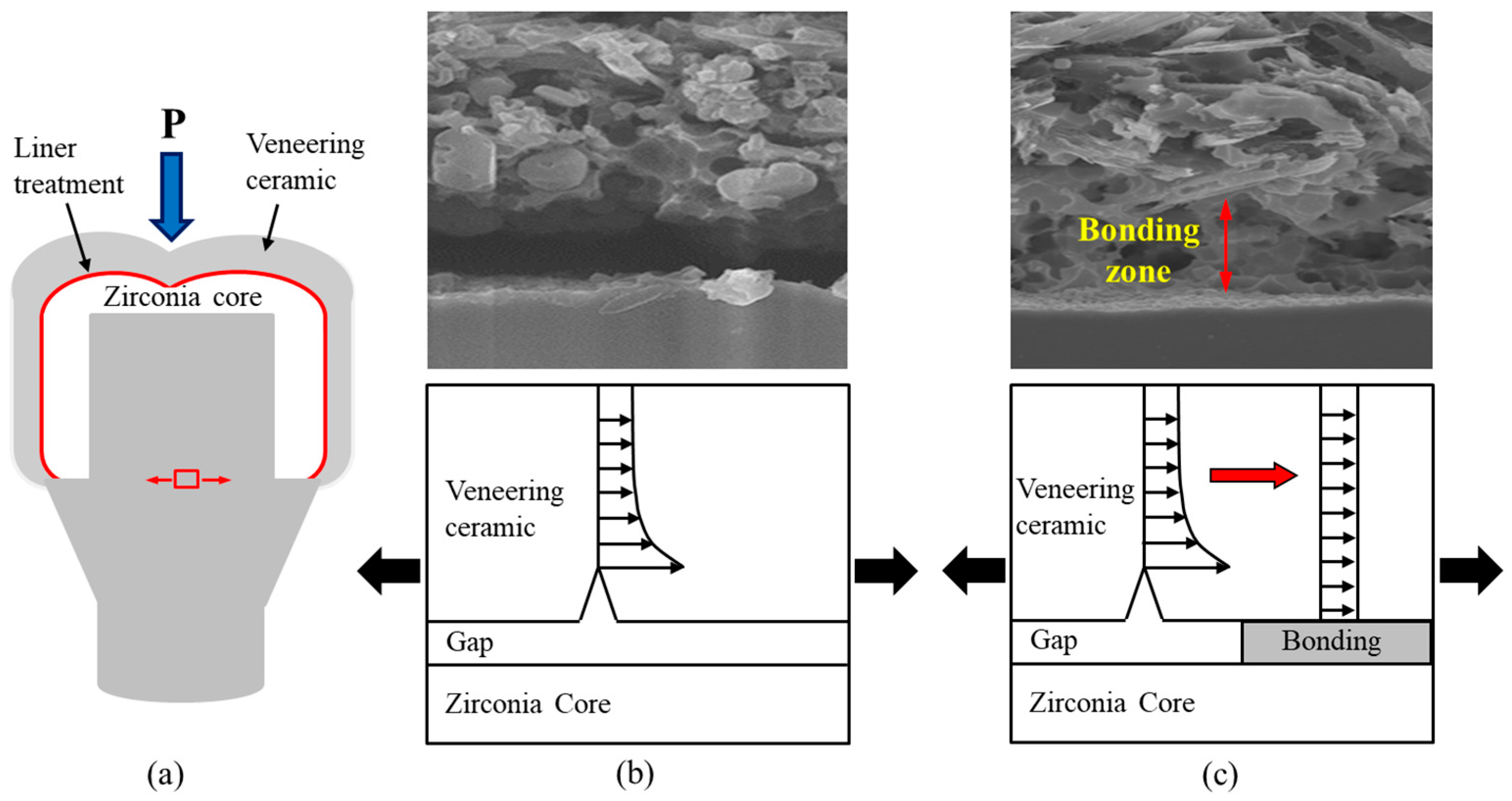
2.1. Measurement of the Bonding Strength via a Microtensile Test
2.2. Measurement of the Fracture Strength via a Compression Test
3. Discussion
4. Materials and Methods
4.1. Measurement of Bonding Strength
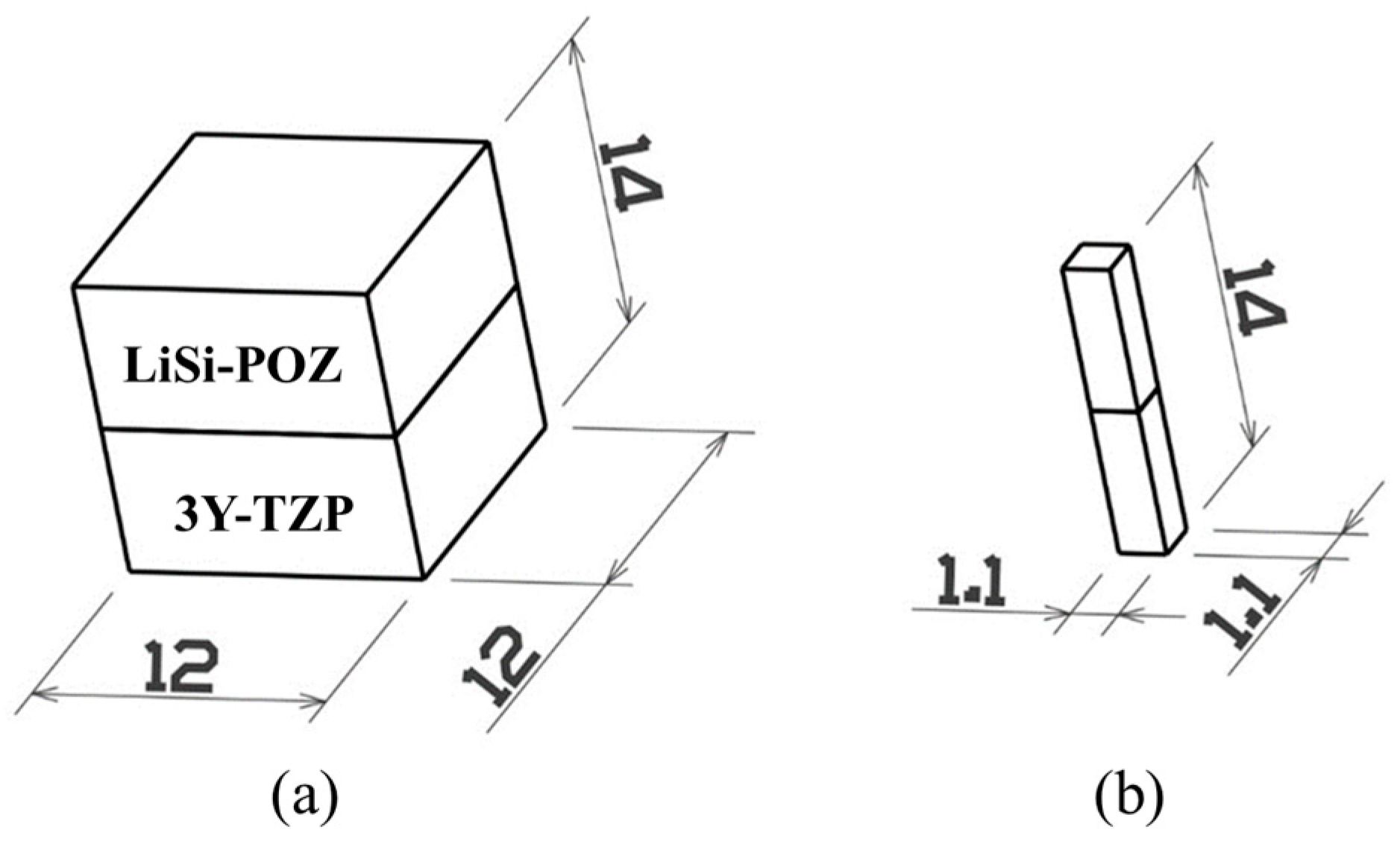
4.1.1. Preparation of Specimens
4.1.2. Microtensile Test
4.2. Fracture Test of All-Ceramic Zirconia Crown
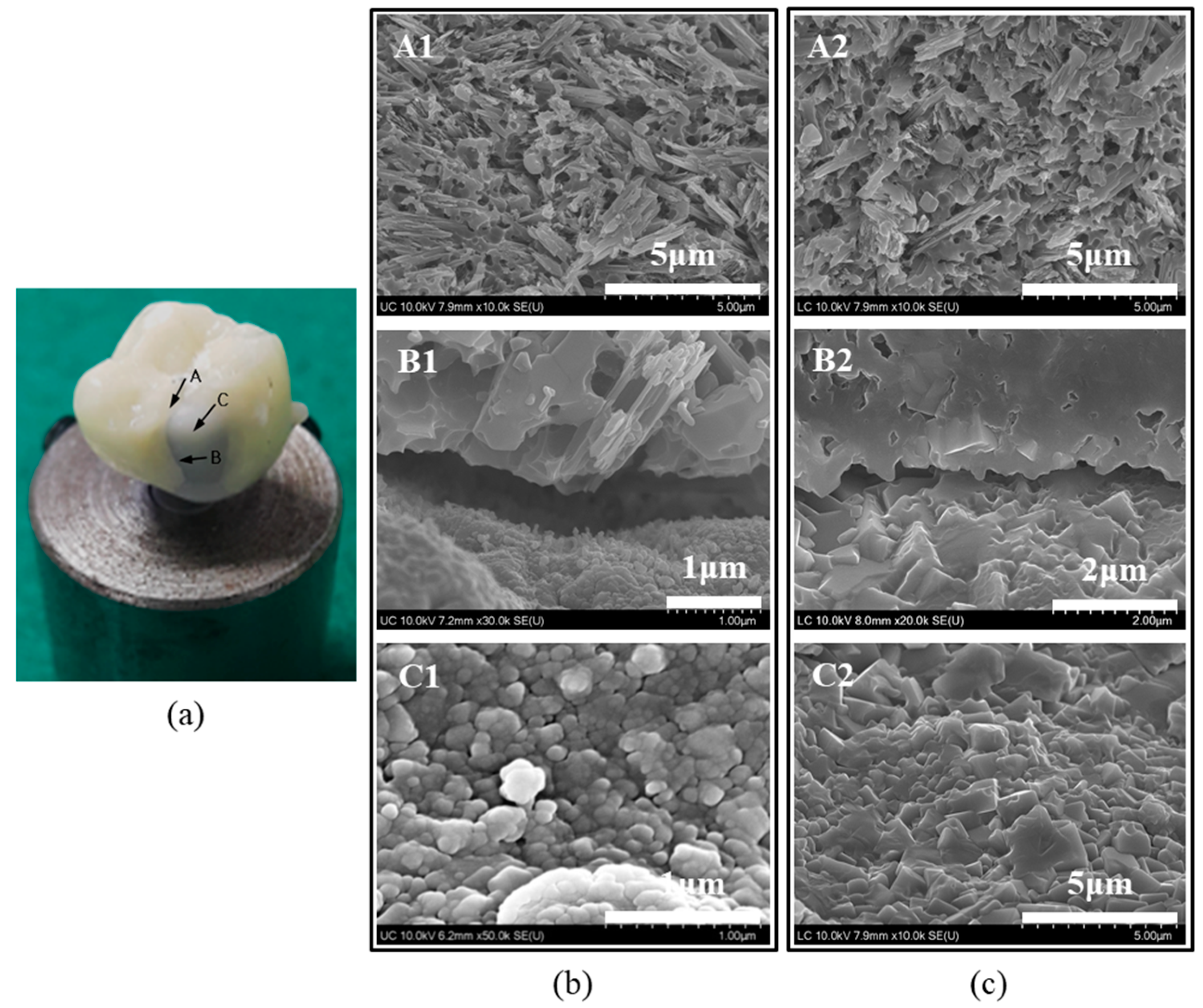
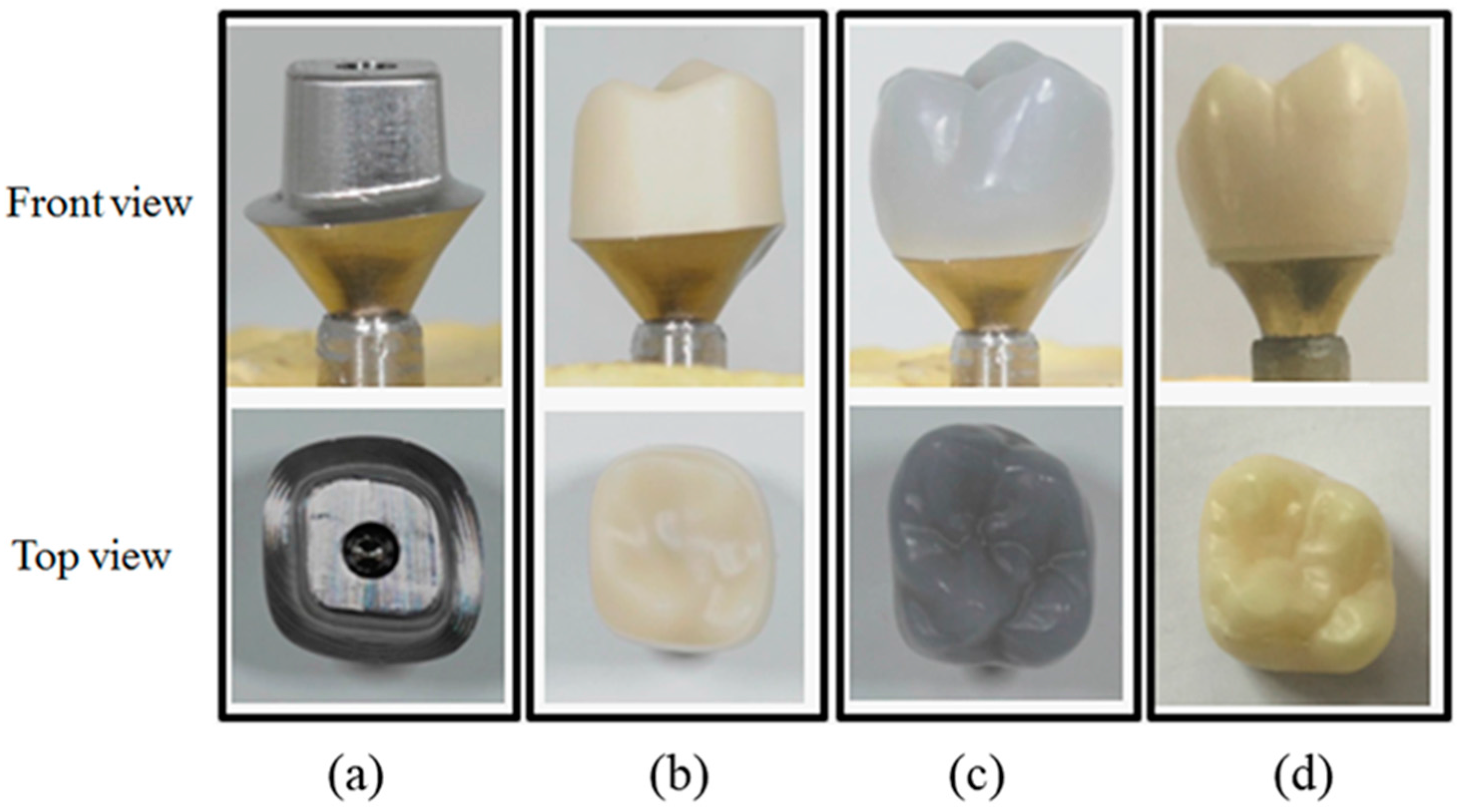
4.2.1. Preparation of Specimens
4.2.2. Cementation
4.2.3. Crown Fracture Test
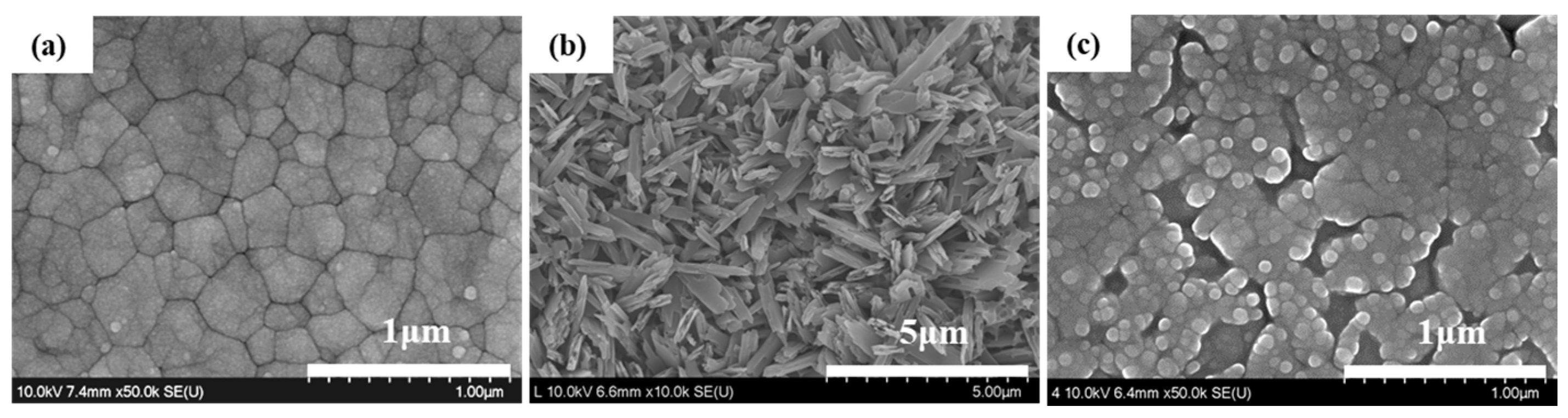
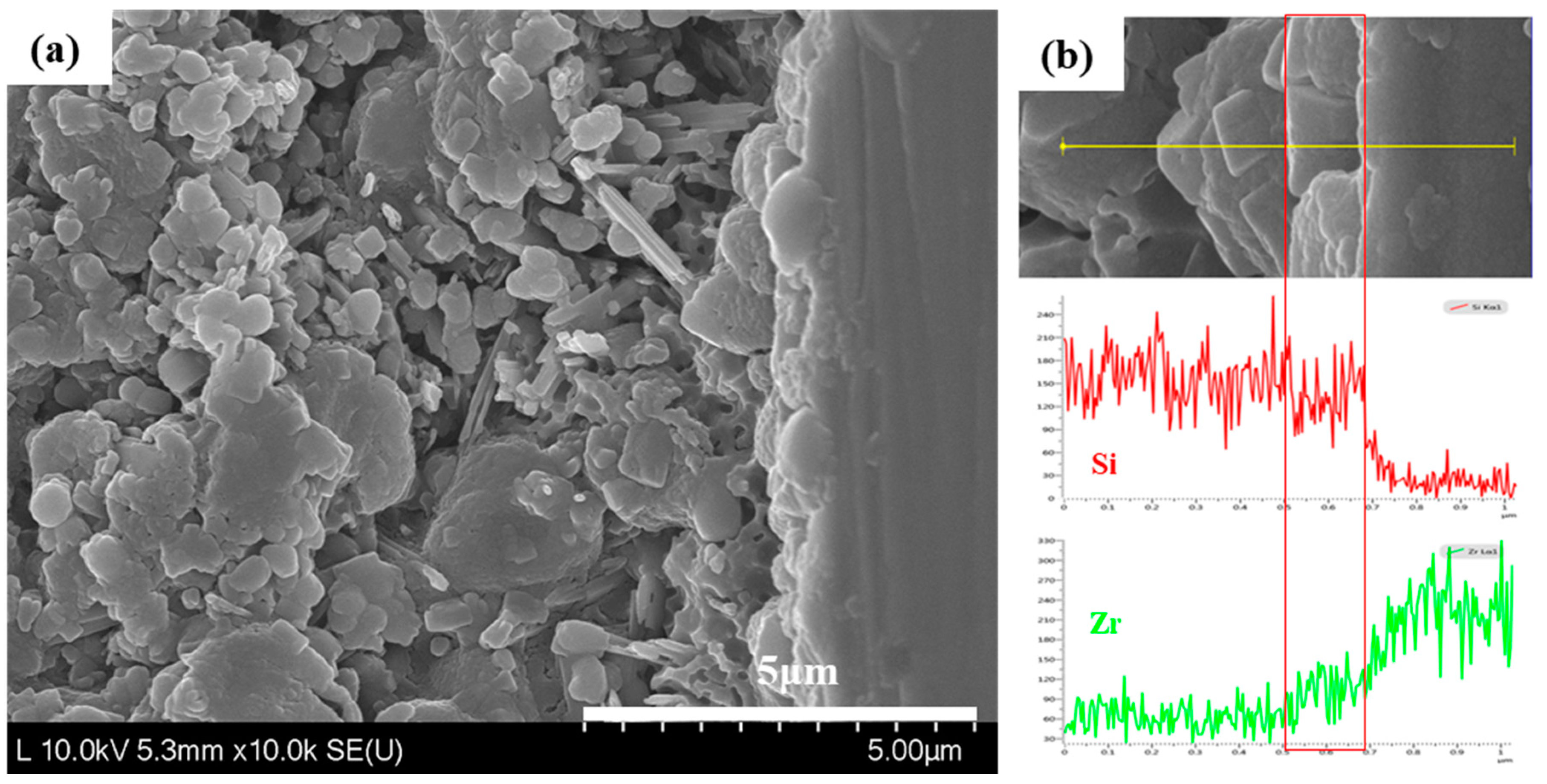
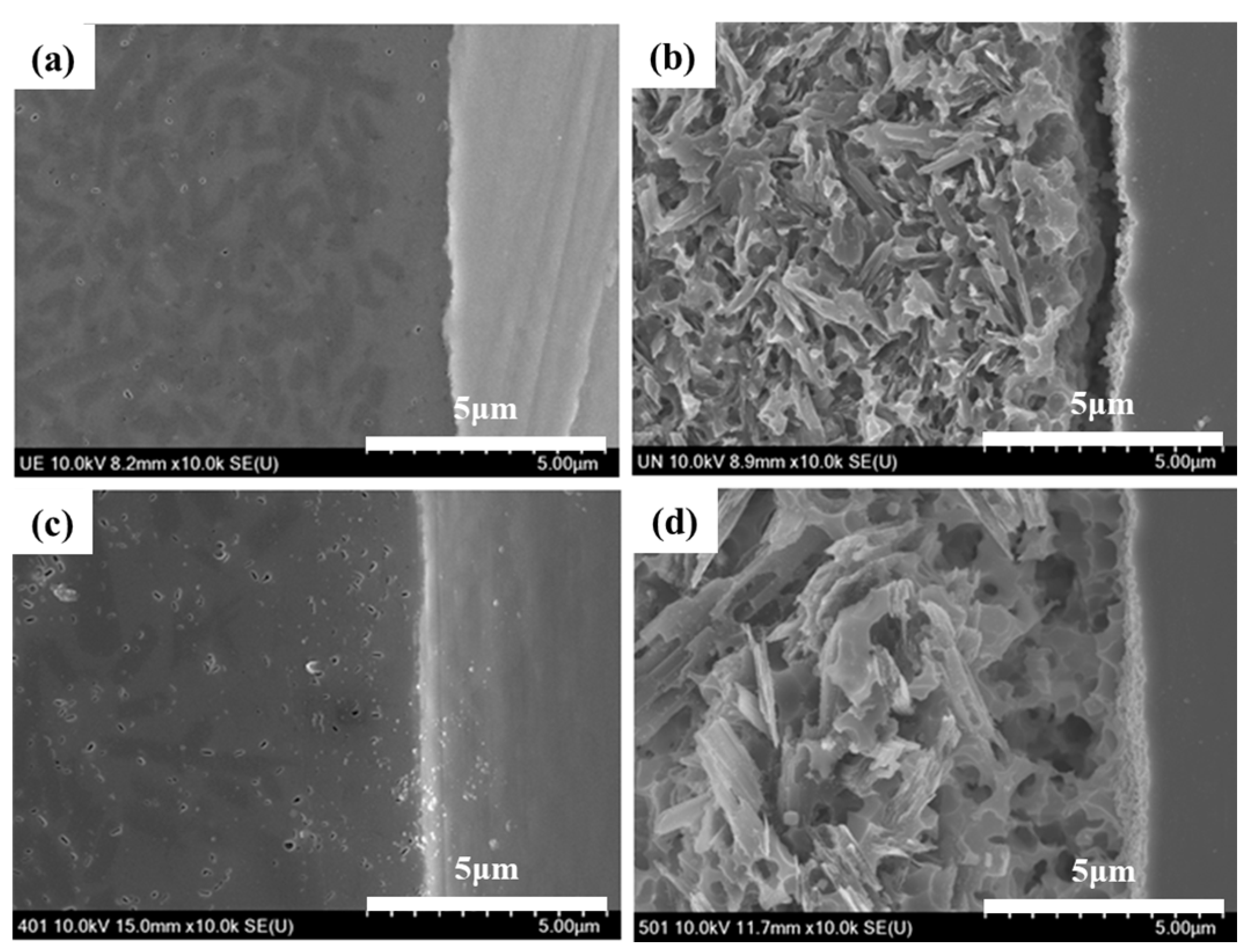
4.3. Surface Characterization
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yamamoto, M. Metal-Ceramics: Principles and Methods of Makoto Yamamoto; Quintessence Publishing Co.: Chicago, IL, USA, 1985; pp. 219–291. [Google Scholar]
- Piddock, V.; Qualtrough, A. Dental ceramics—An update. J. Dent. 1990, 18, 227–235. [Google Scholar] [CrossRef]
- Vichi, A.; Louca, C.; Corciolani, G.; Ferrari, M. Color related to ceramic and zirconia restorations: A review. Dent. Mater. 2011, 27, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Garvie, R.; Hannink, R.; Pascoe, R. Ceramic steel? Nature 1975, 258, 703–704. [Google Scholar] [CrossRef]
- Christel, P.; Meunier, A.; Heller, M.; Torre, J.; Peille, C. Mechanical properties and short-term in vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J. Biomed. Mater. Res. Part A 1989, 23, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Heuer, A. Transformation Toughening in Ceramics: Martensitic Transformations in Crack-Tip Stress Fields. J. Am. Ceram. Soc. 1980, 63, 241–248. [Google Scholar] [CrossRef]
- Messing, G.L.; Hirano, S.I.; Gauckler, L. Ceramic processing science. J. Am. Ceram. Soc. 2006, 89, 1769–1770. [Google Scholar] [CrossRef]
- Kelly, J.; Tesk, J.; Sorensen, J. Failure of all-ceramic fixed partial dentures in vitro and in vivo: Analysis and modeling. J. Dent. Res. 1995, 74, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Larsson, C.; Vult von Steyern, P.; Sunzel, B.; Nilner, K. All-ceramic two-to five-unit implant-supported reconstructions. A randomized, prospective clinical trial. Swed. Dent. J. 2006, 30, 45–53. [Google Scholar] [PubMed]
- Sundh, A.; Sjögren, G. A comparison of fracture strength of yttrium-oxide-partially-stabilized zirconia ceramic crowns with varying core thickness, shapes and veneer ceramics. J. Oral Rehabilit. 2004, 31, 682–688. [Google Scholar] [CrossRef] [PubMed]
- De Jager, N.; Pallav, P.; Feilzer, A.J. The influence of design parameters on the FEA-determined stress distribution in CAD–CAM produced all-ceramic dental crowns. Dent. Mater. 2005, 21, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M.N.; Kleverlaan, C.J.; Feilzer, A.J. Selective infiltration-etching technique for a strong and durable bond of resin cements to zirconia-based materials. J. Prosthet. Dent. 2007, 98, 379–388. [Google Scholar] [CrossRef]
- Fischer, J.; Grohmann, P.; Stawarczyk, B. Effect of zirconia surface treatments on the shear strength of zirconia/veneering ceramic composites. Dent. Mater. J. 2008, 27, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Matinlinna, J.P.; Pow, E.H. Insights into porcelain to zirconia bonding. J. Adhes. Sci. Technol. 2012, 26, 1249–1265. [Google Scholar]
- Cevik, P.; Cengiz, D.; Malkoc, M.A. Bond strength of veneering porcelain to zirconia after different surface treatments. J. Adhes. Sci. Technol. 2016, 30, 2466–2476. [Google Scholar] [CrossRef]
- Yoon, H.-I.; Yeo, I.-S.; Yi, Y.-J.; Kim, S.-H.; Lee, J.-B.; Han, J.-S. Effect of surface treatment and liner material on the adhesion between veneering ceramic and zirconia. J. Mech. Behav. Biomed. Mater. 2014, 40, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Silva, F.; Nascimento, R.; Souza, J.; Motta, F.; Carvalho, O.; Henriques, B. Shear bond strength of veneering porcelain to zirconia: Effect of surface treatment by CNC-milling and composite layer deposition on zirconia. J. Mech. Behav. Biomed. Mater. 2016, 60, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Kirmali, O.; Kapdan, A.; Kustarci, A.; Er, K. Veneer Ceramic to Y-TZP Bonding: Comparison of Different Surface Treatments. J. Prosthodont. 2016, 25, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Balkenhol, M.; Nothdurft, F.P.; Hannig, M.; Schindler, A.; Lehmann, A.; Arnold, T.; Knauber, A.; Rupf, S. Bonding to zirconia ceramic: The effect of cold plasma treatment and 4-META. Clin. Plasma Med. 2017, 5, 8–13. [Google Scholar] [CrossRef]
- Yoon, H.-I.; Yeo, I.-S.; Han, J.-S. Effect of various surface treatments on the interfacial adhesion between zirconia cores and porcelain veneers. Int. J. Adhes. Adhes. 2016, 69, 79–85. [Google Scholar] [CrossRef]
- Alghazzawi, T.F.; Janowski, G.M. Effect of liner and porcelain application on zirconia surface structure and composition. Int. J. Oral Sci. 2016, 8, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Kwon, T.-Y. The Application of a Novel Ceramic Liner Improves Bonding between Zirconia and Veneering Porcelain. Materials 2017, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Zaher, A.M.; Hochstedler, J.; Rueggeberg, F.A.; Kee, E.L. Shear bond strength of zirconia-based ceramics veneered with 2 different techniques. J. Prosthet. Dent. 2017, 118, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, E.; Sinescu, C.; Negrutiu, M.L.; Pop, D.M.; Lauer, H.-C.; Rominu, M.; Hutiu, G.; Bunoiu, M.; Duma, V.-F.; Antoniac, I. Shear bond strength tests of zirconia veneering ceramics after chipping repair. J. Adhes. Sci. Technol. 2016, 30, 666–676. [Google Scholar] [CrossRef]
- Dündar, M.; Özcan, M.; Gökçe, B.; Çömlekoğlu, E.; Leite, F.; Valandro, L.F. Comparison of two bond strength testing methodologies for bilayered all-ceramics. Dent. Mater. 2007, 23, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, S.; Bian, C.; Kong, H. Interface toughness of a zirconia-veneer system and the effect of a liner application. J. Prosthet. Dent. 2014, 112, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-W.; Kim, S.-Y.; Bae, J.-H.; Bae, E.-B.; Huh, J.-B. In vitro study of the fracture resistance of monolithic lithium disilicate, monolithic zirconia, and lithium disilicate pressed on zirconia for three-unit fixed dental prostheses. J. Adv. Prosthodont. 2017, 9, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Bakitian, F.; Seweryniak, P.; Papia, E.; Larsson, C.; Vult von Steyern, P. Fracture strength of veneered translucent zirconium dioxide crowns with different porcelain thicknesses. Acta Biomater. Odontol. Scand. 2017, 3, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Vohra, F.; Bin Ismail, I.H.; Al-Rifaiy, M.Q.; Abduljabbar, T.; Abualsaud, H.; Ali, M.; Abu Hasan, M.I. Influence of veneering technique and veneer-coping thickness on fracture toughness of implant retained veneered Y-TZP zirconia crowns. J. Adhes. Sci. Technol. 2017, 31, 1758–1767. [Google Scholar] [CrossRef]
- Zhao, K.; Pan, Y.; Guess, P.C.; Zhang, X.-P.; Swain, M.V. Influence of veneer application on fracture behavior of lithium-disilicate-based ceramic crowns. Dent. Mater. 2012, 28, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Stawarczyk, B.; Tomic, M.; Strub, J.R.; Haemmerle, C.H. Effect of thermal misfit between different veneering ceramics and zirconia frameworks on in vitro fracture load of single crowns. Dent. Mater. J. 2007, 26, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Göstemeyer, G.; Jendras, M.; Borchers, L.; Bach, F.-W.; Stiesch, M.; Kohorst, P. Effect of thermal expansion mismatch on the Y-TZP/veneer interfacial adhesion determined by strain energy release rate. J. Prosthodont. Res. 2012, 56, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M.N.; Kleverlaan, C.J.; Feilzer, A.J. Microtensile bond strength of different components of core veneered all-ceramic restorations: Part II: Zirconia veneering ceramics. Dent. Mater. 2006, 22, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M.N.; Feilzer, A.J.; de Jager, N.; Kleverlaan, C.J. Prestresses in bilayered all-ceramic restorations. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 139–145. [Google Scholar] [CrossRef] [PubMed]




| Group | Untreated | Liner-Treated | |
|---|---|---|---|
| Parameter | |||
| m | 2.87 | 4.68 | |
| σo | 2.45 | 3.77 | |
| r2 | 0.86 | 0.91 | |
| σf(avg) ± SD | 2.18 ± 0.85 | 3.45 ± 0.80 | |
| N | 15 | 15 | |
| Veneering Materials | ST (°C) | DT (min) | TRI (°C/min) | FT (°C) | V1 (°C) | V2 (°C) | HT (min) |
|---|---|---|---|---|---|---|---|
| Amber LiSi-POZ | 650 | 1 | 60 | 925 | 650 | 925 | 20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, Y.-S.; Noh, H.-R.; Lee, M.-H.; Lim, M.-J.; Bae, T.-S. Effect of Lithium Disilicate Reinforced Liner Treatment on Bond and Fracture Strengths of Bilayered Zirconia All-Ceramic Crown. Materials 2018, 11, 77. https://doi.org/10.3390/ma11010077
Jang Y-S, Noh H-R, Lee M-H, Lim M-J, Bae T-S. Effect of Lithium Disilicate Reinforced Liner Treatment on Bond and Fracture Strengths of Bilayered Zirconia All-Ceramic Crown. Materials. 2018; 11(1):77. https://doi.org/10.3390/ma11010077
Chicago/Turabian StyleJang, Yong-Seok, Hyeong-Rok Noh, Min-Ho Lee, Myung-Jin Lim, and Tae-Sung Bae. 2018. "Effect of Lithium Disilicate Reinforced Liner Treatment on Bond and Fracture Strengths of Bilayered Zirconia All-Ceramic Crown" Materials 11, no. 1: 77. https://doi.org/10.3390/ma11010077




