Formation Mechanism and Dispersion of Pseudo-Tetragonal BaTiO3-PVP Nanoparticles from Different Titanium Precursors: TiCl4 and TiO2
Abstract
:1. Introduction
2. Results and Discussion
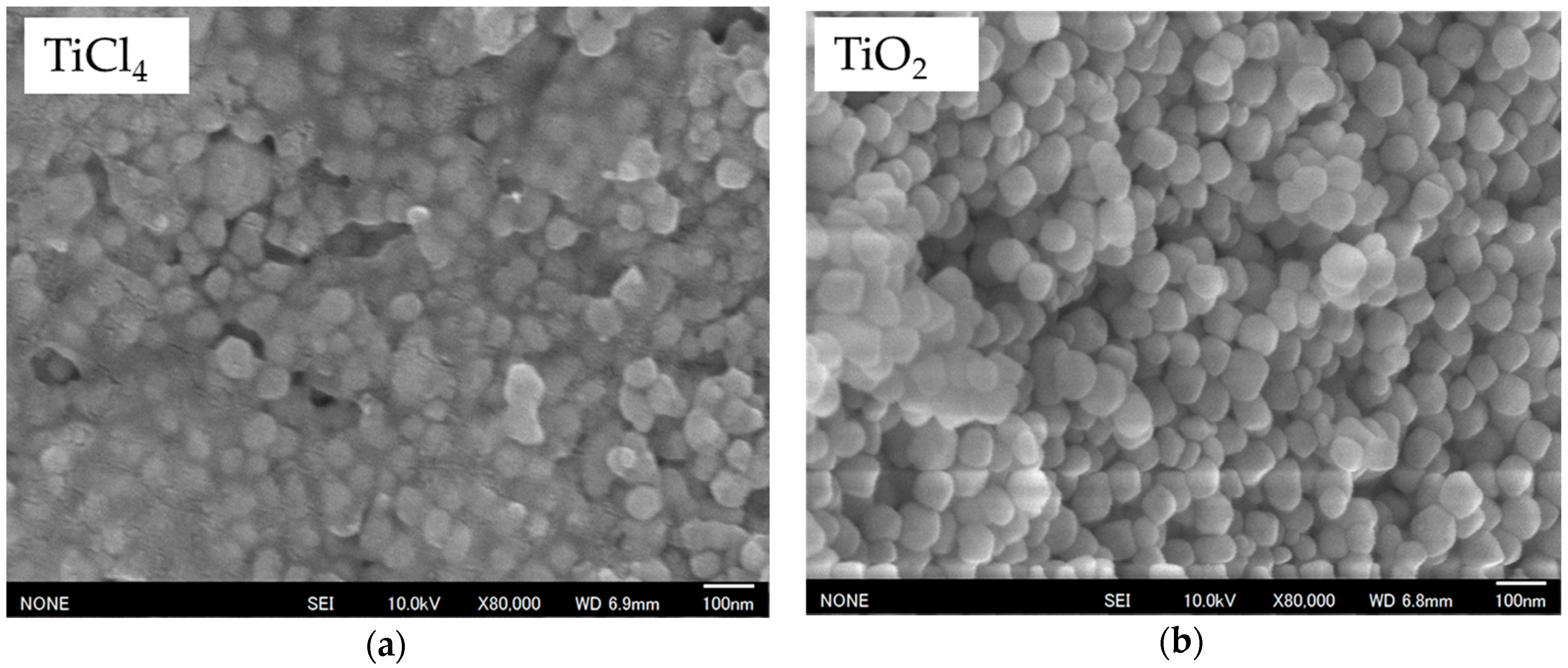
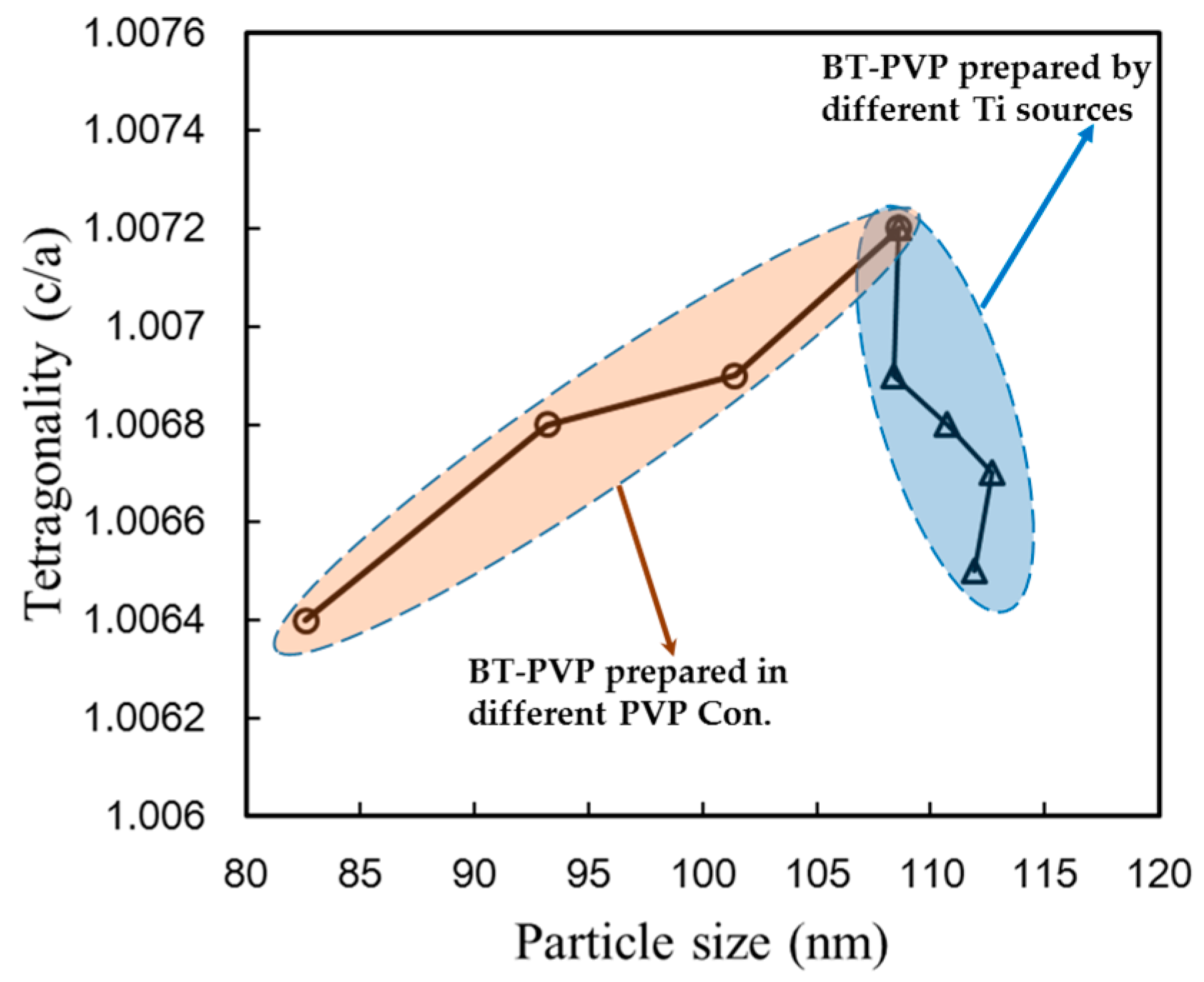
2.1. Enhanced Tetragonality by TiO2 Substitution
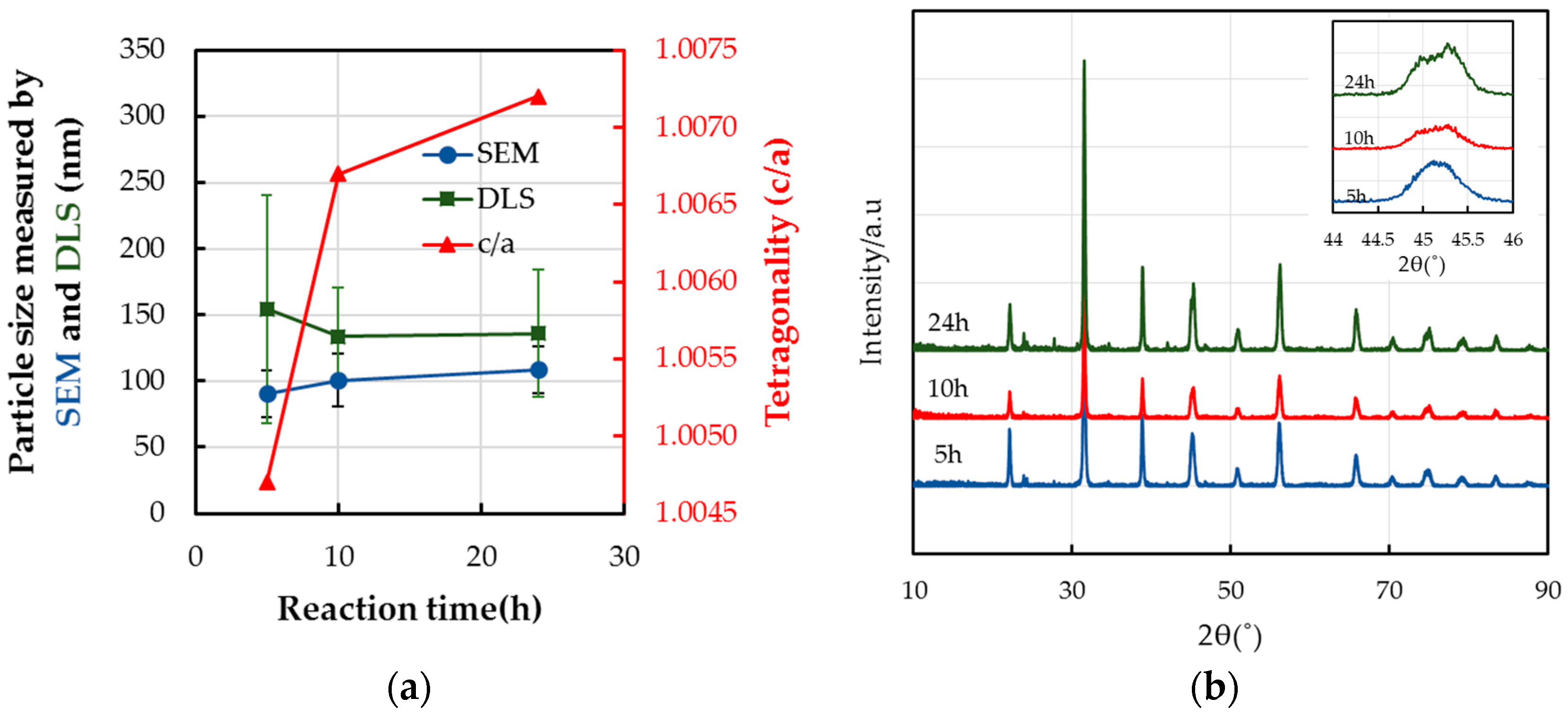
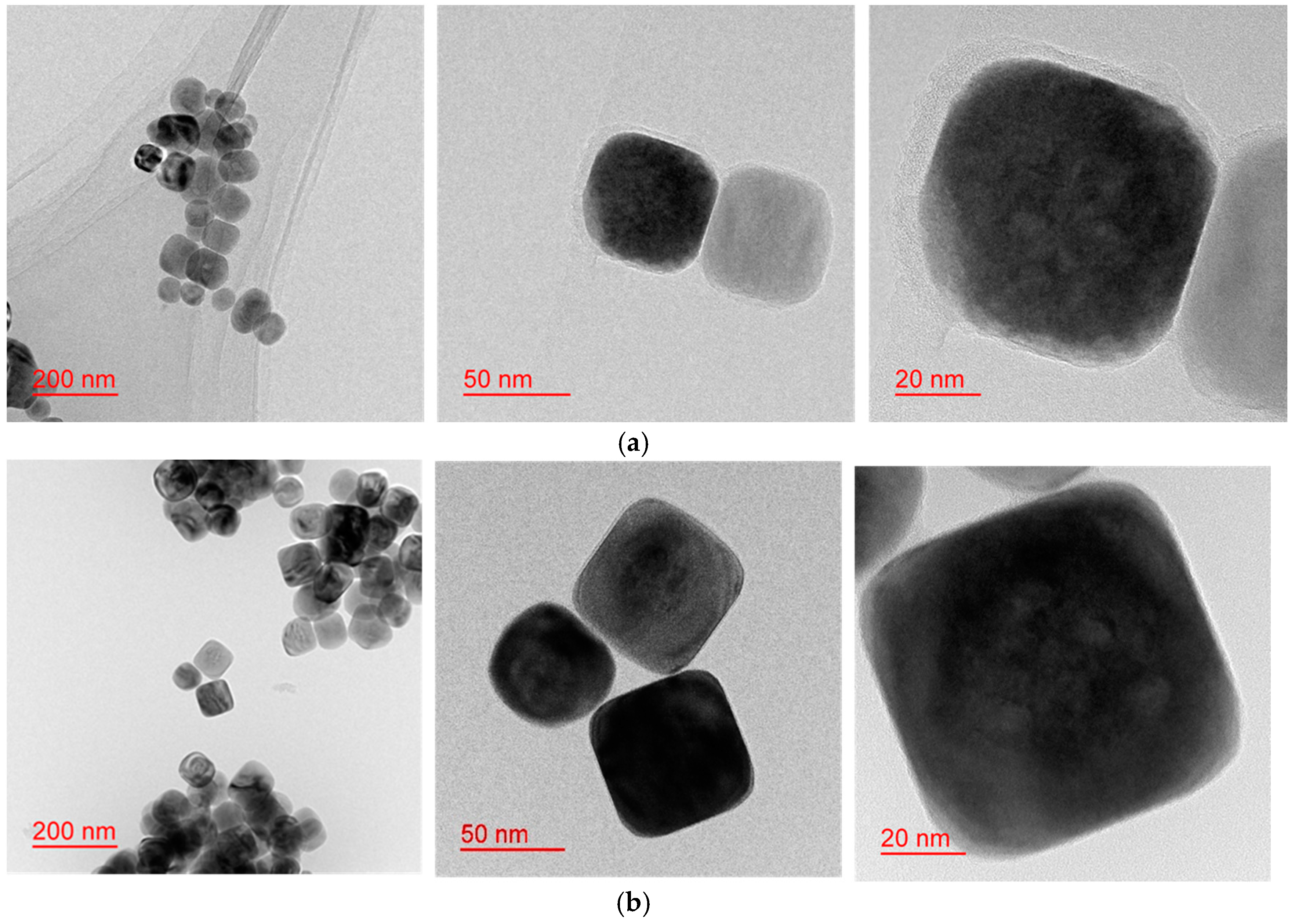
2.2. Dependency of the Formation Mechanism and Morphological Changes on the Reaction Time
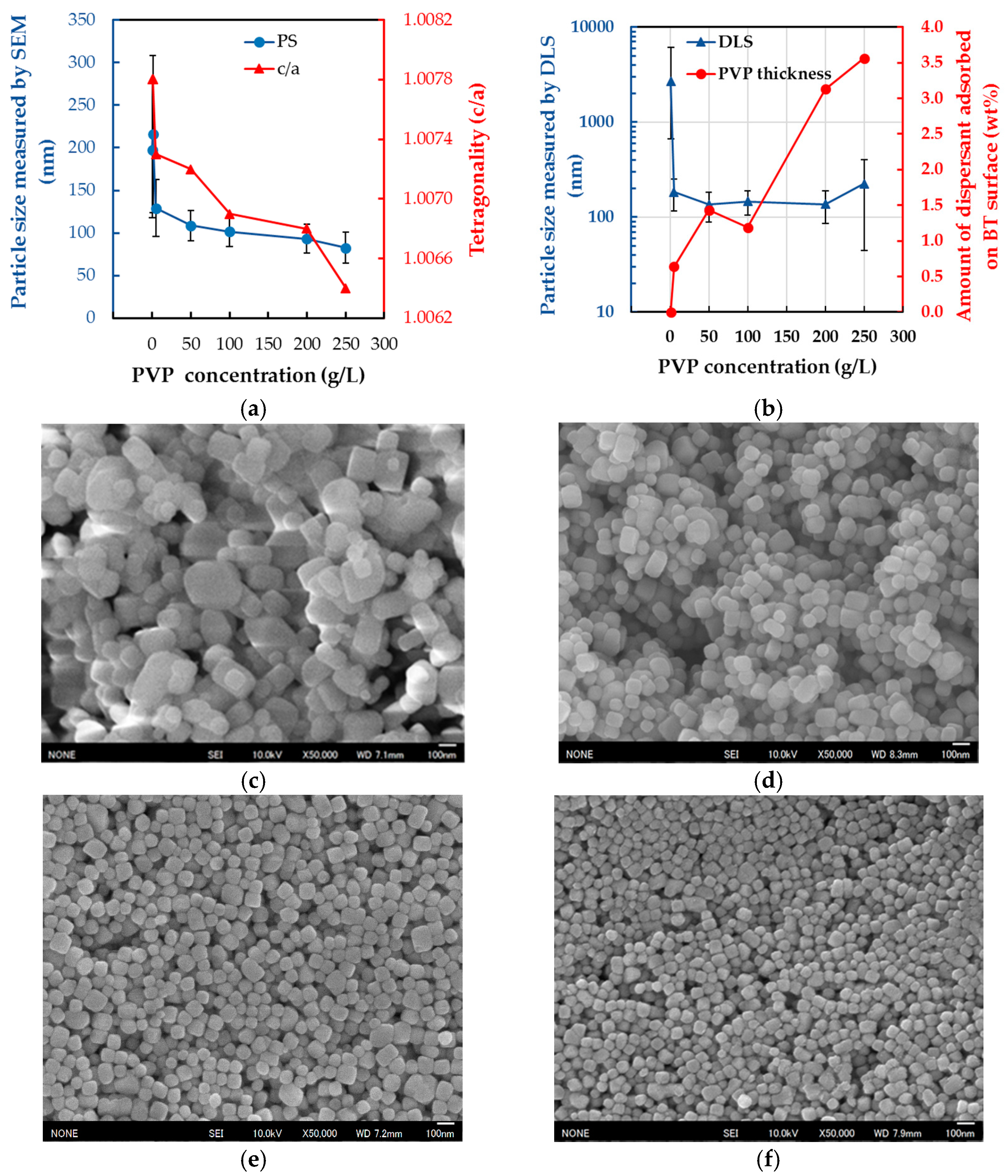
2.3. Dispersion of BT-PVP and the Effect of PVP Concentration
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, L.; Chen, Z.; Wilson, J.D.; Banerjee, S.; Robinson, R.D.; Herman, I.P. Barium titanate nanocrystals and nanocrystal thin films: Synthesis, ferroelectricity, and dielectric properties. J. Appl. Phys. 2006, 100, 034316. [Google Scholar] [CrossRef]
- Varghese, J.; Whatomore, R.W.; Holmes, J.D. Ferroelectric nanoparticles, wires and tubes: Synthesis, characterisation and applications. J. Mater. Chem. 2013, 1, 2618–2638. [Google Scholar] [CrossRef]
- Philippot, G.; Elissalde, C.; Maglione, M.; Aymonier, C. Supercritical fluid technology: A reliable process for high quality BaTiO3 based nanomaterials. Adv. Powder Technol. 2014, 25, 1415–1429. [Google Scholar] [CrossRef]
- Ciofani, G.; Ricotti, L.; Canale, C.; D’Alessandro, D.; Berrettini, S.; Mazzolai, B.; Mattoli, V. Effects of barium titanate nanoparticles on proliferation and differentiation of rat mesenchymal stem cells. Colloids Surf. B 2013, 102, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Ma, C.; Zhu, Y.; Li, S.; Shao, Y.; Wang, Y.; Xiao, Z. Enhanced specificity of multiplex polymerase chain reaction via CdTe quantum dots. Nanoscale Res. Lett. 2010, 5, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Z.; Zhu, J.; Zhou, S.; Liu, Z. Morphology and atomic-scale surface structure of barium titanate nanocrystals formed at hydrothermal conditions. J. Cryst. Growth 2009, 311, 2437–2442. [Google Scholar] [CrossRef]
- Hoshina, T.; Tsurumi, T. Size effect of barium titanate: Fine particles and ceramics. J. Ceram. Soc. Jpn. 2013, 121, 156–161. [Google Scholar] [CrossRef]
- Hennings, D.F.K.; Metzmacher, C.; Schreinemacher, B.S. Defect chemistry and microstructure of hydrothermal barium titanate. J. Am. Ceram. Soc. 2001, 84, 179–182. [Google Scholar] [CrossRef]
- Xu, H.; Gao, L.; Guo, J. Preparation and characterizations of tetragonal barium titanate powders by hydrothermal method. J. Eur. Ceram. Soc. 2002, 22, 1163–1170. [Google Scholar] [CrossRef]
- Kwon, S.; Park, B.; Choi, K.; Choi, E.; Nam, S.; Kim, J. Solvothermally synthesized tetragonal barium titanate powders using H2O/EtOH solvent. J. Eur. Ceram. Soc. 2006, 26, 1401–1404. [Google Scholar] [CrossRef]
- Habbib, A.; Stelzer, N.; Angerer, P.; Haubner, R. Effect of temperature and time on solvothermal synthesis of tetragonal BaTiO3. Bull. Mater. Sci. 2011, 34, 19–23. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Zhang, Z.; Zhu, J.; Zhou, S.; Liu, Z.; Ming, N. Pervoskite nanoparticles and nanowires: Microwave-hydrothermal synthesis and structural characterization by high-resolution transmission electron microscopy. J. Am. Ceram. Soc. 2008, 91, 2683–2689. [Google Scholar] [CrossRef]
- Guzenko, N.V.; Voronina, O.E.; Vlasova, N.N. Absorption of iodine on the surface of silica modified by polyvinylpyrrolidone and albumin. J. Appl. Spectrosc. 2004, 71, 151–155. [Google Scholar] [CrossRef]
- Hai, C.; Inukai, K.; Takahashi, Y.; Izu, N.; Akamasu, T.; Itoh, T.; Shin, W. Surfactant-assisted synthesis of mono-dispersed cubic BaTiO3 nanoparticles. Mater. Res. Bull. 2014, 57, 103–109. [Google Scholar] [CrossRef]
- Izu, N.; Matsubara, I.; Itoh, T.; Shin, W.; Nishibori, M. Controlled synthesis of monodispersed cerium oxide nanoparticle sols applicable to preparing ordered self-assemblies. Bull. Chem. Soc. Jpn. 2008, 81, 761–766. [Google Scholar] [CrossRef]
- Li, J.; Inukai, K.; Takahashi, Y.; Shin, W. Synthesis and size control of monodispersed BaTiO3–PVP nanoparticles. J. Asian Ceram. Soc. 2016, 4, 394–402. [Google Scholar] [CrossRef]
- Li, J.; Inukai, K.; Takahashi, Y.; Shin, W. Effect of PVP on the synthesis of high-dispersion core–shell barium-titanate–polyvinylpyrrolidone nanoparticles. J. Asian Ceram. Soc. 2017, 5, 216–225. [Google Scholar] [CrossRef]
- Eckert, J.O., Jr.; Hung-Houston, C.C.; Gersten, B.L.; Lencka, M.M.; Riman, R.E. Kinetics and mechanism of hydrothermal synthesis of barium titanate. J. Am. Ceram. Soc. 1996, 79, 2929–2939. [Google Scholar] [CrossRef]
- Lee, J.J.; Park, K.J.; Hur, K.H.; Yi, S.C.; Koo, S.M. Synthesis of ultrafine and spherical barium titanate powders using a titania nano-sol. J. Am. Ceram. Soc. 2006, 89, 3299–3301. [Google Scholar] [CrossRef]
- Li, J.; Inukai, K.; Takahashi, Y.; Shin, W. Synthesis of highly disperse tetragonal BaTiO3 nanoparticles with core-shell by a hydrothermal method. J. Asian Ceram. Soc. 2017, 5, 444–451. [Google Scholar] [CrossRef]
- Wada, S.; Nozawa, A.; Ohno, M.; Kakemoto, H.; Tsurumi, T.; Kameshima, Y.; Ohba, Y. Preparation of barium titanate nanocube particles by solvothermal method and their characterization. J. Mater. Sci. 2009, 44, 5161–5166. [Google Scholar] [CrossRef]
- Joung, M.-R.; Kim, J.-S.; Sing, M.; Choi, J.-H.; Nahm, S.; Choi, C.-H.; Sung, T.-H. Synthesis of highly tetragonal BaTiO3 nanopowders by a two-step alkoxide–hydroxide route. J. Alloys Compd. 2011, 509, 9089–9092. [Google Scholar] [CrossRef]
- Han, J.-M.; Joung, M.-R.; Kim, J.-S.; Lee, Y.-S.; Nahm, S.; Choi, Y.-K.; Paik, J.-H. Hydrothermal synthesis of BaTiO3 nanopowders using TiO2 nanoparticles. J. Am. Ceram. Soc. 2014, 97, 346–349. [Google Scholar] [CrossRef]
- Izu, N.; Uchida, T.; Matsubara, I.; Itoh, T.; Shin, W.; Nishibori, M. Formation mechanism of monodispersed spherical core–shell ceria/polymer hybrid nanoparticles. Mater. Res. Bull. 2011, 46, 1168–1176. [Google Scholar] [CrossRef]
- Lee, H.W.; Moon, S.; Choi, C.H.; Kim, D.K. Synthesis and size control of tetragonal barium titanate nanopowders by facile solvothermal method. J. Am. Ceram. Soc. 2012, 95, 2429–2434. [Google Scholar] [CrossRef]
- Hoshina, T.; Kakemoto, H.; Tsurumi, T.; Wada, S.; Yashima, M. Size and temperature induced phase transition behaviors of barium titanate nanoparticles. J. Appl. Phys. 2006, 99, 054311. [Google Scholar] [CrossRef]



| Reaction Parameter | TiO2 (mol %) | Reaction Time (h) | PVP Concentration (g/L) |
|---|---|---|---|
| Content of TiO2 in TiCl4 | 0, 1, 2, 10, 50, 100 | 24 | 50 |
| Reaction time | 100 (TiO2) | 5, 10, 24 | 50 |
| 0 (TiCl4) | 5, 24 | ||
| PVP concentration | 100 | 24 | 0, 1, 5, 50, 100, 200, 250 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Inukai, K.; Takahashi, Y.; Tsuruta, A.; Shin, W. Formation Mechanism and Dispersion of Pseudo-Tetragonal BaTiO3-PVP Nanoparticles from Different Titanium Precursors: TiCl4 and TiO2. Materials 2018, 11, 51. https://doi.org/10.3390/ma11010051
Li J, Inukai K, Takahashi Y, Tsuruta A, Shin W. Formation Mechanism and Dispersion of Pseudo-Tetragonal BaTiO3-PVP Nanoparticles from Different Titanium Precursors: TiCl4 and TiO2. Materials. 2018; 11(1):51. https://doi.org/10.3390/ma11010051
Chicago/Turabian StyleLi, Jinhui, Koji Inukai, Yosuke Takahashi, Akihiro Tsuruta, and Woosuck Shin. 2018. "Formation Mechanism and Dispersion of Pseudo-Tetragonal BaTiO3-PVP Nanoparticles from Different Titanium Precursors: TiCl4 and TiO2" Materials 11, no. 1: 51. https://doi.org/10.3390/ma11010051




