Thermal Peak Management Using Organic Phase Change Materials for Latent Heat Storage in Electronic Applications
Abstract
:1. Introduction
2. Fundamentals
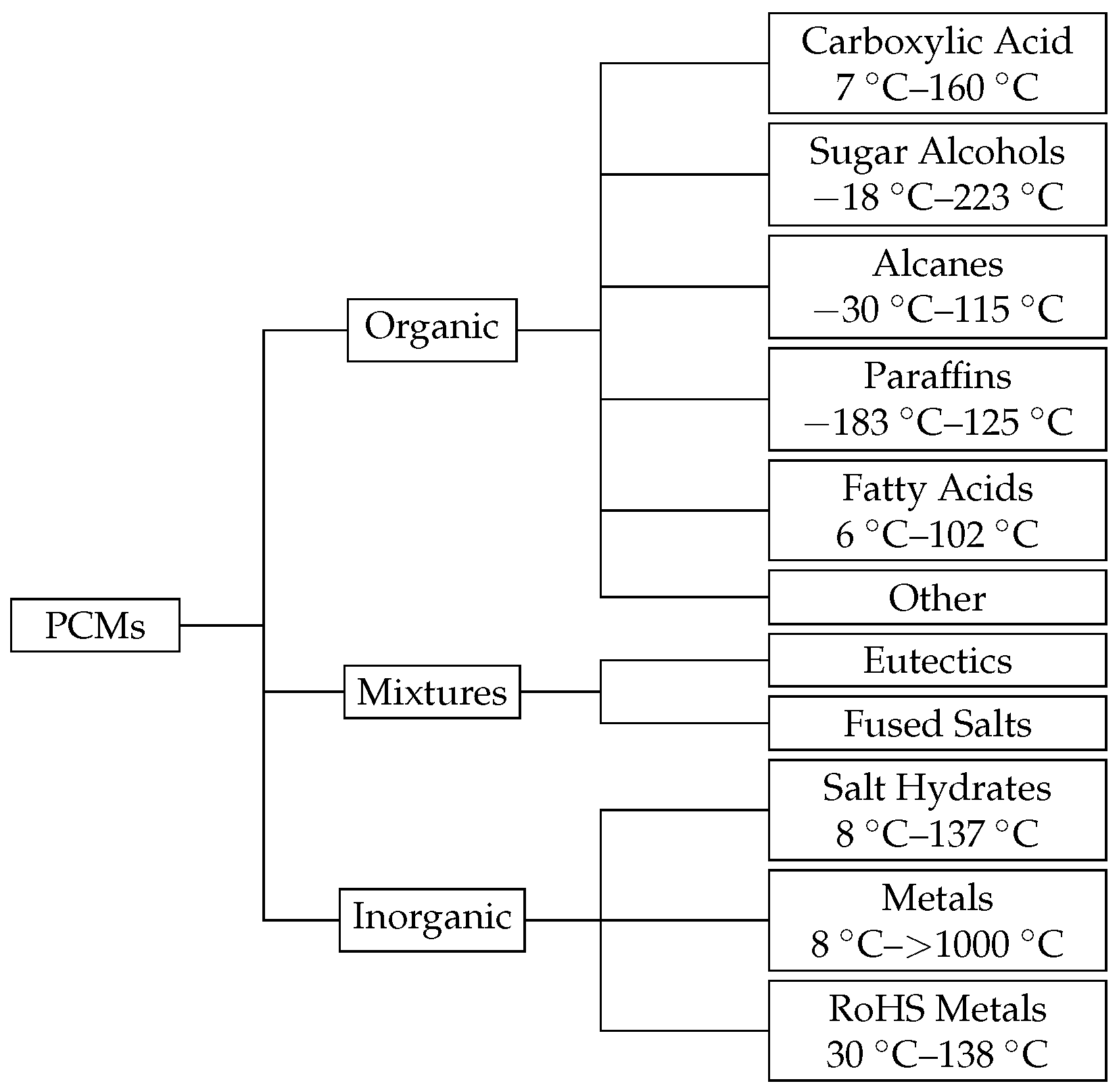
2.1. Phase Change Materials
- solid-liqid at 0 (1 bar): ice melting to water
- liquid-gas at 100 (1 bar): water boiling to vapour
- solid-gas at ( bar) ice sublimating to vapour
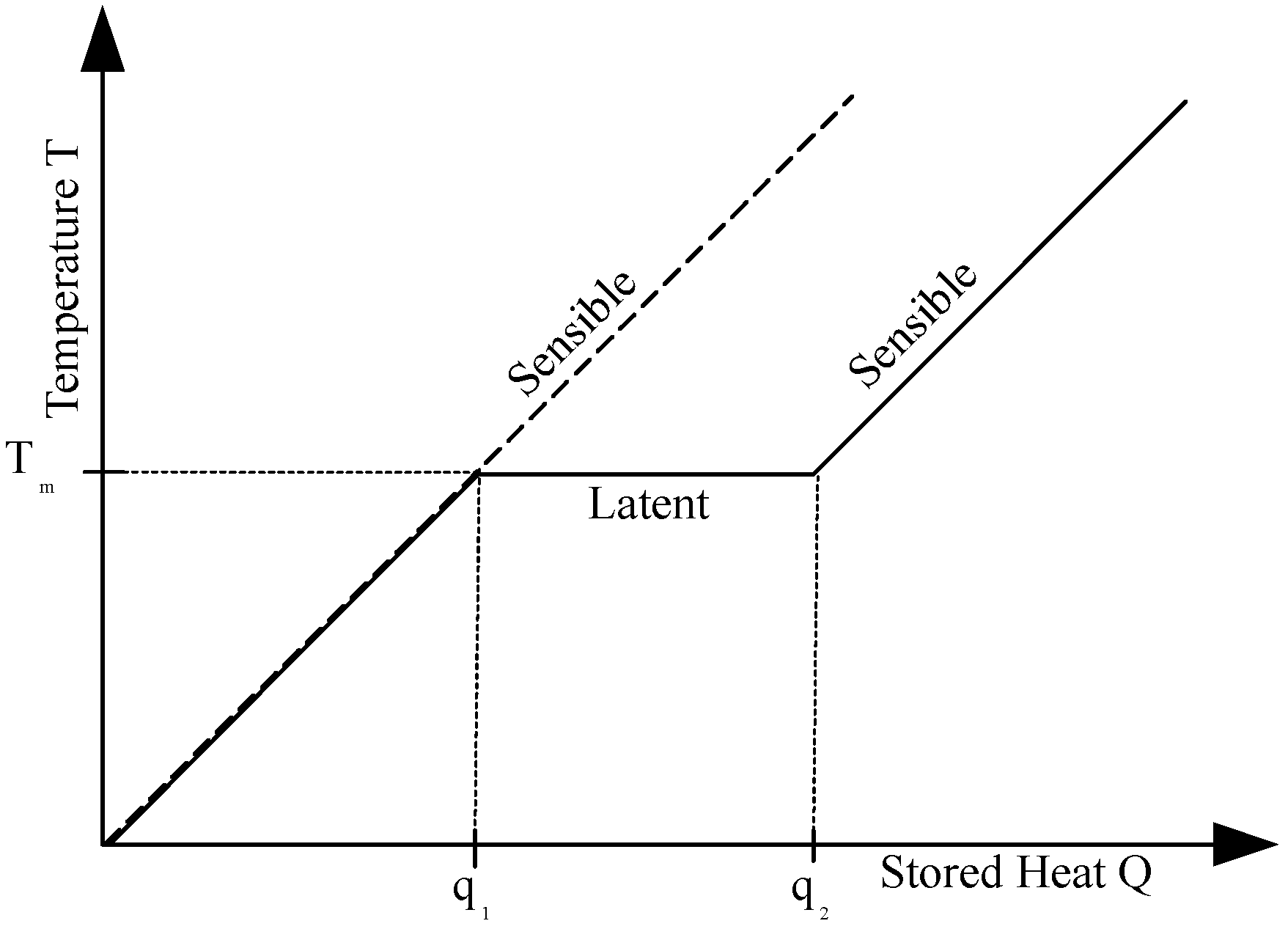
2.2. Latent Heat Storage
2.3. Previous Research and Research Objectives
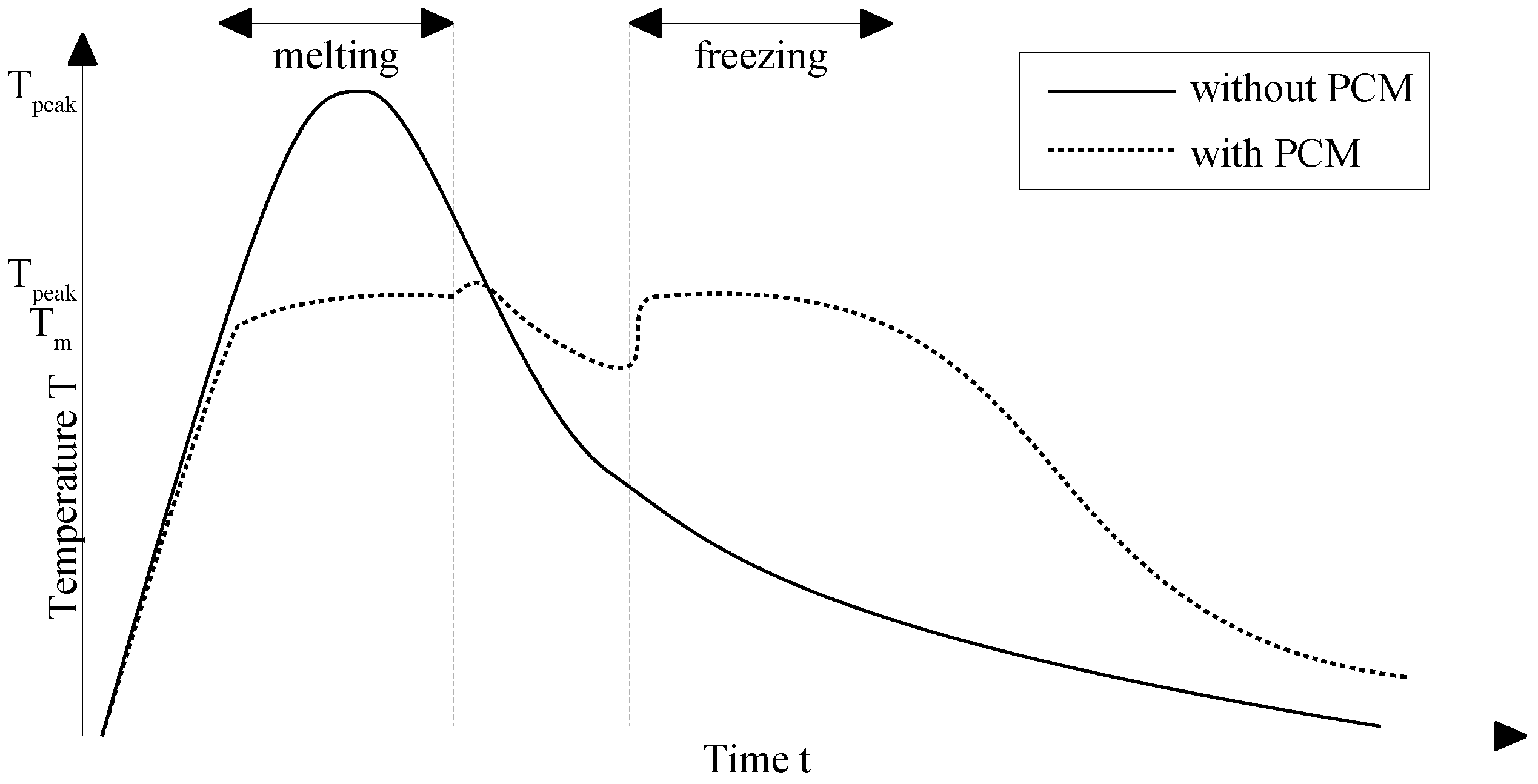
3. Cooling Concept
3.1. Requirements
3.2. General Description
4. Material Evaluation
4.1. Sugar Alcohols
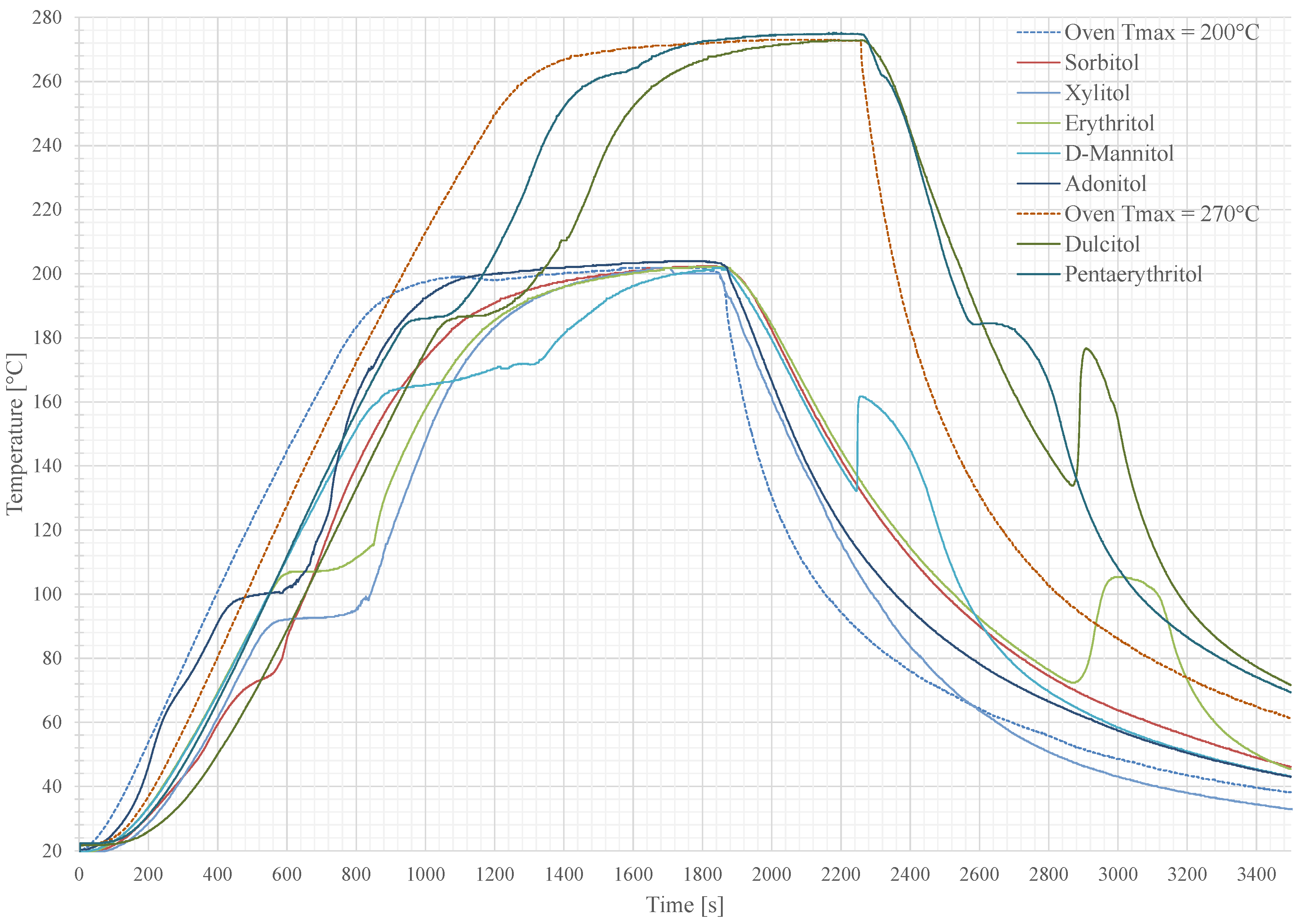
4.1.1. Simulation
4.1.2. Measurement
4.2. Additives
4.2.1. Powders
4.2.2. Liquids
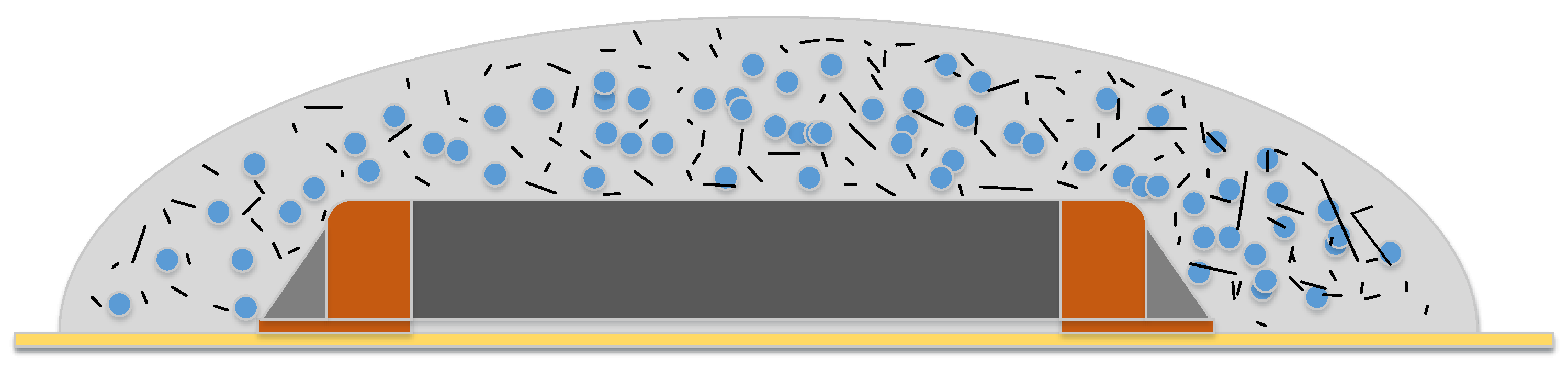
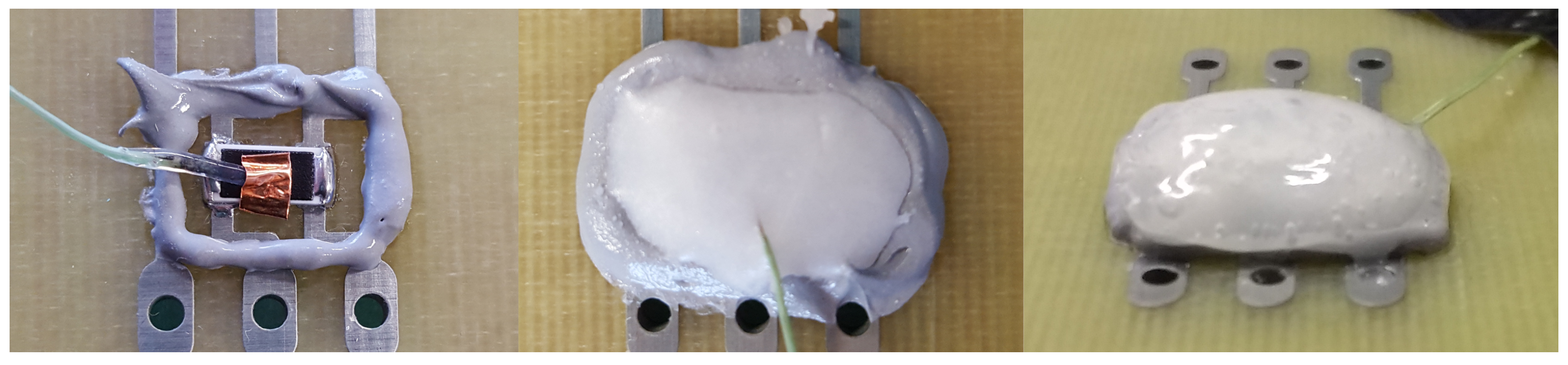
5. PCM Enclosures
5.1. Resin Matrix
5.2. Dam and Fill
5.3. Cooling Moulding
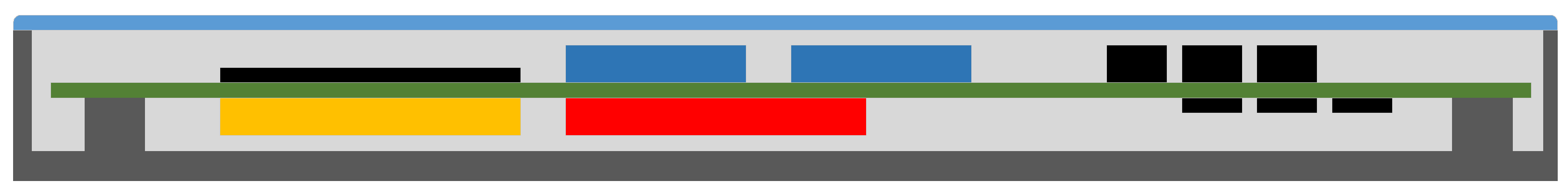
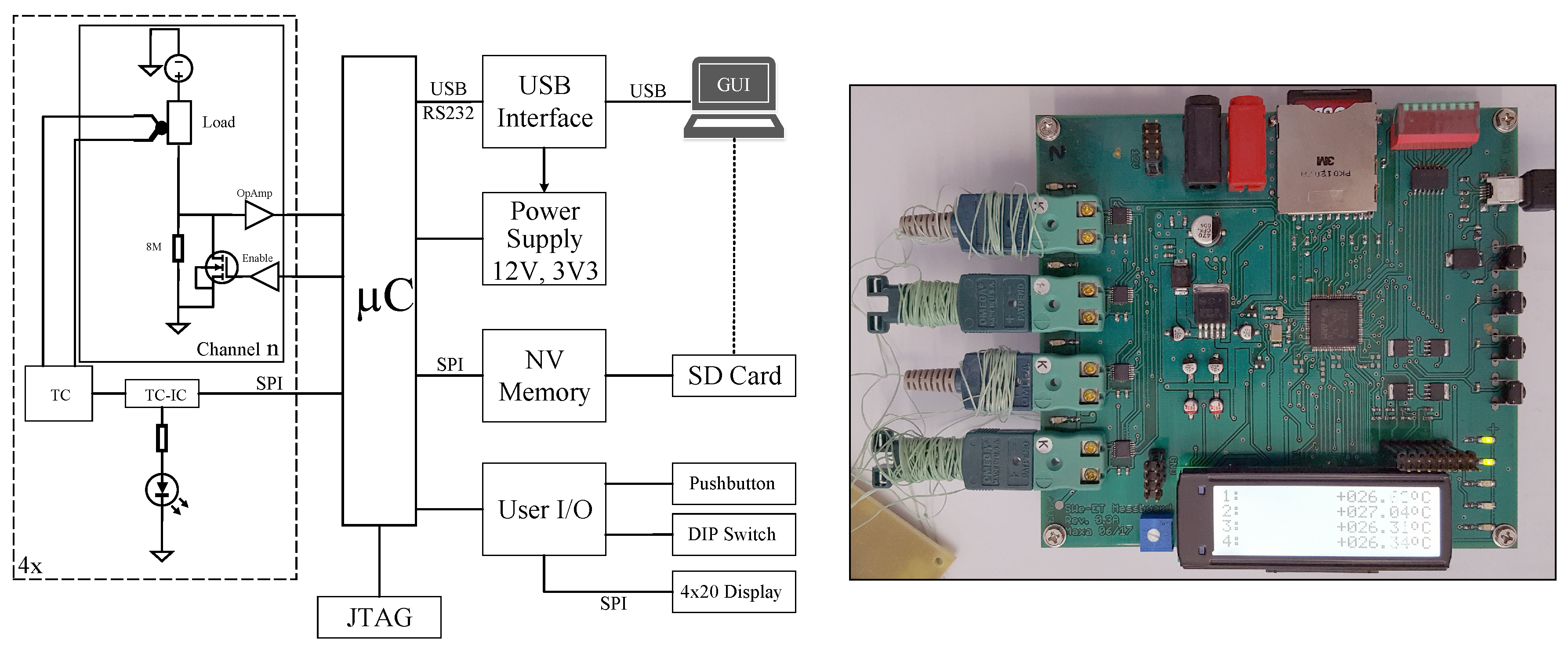
6. Measurement Circuit
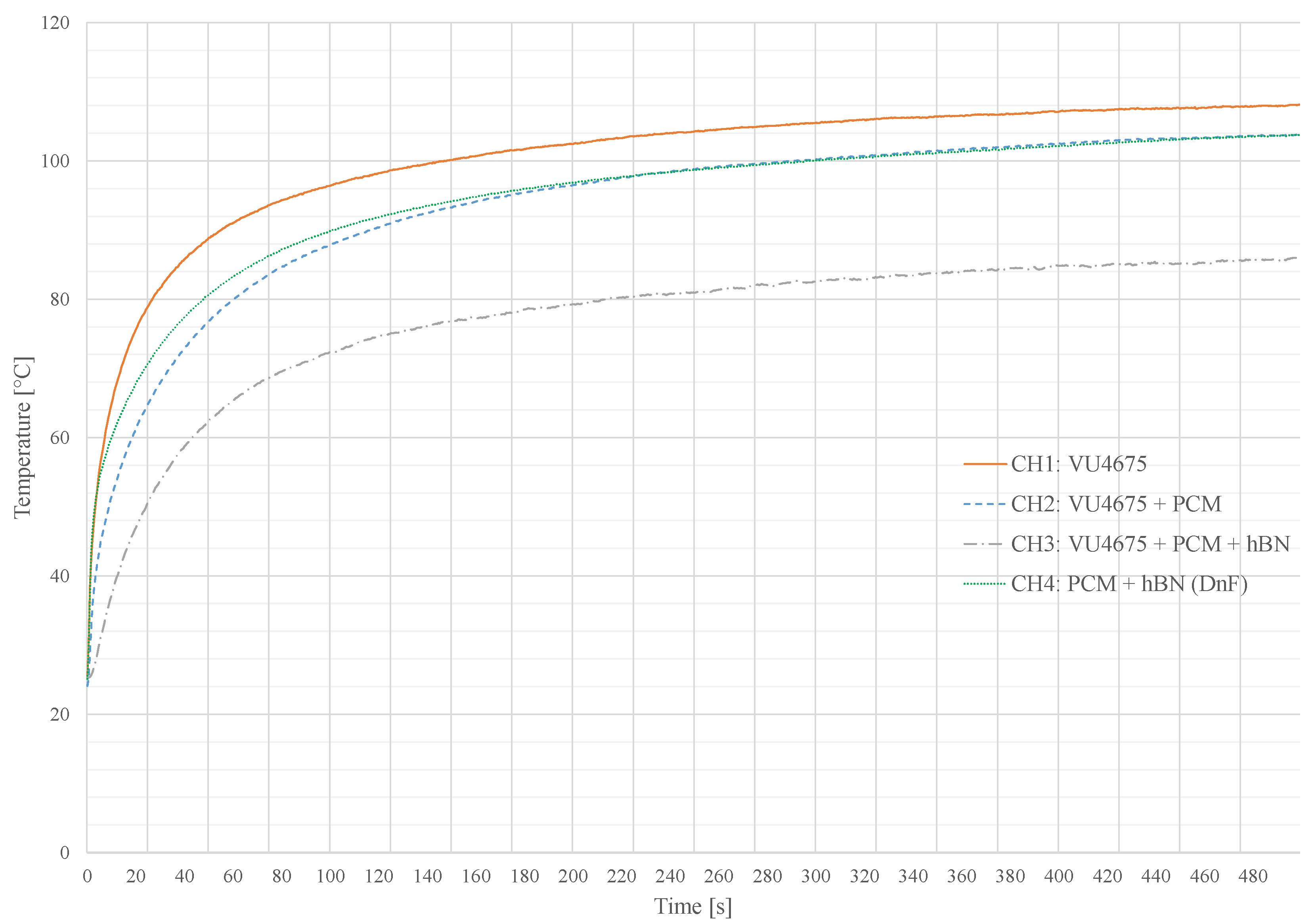
7. Measurements and Recommendations
8. Conclusions and Future Work
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| A | B | Mol. Fract. A | C | J g | Mol. Mass g mol | Density g cm | J cm |
|---|---|---|---|---|---|---|---|
| Isomaltol | Pentaerythritol | 0.345 | 68 | 108,299 | 207,907 | 1.497 | 77,997 |
| Pentaerythritol | Xylitol | 0.538 | 74 | 203.562 | 143.540 | 1.453 | 206.097 |
| Sorbitol | Pentaerythritol | 0.457 | 75 | 181.569 | 157.202 | 1.444 | 166.733 |
| Isomaltol | Xylitol | 0.386 | 75 | 318.695 | 226.328 | 1.586 | 223.273 |
| Sorbitol | Xylitol | 0.493 | 76 | 359.779 | 166.944 | 1.510 | 325.449 |
| Pentaerythritol | Ribitol | 0.550 | 78 | 191.598 | 143.347 | 1.456 | 194.646 |
| Lactitol-Monohydr. | Pentaerythritol | 0.425 | 78 | 277.390 | 232.197 | 1.521 | 181.685 |
| Xylitol | Ribitol | 0.543 | 78 | 373.817 | 152.150 | 1.525 | 374.570 |
| Lactitol-Monohydr. | Xylitol | 0.425 | 78 | 468.105 | 241.450 | 1.592 | 308.689 |
| Isomaltol | Sorbitol | 0.381 | 79 | 291.766 | 243.903 | 1.572 | 188.089 |
| Isomaltol | Ribitol | 0.398 | 79 | 306.057 | 228.661 | 1.594 | 213.314 |
| Sorbitol | Ribitol | 0.525 | 79 | 349.925 | 167.914 | 1.514 | 315.562 |
| Arabinitol | Xylitol | 0.413 | 80 | 400.135 | 152.150 | 1.522 | 400.284 |
| Lactitol-Monohydr. | Sorbitol | 0.442 | 80 | 446.612 | 261.856 | 1.584 | 270.168 |
| Lactitol-Monohydr. | Ribitol | 0.477 | 80 | 469.447 | 252.426 | 1.606 | 298.737 |
| Sorbitol | Arabinitol | 0.569 | 81 | 375.376 | 169.231 | 1.511 | 335.109 |
| Arabinitol | Pentaerythritol | 0.439 | 82 | 215.240 | 143.169 | 1.453 | 218.382 |
| Isomaltol | Arabinitol | 0.422 | 82 | 338.373 | 233.181 | 1.595 | 231.392 |
| Lactitol-Monohydr. | Arabinitol | 0.519 | 82 | 508.401 | 261.199 | 1.611 | 313.491 |
| Arabinitol | Ribitol | 0.456 | 83 | 392.392 | 152.150 | 1.528 | 393.997 |
| Erythritol | Xylitol | 0.295 | 85 | 391.739 | 143.277 | 1.499 | 409.935 |
| Isomaltol | Lactitol-Monohydr. | 0.393 | 85 | 440.703 | 355.248 | 1.690 | 209.653 |
| Lactitol-Monohydr. | Erythritol | 0.631 | 86 | 522.224 | 273.697 | 1.601 | 305.561 |
| Sorbitol | Erythritol | 0.665 | 87 | 363.171 | 162.083 | 1.483 | 332.351 |
| Lactitol-Monohydr. | Maltol | 0.671 | 87 | 455.519 | 284.504 | 1.667 | 266.893 |
| Maltol | Xylitol | 0.227 | 88 | 338.919 | 146.244 | 1.543 | 357.514 |
| Erythritol | Ribitol | 0.354 | 88 | 382.239 | 141.530 | 1.502 | 405.576 |
| Isomaltol | Erythritol | 0.478 | 89 | 321.040 | 228.308 | 1.565 | 220.024 |
| Sorbitol | Maltol | 0.737 | 90 | 298.911 | 167.399 | 1.532 | 273.489 |
| Arabinitol | Erythritol | 0.633 | 90 | 422.052 | 141.129 | 1.497 | 447.825 |
| Maltol | Ribitol | 0.277 | 92 | 316.570 | 144.931 | 1.555 | 339.646 |
| Erythritol | Pentaerythritol | 0.405 | 93 | 198.381 | 130.461 | 1.418 | 215.608 |
| Isomaltol | Maltol | 0.499 | 93 | 205.255 | 234.954 | 1.655 | 144.573 |
| Mannitol | Xylitol | 0.037 | 94 | 389.156 | 153.251 | 1.520 | 385:980 |
| Lactitol-Monohydr. | Mannitol | 0.956 | 94 | 584.582 | 354.371 | 1.682 | 277.549 |
| Arabinitol | Maltol | 0.718 | 95 | 360.194 | 144.813 | 1.552 | 385.973 |
| Lactitol-Monohydr. | Myo-Inositol | 0.979 | 95 | 580.763 | 358.551 | 1.697 | 274.910 |
| Lactitol-Monohydr. | Dulcitol | 0.985 | 95 | 585.714 | 359.598 | 1.687 | 274.724 |
| Sorbitol | Mannitol | 0.947 | 98 | 349.826 | 182.170 | 1.501 | 288.251 |
| Sorbitol | Myo-Inositol | 0.978 | 99 | 338.200 | 182.125 | 1.512 | 280.779 |
| Sorbitol | Dulcitol | 0.982 | 99 | 342.700 | 182.170 | 1.499 | 282.077 |
| Mannitol | Ribitol | 0.047 | 100 | 374.419 | 153.573 | 1.530 | 372.908 |
| Myo-Inositol | Xylitol | −0.156 | 100 | 380.615 | 147.794 | 1.439 | 370.661 |
| Myo-Inositol | Ribitol | 0.017 | 101 | 364.648 | 152.634 | 1.539 | 367.622 |
| Arabinitol | Mannitol | 0.937 | 101 | 435.779 | 154.054 | 1.525 | 431.292 |
| Arabinitol | Myo-Inositol | 0.974 | 102 | 425.090 | 152.876 | 1.538 | 427.749 |
| Arabinitol | Dulcitol | 0.977 | 102 | 430.973 | 152.829 | 1.524 | 429.695 |
| Dulcitol | Xylitol | −0.300 | 103 | 302.990 | 143.149 | 1.535 | 324.897 |
| Dulcitol | Ribitol | −0.046 | 103 | 351.251 | 150.766 | 1.533 | 357.100 |
| Maltol | Pentaerythritol | 0.387 | 106 | 105.438 | 132.263 | 1.483 | 118.200 |
| Erythritol | Maltol | 0.644 | 107 | 335.891 | 123.540 | 1.511 | 410.691 |
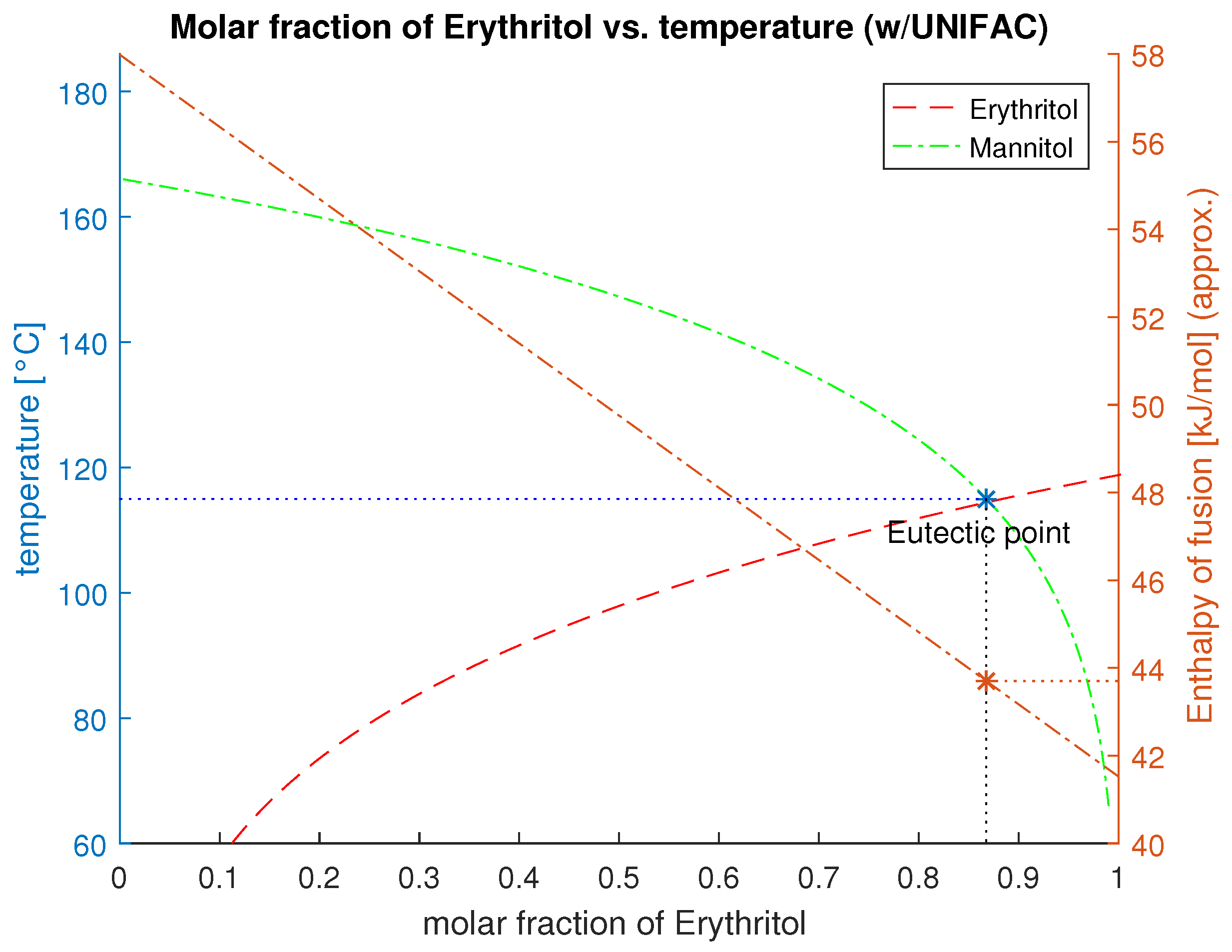
| Erythritol | Mannitol | 0.868 | 115 | 437.013 | 130.073 | 1.459 | 490.278 |
| Erythritol | Myo-Inositol | 0.952 | 117 | 414.003 | 124.909 | 1.478 | 489.974 |
| Erythritol | Dulcitol | 0.946 | 117 | 427.683 | 125.379 | 1.451 | 494.982 |
| Isomaltol | Mannitol | 0.761 | 123 | 304.625 | 305.552 | 1.649 | 164.436 |
| Isomaltol | Dulcitol | 0.854 | 132 | 280.354 | 320.692 | 1.658 | 144.941 |
| Isomaltol | Myo-Inositol | 0.871 | 133 | 240.361 | 323.128 | 1.735 | 129.062 |
| Mannitol | Pentaerythritol | 0.292 | 134 | 205.095 | 149.590 | 1.432 | 196.364 |
| Maltol | Mannitol | 0.687 | 138 | 313.645 | 143.653 | 1.589 | 346.870 |
| Maltol | Myo-Inositol | 0.831 | 148 | 225.757 | 135.228 | 1.691 | 282.252 |
| Maltol | Dulcitol | 0.821 | 148 | 273.372 | 136.138 | 1.593 | 319.917 |
| Dulcitol | Pentaerythritol | 0.249 | 151 | 198.540 | 147.608 | 1.414 | 190.246 |
| Mannitol | Dulcitol | 0.696 | 156 | 599.643 | 182.170 | 1.505 | 495.338 |
| Mannitol | Myo-Inositol | 0.829 | 161 | 547.367 | 181.826 | 1.609 | 484.329 |
| Myo-Inositol | Pentaerythritol | 0.238 | 164 | 131.276 | 146.617 | 1.549 | 138.685 |
| Dulcitol | Myo-Inositol | 0.711 | 178 | 571.307 | 181.588 | 1.635 | 514.280 |
References
- Mohan, N.; Undeland, T.M.; Robbins, W.P. Power Electronics: Converters, Applications, and Design, 3rd ed.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Mehling, H.; Cabeza, L.F. Heat and Cold Storage with PCM: An up to Date Introduction into Basics and Applications with 28 Tables; Heat and Mass Transfer; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Jankowski, N.R.; McCluskey, F.P. A review of phase change materials for vehicle component thermal buffering. Appl. Energy 2014, 113, 1525–1561. [Google Scholar] [CrossRef]
- Sharma, S.D.; Kitano, H.; Sagara, K. Phase Change Materials for Low Temperature Solar Thermal Applications. Ph.D. Thesis, Mie University, Tsu, Japan, 2004. [Google Scholar]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.; Sandaran, S.C. Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Convers. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef]
- Diarce, G.; Gandarias, I.; Campos-Celador, Á.; García-Romero, A.; Griesser, U.J. Eutectic mixtures of sugar alcohols for thermal energy storage in the 50–90 °C temperature range. Sol. Energy Mater. Sol. Cells 2015, 134, 215–226. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Kandasamy, R.; Wang, X.Q.; Mujumdar, A.S. Transient cooling of electronics using phase change material (PCM)-based heat sinks. Appl. Therm. Eng. 2008, 28, 1047–1057. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Das, M.K.; Rath, P. Application of TCE-PCM based heat sinks for cooling of electronic components: A review. Renew. Sustain. Energy Rev. 2016, 59, 550–582. [Google Scholar] [CrossRef]
- Baby, R.; Balaji, C. Thermal management of electronics using phase change material based pin fin heat sinks. J. Phys. Conf. Ser. 2012, 395, 012134. [Google Scholar] [CrossRef]
- Yoo, D.W.; Joshi, Y.K. Energy Efficient Thermal Management of Electronic Components Using Solid–Liquid Phase Change Materials. IEEE Trans. Device Mater. Reliab. 2004, 4, 641–649. [Google Scholar] [CrossRef]
- Green, C.E.; Fedorov, A.G.; Joshi, Y.K. Time Scale Matching of Dynamically Operated Devices Using Composite Thermal Capacitors. Microelectron. J. 2014, 45, 1069–1078. [Google Scholar] [CrossRef]
- Oya, T.; Nomura, T.; Okinaka, N.; Akiyama, T. Phase change composite based on porous nickel and erythritol. Appl. Therm. Eng. 2012, 40, 373–377. [Google Scholar] [CrossRef]
- Dietrich, B. Thermische Charakterisierung von Keramischen Schwammstrukturen für Verfahrenstechnische Apparate: Zugl.: Karlsruhe, KIT, Diss., 2010; KIT Scientific Publishing: Karlsruhe, Germany, 2010. [Google Scholar]
- Zhang, H.; Rindt, C.C.M.; Smeulders, D.M.J.; Nedea, S.V. Nanoscale Heat Transfer in Carbon Nanotubes—Sugar Alcohol Composite as Heat Storage Materials. J. Phys. Chem. C 2016, 120, 21915–21924. [Google Scholar] [CrossRef]
- Harish, S.; Orejon, D.; Takata, Y.; Kohno, M. Thermal conductivity enhancement of lauric acid phase change nanocomposite with graphene nanoplatelets. Appl. Therm. Eng. 2015, 80, 205–211. [Google Scholar] [CrossRef]
- Qi, G.; Yang, J.; Bao, R.; Xia, D.; Cao, M.; Yang, W.; Yang, M.; Wei, D. Hierarchical graphene foam-based phase change materials with enhanced thermal conductivity and shape stability for efficient solar-to-thermal energy conversion and storage. Nano Res. 2017, 10, 802–813. [Google Scholar] [CrossRef]
- ADLER, J.; Standke, G. Offenzellige Siliciumcarbid-Schaumkeramik und Verfahren zu Ihrer Herstellung. Patent No. DE10044656 B4, 29 December 2005. [Google Scholar]
- Reitzmann, A.; Patcas, F.C.; Kraushaar-Czarnetzki, B. Keramische Schwämme—Anwendungspotenzial monolithischer Netzstrukturen als katalytische Packungen. Chem. Ing. Tech. 2006, 78, 885–898. [Google Scholar] [CrossRef]
- Palomo Del Barrio, E.; Cadoret, R.; Daranlot, J.; Achchaq, F. New sugar alcohols mixtures for long-term thermal energy storage applications at temperatures between 70 °C and 100 °C. Sol. Energy Mater. Sol. Cells 2016, 155, 454–468. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Pan, R.; Chiu, J.N.; Martin, V. Polyols as Phase Change Materials for Low-grade Excess Heat Storage. Energy Procedia 2014, 61, 664–669. [Google Scholar] [CrossRef]
- Wittig, R. Experimentelle und Theoretische Untersuchungen zur Erweiterung der Gruppenbeitragsmethoden UNIFAC und Modified UNIFAC (Dortmund). Ph.D. Thesis, Universität Oldenburg, Oldenburg, Germany, 2002. [Google Scholar]
- Klemens, P.G.; Williams, R.K. Thermal conductivity of metals and alloys. Int. Mater. Rev. 1986, 31, 197–215. [Google Scholar] [CrossRef]
- Samani, M.K.; Khosravian, N.; Chen, G.; Shakerzadeh, M.; Baillargeat, D.; Tay, B.K. Thermal conductivity of individual multiwalled carbon nanotubes. Int. J. Therm. Sci. 2012, 62, 40–43. [Google Scholar] [CrossRef]
- Pflug, G.; Gladitz, M.; Reinemann, S. Wärme besser leiten: Nanoskalige Füllstoffe. Kunststoffe 2009, 12, 54–60. [Google Scholar]
- Strehse, C. Zuverlässigkeitsanalyse von Hochleistungsfähigen PCM-basierten Temperaturschutzschichten. Master’s Thesis, Universität Rostock, Rostock, Germany, 2015. [Google Scholar]
- Borisenko, E. (Ed.) Crystallization and Materials Science of Modern Artificial and Natural Crystals; InTech: London, UK, 2012. [Google Scholar]
- Seppälä, A.; Meriläinen, A.; Wikström, L.; Kauranen, P. The effect of additives on the speed of the crystallization front of xylitol with various degrees of supercooling. Exp. Therm. Fluid Sci. 2010, 34, 523–527. [Google Scholar] [CrossRef]
- Nomura, T.; Zhu, C.; Sagara, A.; Okinaka, N.; Akiyama, T. Estimation of thermal endurance of multicomponent sugar alcohols as phase change materials. Appl. Therm. Eng. 2015, 75, 481–486. [Google Scholar] [CrossRef]
| Property | Value | Unit |
|---|---|---|
| Enthalpy | >200 | J g |
| Melting Point | 80–100, 140–150 | C |
| Reuseability n | >5000 | cycles |
| Thermal Stability | + 50 | C |
| Thermal Conductivity | >1.5 | W mK |
| Electrical Resistance R | >10 | |
| Hold Time | s |
| Name | Formula | Melting Range | Enthalpy | Density |
|---|---|---|---|---|
| Unit | - | °C | J g−1 | g cm−3 |
| Sorbitol | ||||
| Xylitol | ||||
| Adonitol | ||||
| Erythritol | ||||
| D-Mannitol | ||||
| Pentaerythritol | ||||
| Dulcitol |
| Additive | (W m K) | Miscibility | Crystallisation | Source |
|---|---|---|---|---|
| Copper | 401 | + | + | [24] |
| MgCl + 6HO | 0.704 | − | o | [8] |
| Zinc | 110 | + | + | [24] |
| MWCNT | >2000 | − | − | [25,26] |
| Nickel | 85 | + | + | [24] |
| Palmitic Acid | 0.162 | − | − | [8] |
| Ceramic | 40 | + | + | [26] |
| Hex-Boron Nitride | 40 | + | + | [26] |
| Channel | Application | T | PCM | Additive | T | T |
|---|---|---|---|---|---|---|
| 1 | Matrix | 22 | - | - | 108 | 0 |
| 2 | Matrix | 22 | 30 wt. % 80 C | - | 103 | |
| 3 | Matrix | 22 | 30 wt. % 80 C | 10 wt. % hBN | 86 | |
| 4 | Dam and Fill | 22 | 90 wt. % 80 C | 10 wt. % hBN | 103 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maxa, J.; Novikov, A.; Nowottnick, M. Thermal Peak Management Using Organic Phase Change Materials for Latent Heat Storage in Electronic Applications. Materials 2018, 11, 31. https://doi.org/10.3390/ma11010031
Maxa J, Novikov A, Nowottnick M. Thermal Peak Management Using Organic Phase Change Materials for Latent Heat Storage in Electronic Applications. Materials. 2018; 11(1):31. https://doi.org/10.3390/ma11010031
Chicago/Turabian StyleMaxa, Jacob, Andrej Novikov, and Mathias Nowottnick. 2018. "Thermal Peak Management Using Organic Phase Change Materials for Latent Heat Storage in Electronic Applications" Materials 11, no. 1: 31. https://doi.org/10.3390/ma11010031