Effect of the Heat-Treated Ti6Al4V Alloy on the Fibroblastic Cell Response
Abstract
:1. Introduction
2. Results and Discussion
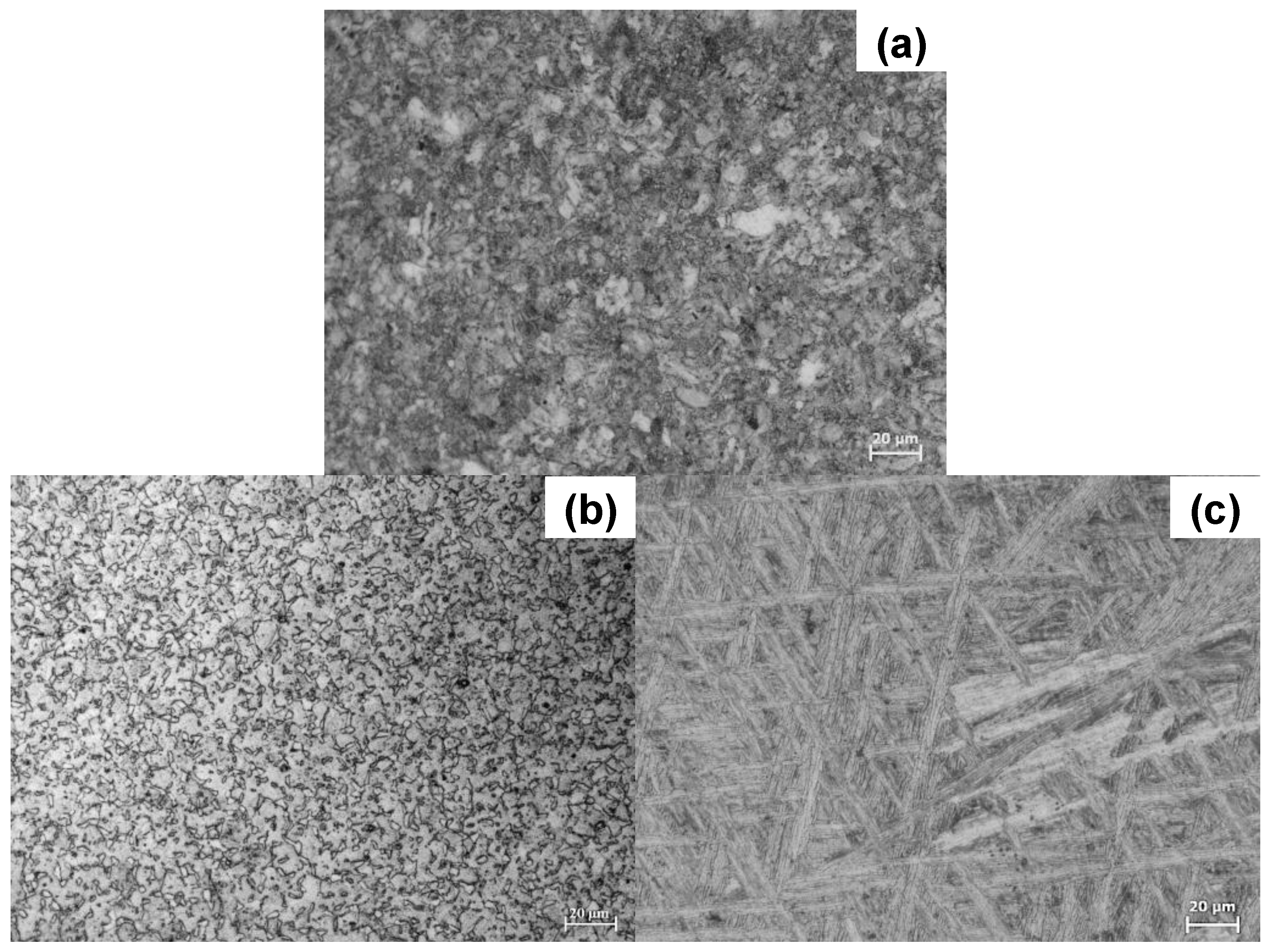
2.1. Microstructural Characterization
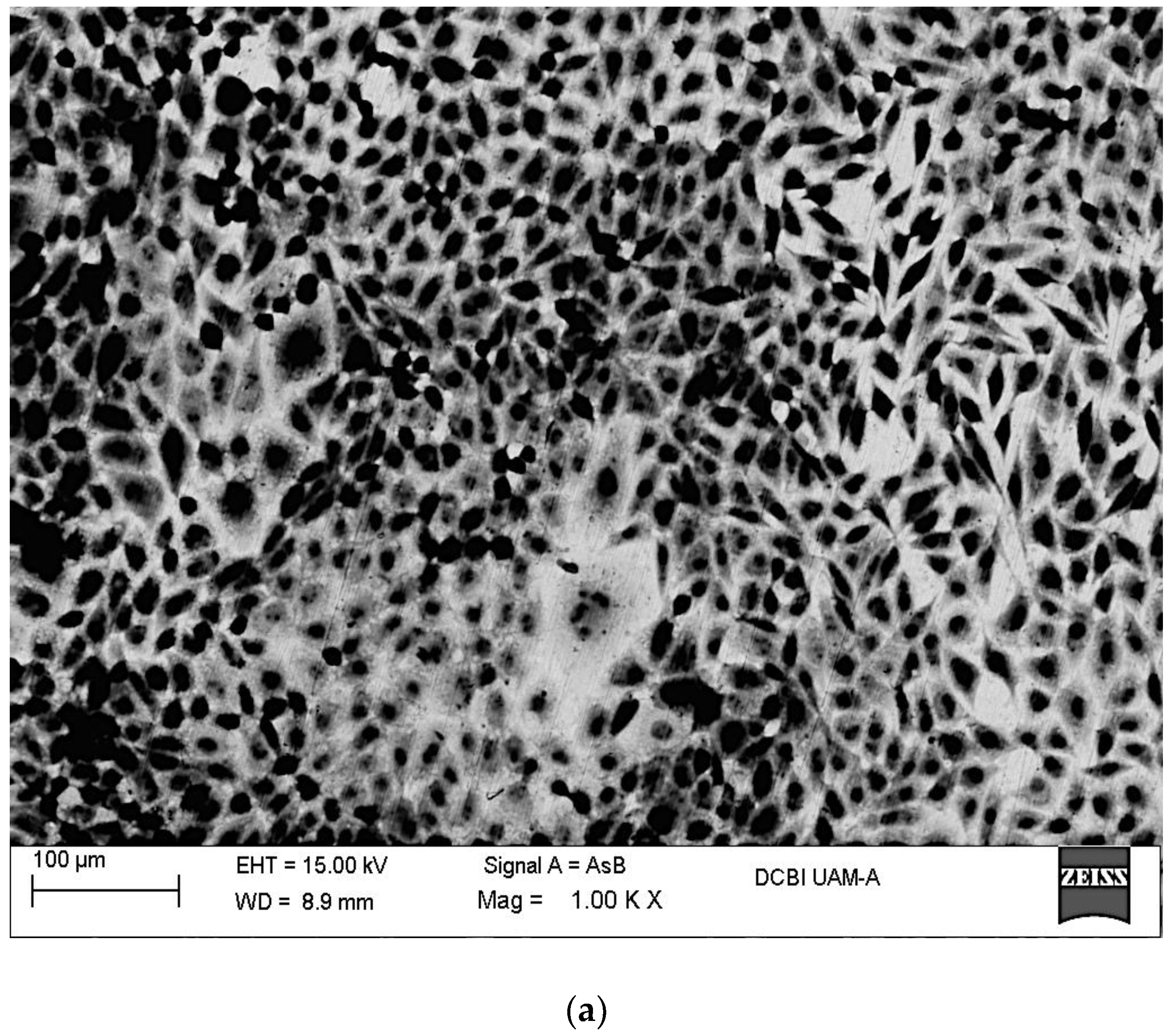
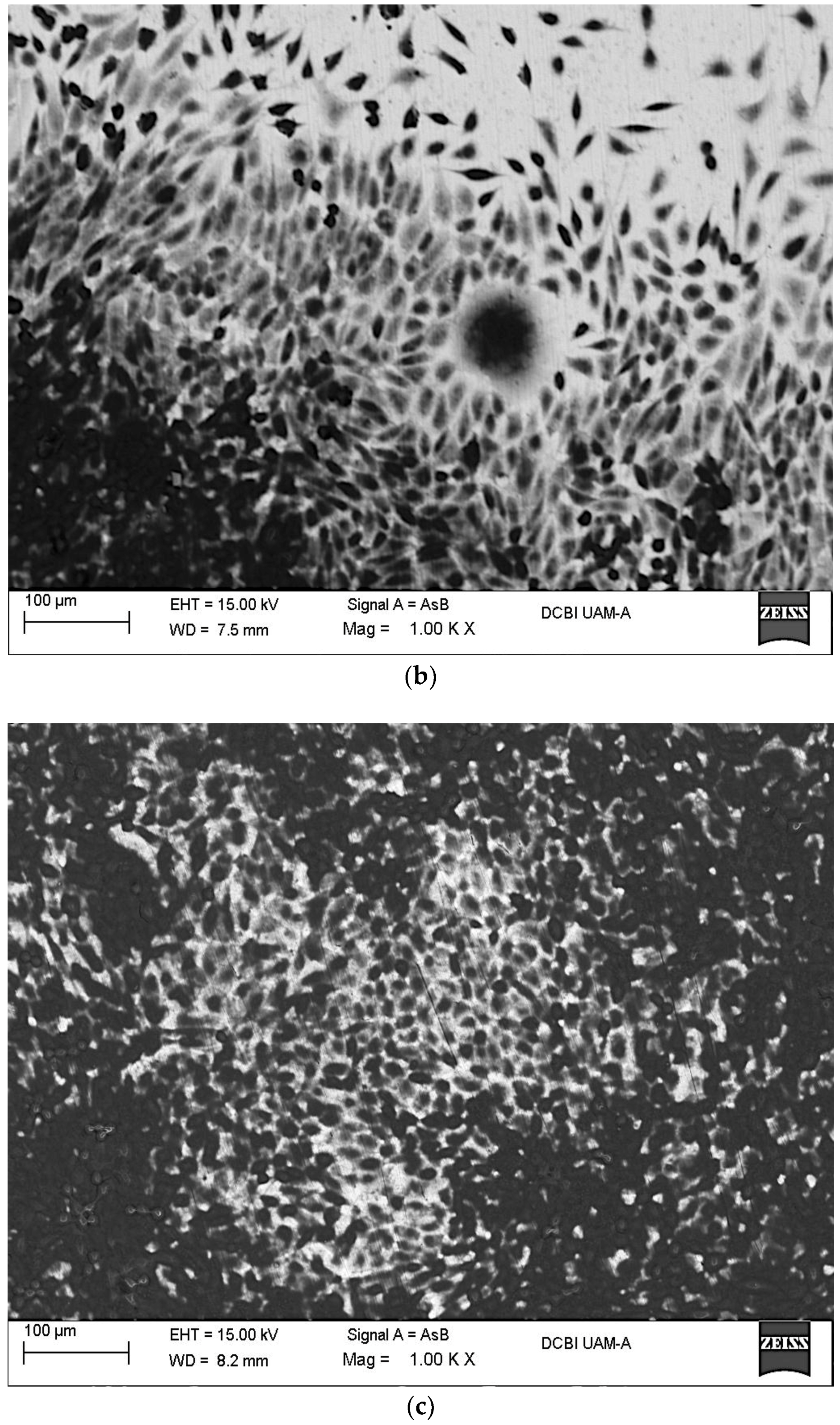
2.2. In Vitro Assays
2.3. Electrochemical Characterization
2.3.1. Evolution of Open Circuit Potentials (EOCP)
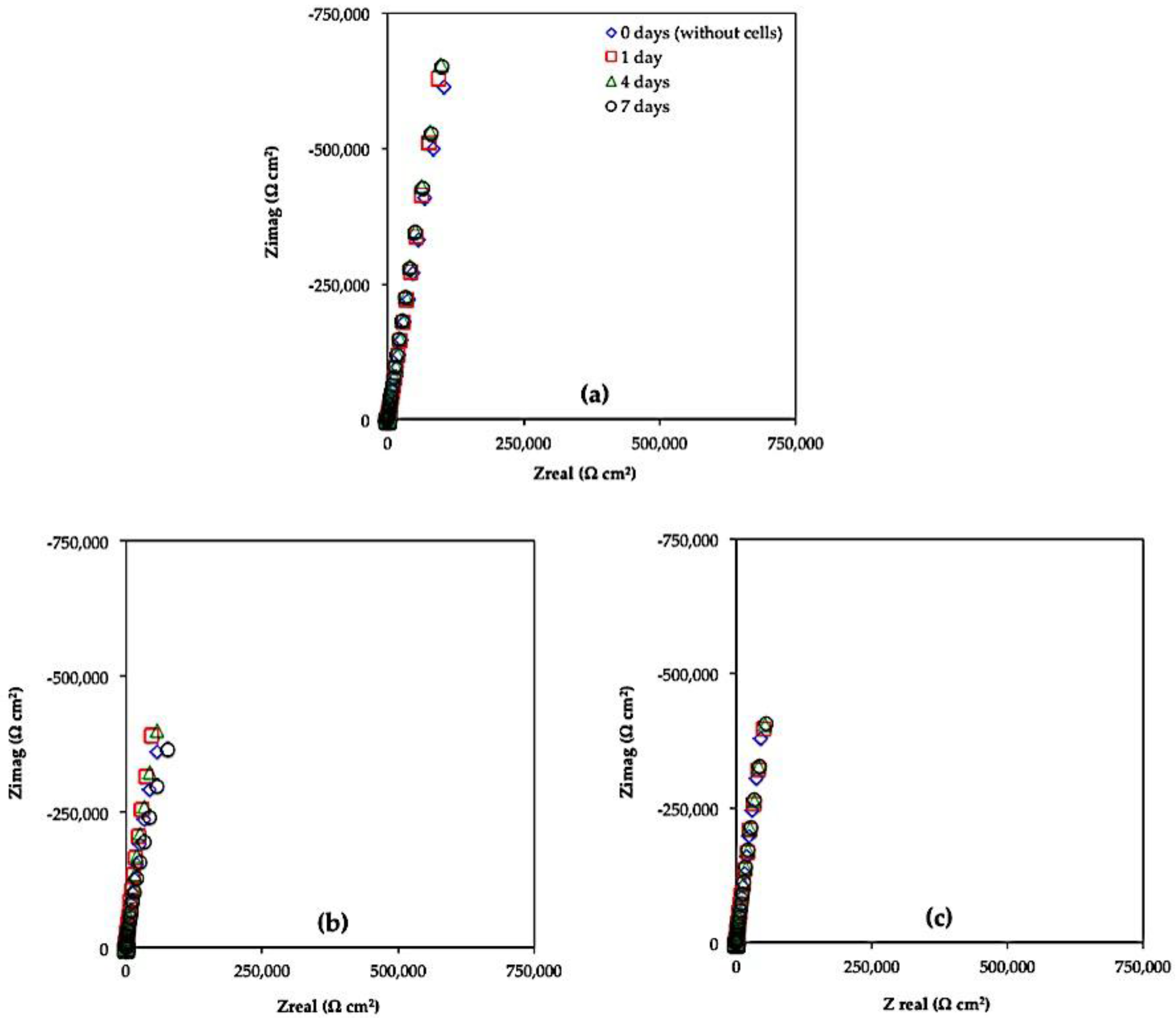
2.3.2. Electrochemical Impedance Spectroscopy (EIS)
3. Materials and Methods
3.1. Heat Treatments
3.2. Metallography
3.3. X-ray Diffraction and XPS Characterizations
3.4. In Vitro Assays
3.5. Cell Fixation
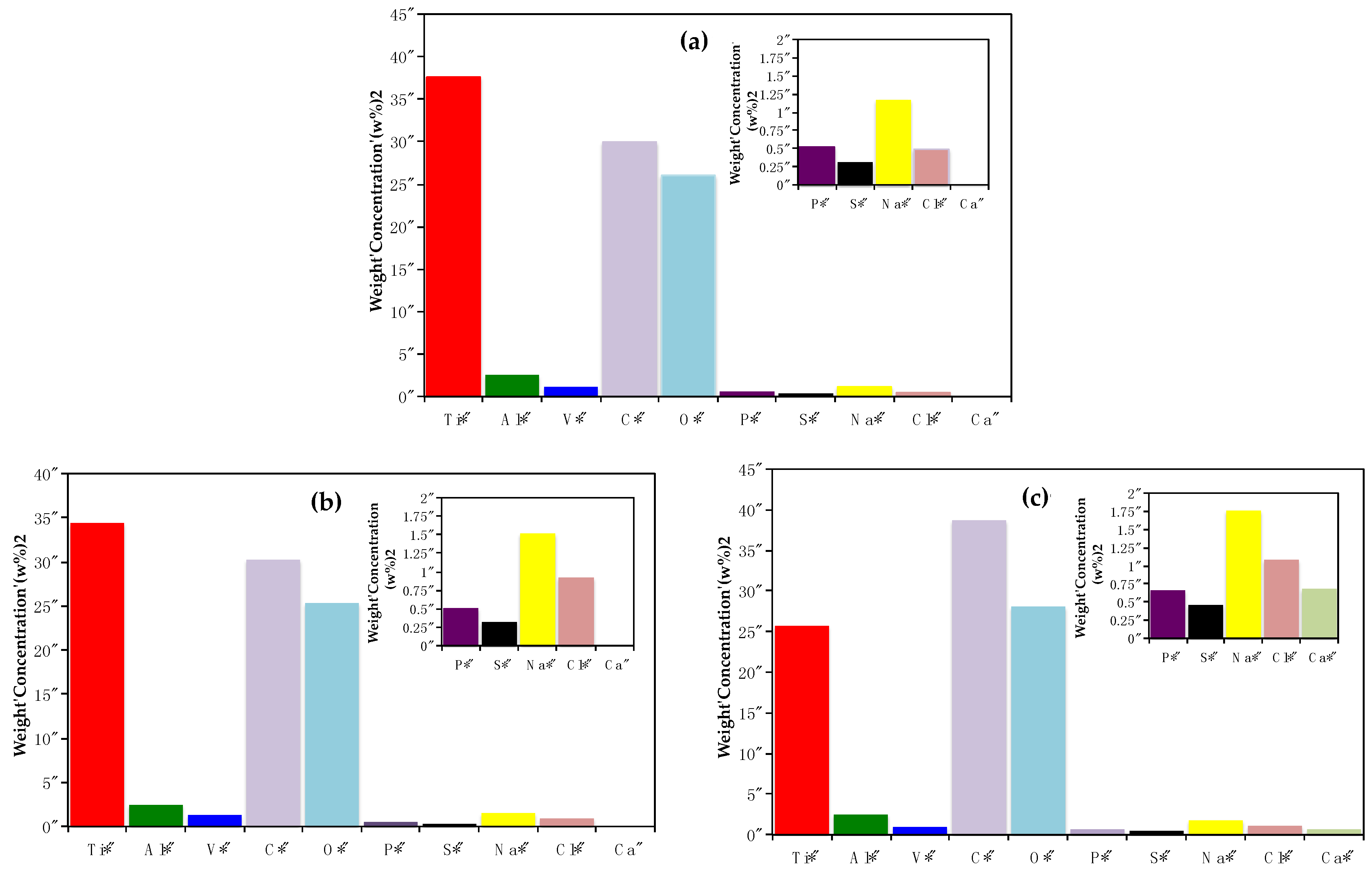
3.6. SEM-EDX Surface Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Scholz, M.S.; Blanchfield, J.P.; Bloom, L.D.; Coburn, B.H.; Elkington, M.; Fuller, J.D.; Gilbert, M.E.; Muflahi, S.A.; Pernice, M.F.; Rae, S.I.; et al. The use of composite materials in modern orthopedic medicine and prosthetic device. Compos. Sci. Technol. 2011, 71, 1791–1803. [Google Scholar] [CrossRef]
- Waite, D.E. Overview and historical perspective of oral reconstructive surgery. Oral Surg. Oral Med. Oral Pathol. 1989, 68, 495–498. [Google Scholar] [CrossRef]
- Lizuca, T.; Hallermann, W.; Seto, I.; Smolka, W. A titanium arch bar for maxillomandibular fixation in oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 2006, 64, 989–992. [Google Scholar]
- Castner, D.G.; Ratner, B.D. Biomedical surface science: Foundations to frontiers. Surf. Sci. 2002, 500, 28–60. [Google Scholar] [CrossRef]
- Sumita, M. Present status and future trend of metallic materials used in orthopedics. Orthop. Surg. 1997, 48, 927–934. [Google Scholar]
- Vydehi Arun, J. Titanium Alloys: An Atlas of Structures and Fracture Features, Physical Metallurgy of Titanium Alloys; CRC Press: Abingdon, UK, 2017; pp. 7–15. [Google Scholar]
- Donachie, M.J. Titanium a Technical Guide. Understanding the Metallurgy of Titanium; ASM International: Materials Park, OH, USA, 2000; pp. 13–24. [Google Scholar]
- Vrancken, B.; Thijs, L.; Kruth, J.P.; van Humbeeck, J. Heat treatment of Ti6Al4V produced by Selective Laser Melting: Microstructure and mechanical properties. J. Alloys Compd. 2012, 541, 177–185. [Google Scholar] [CrossRef]
- Hanawa, T.; Asami, K.; Asaoka, K. Repassivation of titanium and surface oxide film regenerated in simulated bioliquid. J. Biomed. Mater. Res. 1997, 40, 530–538. [Google Scholar] [CrossRef]
- Li, S.J.; Yang, R.; Niinomi, M.; Hao, Y.L.; Cui, Y.Y. Formation and growth of calcium phosphate on the surface of oxided Ti-29Nb-13Ta-4.6Zr alloy. Biomaterials 2004, 25, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Rafieerad, A.R.; Ashra, M.R.; Mahmoodian, R.; Bushroa, A.R. Surface characterization and corrosion behavior of calcium phosphate-base composite layer on titanium and its alloys via plasma electrolytic oxidation: A review paper. Mater. Sci. Eng. C 2015, 57, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Fatigue performance and cytotoxicity of low rigidity titanium alloy Ti-29Nb-13Ta-4.6Zr. Biomaterials 2003, 24, 2673–2683. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakai, M.; Akahori, T.; Niinomi, M.; Tsutsumi, Y.; Doi, H.; Hanawa, T. Characterization of air-formed surface oxide film on Ti-29Nb-13Ta-4.6Zr alloy surface using XPS and AES. Corros. Sci. 2008, 50, 2111–2116. [Google Scholar] [CrossRef]
- Variola, F.; Yi, J.H.; Richert, L.; Wuest, J.D.; Rosei, F.; Nanci, A. Tailoring the surface properties of Ti6Al4V by controlled chemical oxidation. Biomaterials 2008, 29, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- De Assis, S.L.; Wolynec, S.; Costa, I. Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim. Acta 2006, 51, 1815–1819. [Google Scholar] [CrossRef]
- Lausmaa, J.; Kasemo, B. Surface Spectroscopic Characterization on Titanium Implant Materials. Appl. Surf. Sci. 1990, 44, 133–146. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Hee, A.C.; Zhao, Y.; Jamali, S.S.; Martin, P.J.; Bendavid, A.; Peng, H.; Cheng, X. Corrosion behaviour and microstructure of tantalum film on Ti6Al4V substrate by filtered cathodic vacuum arc deposition. Thin Solid Films. 2017, 636, 54–62. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys and related materials for biomedical applications. Mater. Sci. Eng. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Williams, D.F. Titanium and Titanium Alloys, Biocompatibility of Clinical Implant Materials; CRC Press: Boca Raton, FL, USA, 1981. [Google Scholar]
- Goldberg, J.R.; Gilbert, J.L. The electrochemical and mechanical behavior of passivated and TiN/AlN coated CoCrMo and Ti6Al4V alloys. Biomaterials 2004, 25, 851–864. [Google Scholar] [CrossRef]
- Ramires, J.; Guastaldi, A.C. Study of Ti-6Al-4V biomaterial using electrochemistry and XPS techniques. Quim. Nova 2002, 25, 10–14. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Chávez-Díaz, M.P.; Escudero-Rincón, M.L.; Arce-Estrada, E.M.; Cabrera-Sierra, R. Osteoblast Cell Response on the Ti6Al4V Alloy Heat-Treated. Materials 2017, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Yang, M.; Zhu, Y.; Tomsia, A.; Mao, C. Untangling the Effects of Peptide Sequences and Nanotopographies in a Biomimetic Niche for Directed Differentiation of iPSCs by Assemblies of Genetically Engineered Viral Nanofibers. Nano Lett. 2014, 14, 6850–6856. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Yang, M.; Mao, C. Phage as a Genetically Modifiable Supramacromolecule in Chemistry, Materials and Medicine. Acc. Chem. Res. 2016, 49, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Q.; Mao, C.; Zhang, S. Osteogenic differentiation of bone marrow mesenchymal stem cells on the collagen/silk fibroin bitemplate induced biomimetic bone substitutes. J. Biomed. Mater. Res. Part A 2012, 100, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Vetsch, J.R.; Betts, D.C.; Müller, R.; Hofmann, S. Flow velocity-driven differentiation of human mesenchymal stromal cells in silk fibroin scaffolds: A combined experimental and computational approach. PLoS ONE 2017, 12, e0180781. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cao, I.; Liu, Y.; Zheng, A.; Wu, J.; Jiang, X.; Ji, P. Evaluation of synergistic osteogenesis between icariin and BMP2 through a micro/meso hierarchical porous delivery system. Int. J. Nanomed. 2017, 12, 7721–7735. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, M.C.; Saldaña, L.; Alonso, C.; Barranco, V.; Muñoz-Morris, M.A.; Escudero, M.L. In situ cell culture monitoring on a Ti-6Al-4V surface by electrochemical techniques. Acta Biomater. 2009, 5, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Sallica-Leva, E.; Jardini, A.L.; Fogagnolo, J.B. Microstructure and mechanical behavior of porous Ti-6Al-4V parts obtained by selective laser melting. J. Mech. Behav. Biomed. Mater. 2013, 26, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Elmer, J.W.; Palmer, T.A.; Babu, S.S.; Specht, E.D. In situ observations of lattice expansion and transformation rates of and phases in Ti-6Al-4V. Mater. Sci. Eng. A 2005, 391, 104–113. [Google Scholar] [CrossRef]
- Malinov, S.; Guo, Z.; Sha, W.; Wilson, A. Differential Scanning Calorimetry Study and Computer Modelling of β-α Phase Transformation in Ti-6Al-4VAlloy. Metall. Mater. Trans. A 2001, 32, 879–887. [Google Scholar] [CrossRef]
- Malinov, S.; Sha, W.; Guo, Z.; Tang, C.C.; Long, A.E. Synchrotron X-ray diffraction study of the phase transformations in titanium alloys. Mater. Charact. 2002, 48, 279–295. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, N.R.; Quinn, R.D. Auger and X-ray photoelectron spectroscopic and electrochemical characterization of titanium thin film electrodes. Surf. Sci. 1977, 67, 451–468. [Google Scholar] [CrossRef]
- Strohmeier, B.R. An ESCA method for determining the oxide thickness on aluminium-alloys. Surf. Interface Anal. 1990, 15, 51–56. [Google Scholar] [CrossRef]
- Milosev, M.; Metikos-Hukovic, M.; Strehblow, H.H. Passive film on orthopaedic TiAlV alloy formed in physiological solution investigated by X-ray photoelectron spectroscopy. Biomaterials 2000, 21, 2103–2113. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. An X-ray photoelectron spectroscopy sputter profile study of the native air-formed oxide film on titanium. Appl. Surf. Sci. 1999, 143, 92–100. [Google Scholar] [CrossRef]
- Feliu, S., Jr.; Barranco, V. XPS study of the surface chemistry of conventional hot-dip galvanised pure Zn, galvanneal and Zn–Al alloy coatings on steel. Acta Mater. 2003, 51, 5413–5424. [Google Scholar] [CrossRef]
- Bunker, B.C.; Nelson, G.C.; Zavadil, K.R.; Barbour, J.C.; Wall, F.D.; Sullivan, J.P. Hydration of Passive Oxide Films on Aluminum. J. Phys. Chem. B 2002, 106, 4705–4713. [Google Scholar] [CrossRef]
- Popa, M.V.; Demetrescu, I.; Vasilescu, E.; Drob, P.; Santana López, A.; Mirza-Rosca, J.; Vasilescu, C.; Ionita, D. Corrosion susceptibility of implant materials. Ti-5Al-4V and Ti-6Al-4Fe in artificial extra-cellular fluids. Electrochim. Acta 2004, 49, 2113–2121. [Google Scholar] [CrossRef]
- Chikarakara, E.; Fitzpatrick, P.; Moore, E.; Levingstone, T.; Grehan, L.; Higginbotham, C.; Vázquez, M.; Bagga, K.; Naher, S.; Brabazon, D. In vitro fibroblast and pre-osteoblastic cellular responses on laser surface modified Ti-6Al-4V. Appl. Surf. Sci. 2016, 366, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Lin, L.F.; Macdonald, D.D. A Point Defect model for anodic passive films, I. Film growth kinetics. J. Electrochem. Soc. 1981, 128, 1187–1193. [Google Scholar] [CrossRef]
- Cabrera-Sierra, R.; Hallen, J.M.; Vázquez-Arenas, J.; Vázquez, G.; González, I. EIS characterization of tantalum and niobium oxide films based on a modification of the point defect model. J. Electroanal. Chem. 2010, 638, 51–58. [Google Scholar] [CrossRef]
- Cabrera-Sierra, R.; Vázquez-Arenas, J.; Cardoso, S.; Luna-Sánchez, R.M.; Trejo, M.A.; Marín-Cruz, J.; Hallen, J.M. Analysis of the formation of Ta2O5 passive films in acid media through mechanistic modeling. Electrochim. Acta 2011, 56, 8040–8047. [Google Scholar] [CrossRef]
- Göpel, W.; Anderson, J.A.; Frenkel, D.; Jeaning, M.; Philips, K.; Schäfer, J.A.; Rocker, G. Surface defects of TiO2 (1 1 0): A combined XPS, XAES and ELS study. Surf. Sci. 1984, 67, 333–346. [Google Scholar] [CrossRef]
- Castellani, C.; Lindtner, R.A.; Hausbrandt, P.; Tschegg, E.; Stanzl-Tschegg, S.E.; Zanoni, G.; Beck, S.; Weinberg, A.M. Bone-implant interface strength and osseointegration: Biodegradable magnesium alloy versus standard titanium control. Acta Biomater. 2011, 1, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Contu, F.; Elsener, B.; Bohni, H. Characterization of implant materials in fetal bovine serum and sodium-sulfate by electrochemical impedance spectroscopy-I-Mechanically polished samples. J. Biomed. Mater. Res. 2002, 62, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Lee, T.M. Effect of surface chemistry and characteristics of Ti6Al4V on the Ca and P adsorption and ion dissolution in Hanks ethylene diamine tetraacetic acid solution. Biomaterials 2002, 23, 2917–2995. [Google Scholar] [CrossRef]
- Roessler, S.; Zimmermann, R.; Scharnweber, D.; Werner, C.; Worch, H. Characterization of oxide layers on Ti6Al4V and titanium by streaming potential and streaming current measurements. Colloids Surf. B 2002, 26, 87–395. [Google Scholar] [CrossRef]
- Einsenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of beta-stabilizing of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef] [PubMed]
- Frauchiger, L.; Taborelli, M.; Aronsson, B.O.; Descouts, P. Ion adsorption on titanium surface exposed to a physiological solution. Appl. Surf. Sci. 1999, 143, 67–77. [Google Scholar] [CrossRef]
- Khan, M.A.; Williams, R.L.; Williams, D.F. The corrosion behavior of Ti-6Al-4V, Ti-6Al-7Nb and Ti-13Nb-13Zr in protein solutions. Biomaterials 1999, 20, 631–637. [Google Scholar] [CrossRef]
- Barranco, V.; Escudero, M.L.; García-Alonso, M.C. Influence of the microstructure and topography on the barrier properties of oxide scales generated on blasted Ti6Al4V surfaces. Acta Biomaterialia. 2011, 7, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.-C.; Wu, E.-Y.; Pan, Y.-N.; Ou, K.-L. Effects of Chemical and Heat Treatments on Surface Characteristics and Biocompatibility of Titanium-Niobium Alloys. Mater. Trans. 2007, 48, 2978–2985. [Google Scholar] [CrossRef]
- Lavos-Valereto, I.C.; Wolynec, S.; Ramires, I.; Guastaldi, A.C.; Costa, I. Electrochemical impedance spectroscopy characterization of passive film formed on implant Ti6Al7Nb alloy in Hank’s solution. J. Mater. Sci. 2004, 15, 55–59. [Google Scholar]
- Kubies, D.; Himmlová, L.; Riedel, T.; Chánová, E.; Balík, K.; Doudeˇrová, M.; Bártová, J.; Pešáková, V. The Interaction of Osteoblasts with Bone-Implant Materials: 1. The effects of physicochemical surface properties of implant materials. Physiol. Res. 2011, 60, 95–111. [Google Scholar] [PubMed]
- Chesmel, K.D.; Clark, C.C.; Brighton, C.T.; Black, J. Cellular responses to chemical and morphologic aspects of biomaterial surfaces. II. The biosynthetic and migratory response of bone cell populations. J. Biomed. Mater. Res. 1995, 29, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Oates, C.J.; Wen, W.; Hamilton, D.W. Role of Titanium Surface Topography and Surface Wettability on Focal Adhesion Kinase Mediated Signaling in Fibroblasts. Materials 2011, 4, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Szymonowicz, M.; Korczynski, M.; Dobrzynski, M.; Zawisza, K.; Mikulewicz, M.; Karuga-Kuzniewska, E.; Zywicka, B.; Rybak, Z.; Wiglusz, R.J. Cytotoxicity Evaluation of High-Temperature Annealed Nanohydroxyapatite in Contact with Fibroblast Cells. Materials 2017, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Cabrera-Sierra, R.; Pech-Canul, M.A.; González, I. The Role of Hydroxide in the Electrochemical Impedance Response of Passive Films in Corrosion Environments. J. Electrochem. Soc. 2006, 153, B101–B107. [Google Scholar] [CrossRef]
- Takana, Y.; Kobayashi, E.; Hiromoto, K.; Asami, H.; Imai, H.; Hanawa, T. Calcium phosphate formation on titanium by low-voltage electrolytic treatments. J. Mater. Sci. 2007, 18, 797–806. [Google Scholar]
- Ban, S.; Maruno, S. Deposition of calcium phosphate on titanium by electrochemical process in simulated body fluid. Jpn. J. Appl. Phys. 1993, 32, L1577. [Google Scholar] [CrossRef]
- Cremasco, A.; Dutra Messias, A.; Rodrigues Esposito, E. Aparecida de Rezende Duek, R. Caram. Effects of alloying elements on the cytotoxic response of titanium alloys. Mater. Sci. Eng. C 2011, 31, 833–839. [Google Scholar] [CrossRef]
- Bargeron, C.B.; Givens, R.B. Precursive blistering in the localized corrosion of aluminum. Corrosion 1980, 36, 618–625. [Google Scholar] [CrossRef]
- Bargeron, C.B.; Givens, R.B. Localized Corrosion of Aluminum: Blister Formation as a Precursor of Pitting. J. Electrochem. Soc. 1977, 124, 1845–1848. [Google Scholar] [CrossRef]
- Puleo, D.A.; Nanci, A. Understanding and controlling the bone–implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Thompson, G.J.; Puleo, D.A. Ti-6Al-4V ion solution inhibition of osteogenic cell phenotype as a function of differentiation time course in vitro. Biomaterials 1996, 17, 54–1949. [Google Scholar] [CrossRef]
- Nichols, K.G.; Puleo, D.A. Effect of metal ions on the formation and function of osteoclastic cells in vitro. J. Biomed. Mater. Res. 1997, 35, 71–265. [Google Scholar] [CrossRef]
- Neupane, M.P.; Kim, Y.K.; Park, S.I.; Lee, S.J.; Lee, M.H.; Bae, T.S. Effect of pH on the Structure and in vitro Osteoblasts Response to Anodic Titanium Oxide. Met. Mater. Int. 2008, 14, 607–613. [Google Scholar] [CrossRef]
- Geetha, M.; Kamachi Mudali, U.; Gogia, A.K.; Asokamani, R.; Raj, B. Influence of microstructure and alloying elements on corrosion behavior of Ti-13Nb-13Zr alloy. Corros. Sci. 2004, 46, 877–892. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, C.; Kim, D.G.; Choi, K.; Lee, K.H.; Kim, Y.D. Effect of surface structure on biomechanical properties and osseointegration. Mater. Sci. Eng. C 2008, 28, 1448–1461. [Google Scholar] [CrossRef]
- Geetha, M.; Durgalaksshmi, D.; Asokamani, R. Biomedical Implants: Corrosion and its Prevention-A Review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar]
- Mustafa, K.; Pan, J.; Wroblewsli, J.; Leygraf, C.; Arvidson, K. Electrochemical impedance spectroscopy and X-ray photoelectron spectroscopy analysis of titanium surfaces cultured with osteoblast-like cells derived from human mandibular bone. J. Biomed. Mater. Res. 2002, 59, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Mróz, W.; Bunder, B.; Syroka, R.; Niedzielski, K.; Golanski, G.; Slósarczyk, A.; Schwarz, D.; Douglas, T.E. In vivo implantation of porous titanium alloy implants coated with magnesium-doped octacalcium phosphate and hydroxyapatite thin films using pulsed laser deposition. J. Biomed. Mater. Res. Part B 2014, 103, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Linez, P.; Bigerelle, M.; Le Maguer, D.; Le Maguer, A.; Hardouin, P.; Hildebrand, H.F.; Iost, A.; Leroy, J.M. The relative influence of the topography and chemistry of TiAl6V4 surfaces on osteoblastic cell behaviour. Biomaterials 2000, 21, 1567–1577. [Google Scholar] [CrossRef]
- Karimzadeh, F.; Heidarbeigy, M.; Saatchi, A. Effect of heat treatment on corrosion behavior of Ti-6Al-4V alloy weldments. J. Mater. Process. Technol. 2008, 206, 388–394. [Google Scholar] [CrossRef]
- Wang, X.J.; Li, Y.C.; Lin, J.G.; Hodgson, P.D.; Wen, C. Effect of heat-treatment atmosphere on the bond strength of apatite layer on Ti substrate. Dent. Mater. 2008, 24, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Farhang, P.; Pupak, A.; Sirous, A. Influence of mechanical and chemical surface treatments on the formation of bone-like structure in cpTi for endosseous dental implants. Appl. Surf. Sci. 2012, 259, 283–287. [Google Scholar]
- He, X.; Zhang, G.; Wang, X.; Hang, R.; Huang, X.; Qin, L.; Tang, B.; Zhang, X. Biocompatibility, corrosion resistance and antibacterial activity of TiO2/CuO coating on titanium. Ceram. Int. 2017, 43, 16185–16195. [Google Scholar] [CrossRef]
- Burgos-Asperilla, L.; Garcia Fierro, J.L.; Gamero, M.; Escudero, M.L.; Alonso, C.; García-Alonso, M.C. In situ electrochemical study of the interaction of cells with thermally treated titanium. Biointerphases 2015, 10, 021006. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Asperilla, L.; García-Alonso, M.C.; Escudero, M.L.; Alonso, C. Cell adhesion on Ti surface with controlled roughness. Rev. Metal. 2015, 51. [Google Scholar] [CrossRef]



| --- | Time (Days) | Re (Ω cm2) | Rextra (Ω cm2) | Qcell (Siemens sn) (cm−2) | n | Rf (Ω cm2) | Qf (Siemens sn) (cm−2) | N | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| Ti6Al4V | 0 | 49.82 | --- | --- | --- | 1.07 × 108 | 1.84 × 10−5 | 0.859 | 4.36 × 10−3 |
| 1 | 67.29 | 6661 | 1.79 × 10−5 | 0.887 | 1.29 × 108 | 8.47 × 10−7 | 0.978 | 4.40 × 10−4 | |
| 4 | 59.11 | 1995 | 1.79 × 10−5 | 0.900 | 1.34 × 108 | 8.56 × 10−7 | 0.977 | 8.87 × 10−4 | |
| 7 | 66.33 | 1177 | 1.82 × 10−5 | 0.903 | 1.61 × 108 | 8.62 × 10−7 | 0.993 | 4.84 × 10−4 | |
| Ti6Al4V800 | 0 | 34.33 | --- | --- | --- | 1.17 × 108 | 3.59 × 10−5 | 0.915 | 6.70 × 10−3 |
| 1 | 45.14 | 8515 | 3.39 × 10−5 | 0.929 | 1.34 × 108 | 4.47 × 10−7 | 0.991 | 6.7 0 × 10−3 | |
| 4 | 42.14 | 2866 | 3.28 × 10−5 | 0.926 | 1.64 × 108 | 6.09 × 10−7 | 0.956 | 7.69 × 10−3 | |
| 7 | 39.40 | 1686 | 3.45 × 10−5 | 0.921 | 8.40 × 106 | 1.92 × 10−7 | 0.937 | 5.75 × 10−3 | |
| Ti6Al4V1050 | 0 | 58.88 | --- | --- | --- | 1.74 × 108 | 3.46 × 10−5 | 0.927 | 4.77 × 10−3 |
| 1 | 51.03 | 7760 | 3.28 × 10−5 | 0.923 | 1.06 × 108 | 1.41 × 10−7 | 0.940 | 6.67 × 10−3 | |
| 4 | 44.89 | 1277 | 3.22 × 10−5 | 0.922 | 1.76 × 108 | 1.59 × 10−7 | 0.939 | 5.09 × 10−3 | |
| 7 | 55.36 | 945 | 3.20 × 10−5 | 0.924 | 1.96 × 108 | 1.87 × 10−7 | 0.942 | 9.35 × 10−3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Díaz, M.P.; Escudero-Rincón, M.L.; Arce-Estrada, E.M.; Cabrera-Sierra, R. Effect of the Heat-Treated Ti6Al4V Alloy on the Fibroblastic Cell Response. Materials 2018, 11, 21. https://doi.org/10.3390/ma11010021
Chávez-Díaz MP, Escudero-Rincón ML, Arce-Estrada EM, Cabrera-Sierra R. Effect of the Heat-Treated Ti6Al4V Alloy on the Fibroblastic Cell Response. Materials. 2018; 11(1):21. https://doi.org/10.3390/ma11010021
Chicago/Turabian StyleChávez-Díaz, Mercedes Paulina, María Lorenza Escudero-Rincón, Elsa Miriam Arce-Estrada, and Román Cabrera-Sierra. 2018. "Effect of the Heat-Treated Ti6Al4V Alloy on the Fibroblastic Cell Response" Materials 11, no. 1: 21. https://doi.org/10.3390/ma11010021




