The Enhanced Catalytic Performance and Stability of Rh/γ-Al2O3 Catalyst Synthesized by Atomic Layer Deposition (ALD) for Methane Dry Reforming
Abstract
:1. Introduction
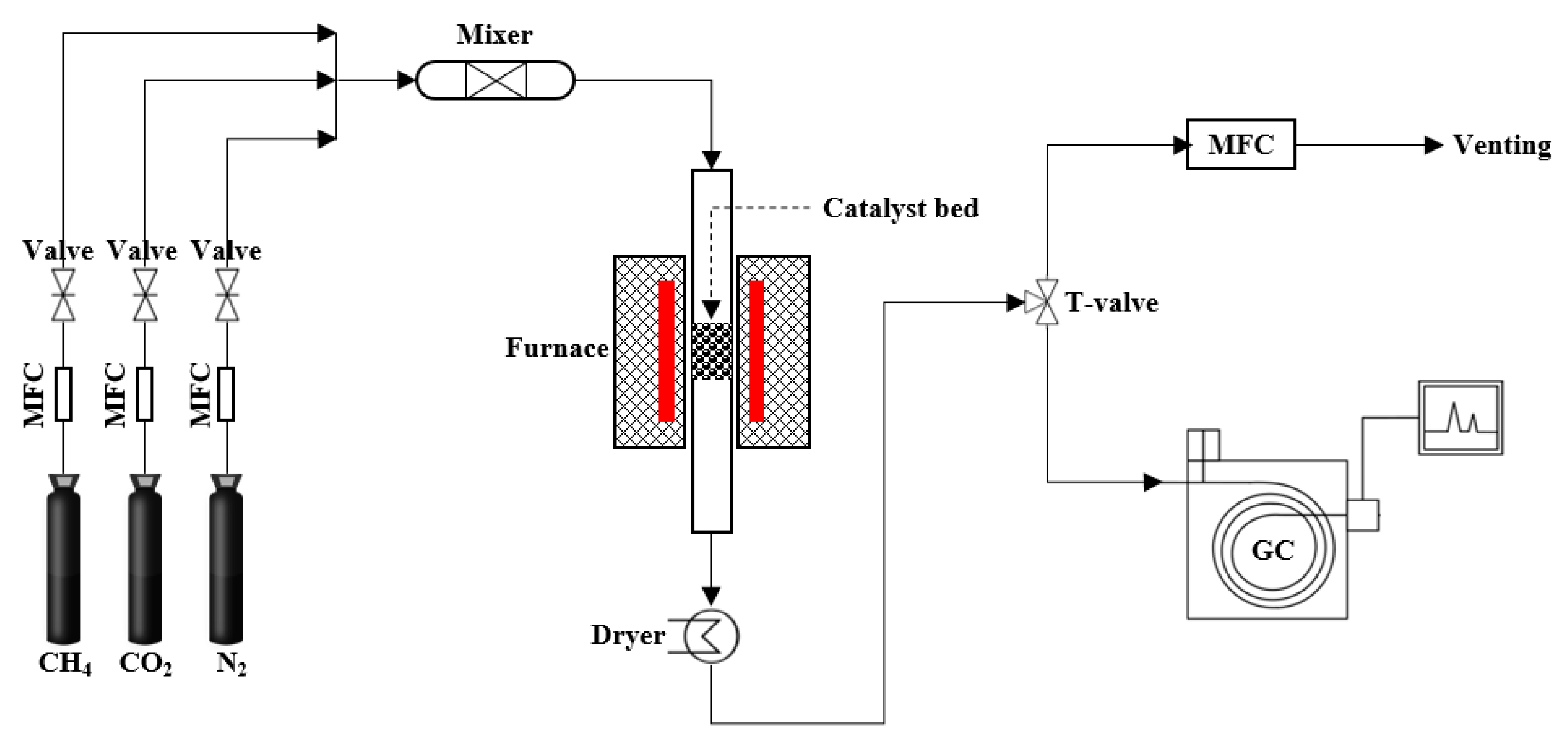
2. Experimental
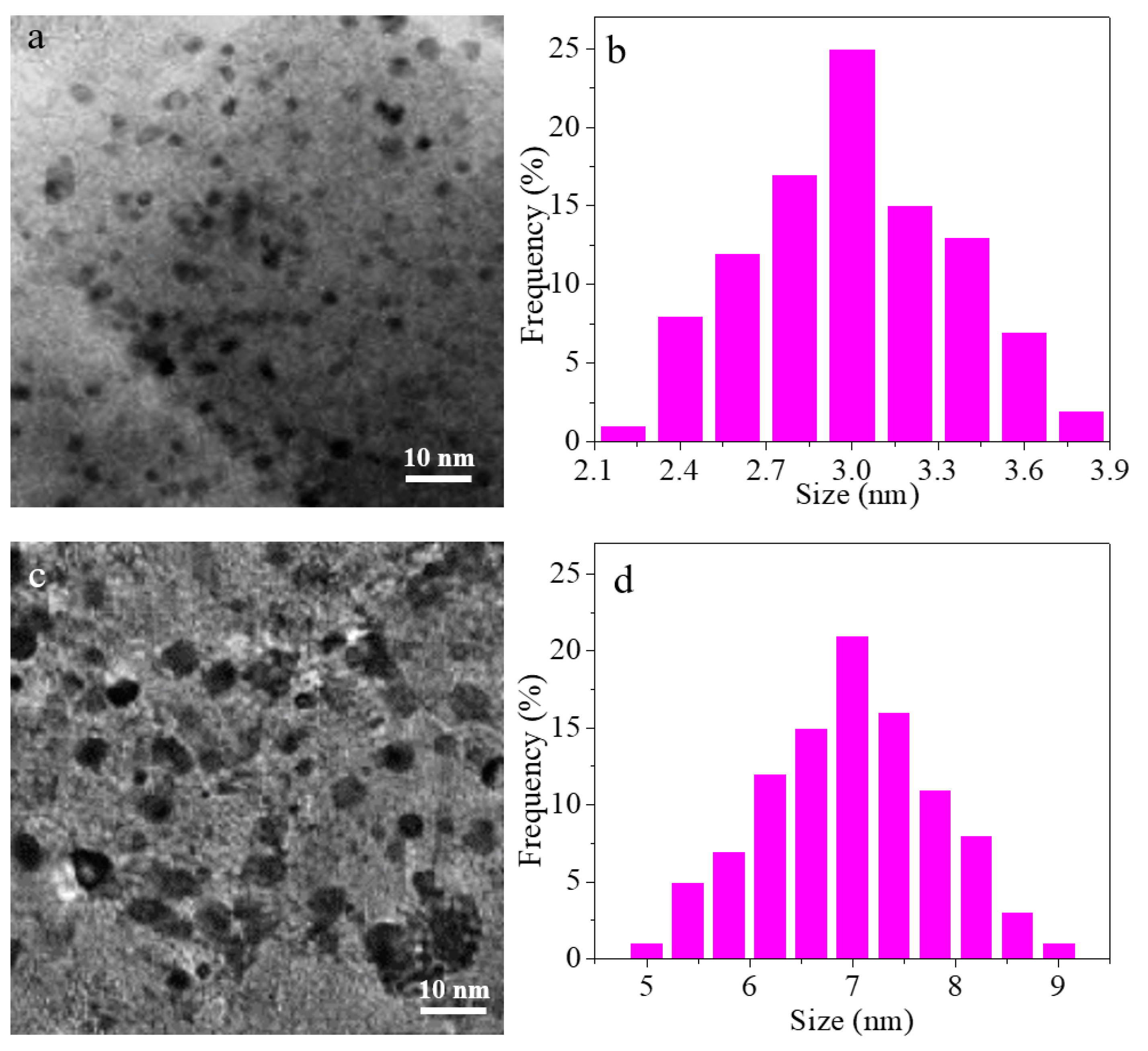
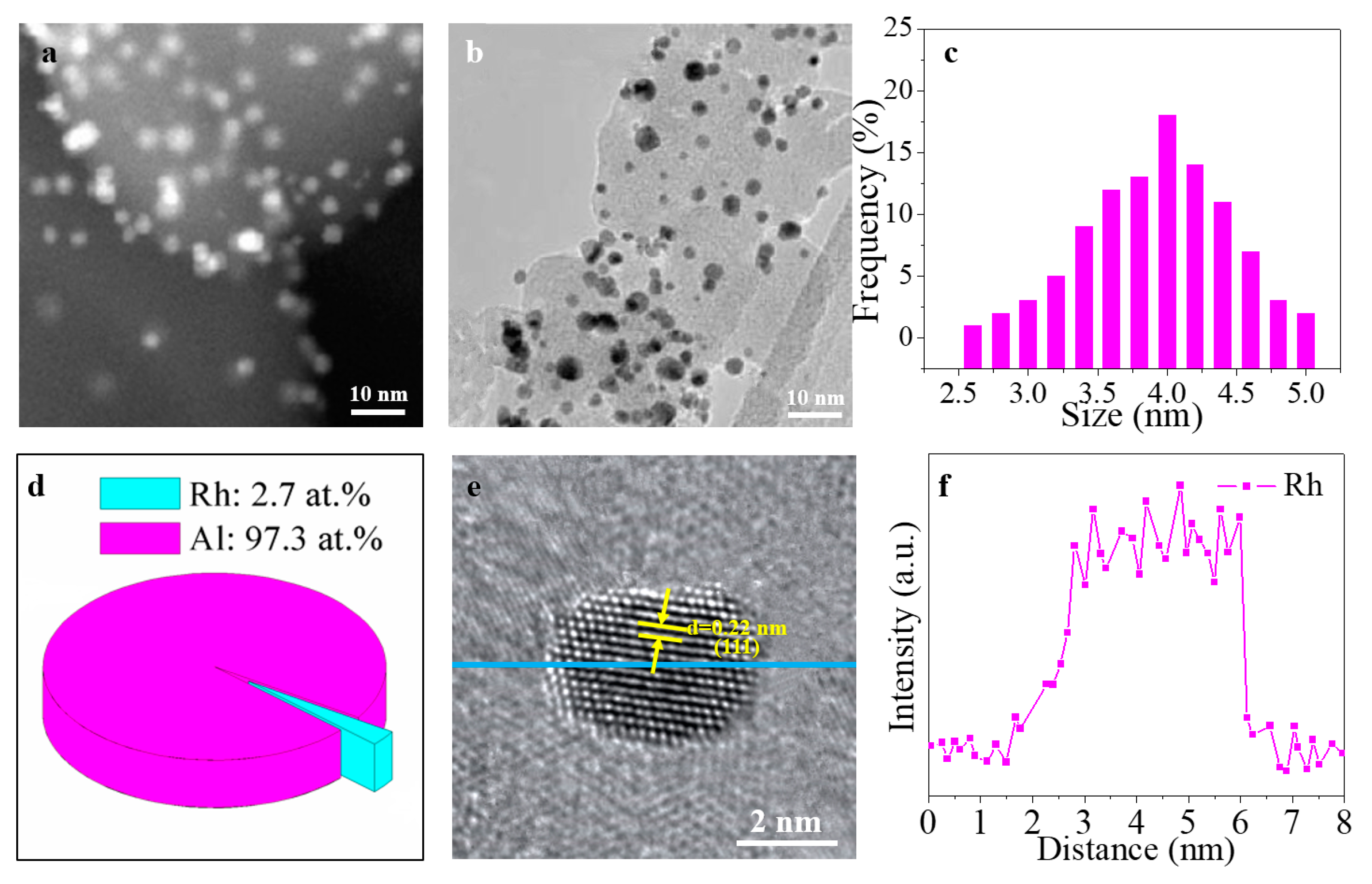
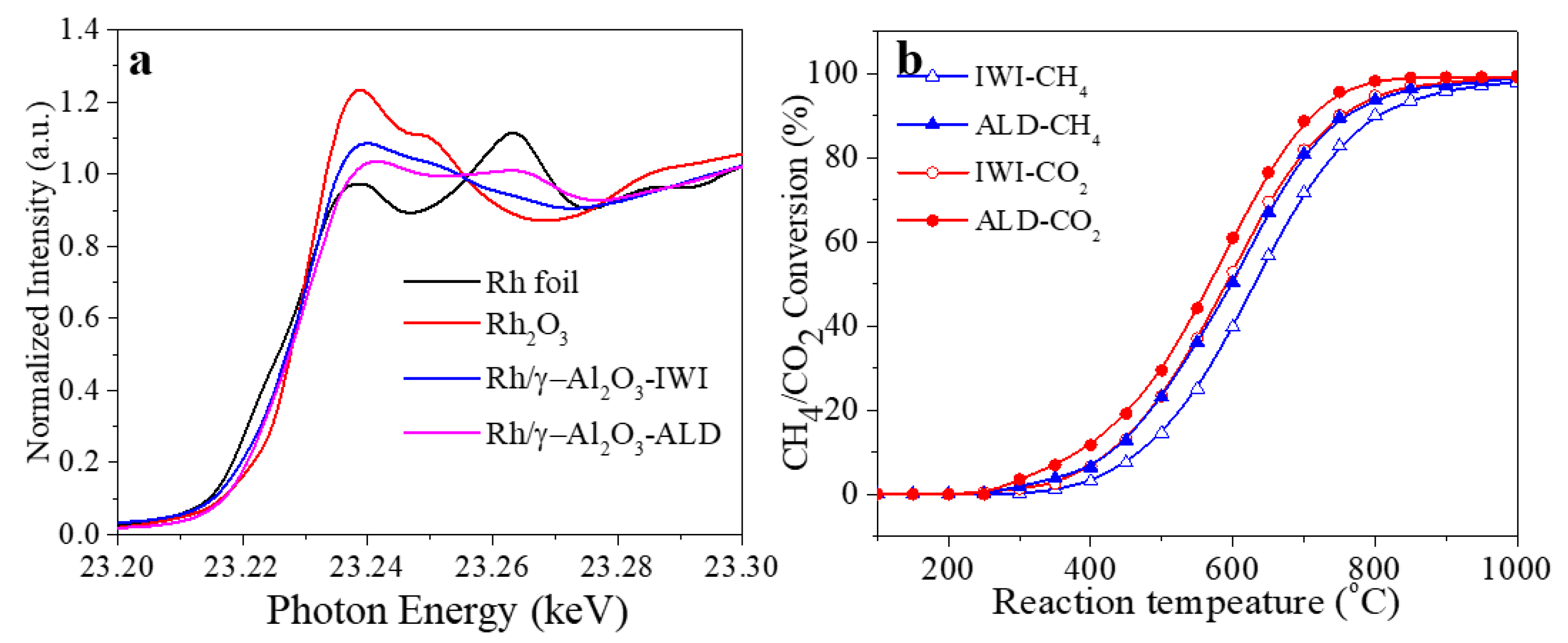
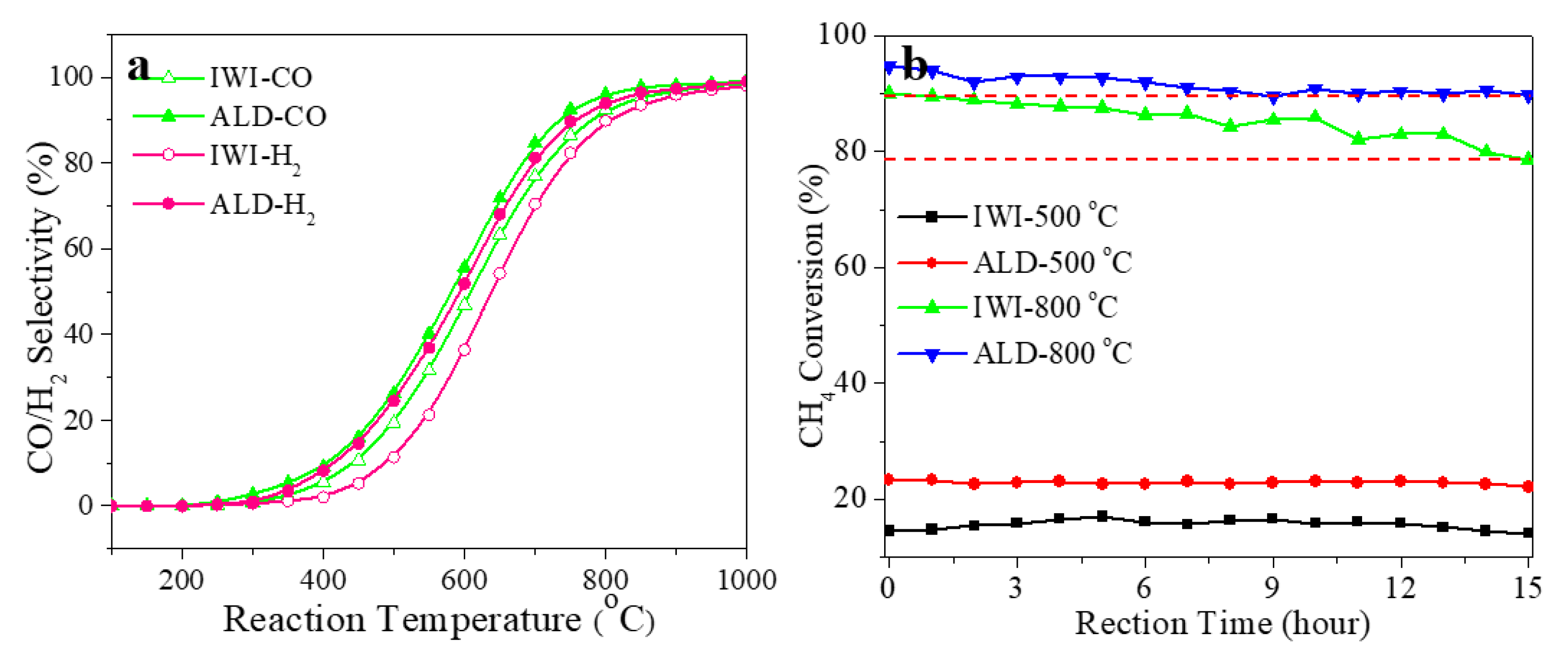
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Khajenoori, M. Combined dry reforming and partial oxidation of methane to synthesis gas on noble metal catalysts. Int. J. Hydrog. Energy 2011, 36, 2969–2978. [Google Scholar] [CrossRef]
- Fan, M.; Liu, Y.; Jha, R.; Dulikravich, G.S.; Schwartz, J.; Koch, C.C. On the evolution of Cu-Ni-rich bridges of Alnico alloys with tempering. J. Magn. Magn. Mater. 2016, 420, 296–302. [Google Scholar] [CrossRef]
- Jha, R.; Dulikravich, G.S.; Colaço, M.J.; Fan, M.; Schwartz, J.; Koch, C.C. Magnetic Alloys Design Using Multi-objective Optimization. In Properties and Characterization of Modern Materials; Öchsner, A., Altenbach, H., Eds.; Springer: Singapore, 2017; pp. 261–284. [Google Scholar]
- Zaddach, A.J.; Niu, C.; Oni, A.A.; Fan, M.; LeBeau, J.M.; Irving, D.L.; Koch, C.C. Structure and magnetic properties of a multi-principal element Ni–Fe–Cr–Co.–Zn–Mn alloy. Intermetallics 2016, 68, 107–112. [Google Scholar] [CrossRef]
- Verykios, X.E. Catalytic dry reforming of natural gas for the production of chemicals and hydrogen. Int. J. Hydrog. Energy 2003, 28, 1045–1063. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer-Tropsch Synthesis: Reaction mechanisms for iron catalysts. Catal. Today 2009, 141, 25–33. [Google Scholar] [CrossRef]
- Fan, M.; Liu, Y.; Jha, R.; Dulikravich, G.S.; Schwartz, J.; Koch, C.C. On the formation and evolution of Cu-Ni-Rich bridges of Alnico alloys with thermomagnetic treatment. IEEE Trans. Magn. 2016, 52, 1–10. [Google Scholar] [CrossRef]
- Jha, R.; Dulikravich, G.S.; Chakraborti, N.; Fan, M.; Schwartz, J.; Koch, C.C.; Colaco, M.J.; Poloni, C.; Egorov, I.N. Algorithms for design optimization of chemistry of hard magnetic alloys using experimental data. J. Alloy Compd. 2016, 682, 454–467. [Google Scholar] [CrossRef]
- Zhang, G.; Fan, M. Synthesis and magnetic properties of double B mixed perovskite series La0.75K0.25Mn1−xFexO3. Chem. Lett. 2011, 40, 244–245. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Ge, Y.; Shen, J.; Zhao, Y.; Zhang, Y.; Yuan, J. Design and synthesis of porous TiO2@C nanotube bundles with enhanced supercapacitive performance. Ceram. Int. 2017, 43, 2876–2880. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G. Biogas Management: Advanced utilization for production of renewable energy and added-value chemicals. Front. Environ. Sci. 2017, 5. [Google Scholar] [CrossRef]
- Lavoie, J.-M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Nguyen, T.; Pomilio, I.; Vitale, M.C.; Velu, S.E. Total synthesis of calothrixins A and B via oxidative radical reaction of cyclohexenone with aminophenanthridinedione. Tetrahedron 2014, 70, 5928–5933. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Nijampatnam, B.; Dutta, S.; Velu, S. Cyanobacterial metabolite calothrixins: recent advances in synthesis and biological evaluation. Mar. Drugs 2016, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst design with atomic layer deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar] [CrossRef]
- Zyulkov, I.; Krishtab, M.; De Gendt, S.; Armini, S. Selective Ru ALD as a catalyst for sub-seven-nanometer bottom-up metal interconnects. ACS Appl. Mater. Interfaces 2017, 9, 31031–31041. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Lubers, A.M.; Neltner, B.T.; Carrier, J.V.; Weimer, A.W.; Falconer, J.L.; Will Medlin, J. Synthesis of supported Ni catalysts by atomic layer deposition. J. Catal. 2013, 303, 9–15. [Google Scholar] [CrossRef]
- Singh, J.A.; Yang, N.; Bent, S.F. Nanoengineering heterogeneous catalysts by atomic layer deposition. Ann. Rev. Chem. Biomol. Eng. 2017, 8, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Sun, X. Single atom catalyst by atomic layer deposition technique. Chin. J. Catal. 2017, 38, 1508–1514. [Google Scholar] [CrossRef]
- Nguyen, T.; Nadkarni, D.; Dutta, S.; Xu, S.; Kim, S.; Murugesan, S.; Velu, S. Synthesis of pyrroloquinones via a can mediated oxidative free radical reaction of 1,3-Dicarbonyl compounds with aminoquinones. J. Chem. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Liu, B.; Lu, J.; Lobo-Lapidus, R.J.; Wu, T.; Feng, H.; Xia, X.; Mane, A.U.; Libera, J.A.; Greeley, J.P.; et al. Synthesis of Pt–Pd Core–Shell nanostructures by atomic layer deposition: application in propane oxidative dehydrogenation to propylene. Chem. Mater. 2012, 24, 3525–3533. [Google Scholar] [CrossRef]
- Christensen, S.T.; Feng, H.; Libera, J.L.; Guo, N.; Miller, J.T.; Stair, P.C.; Elam, J.W. Supported Ru−Pt bimetallic nanoparticle catalysts prepared by atomic layer deposition. Nano Lett. 2010, 10, 3047–3051. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sim, J.K.; Lee, J.; Seo, H.O.; Jeong, M.-G.; Kim, Y.D.; Kim, S.H. Carbon dioxide reforming of methane over mesoporous Ni/SiO2. Fuel 2013, 112, 111–116. [Google Scholar] [CrossRef]
- Seo, H.O.; Sim, J.K.; Kim, K.-D.; Kim, Y.D.; Lim, D.C.; Kim, S.H. Carbon dioxide reforming of methane to synthesis gas over a TiO2–Ni inverse catalyst. Appl. Catal. A Gen. 2013, 451, 43–49. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, A.T.; Cheetham, A.K.; Green, M.L.H.; Vernon, P.D.F. Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 1991, 352, 225–226. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Katsoni, A.; Diamadopoulos, E.; Mantzavinos, D.; Delimitis, A. Dry reforming of methane: catalytic performance and stability of ir catalysts supported on γ-Al2O3, Zr0.92Y0.08O2−δ (YSZ) or Ce0.9Gd0.1O2−δ (GDC) supports. Top. Catal. 2015, 58, 1228–1241. [Google Scholar] [CrossRef]
- Vernoux, P.; Lizarraga, L.; Tsampas, M.N.; Sapountzi, F.M.; De Lucas-Consuegra, A.; Valverde, J.-L.; Souentie, S.; Vayenas, C.G.; Tsiplakides, D.; Balomenou, S.; et al. Ionically conducting ceramics as active catalyst supports. Chem. Rev. 2013, 113, 8192–8260. [Google Scholar] [CrossRef] [PubMed]
- Newton, M.A.; Fiddy, S.G.; Guilera, G.; Jyoti, B.; Evans, J. Oxidation/reduction kinetics of supported Rh/Rh2O3 nanoparticles in plug flow conditions using dispersive EXAFS. Chem. Commun. 2005, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.-Z.; Yuan, Z.-Y.; Su, B.-L. Microwave-Assisted preparation of hierarchical mesoporous−macroporous boehmite AlOOH and γ-Al2O3. Langmuir 2004, 20, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Erdohelyi, A.; Cserenyi, J.; Solymosi, F. Activation of CH4 and its reaction with CO2 over supported Rh catalysts. J. Catal. 1993, 141, 287–299. [Google Scholar] [CrossRef]
- Múnera, J.F.; Cornaglia, L.M.; Cesar, D.V.; Schmal, M.; Lombardo, E.A. Kinetic studies of the dry reforming of methane over the Rh/La2O3−SiO2 catalyst. Ind. Eng. Chem. Res. 2007, 46, 7543–7549. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Jiang, J.; Zhu, C.; Li, L.; Li, Q.; Ding, Y.; Yang, W. The Enhanced Catalytic Performance and Stability of Rh/γ-Al2O3 Catalyst Synthesized by Atomic Layer Deposition (ALD) for Methane Dry Reforming. Materials 2018, 11, 172. https://doi.org/10.3390/ma11010172
Li Y, Jiang J, Zhu C, Li L, Li Q, Ding Y, Yang W. The Enhanced Catalytic Performance and Stability of Rh/γ-Al2O3 Catalyst Synthesized by Atomic Layer Deposition (ALD) for Methane Dry Reforming. Materials. 2018; 11(1):172. https://doi.org/10.3390/ma11010172
Chicago/Turabian StyleLi, Yunlin, Jing Jiang, Chaosheng Zhu, Lili Li, Quanliang Li, Yongjie Ding, and Weijie Yang. 2018. "The Enhanced Catalytic Performance and Stability of Rh/γ-Al2O3 Catalyst Synthesized by Atomic Layer Deposition (ALD) for Methane Dry Reforming" Materials 11, no. 1: 172. https://doi.org/10.3390/ma11010172