Nitrogen-Containing Functional Groups-Facilitated Acetone Adsorption by ZIF-8-Derived Porous Carbon
Abstract
:1. Introduction
2. Experimental Section
2.1. Material Synthesis
2.2. Physicochemica-Characterization of the Samples
2.3. Adsorption Measurement
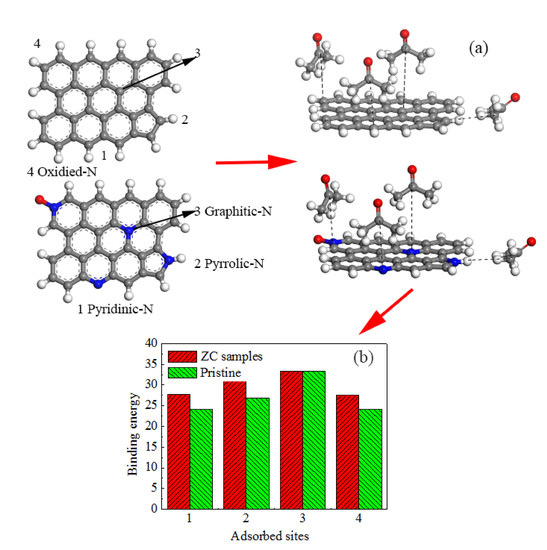
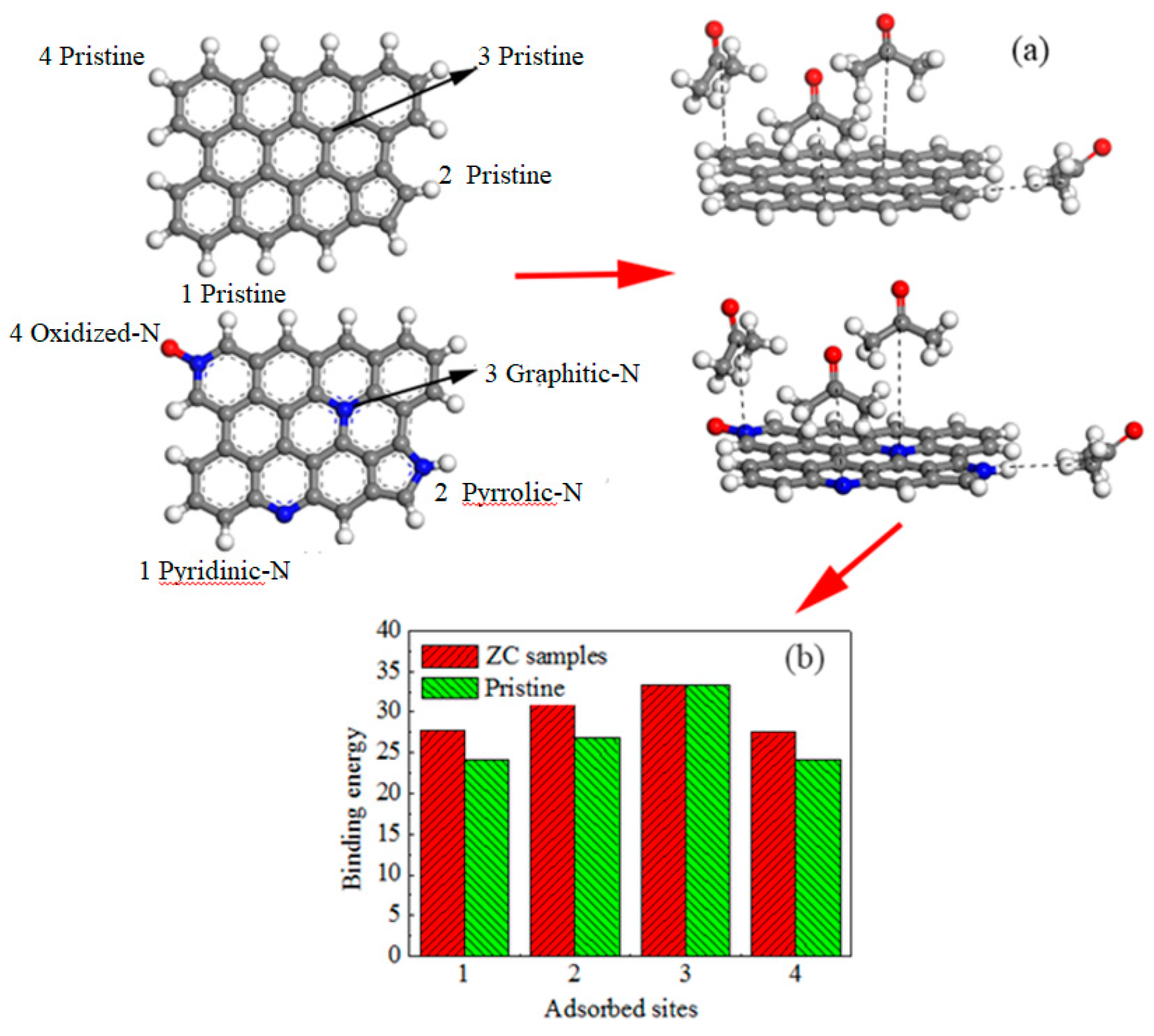
2.4. Computational Details
3. Results and Discussion
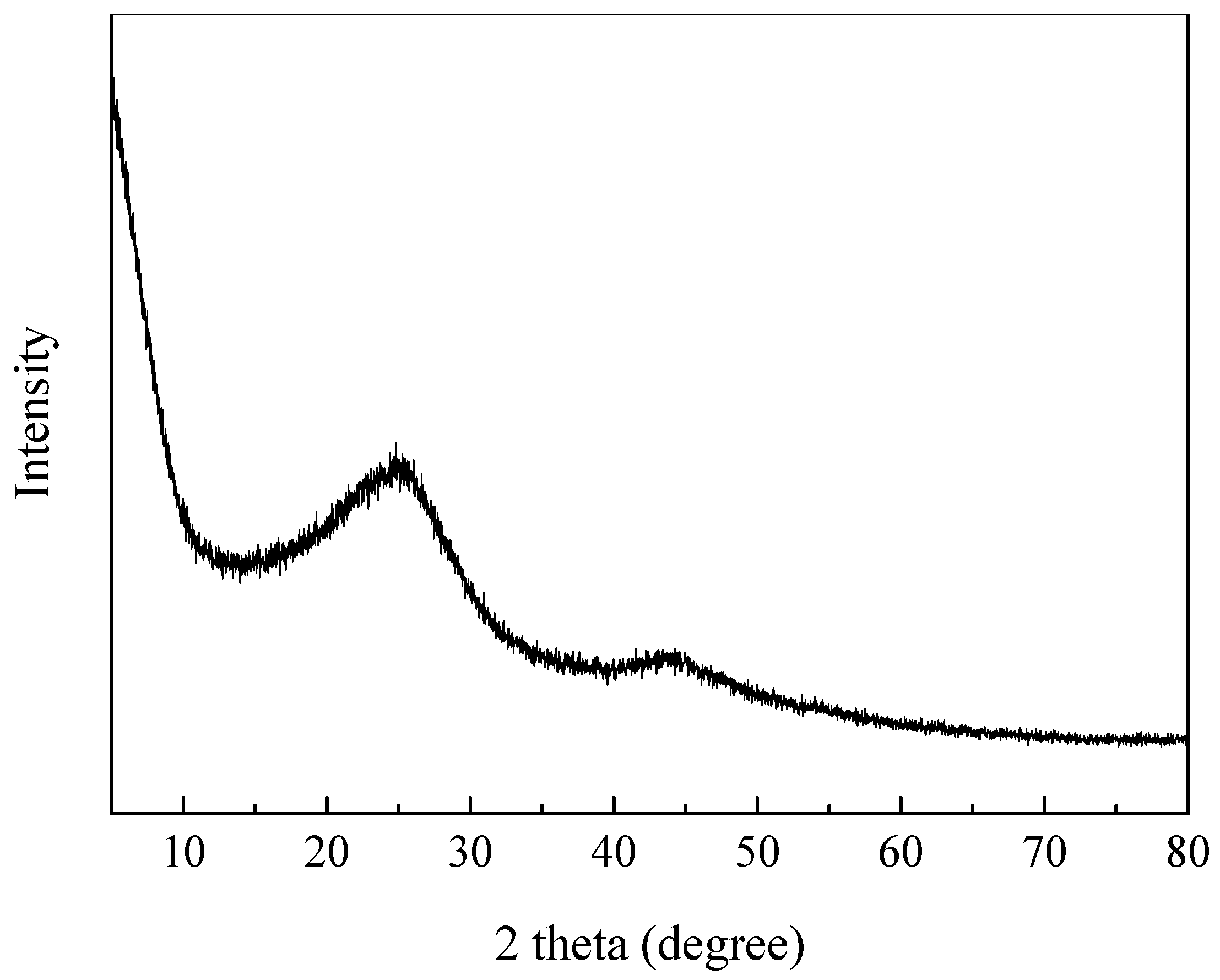
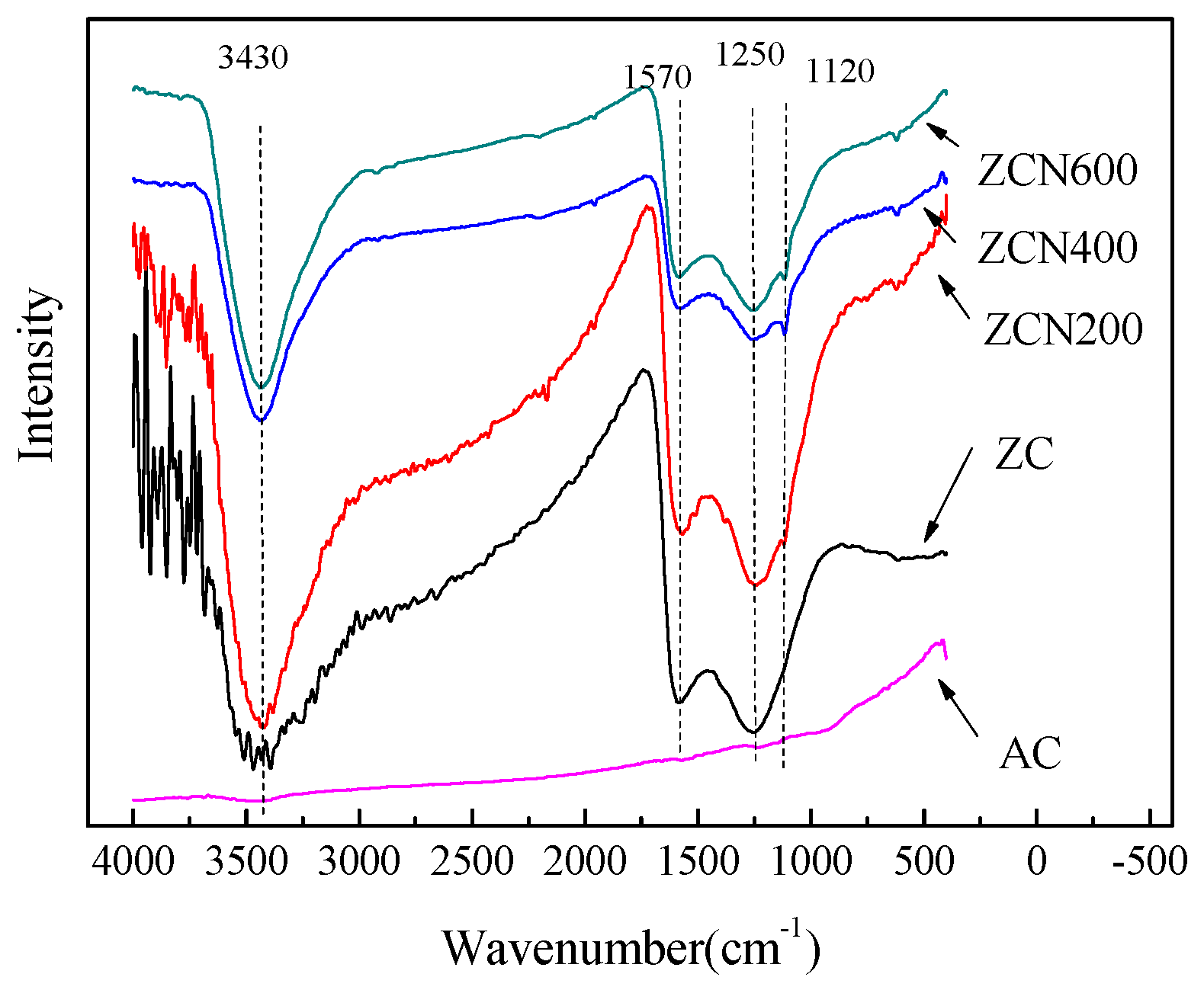
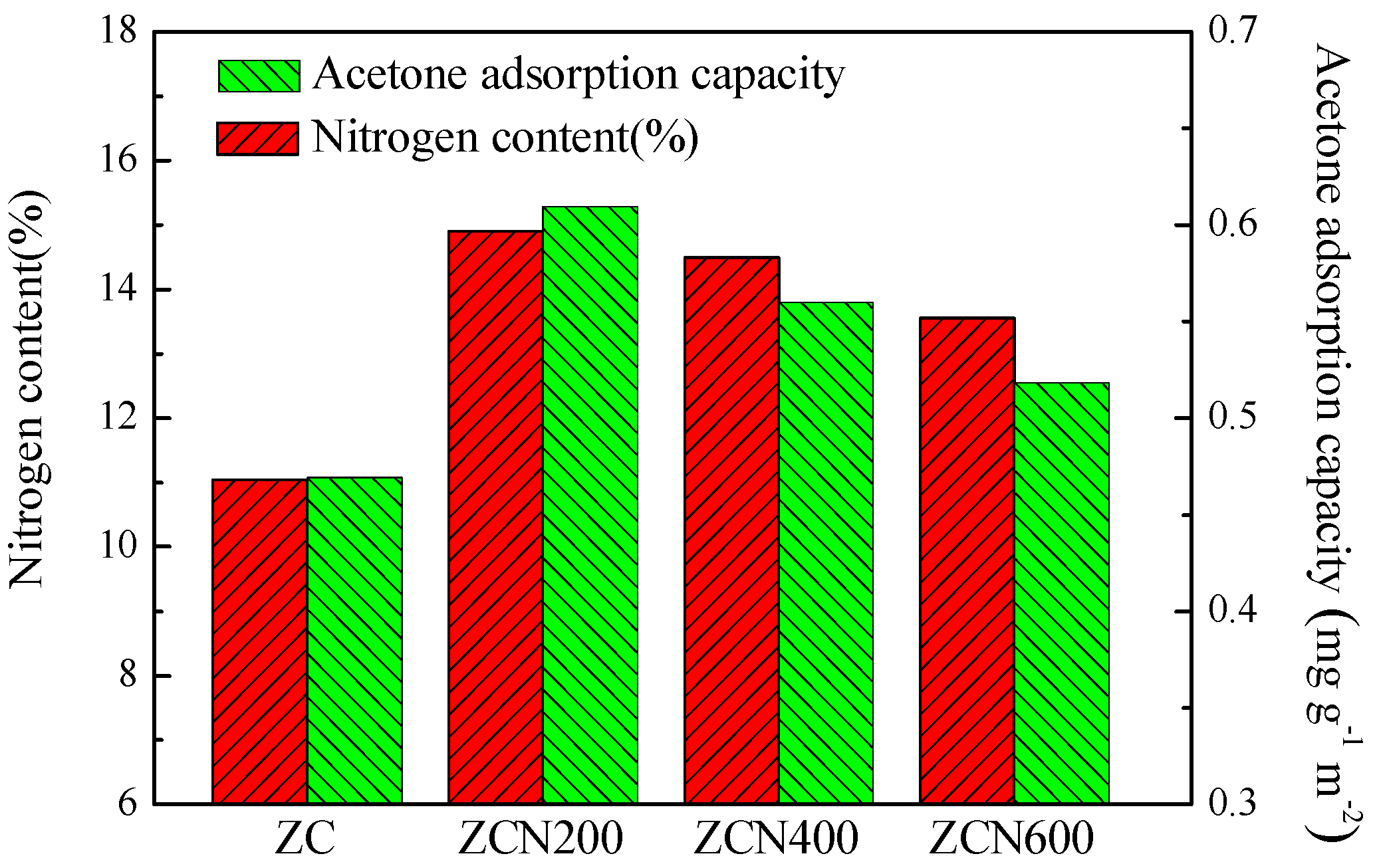
3.1. Textural and Chemical Properties of ZCs and AC
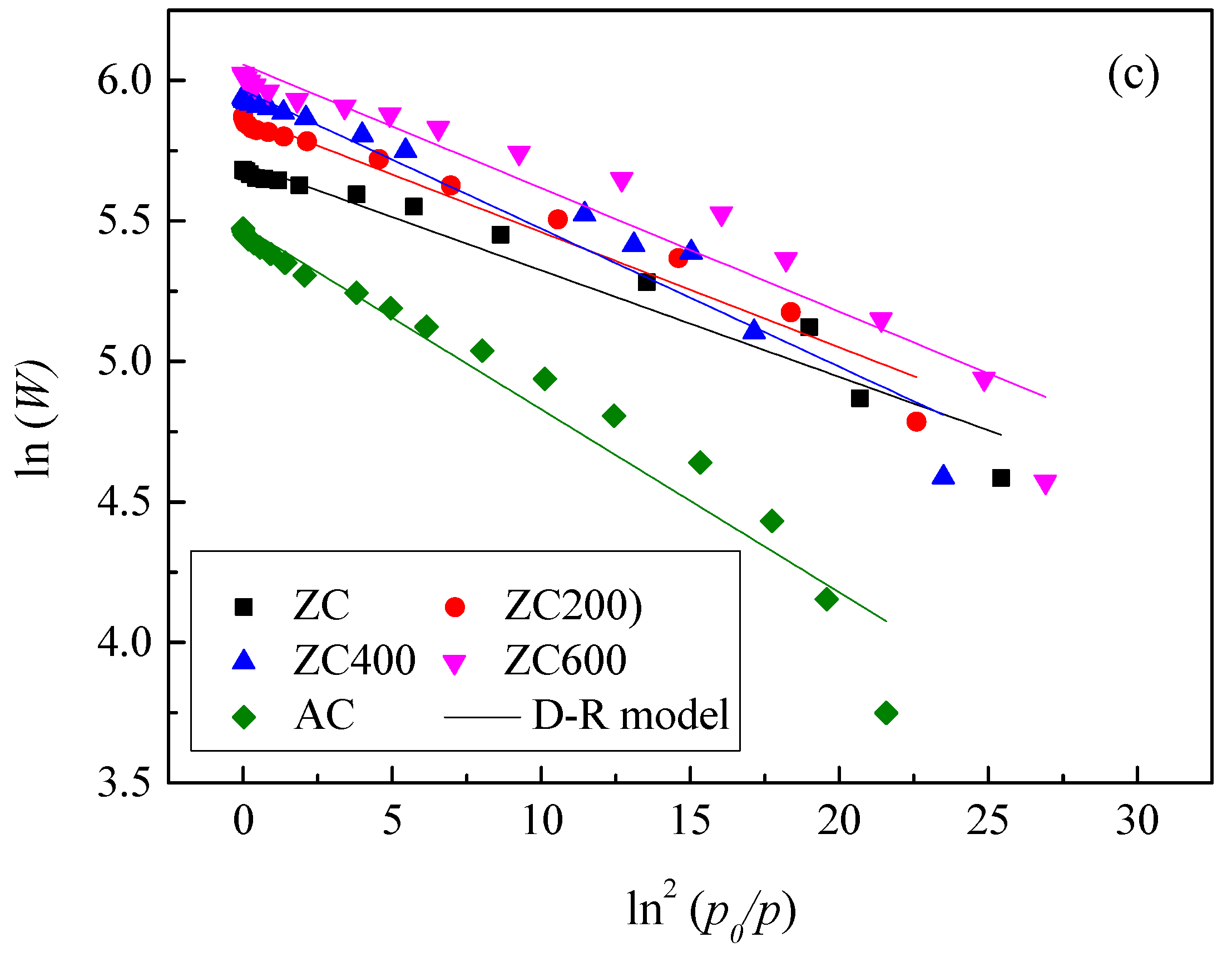
3.2. Acetone Adsorption on Different Carbon Materials
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.; Miao, J.; Sun, X.; Xiao, J.; Li, Y.; Wang, H.; Xia, Q.; Li, Z. Mechanochemical synthesis of Cu-BTC@GO with enhanced water stability and toluene adsorption capacity. Chem. Eng. J. 2016, 298, 191–197. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, T.; Fan, X.; Na, H. Adsorption and desorption of small molecule volatile organic compounds over carbide-derived carbon. Carbon 2014, 67, 712–720. [Google Scholar] [CrossRef]
- Aijaz, A.; Fujiwara, N.; Xu, Q. From metal-organic framework to nitrogen-decorated nanoporous carbons: High CO2 uptake and efficient catalytic oxygen reduction. J. Am. Chem. Soc. 2014, 136, 6790–6793. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Wi, S.; Jeong, S.G.; Kim, S. Evaluation of the adsorption performance and sustainability of exfoliated graphite nanoplatelets (xGnP) for VOCs. Materials 2015, 8, 7615. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, L.; Zhang, X.; Chen, Y. Preparation of zeolitic imidazolate framework-8/graphene oxide composites with enhanced VOCs adsorption capacity. Microporous Mesoporous Mater. 2016, 225, 488–493. [Google Scholar] [CrossRef]
- Zhu, M.; Tong, Z.F.; Zhao, Z.; Jiang, Y.; Zhao, Z. A microporous graphitized biocarbon with high adsorption capacity towards benzene VOCs from humid air at ultra-low pressures. Ind. Eng. Chem. Res. 2016, 55, 3765–3774. [Google Scholar] [CrossRef]
- Tefera, D.T.; Hashisho, Z.; Philips, J.H.; Anderson, J.E.; Nichols, M. Modeling competitive adsorption of mixtures of volatile organic compounds in a fixed-bed of beaded activated carbon. Environ. Sci. Technol. 2014, 48, 5108–5117. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tseng, R.L. Preparation of highly porous carbon from fir wood by KOH etching and CO2 gasification for adsorption of dyes and phenols from water. J. Colloid Interface Sci. 2006, 294, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.P.; Calleja, G.; Botas, J.A.; Gutierrez, F.J. Adsorption and hydrophobic properties of mesostructured MCM-41and SBA-15 materials for VOC removal. Ind. Eng. Chem. Res. 2004, 43, 7010–7018. [Google Scholar] [CrossRef]
- Li, M.S.; Wu, S.C.; Shih, Y.H. Characterization of volatile organic compound adsorption on multiwall carbon nanotubes under different levels of relative humidity using linear solvation energy relationship. J. Hazard. Mater. 2016, 315, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Padial, N.M.; Elsa, Q.P.; Carmen, M.; Elena, L.; Enrique, O.J.; Valentina, C.; Angelo, M.; Norberto, M.; Simona, G.; Irena, S. Highly Hydrophobic Isoreticular Porous Metal-Organic Frameworks for the Capture of Harmful Volatile Organic Compounds. Angew. Chem. Int. Ed. 2013, 125, 8290–8294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xian, S.; Xi, H.; Wang, H.; Li, Z. Improvement of CO2 adsorption on ZIF-8 crystals modified by enhancing basicity of surface. Chem. Eng. Sci. 2011, 66, 4878–4888. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2006, 127, 17998–17999. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kucukkal, M.U.; Clark, A.E. H2 Adsorbed site-to-site electronic delocalization within IRMOF-1: Understanding non-negligible interactions at high pressure. Materials 2016, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, W.P.; Hou, L.; Wang, Y.Y. Four uncommon nanocage-based Ln-MOFs: Highly selective luminescent sensing for Cu2+ ions and selective CO2 capture. Chem. Commun. 2014, 50, 8731–8734. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Song, M.; Gu, Z.Y.; Wang, H.F.; Yan, X.P. Probing the adsorption characteristic of metal-organic framework MIL-101 for volatile organic compounds by quartz crystal microbalance. Environ. Sci. Technol. 2011, 45, 4490–4496. [Google Scholar] [CrossRef] [PubMed]
- Xian, S.; Yu, Y.; Xiao, J.; Zhang, Z.; Xia, Q.; Wang, H.; Li, Z. Competitive adsorption of water vapor with VOCs dichloroethane, ethyl acetate and benzene on MIL-101(Cr) in humid atmosphere. RSC Adv. 2014, 5, 1827–1834. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, F.; Xiang, Z.; Shen, Z.; Yun, J.; Cao, D. ZIF-derived in situ nitrogen-doped porous carbons as efficient metal-free electrocatalysts for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 442–450. [Google Scholar] [CrossRef]
- Sun, J.-K.; Xu, Q. Functional materials derived from open framework templates/precursors: Synthesis and applications. Energy Environ. Sci. 2014, 7, 2071. [Google Scholar] [CrossRef]
- Amali, A.J.; Sun, J.K.; Xu, Q. From assembled metal-organic framework nanoparticles to hierarchically porous carbon for electrochemical energy storage. Chem. Commun. 2014, 50, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, L.; Reboul, J.; Furukawa, S.; Srinivasu, P.; Kitagawa, S.; Yamauchi, Y. Preparation of microporous carbon fibers through carbonization of Al-based porous coordination polymer (Al-PCP) with furfuryl alcohol. Chem. Mater. 2011, 23, 1225–1231. [Google Scholar] [CrossRef]
- Hu, J.; Wang, H.; Gao, Q.; Guo, H. Porous carbons prepared by using metal–organic framework as the precursor for supercapacitors. Carbon 2010, 48, 3599–3606. [Google Scholar] [CrossRef]
- Zhang, L.; Su, Z.; Jiang, F.; Yang, L.; Qian, J.; Zhou, Y.; Li, W.; Hong, M. Highly graphitized nitrogen-doped porous carbon nanopolyhedra derived from ZIF-8 nanocrystals as efficient electrocatalysts for oxygen reduction reactions. Nanoscale 2014, 6, 6590. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, B.; Hu, C.; Cao, M. Nitrogen-doped porous carbon nanofiber webs for efficient CO2 capture and conversion. Carbon 2016, 99, 79–89. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Qi, G.; Li, B.; Song, Z.; Jiang, H.; Zhang, X.; Qiao, J. A novel N-doped porous carbon microsphere composed of hollow carbon nanospheres. Carbon 2016, 96, 864–870. [Google Scholar] [CrossRef]
- Chen, H.; Sun, F.; Wang, J.; Li, W.; Qiao, W.; Ling, L.; Long, D. Nitrogen doping effects on the physical and chemical properties of mesoporous carbons. J. Phys. Chem. C 2013, 117, 8318–8328. [Google Scholar] [CrossRef]
- Yao, M.; Wang, L.; Hu, X.; Hu, G.; Luo, M.; Fan, M. Synthesis of nitrogen-doped carbon with three-dimensional mesostructures for CO2 capture. J. Mater. Sci. 2015, 50, 1221–1227. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Shiraishi, S.; Morallón, E.; Cazorla-Amorós, D. Improvement of carbon materials performance by nitrogen functional groups in electrochemical capacitors in organic electrolyte at severe conditions. Carbon 2015, 82, 205–213. [Google Scholar] [CrossRef]
- Li, S.M.; Yang, S.Y.; Wang, Y.S.; Tsai, H.P.; Tien, H.W.; Hsiao, S.T.; Liao, W.H.; Chang, C.L.; Ma, C.C.M.; Hu, C.C. N-doped structures and surface functional groups of reduced graphene oxide and their effect on the electrochemical performance of supercapacitor with organic electrolyte. J. Power Sources 2015, 278, 218–229. [Google Scholar] [CrossRef]
- Huang, S.; Li, Y.; Feng, Y.; An, H.; Long, P.; Qin, C.; Feng, W. Nitrogen and fluorine co-doped graphene as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 23095–23105. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Y.S.; Li, H.; Wang, Z.; Chen, L. Porous Li4Ti5O12 coated with N-Doped carbon from ionic liquids for Li-Ion batteries. Adv. Mater. 2011, 23, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Liu, Y.; Baek, J.B.; Dai, L. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tang, S.; Yin, Y.; Sun, S.; Qiao, J. Hierarchical porous N-doped graphene foams with superior oxygen reduction reactivity for polymer electrolyte membrane fuel cells. Appl. Energy 2016, 175, 459–467. [Google Scholar] [CrossRef]
- Lu, Z.J.; Xu, M.W.; Bao, S.J.; Tan, K.; Hui, C.; Cai, C.J.; Ji, C.C.; Qiang, Z. Facile preparation of nitrogen-doped reduced graphene oxide as a metal-free catalyst for oxygen reduction reaction. J. Mater. Sci. 2013, 48, 8101–8107. [Google Scholar] [CrossRef]
- Chizallet, C.; Lazare, S.; Bazer-Bachi, D.; Bonnier, F.; Lecocq, V.; Soyer, E.; Quoineaud, A.-A.; Bats, N. Catalysis of transesterification by a nonfunctionalized metal-organic framework: Acido-basicity at the external surface of ZIF-8 probed by FTIR and ab initio calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhou, K.; Sun, X.; Zhao, Z.; Tong, Z.; Zhao, Z. Hydrophobic N-doped Porous Biocarbon from Dopamine for High Selective Adsorption of p-Xylene under Humid Conditions. Chem. Eng. J. 2017, 317, 660–672. [Google Scholar] [CrossRef]
- Yang, L.; Jing, L.; Ming, C.; Zheng, C. Theoretical studies of CO2 adsorption mechanism on linkers of metal-organic frameworks. Fuel 2012, 95, 521–527. [Google Scholar]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Lee, K.B.; Ham, H.C. Effect of N-containing functional groups on CO2 adsorption of carbonaceous materials: A density functional theory approach. J. Phys. Chem. C 2016, 120, 8087–8095. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Ma, X.; Das, S.K.; Ostwal, M.; Gadwal, I.; Yao, K.; Dong, X.; Han, Y.; Pinnau, I. Soluble polymers with intrinsic porosity for flue gas purification and natural gas upgrading. Adv. Mater. 2017, 29, 1605826. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Chang, M.; Zheng, C. Effect of functionalized linker on CO2 binding in zeolitic imidazolate frameworks: Density functional theory study. J. Phys. Chem. C 2012, 116, 16985–16991. [Google Scholar] [CrossRef]
- He, M.; Yao, J.; Li, L.; Zhong, Z.; Chen, F.; Wang, H. Aqueous solution synthesis of ZIF-8 films on a porous nylon substrate by contra-diffusion method. Microporous Mesoporous Mater. 2013, 179, 10–16. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, B.; Boehm, H.P.; Schlögl, R. Enhancement of the catalytic activity of activated carbons in oxidation reactions by thermal treatment with ammonia or hydrogen cyanide and observation of a superoxide species as a possible intermediate. Carbon 1991, 29, 707–720. [Google Scholar]
- Biniak, S.; Szymański, G.; Siedlewski, J.; Światkowski, A. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Przepiorski, J. Enhanced adsorption of phenol from water by ammonia-treated activated carbon. J. Hazard. Mater. 2006, 135, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.P.; Li, W.C.; Qian, D.; Lu, A.H. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Adv. Mater. 2010, 22, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Przepiorski, J.; Skrodzewicz, M.; Morawski, A.W. High temperature ammonia treatment of activated carbon for enhancement of CO2 adsorption. Appl. Surf. Sci. 2004, 225, 235–242. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal stability and reducibility of oxygen-containing functional groups on multiwalled carbon nanotube surfaces: A quantitative high-resolution XPS and TPD/TPR study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Andreoli, E.; Barron, A.R. Correlating Carbon Dioxide Capture and Chemical Changes in Pyrolyzed Polyethylenimine-C60. Energy Fuels 2015, 29, 4479–4487. [Google Scholar] [CrossRef]
- Ramos, M.E.; Bonelli, P.R.; Cukierman, A.L.; Ribeiro Carrott, M.M.; Carrott, P.J. Adsorption of volatile organic compounds onto activated carbon cloths derived from a novel regenerated cellulosic precursor. J. Hazard. Mater. 2010, 177, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Do, D.D. The Dubinin–Radushkevich equation and the underlying microscopic adsorption description. Carbon 2001, 39, 1327–1336. [Google Scholar] [CrossRef]

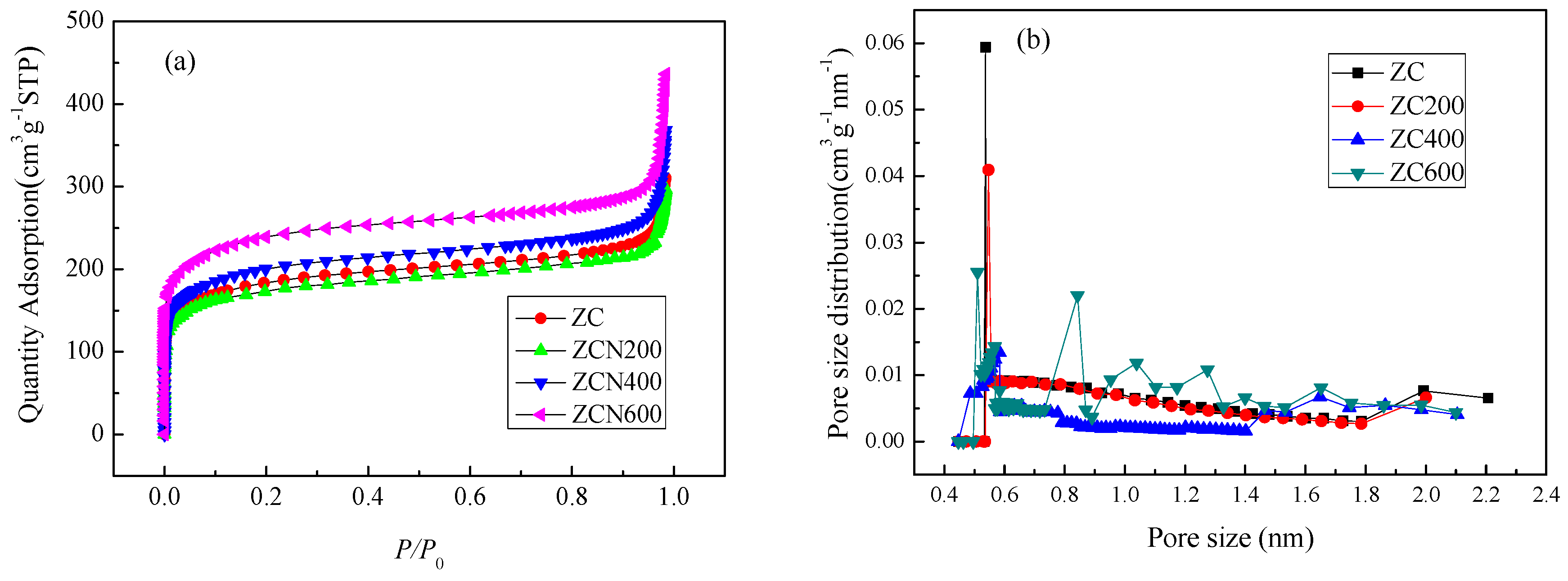
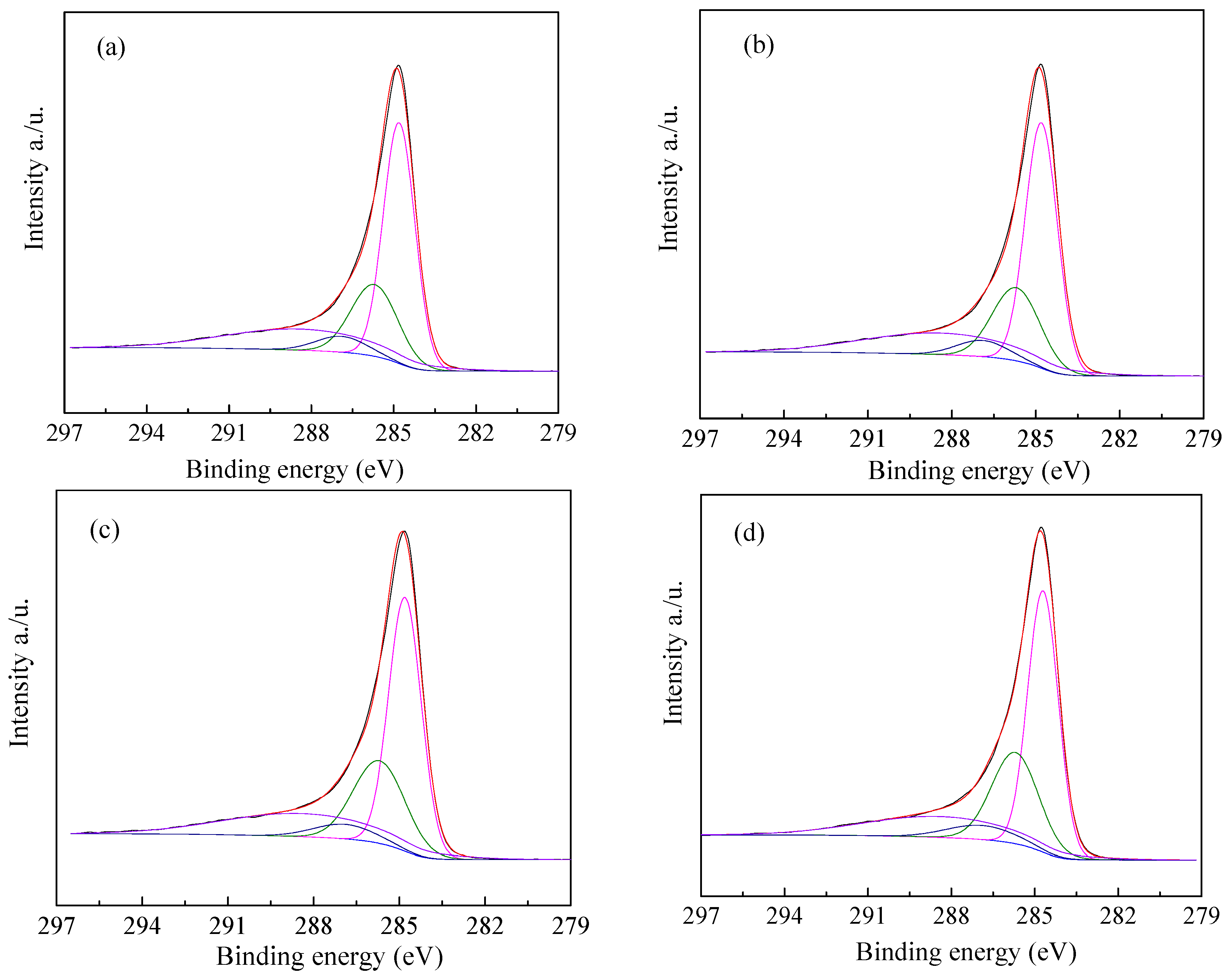
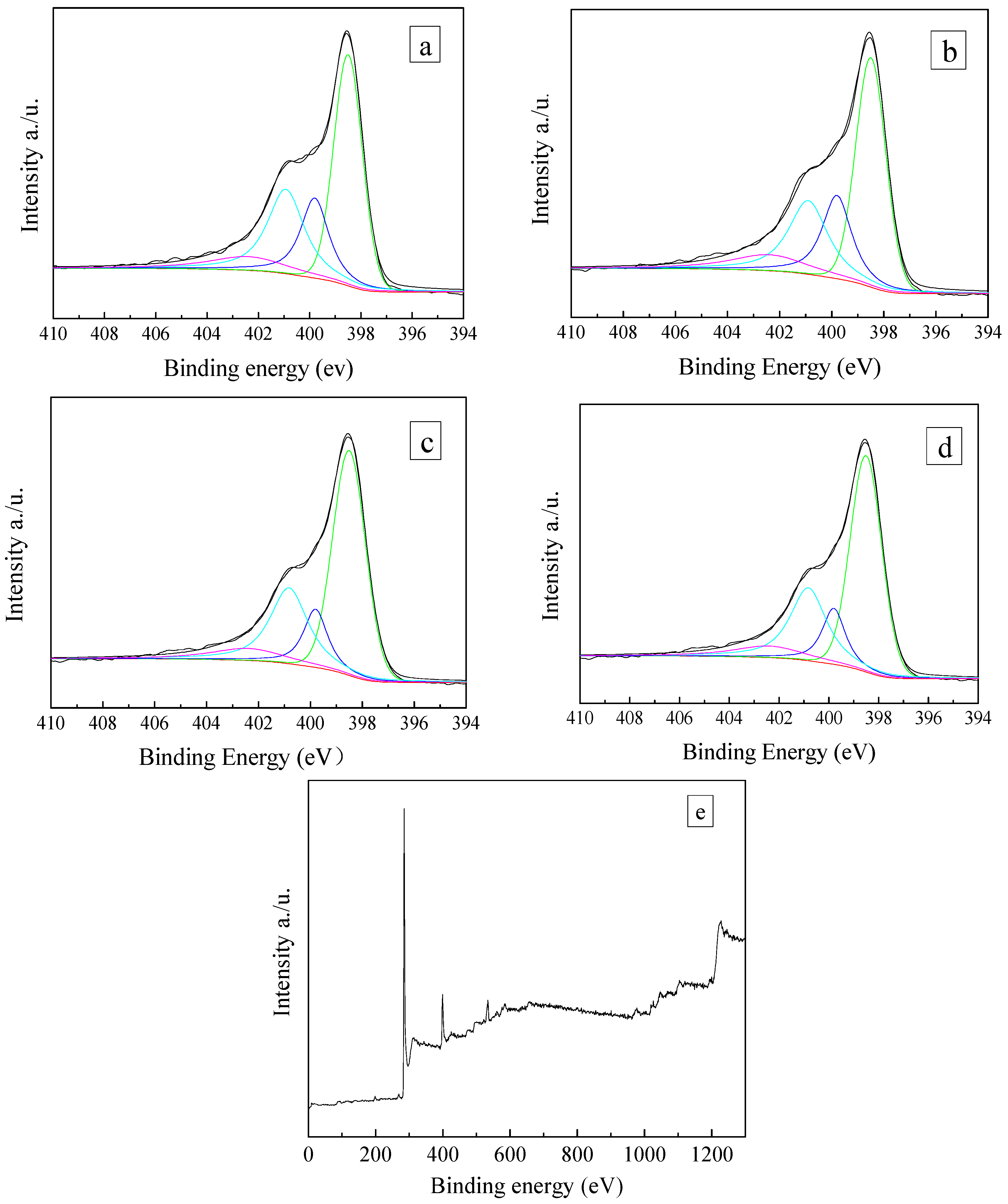


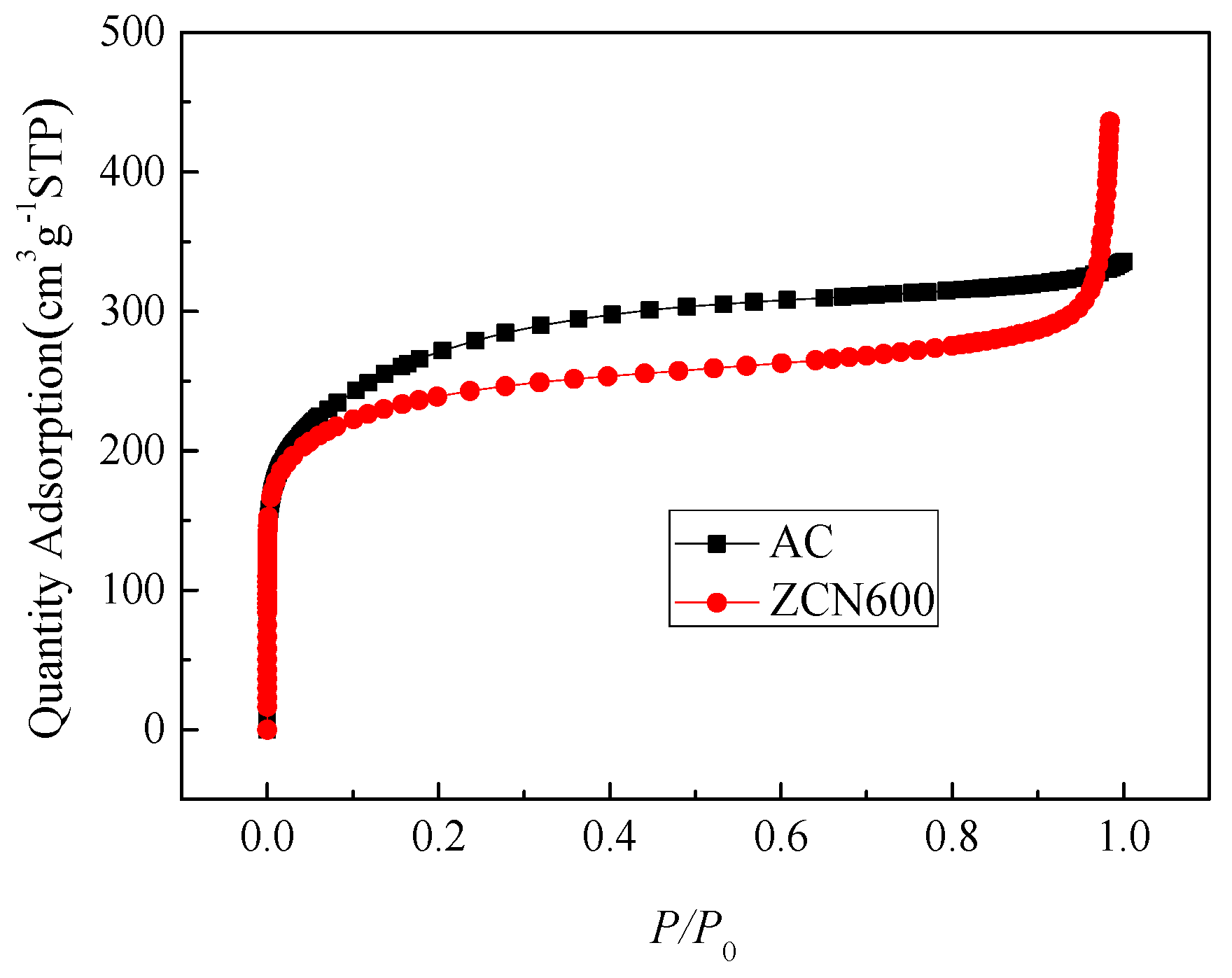
| Samples | SBET | Smic | Smes + Smar | Vtotal | Vmic | Vmes + Vmar | Yield |
|---|---|---|---|---|---|---|---|
| m2 g−1 | m2 g−1 | m2 g−1 | mL g−1 | mL g−1 | mL g−1 | (%) | |
| ZIF-8 | 1400 | 1370 | 30 | 0.71 | 0.65 | 0.06 | - |
| ZC | 624 | 453 | 171 | 0.46 | 0.21 | 0.25 | 31.7 |
| ZCN200 | 592 | 422 | 170 | 0.43 | 0.20 | 0.23 | 31.2 |
| ZCN400 | 686 | 514 | 172 | 0.53 | 0.24 | 0.29 | 30.3 |
| ZCN600 | 805 | 627 | 178 | 0.62 | 0.29 | 0.33 | 26.5 |
| AC | 898 | 605 | 293 | 0.48 | 0.36 | 0.12 | - |
| Sample | Concentration (at. %) | ||||||
|---|---|---|---|---|---|---|---|
| C | O | N | Pyridinic-N | Pyrrolic-N | Graphitic-N | Oxidized-N | |
| ZC | 85.5 | 3.46 | 11.46 | 4.75 | 2.16 | 3.04 | 1.06 |
| ZCN-200 | 83.04 | 2.46 | 14.50 | 6.50 | 3.11 | 3.68 | 1.59 |
| ZCN-400 | 81.92 | 3.18 | 14.90 | 6.84 | 2.65 | 3.59 | 1.42 |
| ZCN-600 | 82.15 | 4.31 | 13.55 | 6.94 | 1.80 | 3.66 | 1.15 |
| Samples | Langmuir | Freundlich | D–R | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qmax/mg g−1 | KL/m3 g−1 | R2 | Kf/mg g−1 | 1/n | R2 | W0/mg g−1 | E/kJ mol−1 | R2 | |
| ZC | 292.7 | 2.59 | 0.9914 | 174.7 | 4.79 | 0.9135 | 300.0 | 12.5 | 0.9549 |
| ZCN200 | 360.9 | 1.86 | 0.9964 | 203.3 | 4.56 | 0.8976 | 354.5 | 12.2 | 0.9636 |
| ZCN400 | 384.2 | 1.59 | 0.9929 | 223.2 | 4.9 | 0.8743 | 389.3 | 11.2 | 0.9480 |
| ZCN600 | 417.2 | 2.14 | 0.9933 | 239.9 | 4.46 | 0.8913 | 426.9 | 11.8 | 0.9321 |
| AC | 237.9 | 1.54 | 0.9937 | 127.1 | 4.10 | 0.9087 | 239.8 | 9.70 | 0.9538 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Ma, X.; Chen, R.; Wang, C.; Lu, M. Nitrogen-Containing Functional Groups-Facilitated Acetone Adsorption by ZIF-8-Derived Porous Carbon. Materials 2018, 11, 159. https://doi.org/10.3390/ma11010159
Li L, Ma X, Chen R, Wang C, Lu M. Nitrogen-Containing Functional Groups-Facilitated Acetone Adsorption by ZIF-8-Derived Porous Carbon. Materials. 2018; 11(1):159. https://doi.org/10.3390/ma11010159
Chicago/Turabian StyleLi, Liqing, Xiancheng Ma, Ruofei Chen, Chunhao Wang, and Mingming Lu. 2018. "Nitrogen-Containing Functional Groups-Facilitated Acetone Adsorption by ZIF-8-Derived Porous Carbon" Materials 11, no. 1: 159. https://doi.org/10.3390/ma11010159