Following this approach, the RTSE data collected in these studies of IGS deposition were analyzed by focusing simultaneously on a large dataset consisting of multiple pairs of ellipsometric (ψ, Δ) spectra acquired at different times over a selected time range of interest [
37,
38]. A Kramers-Kronig consistent b-spline function with a 0.2 eV node spacing was applied in this analysis as an optical model for the (ε
1, ε
2) spectra from 0.74 to 6.0 eV [
39]. From the onset of bulk layer growth to the end of the selected time range for data analysis, the (ε
1, ε
2) spectra of the IGS are assumed to be time independent. As a result, a structural model for the IGS film with an assumed uniform bulk layer is applied. Such a single layer model for the bulk layer is also applicable in the rare circumstances that the (ε
1, ε
2) spectra of the entire uniform bulk layer, from substrate to ambient, evolve with time as in an annealing process. Most often, however, when a time dependence in the bulk layer (ε
1, ε
2) spectra occurs, the dependence is restricted to newly deposited material and an optical property profile is built into the film as it is deposited. Such a profile requires a multilayer or virtual interface model in the analysis of the bulk layer, which then becomes a greater challenge as the overlying surface roughness layer increases in thickness [
40]. In addition to the single uniform bulk layer, a surface roughness layer must be included in the structural model for the IGS film. Typically in this study, the void contents in the surface roughness layers are fixed at 50 vol % for surface roughness layer thicknesses <90 Å and are allowed to vary for such thicknesses >90 Å. An unweighted error function is used to evaluate the quality of each overall fit [
41], for which the final results are the (ε
1, ε
2) spectra of the IGS and the time evolution of the bulk and surface roughness layer thicknesses [
38].
3.1. Variation of IGS Complex Dielectric Function with Ga Composition (x)
The first set of multi-time analysis procedures was applied to RTSE data collected on IGS films fabricated with different Ga contents,
x, on Si/SiO
2/Mo substrates. The goal is to determine simultaneously the structural evolution and the (ε
1, ε
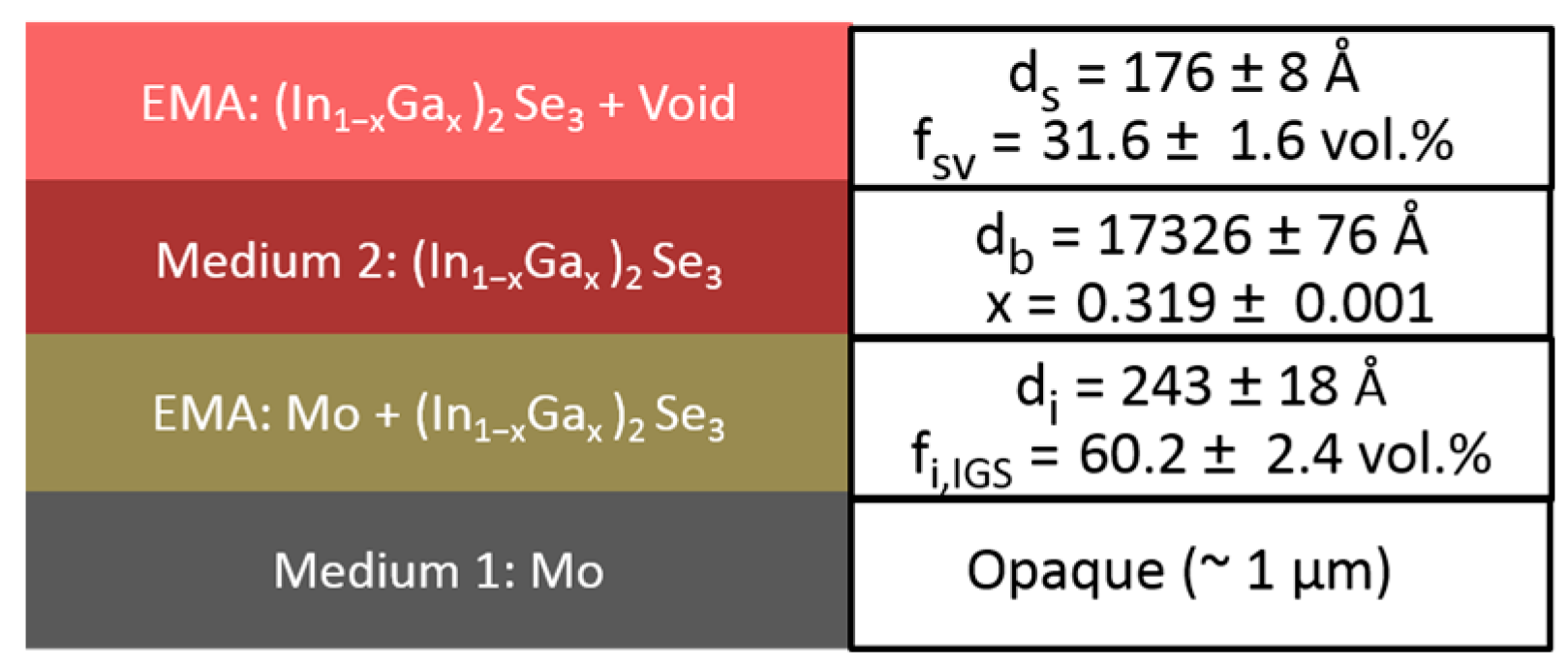
2) spectra of the bulk layer after it has stabilized in the later stage of deposition. In this later stage of deposition, it is assumed that the voids in the underlying Mo surface roughness layer have been completely filled by IGS material, yielding an interface roughness layer with the same thickness as the surface roughness layer on the uncoated Mo. The model applied in this analysis consists of five media and three layers, as shown in
Figure 3, including (i) an opaque Mo film as a substrate, (ii) the Mo/IGS interface roughness layer arising from the roughness on the Mo surface, (iii) the uniform IGS bulk layer, (iv) the IGS surface roughness layer and (v) an ambient medium of vacuum. For these analyses, the Mo/IGS interface layer thickness was fixed at a value deduced from a measurement of the Mo surface roughness thickness at room temperature prior to substrate heating and deposition, with values being in the 69–88 Å range. After the 50 vol % voids of the interface layer are completely filled by depositing IGS, the contents of the two materials in this interface layer are assumed to be 50/50 vol % Mo/IGS. The (ε
1, ε
2) spectra of the interface and surface roughness layers are determined by the Bruggeman effective medium approximation (EMA) as indicated in
Figure 3. The Mo/IGS interface roughness thickness of 75 Å, as deduced optically for IGS with composition
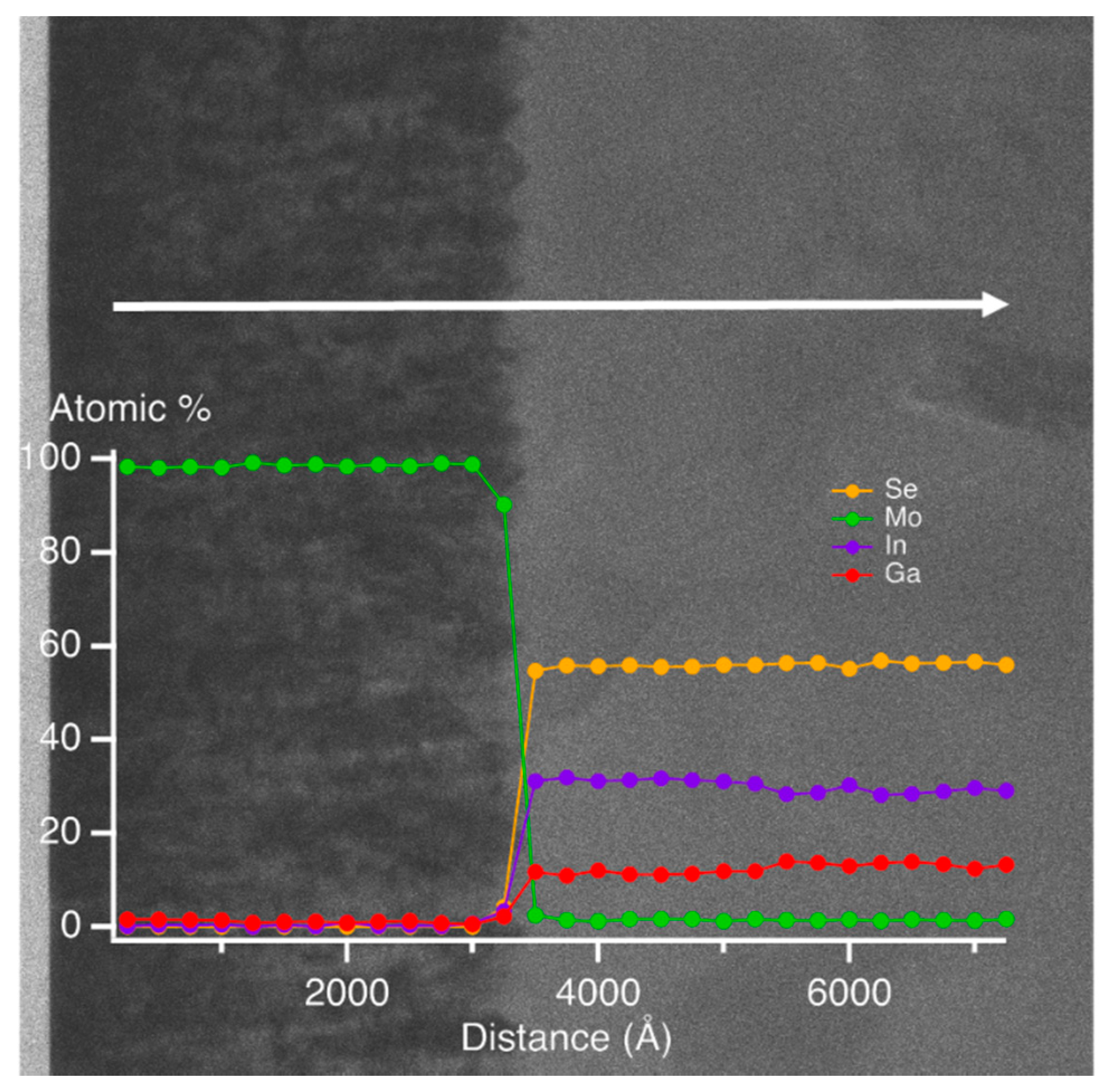
x = 0.31, can be compared to the peak-to-valley result of 200–300 Å from the TEM of
Figure 2. A large difference is possible since it has been found that the roughness thickness deduced by SE analysis is 1.0–1.5 times the root mean-square roughness on semiconductor and metal surfaces as obtained by atomic force microscopy [
23,
25].
Table 1 includes the initial and final times, t
i and t
f, defining the multi-time analysis range, and the center time t*, defining the inversion point for the final (ε
1, ε
2) determination, associated with each of the depositions yielding IGS of different Ga contents
x. The bulk layer thickness values d
b deduced as the best fit results at t
i, t* and t
f are also given in
Table 1 along with the best fit surface roughness layer thickness d
s and the fixed surface roughness void content f
v both at t*. Finally, in the last two columns of
Table 1, the quality of the multi-time best fit, given as the mean square error (MSE), and the instantaneous deposition rate at t*, given as the time derivative of the effective thickness d
eff, are provided. The effective thickness is generally defined as the product of the thickness and the material volume fraction summed over all layers that incorporate the material. Thus, for IGS, the effective thickness is given by d
eff = d
i f
IGS + d
b + d
s(1 − f
v), where d
i is the Mo/IGS interface thickness and f
IGS is its IGS volume fraction as indicated in
Figure 3. As can be observed from
Table 1, the center time in the analysis was chosen for an approximate bulk layer thickness of 4000 Å. Also, it is observed that at t*, the surface roughness thickness is a maximum for In
2Se
3 and a minimum for Ga
2Se
3. For In-rich alloys with 0 <
x ≤ 0.45, the surface roughness thickness lies within the range from 90 to 105 Å and shows no clear trend with
x, whereas for alloys with
x ≥ 0.45, a continuous decrease occurs with increasing
x.
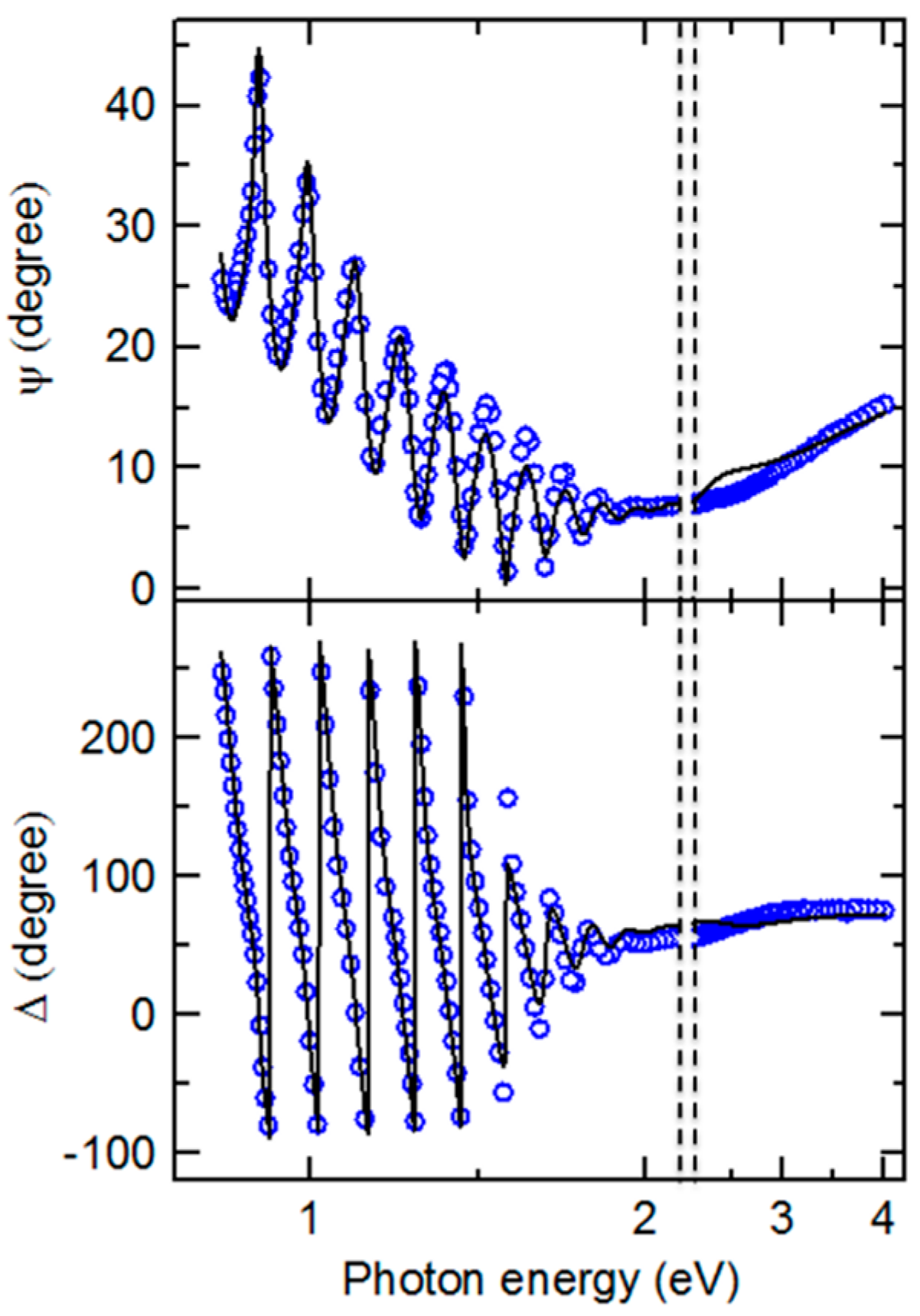
As an example of the fitting achieved continuously versus time in the multi-time analysis, RTSE data in ψ at the specific photon energies of 0.735, 1.620, 2.546 and 5.912 eV are plotted together with the best fit results in
Figure 4 for the IGS deposition with
x = 0.31. Deviations between the data and best fit in
Figure 4 occur not only due to noise in the data (particularly at the lowest and highest photon energies of the range) but also due to limitations in the accuracy of the single bulk layer model and the b-spline dielectric function.
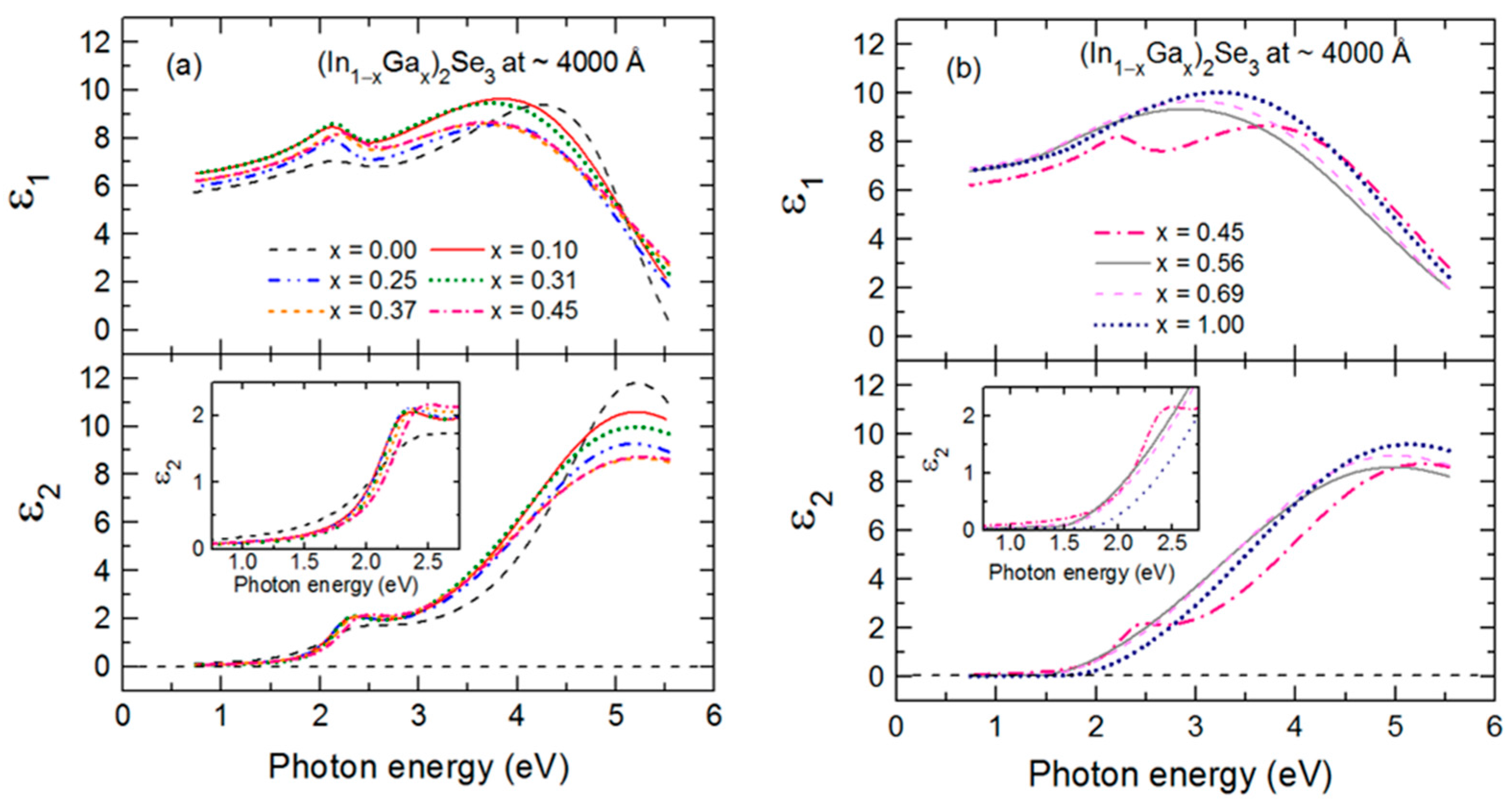
Figure 5 shows the final parametric (ε
1, ε
2) spectra obtained by fitting the inversion results near the center at t* of the multi-time analysis ranges using the oscillator model, which includes single CP and TL oscillators. The resonance energy parameter of the CP oscillator represents the fundamental bandgap
Eg1. In contrast, the bandgap of the TL oscillator described as a Tauc gap
Eg2 serves as a low energy absorption onset for a higher energy Lorentz oscillator with a resonance energy of
E02. The CP and TL oscillators are also defined by their individual amplitudes (
A1,
A2) and their broadening values (Γ
1, Γ
2), respectively. The phase and exponent of the bandgap CP oscillator are fixed at 0° and 0.5, respectively, and the constant contribution to the ε
1 spectra is fixed at unity.
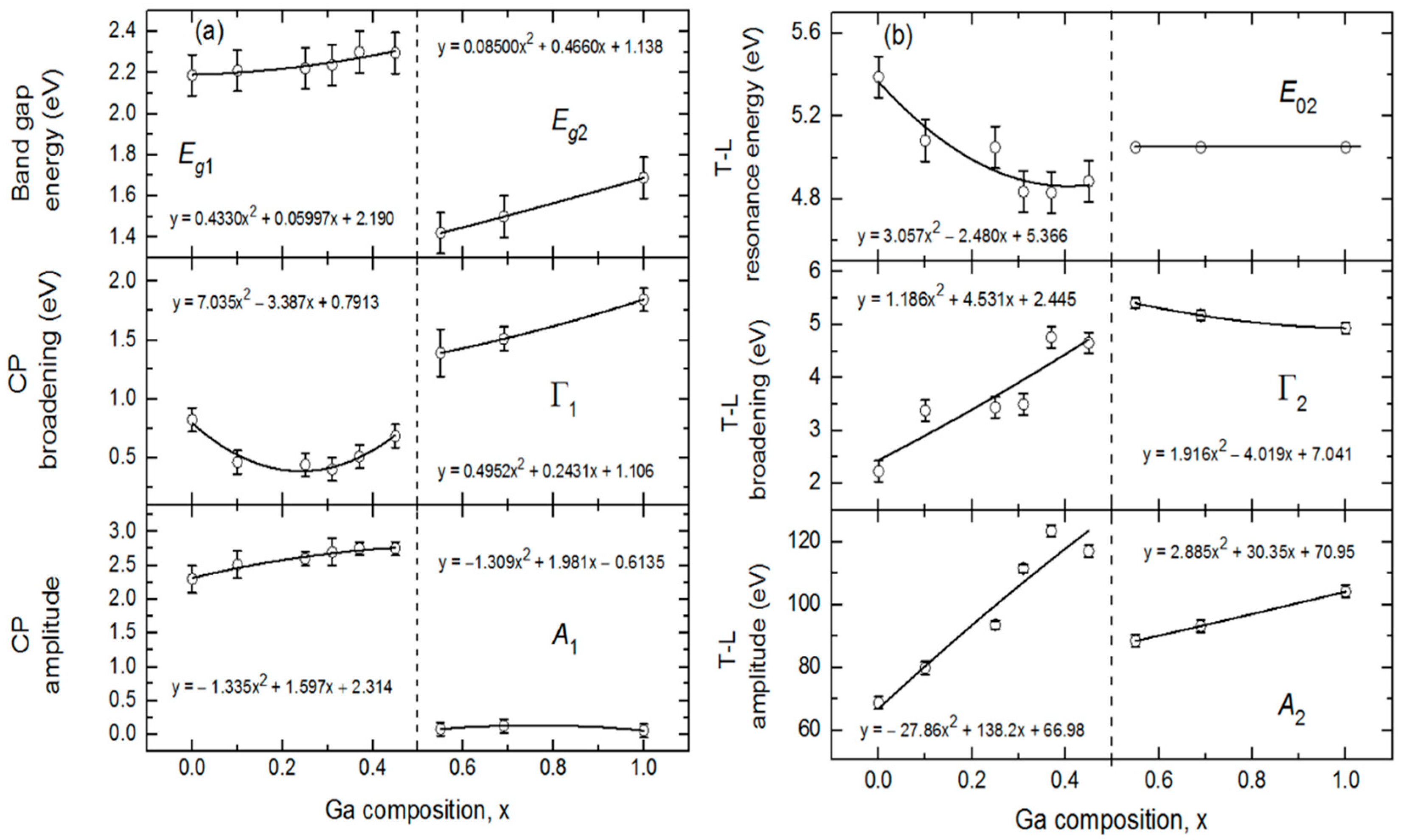
Figure 6 shows the variations of the bandgaps, resonance energies, amplitudes and broadening parameters of the CP and TL oscillators versus alloy composition
x over the full range of
x. The In- and Ga-rich sides of IGS series have different oscillator characteristics and trends with composition as reflected in
Figure 6. For
x ≤ 0.45, the bandgap CP oscillator is clearly visible in (ε
1, ε
2) at
Eg1 ~ 2.2–2.3 eV and exhibits a wide minimum in the broadening parameter Г
1 centered in the range
x = 0.20–0.30. For
x ≤ 0.45, the Tauc gap associated with the high energy TL oscillator is fixed at the bandgap CP energy in order to prevent absorption associated with the TL oscillator from appearing below the bandgap CP energy. In contrast to the behavior for
x ≤ 0.45, the amplitude of the CP oscillator is essentially zero for
x ≥ 0.56, meaning that the signature of the fundamental bandgap disappears from the optical response for the Ga-rich alloys. In its place, a very gradual low energy absorption onset is observed that can be simulated with the low energy tail of the TL oscillator with a Tauc gap that now controls the absorption onset due to the absence of the CP oscillator. This gap
Eg2 increases from 1.42 to 1.69 eV as
x increases from 0.56 to 1.00. In the final analysis for
x ≥ 0.56, the Lorentz oscillator resonance energy associated with the TL is fixed at
E02 = 5.05 eV, an average obtained when all TL parameters are varied for the three samples. This was done to stabilize the fit and obtain a systematic variation in
x for the other TL parameters for
x ≥ 0.56.
Expressions describing the dependence of each variable oscillator parameter on
x for
x ≤ 0.45 and
x ≥ 0.56 were obtained by fitting the results for each parameter to a polynomial function of
x as shown by the solid lines in
Figure 6. These best fit polynomial functions are collected in
Table 2 and
Table 3. The parametric expressions of greatest interest for CIGS solar cells are those relevant to Ga contents with
x ≤ 0.45 in
Figure 6 and
Table 2. These expressions span the composition range
x = 0.20–0.40 where the most efficient solar cells are obtained [
5,
7,
8,
9,
10]. Noteworthy trends in
Figure 6 that are evident from the polynomial fits with increasing
x for
x ≤ 0.45 include (i) a monotonically increasing bandgap CP from 2.19 eV at
x = 0.00 to 2.33 eV at
x = 0.45, (ii) a broad minimum in the CP width centered near
x = 0.20–0.30, (iii) an increasing CP oscillator amplitude that tends to saturate for the depositions at the higher values of
x and (iv) a decreasing resonance energy and an increasing broadening parameter and amplitude associated with the high energy TL oscillator. Additional trends occur in
Figure 6 based on consideration of the full range of composition. First, it should be noted, however, that the amplitude of the CP observed clearly for
x ≤ 0.45 is very small for
x ≥ 0.56, nearly approaching zero. As a result, the increase in CP broadening with increasing Ga content for
x ≥ 0.56 is not a meaningful feature in the interpretation. In contrast, the amplitude of the broad TL oscillator is quite strong for all compositions, and so the increases in the amplitude and bandgap associated with this oscillator and the reduction in its broadening parameter with increasing Ga content for
x ≥ 0.56 in
Figure 6 are obviously meaningful. Although the bandgap feature due to the CP is sharpest for
x = 0.20–0.30, the smallest broadening parameters of the high energy oscillator over the two respective ranges of
x in
Figure 6b occur at
x = 0.00 and
x = 1.00.
It is of interest to compare the bandgaps for In
2Se
3 of 2.19 eV and Ga
2Se
3 of 1.69 eV from the parameters of
Table 2 and
Table 3 with available results in the literature. From the temperature dependence of the free exciton photoluminescence feature, the room-temperature bandgap of γ-phase In
2Se
3 films has been estimated as ~1.95 eV [
43], whereas the bandgap of zinc blende Ga
2Se
3 is estimated from absorption measurements as ~2.0 eV [
44]. The wider bandgap of 2.19 eV in
Table 2 for the In
2Se
3 at 400 °C may be attributed to compositional differences and the narrower bandgap of 1.68 eV in
Table 3 for Ga
2Se
3 also at 400 °C may have components due to the red shift with temperature and to the defects and disorder of the Ga-rich alloys prepared in this study.
The differences in behavior in
Figure 6a,b over the different ranges of
x are consistent with the SEM and XRD results of
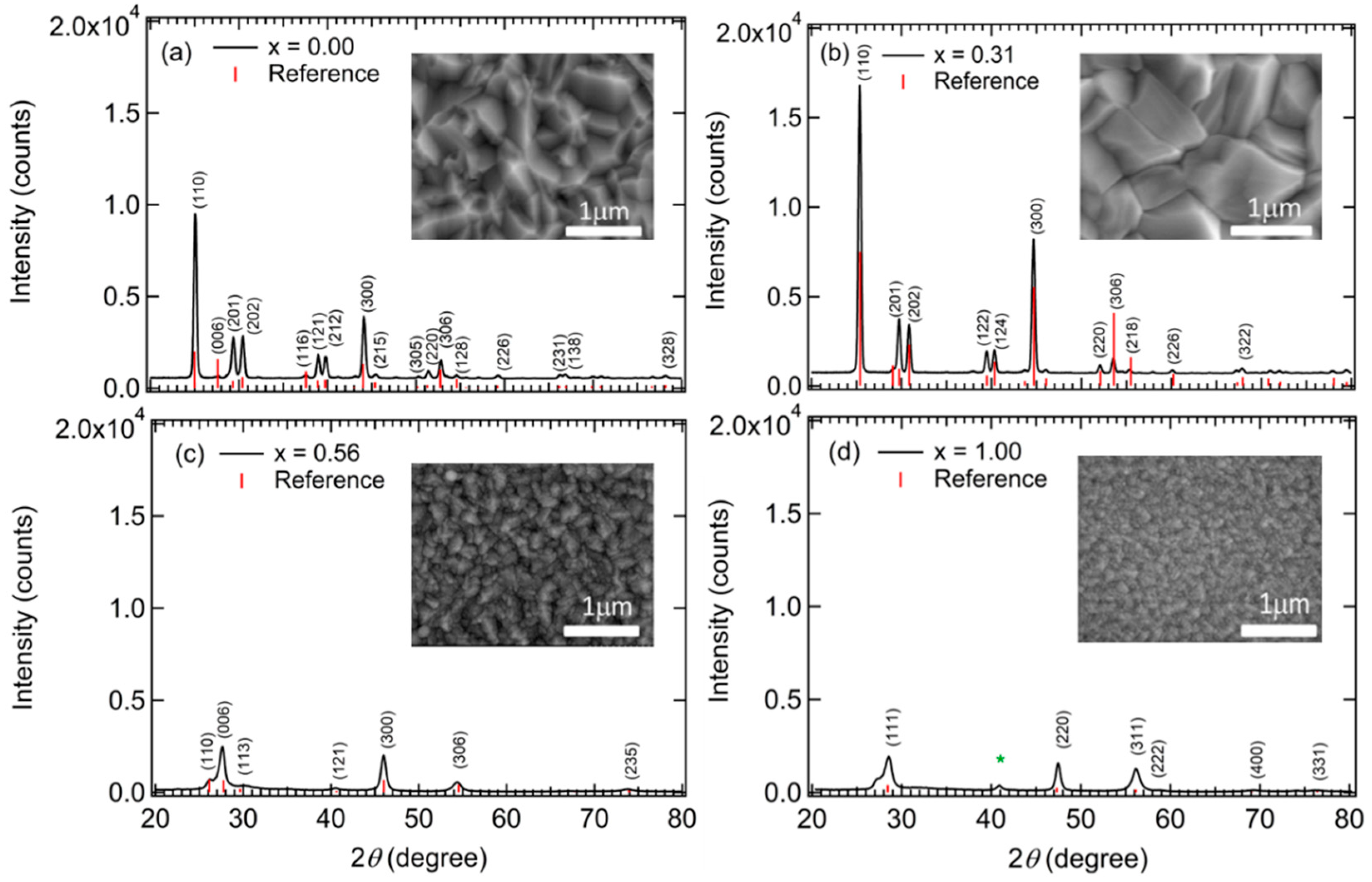
Figure 1 demonstrating that for IGS with
x = 0.00 and 0.31, a large grained structure is found whereas for IGS with
x = 0.56 and 1.00, the grains are much smaller. Evidently, it is the observed transition in grain structure between
x = 0.31 and 0.56, rather than a transition in crystallography, that accounts for the relatively abrupt transition from a well-defined direct bandgap with a strong CP amplitude to a gradual absorption onset with a negligible CP amplitude. Although a gradual absorption onset could be characteristic of an indirect bandgap semiconductor, in this case it is more likely to arise from a defective or nanocrystalline film structure [
45]. Additional observations of the SEM images of selected IGS films as shown in
Figure 1 reveal behavior that supports the interpretation of the results in
Figure 6a, in particular the broadening parameter Г
1 associated with the bandgap CP. The reduced Г
1 value between
x = 0.00 and
x = 0.31 suggests a longer excited state lifetime and a longer excited carrier mean free path. This in turn indicates a larger grain size if grain boundary scattering limits the mean free path, or a lower defect density if defect scattering is the limiting mechanism. Thus, the reduction in Г
1 in
Figure 6a is consistent with the increase in grain size observed from the SEM images in
Figure 1 for an increase in IGS composition from
x = 0.00 to
x = 0.31.
In contrast, the width of the high-energy feature Г
2 represented by the TL oscillator may be controlled by broadening due to alloying over the two composition ranges, with Ga alloying from
x = 0.00 to 0.45 and with In alloying from
x = 1.00 to 0.56. Alternatively, if the TL oscillator serves to simulate multiple transitions, such parameter variations with
x may be due to electronic structure variations. Neither effect would be evident in the SEM images and may explain why Г
2 in
Figure 6b increases with increasing
x from
x = 0.00 to 0.31 and increases as well with decreasing
x from
x = 1.00 to 0.56, even though the grain size from SEM increases with alloying from each endpoint.
The most interesting feature of the trends in
Figure 6a and
Table 2 is the behavior in Г
1, the broadening parameter of the bandgap CP. In fact, the composition range of the minimum in this parameter is close to that yielding the highest efficiency CIGS solar cells. It has been proposed that the origin of the optimum CIGS solar cell efficiency near
x ~ 0.20–0.30 arises from a minimum in the concentration of volume defects due to crystallographic disorder for the ideal lattice parameter ratio of
c/a = 2 near this composition [
10,
46]. The results for the SEM of
Figure 1 and in particular for Г
1 of
Figure 6a, lead to the interesting possibility that the optimum three-stage CIGS at
x ~ 0.20–0.30 is critically linked to the optimum IGS structural properties such as the largest grain size and/or lowest grain boundary related defect concentration associated with the IGS from which CIGS is formed via Cu diffusion in stage II.
For useful applications in IGS deposition monitoring, the IGS (ε
1, ε
2) spectra can be summarized in the form of a database of coefficients. These coefficients generate polynomials in the Ga composition
x in
Table 2 and
Table 3 that in turn describe the parameters to be used in the analytical expressions for the (ε
1, ε
2) spectra. It should be recalled that because RTSE is performed at the IGS deposition temperature, the expressions generated by
Table 2 and
Table 3 are relevant to describe the (ε
1, ε
2) spectra only near 400 °C. Room temperature (ε
1, ε
2) spectra are not useful for this optical property database since the IGS films prepared in stage I of the three stage co-evaporation process are not cooled to room temperature after deposition. Instead, they are heated to the stage II temperature, which is typically in the range of 540 to 620 °C, depending on the softening temperature of the SLG used as a substrate. Because all parameters in the analytical expressions for the (ε
1, ε
2) spectra of IGS at 400 °C are expressed in terms of a single parameter, the composition
x, the (ε
1, ε
2) spectra can be generated for any given composition over these two individual ranges in
x. This capability can be used in conjunction with least squares regression, employing the IGS composition as a free parameter along with the thicknesses d
b and d
s in RTSE analysis.
The results of such an IGS compositional analysis using the parametric expressions of
Figure 6 and
Table 2 are shown in
Figure 7 and
Figure 8. This analysis was applied to a single pair of (ψ, Δ) spectra collected by in-situ SE at the end of the deposition of an IGS layer at 400 °C on the surface of a thicker (~1.0 μm) Mo bilayer as the stage I fabrication step of a three-stage CIGS solar cell.
Figure 7 shows the SE data in the form of (ψ, Δ) along with the best fit using the six-parameter model of
Figure 8, which is based on the schematic structure of
Figure 3. The parameters include the interface, bulk and surface roughness layer thicknesses, the IGS and void contents in the interface and surface roughness layers, respectively, and the IGS composition
x, which defines the (ε
1, ε
2) spectra of the IGS component of all three layers.
Figure 7 shows that the best fit is poor at the high photon energies, possibly a consequence of applying the (ε
1, ε
2) database for 4000 Å IGS to model 1.7 μm IGS. The thicker surface roughness layer on the 1.7 μm IGS may also play a role in the poor fit at high photon energies. In spite of this, the interference fringe patterns in the data at low energies and in particular their damping behavior near the IGS absorption onset are reproduced quite accurately. Aside from the limitations of the best fit, some positive indicators are observed. First, the deduced Mo/IGS interface layer thickness of 243 Å and the IGS content within the interface layer of 60.2 vol % are very close to the surface roughness thickness and void content, respectively, deduced for the underlying Mo before IGS deposition. This suggests that the interface layer derives from the roughness on the Mo and that the voids in this roughness layer are completely filled with IGS in the initial time period of IGS deposition. In addition, the deduced composition
x is within ~0.02 of the intended value of
x = 0.30 for the solar cell and within ~0.01 of the depth averaged value of 0.31, obtained from ex-situ SE of the final solar cell assuming a composition profile consisting of two linear segments [
47].
The results of
Figure 7 and
Figure 8 demonstrate that the IGS composition can be determined from in-situ and real time SE measurements. Once the parametric form for the IGS (ε
1, ε
2) versus
x is available, along with the Mo dielectric function, analysis results such as those in
Figure 8 can be obtained in analysis times on the order of seconds. Thus, if the deposition chamber is fitted with RTSE instrumentation,
x can be determined from measurements during as well as after stage I deposition without removing the sample from the chamber and without even interrupting the deposition process. It should be emphasized, however, that the application of the IGS (ε
1, ε
2) database in
Figure 6 and
Table 2 is restricted to deposition temperatures near 400 °C. A key feature of the IGS (ε
1, ε
2) spectra that provides compositional sensitivity is the shift in the CP to high energy with increasing
x evident in the inset of
Figure 5. Although the shift is relatively weak, ~0.14 eV for 0 ≤
x ≤ 0.45, in the case of a thick film, the CP characteristics control the rapid damping of the interference fringes evident in the transition from the oscillatory pattern in (ψ, Δ) to the smoothly varying spectra with increasing photon energy in
Figure 7. Furthermore, the results of
Figure 7 and
Figure 8 would suggest that the (ε
1, ε
2) database in
Table 2 from the ~3500–4100 Å IGS thickness range is quite robust as it has provided IGS composition even for a thick IGS layer obtained at the end of deposition on a solar cell relevant substrate with a thick Mo back contact layer leading to significant roughness at the Mo/IGS interface. One must further explore the limitations of the
Table 2 database in studies of the effects of thickness on the IGS complex dielectric function. The results of such studies will be described in detail in the next section.
3.2. Evolution of the Complex Dielectric Function with IGS Thickness
The central time for the range of multi-time analysis described in
Section 3.1 was selected as that corresponding to a bulk layer thickness of 3500–4100 Å. This thickness is approaching the mid-way point of the 9000 Å deposition for the series of films prepared with different compositions on Si/SiO
2/Mo substrates. This thickness was also selected to achieve a balance between the highest accuracy and broadest relevance for the parametric expressions of
Figure 6,
Table 2 and
Table 3. As will be described later in this section, for thinner films, the (ε
1, ε
2) spectra are not representative of the thicker films used in devices, possibly due to a smaller grain size or a much higher defect density than the thicker films. For thicker films, however, one must be concerned with the influence of depth non-uniformities on the RTSE analysis results; the effects of these become more pronounced with increasing thickness. In addition, the surface roughness on the IGS increases with increasing thickness, which leads to greater challenges in extracting accurate (ε
1, ε
2) spectra of the bulk layer beneath the surface roughness layer.
For the key IGS layer with
x = 0.30 deposited on SLG coated with an ~8000 Å Mo layer, the structural evolution and (ε
1, ε
2) spectra were obtained by performing a number of multi-time analyses over ranges centered at different times. In these analyses, the three-layer optical model is applied as shown in
Figure 3. In addition, the Mo/IGS interface layer was fixed at the value of 164 Å, as deduced from a measurement of the Mo surface roughness thickness at room temperature prior to substrate heating and deposition. It is also assumed that the voids in the 38/62 vol % Mo/void mixture deduced to characterize the Mo surface roughness layer are completely filled by IGS during interface formation as will be described in
Section 3.3. It should be noted that if the (ε
1, ε
2) spectra evolve with thickness, leaving an optical property depth profile built into the film, then the three-layer model in
Figure 3 is an approximation since it assumes a bulk layer having uniform (ε
1, ε
2) spectra with depth. This approximation becomes closer to reality with increasing photon energy above the bandgap since a small absorption depth implies that the light does not probe the full thickness range of the non-uniformity. Near the bandgap, however, one must be concerned with possible distortions of the deduced (ε
1, ε
2) spectra as a result of neglecting the depth non-uniformity.
In an initial multi-time analysis for each selected thickness range, a Kramers-Kronig consistent b-spline model for the (ε
1, ε
2) spectra is assumed for the bulk layer as described in
Section 3.1. From this analysis, the time evolution of the bulk and surface roughness layer thicknesses is determined. A summary of the analysis details and thickness results are presented in
Table 4 for the IGS deposition with
x = 0.30, listed in order of the initial time t
i of the multi-time range given in the first column. In addition to t
i, the final time t
f and the center time t* are given, as well as the best fit bulk layer thicknesses d
b at t
i, t
f and t* along with the surface roughness thickness d
s and its void content f
v at t*. For the surface roughness layer, a 50/50 vol % mixture of IGS/void is assumed for roughness layer thicknesses <90 Å, whereas the roughness layer composition is allowed to vary for thicker roughness layers. As described in
Section 3.1, the Bruggeman effective medium approximation is used to determine the (ε
1, ε
2) spectra of these roughness layers using the spectra of the IGS/void components and the void fraction values as input. Also shown in the last column of
Table 4 is the IGS deposition rate, given as the derivative of effective thickness d
eff versus time. The deposition rate in terms of d
eff should be constant over the deposition time if the fluxes from the In and Ga evaporation sources are stable. The results in
Table 4 suggest that the rate is constant at 10.2 Å/s within ~±3% over the first 10 min of deposition. A ~7% higher rate than the average over the initial 10 min is determined at the end of deposition. This higher rate is outside the confidence limits of the measurement. Such fluctuations in rate during longer depositions due to variations in the source fluxes may lead to compositional non-uniformities in the IGS film.
The structural parameters of
Table 4 including d
b, d
s and f
v deduced at t* are used in an inversion procedure to extract the (ε
1, ε
2) spectra from the (ψ, Δ) spectra at t*. Such inversions at the five center times in
Table 4 lead to a series of (ε
1, ε
2) spectra at different bulk layer thicknesses. Each set of (ε
1, ε
2) spectra obtained by inversion is fitted assuming the same parametric expression that was applied for the IGS composition series within the narrow 3500–4100 Å range of thickness. In addition to the expression, the constraints on the parameters in the analyses were the same as well. The best fit parametric forms of the (ε
1, ε
2) spectra of IGS for the different bulk layer thicknesses of
Table 4 are presented together for comparison in
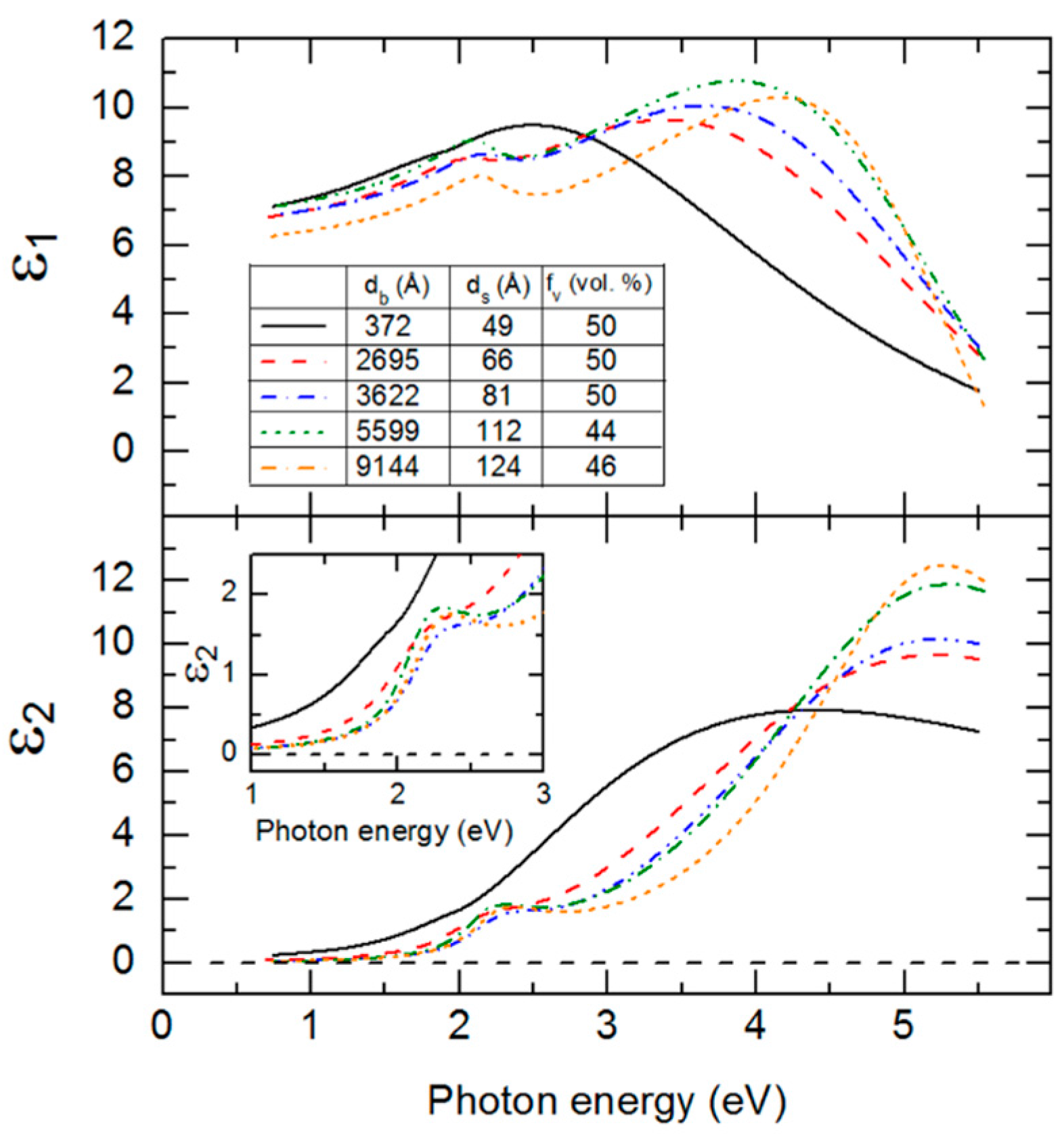
Figure 9.
Among the ten parameters that could be varied, namely five for the CP oscillator, four for the TL oscillator and the constant ε
1∞, only six are varied. The CP phase and exponent are fixed at 0° and 0.5, respectively and ε
1,∞ is fixed at unity. In addition, the TL bandgap energy is equated to the CP resonance energy. The best fit variable parameters in the analytical expression for these (ε
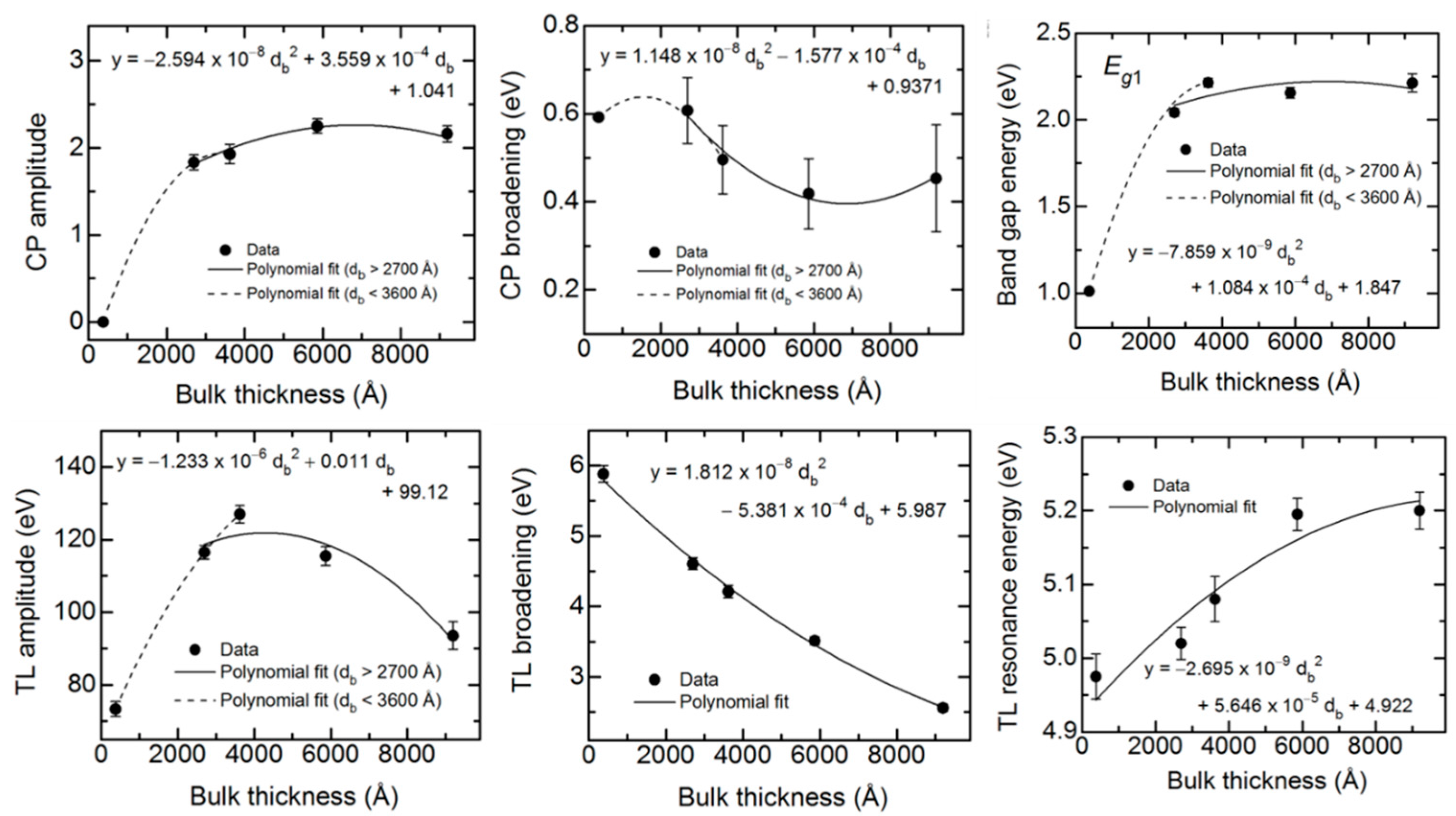
1, ε
2) spectra are plotted versus d
b at t* in
Figure 10. In order to quantify the observed thickness effects, polynomial functions describing the dependence of each variable oscillator parameter on bulk layer thickness were obtained in as many as two segments. The resulting polynomial functions associated with each parameter, shown by the lines in
Figure 10, are summarized together in
Table 5.
As is clear from the results in
Figure 9, the (ε
1, ε
2) spectra at an IGS bulk layer thickness of 372 Å are significantly different from the spectra at later times. The thin layer (ε
1, ε
2) spectra exhibit only the high energy TL oscillator with no evidence of the lower energy CP oscillator, the latter identifying the crystalline phase bandgap. Because of the abruptness of the Mo/IGS interface indicated by the EDS profile in
Figure 2, the different behavior of the (ε
1, ε
2) spectra at 372 Å is not attributed to a different initial growth material such as MoSe
2 that could be generated by reaction of the initial deposition flux with the Mo surface. The lower temperature of the stage I process may account for the proposed absence of a reaction as indicated previously [
36]. Instead, the thin layer material appears to be highly defective or disordered IGS with a TL oscillator bandgap of ~1 eV, which is much lower than the CP energy of the IGS determined at the later times. As a result of the two different characteristics of the (ε
1, ε
2) spectra, the polynomials in
Figure 10 and
Table 5 are given over the two overlapping thickness ranges of 370–3600 Å and 2700–9100 Å, indicated by the broken and solid lines, respectively, in
Figure 10. For the range of smaller thickness values, it is clear that the database for the (ε
1, ε
2) spectra in
Figure 5 and
Table 2 obtained over the 3600–4100 Å range cannot be used. More detailed studies spanning the 370–3600 Å range are needed to provide an appropriate database that may describe the dependence of the (ε
1, ε
2) spectra on
x and d
b when the CP is suppressed and a single TL oscillator may be used to fit the inversions. Further study of the structural evolution and (ε
1, ε
2) spectra over this range of thickness for the different IGS compositions will be presented in
Section 3.3.
For the 2700–9100 Å range of larger thicknesses where the bandgap CP is clearly observed, the associated CP parameter variations with thickness are relatively weak at least within the confidence limits, as indicated in
Figure 10. This is also observed in the similarity of the absorption onsets given in the
Figure 9 inset, particularly for thicknesses ≥3600 Å. In contrast, the TL oscillator parameters show larger variations over the 2700–9100 Å thickness range. The TL oscillator amplitude in
Figure 10 decreases systematically with thickness above 3600 Å with a variation outside of the confidence limits. In contrast to expectations from
Figure 10, the high energy TL oscillator is increasing in peak height with increasing thickness in the (ε
1, ε
2) spectra of
Figure 9. This apparent inconsistency arises from the fact that the prefactor in the TL oscillator equation for ε
2 incorporates not only the amplitude but also the broadening parameter and the resonance energy. As a result, the amplitude
A2 more closely reflects the integral of the oscillator, rather than its peak height. The strong systematic decrease with thickness in the broadening parameter Г
2 leads to a decrease in the TL oscillator integral in spite of the increase in peak height in
Figure 9 and, thus, a decrease in the value of
A2 in
Figure 10.
An apparent decrease in the broadening from thin (2700 Å) to thick (5900 Å) films is also observable for the CP, whereas at the largest thickness of 9100 Å the continuation of this trend is unclear. A decrease in the broadening parameter with thickness is a general indicator of a reduction in defect density or disorder, or an increase in grain size. It may be possible to couple the two broadening parameters and other CP and TL oscillator parameters as needed, to a single excited electron mean free path λ. In fact, this second parameter λ may serve along with
x as a two-parameter description of the (ε
1, ε
2) spectra for both compositional and grain size analysis of IGS films by RTSE. Such a coupling of broadening parameters has been demonstrated for the multiple CPs in the (ε
1, ε
2) spectra of CdS and CdTe thin films [
48]. In addition, a simultaneous change in void content f
bv may occur with increasing d
b, and this could be added as a third parameter in addition to
x and λ for the simulation of the (ε
1, ε
2) spectra of IGS films to account for possible density variations with thickness. These approaches are restricted to thicker films with d
b > 2700 Å where the same form of the complex dielectric function is obtained.
A key observation of this section is the systematic thickness dependence in
Figure 9 over the high energy range where the TL oscillator dominates, which then appears to suggest that the polynomials in
Figure 6 and
Table 2 are relevant strictly for thicknesses in the range of 3500–4100 Å. Variations in the ε
1 spectra in
Figure 9 also occur over the low photon energy range. In fact, the differences in the overall shapes of the (ε
1, ε
2) spectra for different thicknesses in
Figure 9 motivate a need for adjusting the parameters of
Table 2 and
Table 3 if the parametric expressions are to be valid for IGS films with largely different thicknesses. On this basis, an explanation for the apparent robustness of the fitting as indicated in
Figure 7 and
Figure 8 for a 1.7 μm thick IGS layer must be provided. It is evident from the inset in
Figure 9 that no variation in ε
2 with thickness occurs for thicknesses ≥3600 Å when 0 < ε
2 < 0.5, which is the case for photon energies 0.74 <
E < 1.9 eV. It is this range that controls the variation in
Figure 7 from undamped fringe pattern to full opacity with increasing photon energy. This conclusion is drawn from the fact that ε
2 ~ 0.2 generates an absorption depth
d0 ~
nλ/2πε
2 ~ 1.7 μm, where λ is the wavelength and
n is the index of refraction. In conclusion, an unchanging ε
2 spectrum with thickness near the absorption onset in
Figure 9 may help to explain the success of the analysis of
Figure 7 and
Figure 8. Finally, the thickness dependence in the ε
1 spectra in
Figure 9 over this same low photon energy range may be compensated by variations in the film thickness in the modeling of
Figure 7 and
Figure 8, again accounting for the robustness of the database of
Section 3.1. A more advanced database including variations in parameters that account for the dependence on thickness, however, may lead to improved fits in the high energy range of
Figure 7.
3.3. Early Stage Dielectric Function and Structural Evolution
RTSE is sensitive to monolayer level nucleation, coalescence and growth processes through precise measurements of bulk and surface roughness layer thicknesses. It also provides high sensitivity to the (ε
1, ε
2) spectra of materials even in ultrathin (~10 Å) layers [
38,
49,
50]. Through measurements of the (ε
1, ε
2) spectra, RTSE has the potential to distinguish small differences in the alloy compositions of deposited thin film materials and can detect small density deficits in the films, which affect (ε
1, ε
2) as well. Considering the results of
Section 3.1 and
Section 3.2, it is relevant to explore the sensitivity of the (ε
1, ε
2) spectra to IGS composition in the early stage of growth and thus the potential capability of real time SE for compositional analysis in this early stage.
The multi-time and inversion approaches for RTSE data analysis have been adapted to describe the early stages of IGS film growth, focusing on the specific depositions using Mo-coated, thermally-oxidized Si wafer substrates as presented in
Section 3.1. Distinct structural models depicted in the upper and lower panels in
Figure 11 are used in two time regimes. In the first regime (upper panel), the voids in the Mo surface roughness layer are filled in by depositing IGS material as substrate-induced IGS surface roughness develops simultaneously. In this regime, no IGS bulk layer is observed and as a result, the regime is characterized by only two layers as shown in the upper panel of
Figure 11. These two layers consist of (i) a three-component Mo/IGS interface roughness layer having a fixed thickness and Mo content, assumed equal to those of the roughness layer determined for the uncoated Mo surface, along with a variable IGS content f
IGS, and (ii) an IGS surface roughness layer of thickness d
s with a variable void content f
v. Here, the thickness and void content of the surface roughness layer and the IGS content in the interface roughness layer are determined individually at each time point in the analysis. In the second regime (lower panel of
Figure 11), the Mo surface roughness layer has been completely converted to a Mo/IGS interface layer. The IGS bulk layer then develops and increases in thickness with a surface roughness layer on top consisting of an assumed 50/50 vol % mixture of IGS and void, resulting in a three-layer model. The three layers from the substrate to ambient medium consist of (i) a Mo/IGS interface roughness layer, having a fixed thickness and Mo content assumed equal to those of the Mo roughness layer, (ii) an IGS bulk layer of thickness d
b and (iii) an IGS surface roughness layer of thickness d
s. In this regime, the bulk and roughness layer thicknesses are determined individually at each time point in the analysis.
The complete multi-time analysis procedure has been performed on RTSE data acquired for samples of different IGS composition in studies of the nucleation and growth of the films, in addition to determination of the early stage (ε
1, ε
2) spectra. The data analysis applied in this subsection centers on bulk layer thicknesses in the range of 350–410 Å. The strategy is similar to those applied to the IGS films with different compositions and bulk layer thicknesses in the range of 3500–4100 Å as described in
Section 3.1 and to a single film of composition
x = 0.30 and different thicknesses as described in
Section 3.2.
Table 6 summarizes the selected initial time (t
i), center time (t*) and final time (t
f) in multi-time analyses performed to obtain the early stage structural evolution and (ε
1, ε
2) spectra for IGS depositions with different Ga contents. In these analyses, a Kramers-Kronig consistent b-spline model is used initially for the (ε
1, ε
2) spectra with a node spacing of 0.1 eV. The best fit bulk layer thicknesses at t
i, t* and t
f, as well as the surface roughness layer thickness and void volume percentage in the roughness layer at t*, are also summarized in
Table 6. The mean square error for each of the best fit multi-time analyses is given in the second-to-last column of the table, with the deposition rate in terms of effective thickness d
eff in the last column. A comparison of the deposition rates in
Table 1 and
Table 6 suggests that a systematic increase in the rates up to 10% occurs from the early stage to the 3500–4100 Å thickness range for these IGS depositions that was not evident from the
x = 0.30 deposition of
Table 4. The average of these effective thickness rate increases is ~7%.
The deduced structural parameters at t* in
Table 6 enable exact inversion of the (ε
1, ε
2) spectra at this time, and these representations of the data are fitted using an analytical expression given by a single TL oscillator. An additional CP term in the expression, as required for larger bulk layer thicknesses and compositions
x ≤ 0.45, does not provide a significant improvement in the fit for these analyses.
Figure 12 presents the complete set of (ε
1, ε
2) spectra for IGS depositions of different
x, relevant for a measurement temperature of 400 °C and for IGS bulk thicknesses near ~400 Å. The best fit parameters in the single TL model for the inverted (ε
1, ε
2) spectra are given in
Table 7. Various weak trends appear in
Table 7. The resonance energy
E02 increases from 4.0 to 4.9 eV between
x = 0 and
x = 0.37, followed by a decrease and a saturation in the range from 4.6 to 4.8 eV at higher
x values. The Tauc gap
Eg2 shows the minimum and maximum values of ~1.0 and 1.6 at
x = 0 and 1, respectively, but with a rather weak variation for 0.1 ≤
x ≤ 0.45 with
x values between 1.11 and 1.16 eV. The broadening parameter Г
2 shows minimum values for
x = 0.00 and 1.00, with an abrupt increase with
x for 0.00 ≤
x ≤ 0.10 and a gradual decrease for
x ≥ 0.56. Based on the trends in
Table 7, one can conclude that (ε
1, ε
2) spectra obtained near ~400 Å do not exhibit a sufficiently sharp absorption onset to provide high compositional sensitivity in the early stage of IGS growth. Only after the CP oscillator associated with larger grain polycrystalline IGS emerges does compositional analysis become possible as demonstrated in
Section 3.1.
The initial nucleation and early stage growth behavior of the IGS can be characterized according to the structural evolution in
Figure 11 by applying the best fit early stage (ε
1, ε
2) spectra in the two models. These (ε
1, ε
2) spectra are modeled as Kramers-Kronig consistent b-splines and deduced over the range of 350–410 Å. It is clear from the results for the composition
x = 0.30, as studied in depth in
Section 3.2, that the thin layer (ε
1, ε
2) spectra are needed, e.g., those at d
b* = 408 Å as indicated in
Table 6 for the analysis of the
x = 0.31 composition, because they differ significantly from those obtained near 3600 Å.
Figure 13 shows the final results of this structural analysis for the
x = 0.31 IGS film. The time evolution of the best fit MSE is presented in
Figure 13a and the evolution of the best fit structural parameters according to the model of
Figure 11 is presented in
Figure 13b–e. The structural evolution panels depict the basic characteristics of interface filling, surface roughness development and bulk layer growth.
Figure 13b shows the early stage evolution in which the assumed 50 vol % void content associated with the 75 Å thick roughness layer on the underlying Mo is replaced by IGS, leading to a rapid increase in the IGS content (f
IGS) of the layer. Simultaneously, the IGS surface roughness layer thickness d
s increases and its void content f
v decreases as shown in
Figure 13c,d, respectively, as the Mo surface roughness is conformally covered. After the void volume in the Mo surface roughness is completely filled by IGS to form a stable Mo/IGS interface roughness layer of composition 50/50 vol %, a bulk layer of thickness d
b can be incorporated into the model. In
Figure 13e, the bulk layer is observed to follow expectations, increasing linearly with time from the onset of its growth. During initial bulk layer growth, the surface roughness on the IGS decreases rapidly in thickness as observed in
Figure 13c, indicating that the substrate induced roughness is suppressed due to thin film structural coalescence. During bulk layer growth,
Figure 13b,d are no longer interesting, as they simply show that the IGS content in the Mo/IGS interface roughness and the void content in the IGS surface roughness, respectively, are both fixed at 50 vol %. These assumptions are made for all analyses after the onset of bulk layer growth due to the complete filling of the Mo roughness and the relatively thin or in some cases negligible roughness layer during initial bulk layer growth. Interface filling, surface roughness development and a linearly increasing bulk layer thickness, as well as structural coalescence, are observed similarly for all other Ga compositions.
Next the focus of the discussion turns to the details of the IGS nucleation and growth process as depicted in
Figure 13 for the composition
x = 0.31 and in
Figure 14 for all compositions. In the interface filling stage, IGS surface roughness development is interpreted as resulting from the IGS layer conformally covering the roughness on the Mo surface. Due to the presence of the relatively thick Mo roughness layer, in the range 69–88 Å, it cannot be determined whether interface filling occurs conformally layer-by-layer or as isolated nuclei that coalesce. In contrast, if the Mo was atomically smooth, any development of roughness in the initial stage could be attributed to a nucleation process. For the depositions of
Figure 14, a roughness layer of thickness ranging from ~10 Å for In
2Se
3 to 40 Å for Ga
2Se
3 forms at the onset of bulk layer growth. Thus, for all depositions in
Figure 14, the IGS roughness values do not approach those typical of the underlying Mo. This indicates that in the interface formation process, the evolving IGS surface smoothens in the transition from uncoated to completely coated Mo, and for all IGS depositions with
x > 0.00, either the smoothening process or an atomically smooth surface is observed at least until a bulk layer thickness of 100 Å has been reached.
Although non-monotonic behavior versus composition occurs in
Figure 14, some observations appear conclusive. The minimum and maximum IGS surface roughness thicknesses of 10 Å and 40 Å at the onset of bulk layer growth occur for In
2Se
3 and Ga
2Se
3, respectively. The weakest smoothening effects subsequent to these onsets are observed for the In
2Se
3 deposition and for the three depositions with Ga compositions
x ≥ 0.56. In fact, for In
2Se
3, roughening occurs after ~70 Å of bulk layer growth in
Figure 14 whereas for Ga
2Se
3, weak smoothening continues over the 70–100 Å range of bulk layer growth. For IGS with 0.10 ≤
x ≤ 0.45, surface smoothening after the onset of bulk layer growth is not only rapid but also sufficient to generate atomically smooth surfaces. It is also interesting to note that for the samples with
x = 0.31 and 0.37, the coalescence process occurs most rapidly, yielding a film with an atomically smooth surface after ~30 Å of bulk layer deposition. These same films also show longest time-period of atomically smooth surfaces. The behavior for all IGS depositions in
Figure 14 is consistent with the single TL nature of the complex dielectric function of these films determined at bulk layer thicknesses of 350–410 Å in
Figure 12. Consistency is suggested because highly disordered or even liquid films are expected to exhibit a single TL and can form atomically smooth surfaces upon coalescence [
51]. A liquid film is a possibility due to the reduction in the melting point from the bulk that can occur in clusters and very thin films, as observed in RTSE studies of Ag thin film deposition [
52]. It is of interest to note that the compositions with the most rapid smoothening effect during coalescence are those that exhibit the highest performance when incorporated into devices.
3.4. Intermediate and Later Stage Structural Evolution
In this section, the discussion will focus on the intermediate and later stage surface roughness evolution for the IGS thin films of different compositions. For the nine samples of the composition series, these stages are defined as spanning the thickness ranges over which the (ε
1, ε
2) spectra deduced at 3500–4100 Å and at the endpoint near 9000 Å, respectively, are relevant [
53]. The results for the two stages can be compared with the roughness evolution associated with initial growth, which uses the (ε
1, ε
2) spectra deduced at thicknesses within the range of 350–410 Å. For the five different thickness ranges of the thicker IGS deposition with
x = 0.30, the (ε
1, ε
2) spectra obtained by multi-time analysis using a b-spline model were applied, as described in
Section 3.2. Although the complex dielectric function is varying with thickness throughout the deposition, the results for the surface roughness evolution presented in this subsection show clear trends with composition that support the validity of the overall analyses.
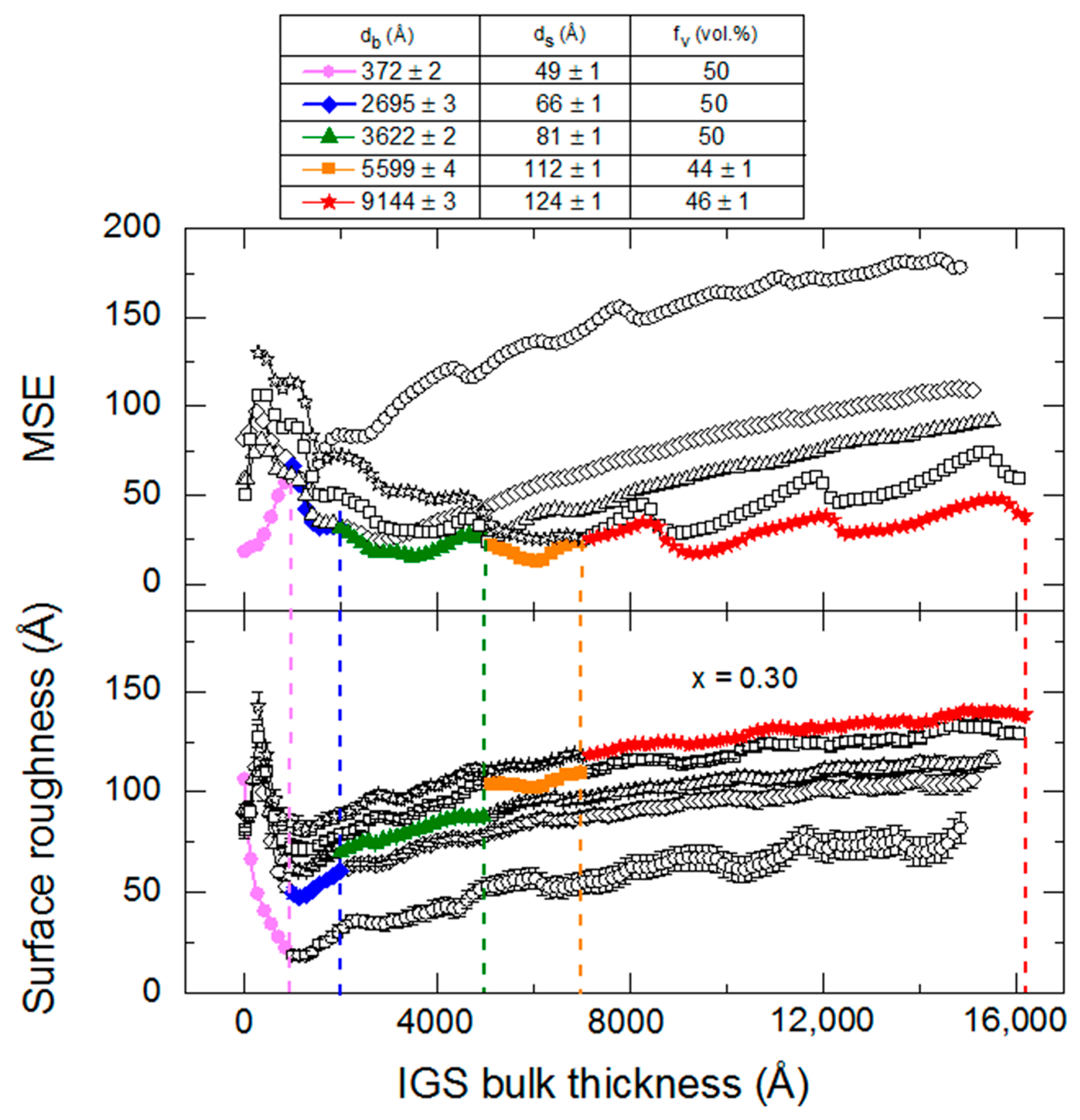
In order to evaluate the effect of the thickness dependent (ε
1, ε
2) spectra on the deduced surface roughness evolution for an IGS film, the film with
x = 0.30 from the thickness series of
Section 3.2 was studied using five different pairs of (ε
1, ε
2) spectra in the structural analyses. For this film, the time evolution of the surface roughness and MSE, as determined using the five sets of (ε
1, ε
2) spectra, are shown in the lower and upper panels of
Figure 15, respectively. A table of the bulk and surface roughness layer thicknesses and the surface roughness void contents at the center times of the multi-time analyses are reproduced from
Table 4 above the panels in
Figure 15. These structural parameters are obtained simultaneously with the five sets of (ε
1, ε
2) spectra modeled as b-splines. Considering the main part of
Figure 15, clear offsets in the resulting values of surface roughness can be seen (lower panel), depending on the (ε
1, ε
2) spectra selected for analysis; however, the roughening trends are consistent among the five data sets. On the basis of the MSE (upper panel), the (ε
1, ε
2) spectra associated with the bulk thickness of 372 Å gives the lowest MSE from the onset of bulk layer growth to a thickness of only ~1000 Å. Because these (ε
1, ε
2) spectra are significantly different from those obtained in the analyses centered at 2695 and 3622 Å, as shown in
Figure 9, the MSE obtained using the (ε
1, ε
2) spectra from the 372 Å center thickness (open circles) rapidly increases over this thickness range, and the resulting roughness evolution for IGS thicknesses above 1000 Å should not be considered accurate in this analysis. For the results using the (ε
1, ε
2) spectra from the four larger center thicknesses, the MSE increases are not as large. Selecting the lowest MSE results from among the five possible analysis results, the best fitting roughness evolution is expected to vary from ~110 Å at the lower thickness [results obtained with the (ε
1, ε
2) spectra deduced at the lowest thickness] to ~140 Å at the highest thickness [results obtained with the (ε
1, ε
2) spectra deduced at the endpoint]. The lowest MSE results are highlighted in the lower and upper panels of
Figure 15 as the filled points. The larger roughness layer thicknesses in the early stage of growth for this
x = 0.30 deposition compared with those for
x = 0.31 in
Figure 14 is substrate induced, i.e., caused by the much larger roughness on the surface of the thicker Mo film deposited as a solar cell back contact on SLG.
Figure 15 suggests that in a comparison of results for ~9000 Å thick IGS depositions of different compositions from
Section 3.1, a reasonable approximation to the surface roughness evolution can be obtained over the bulk layer thickness range from 1000 Å to the end of the deposition using the (ε
1, ε
2) spectra deduced from multi-time analysis centered at 3500–4100 Å.
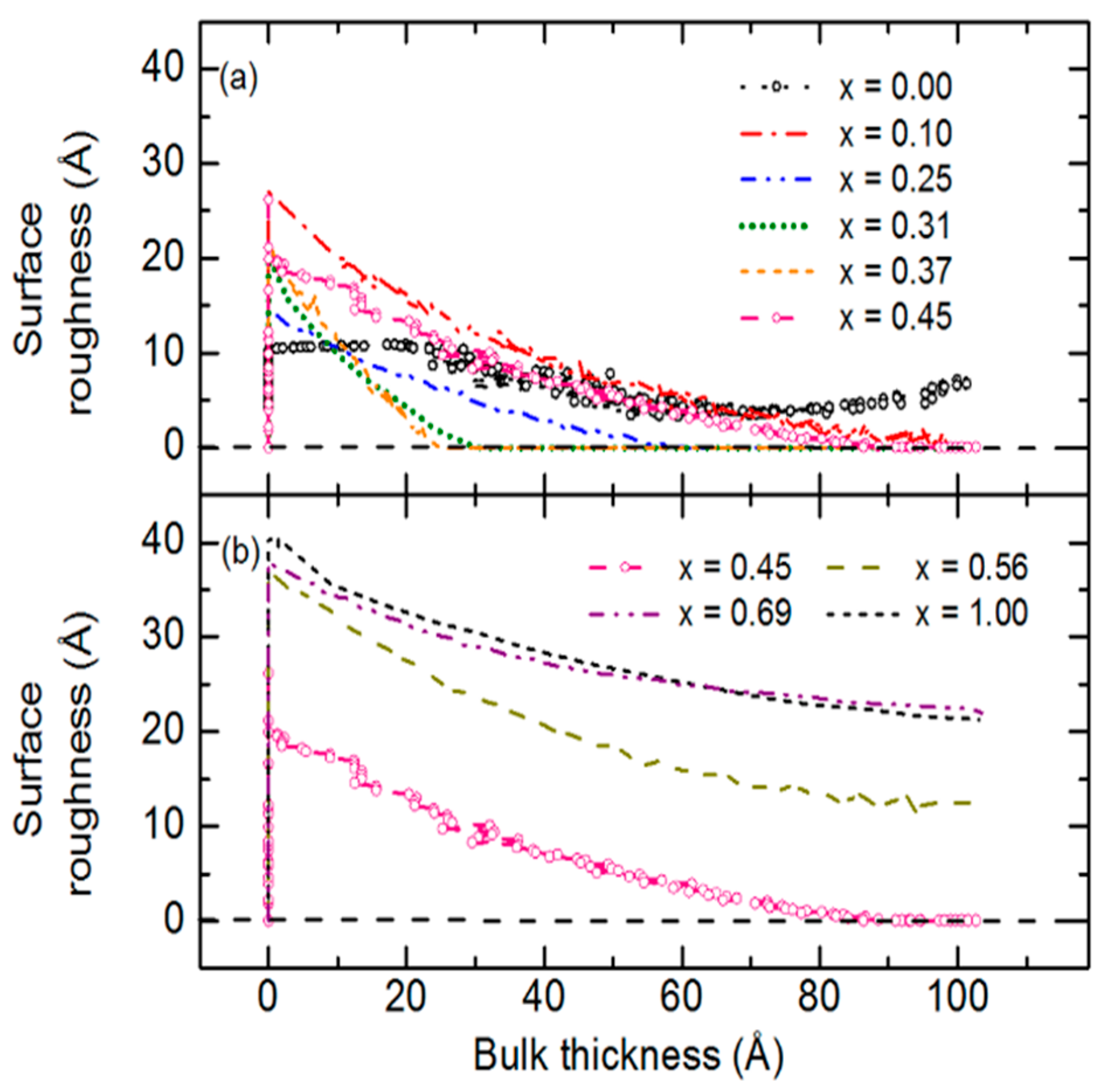
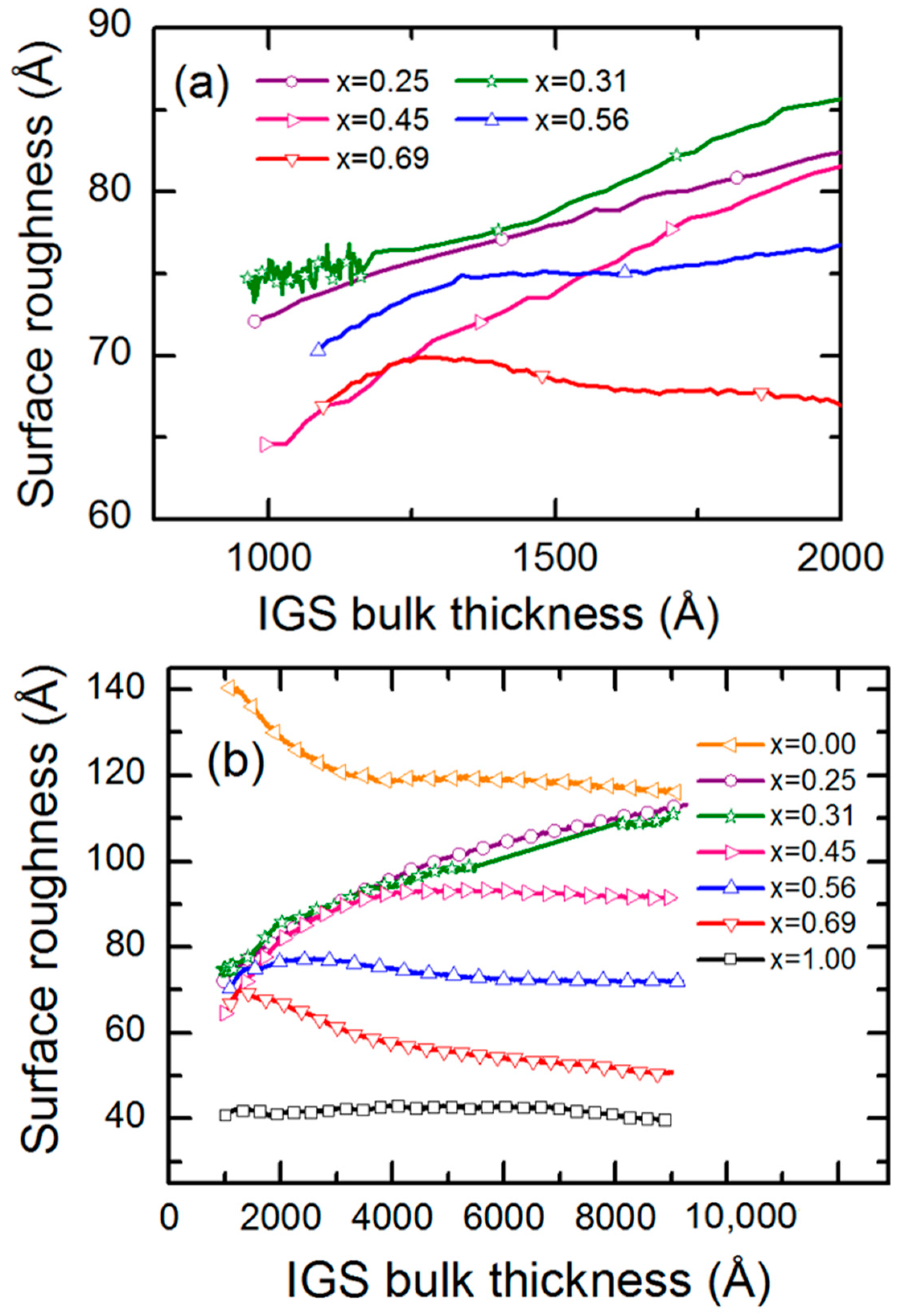
Figure 16a shows these results for Ga compositions
x = [Ga]/{[In] + [Ga]} selected for clarity over the bulk layer thickness range from 1000 to 2000 Å, and
Figure 16b shows such results for a larger sample set and covering a wider range of bulk layer thickness to the deposition endpoint. For
x ≤ 0.45, continuous roughening from 1000 to 2000 Å in
Figure 16a suggests continuous crystallite evolution which generates growing protrusions at the surface, whereas for
x = 0.69, a smoothening effect is observed, yielding a flatter surface characteristic of the stabilization of a fine-grained structure [
53,
54]. During the later stage of growth in
Figure 16b, only the films with
x = 0.25 and 0.31 show continued roughening throughout the growth process. All other films show either stabilized or smoothening surfaces at the end of deposition.
The multi-time approach as described in
Section 3.1 was also used for the determination of (ε
1, ε
2) spectra at the endpoint thickness near 9000 Å for the IGS depositions of the composition series of
Section 3.1. The endpoint surface roughness layer thicknesses deduced from these (ε
1, ε
2) spectra are presented in
Figure 17. Although these results exhibit a clear monotonic trend with composition, they also tend to show different magnitudes over the two ranges of composition
x ≤ 0.45 and
x ≥ 0.56. In fact, the surface roughness thickness shows a more rapid decrease when the
x value increases above
x ~ 0.45, which is likely to suggest a rapid reduction in the grain size, leading to smaller, stable protrusions above the surface. It is of interest to consider the results in
Figure 17, in view of the (ε
1, ε
2) spectra versus
x in
Figure 5 and also in view of the SEM images and XRD patterns of the IGS films in
Figure 1, obtained for the same set of IGS films. The rapid change in structure above
x = 0.45 in
Figure 17 is consistent with the loss of the bandgap CP in
Figure 5 and its replacement by a broad absorption onset typical of a disordered or nanocrystalline semiconductor. From the SEM images and XRD patterns, a strong reduction in grain size is observed between the available results for
x = 0.31 and
x = 0.56, which is consistent with the reduction in surface roughness in
Figure 17.