Effects of Polypropylene Orientation on Mechanical and Heat Seal Properties of Polymer-Aluminum-Polymer Composite Films for Pouch Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
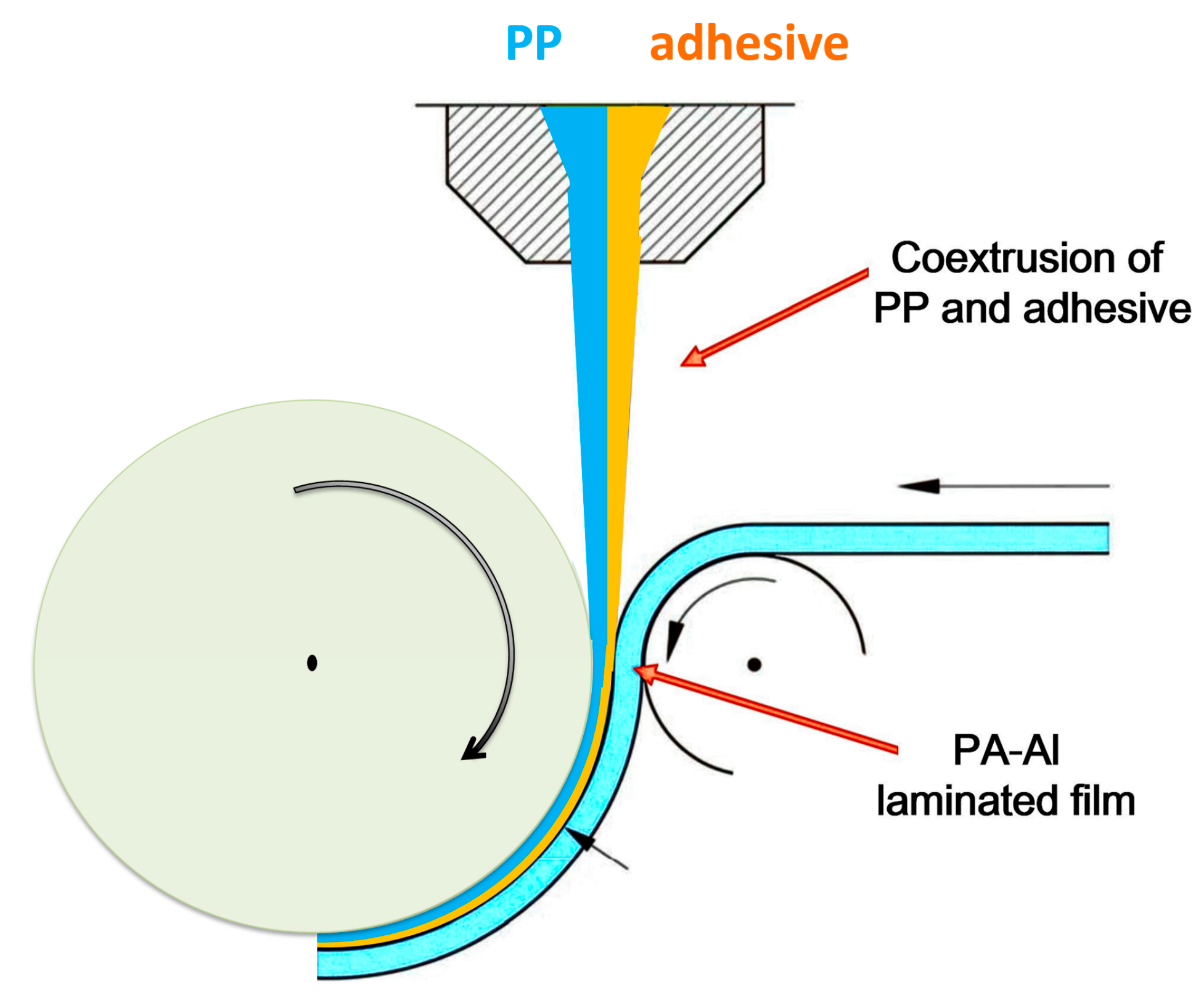
2.2. Sample Preparation
2.3. Characterizations
2.3.1. Scanning Electron Microscope (SEM)
2.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.3. Differential Scanning Calorimeter (DSC)
2.3.4. Mechanical Properties Measurement
3. Results and Discussion
3.1. Overview
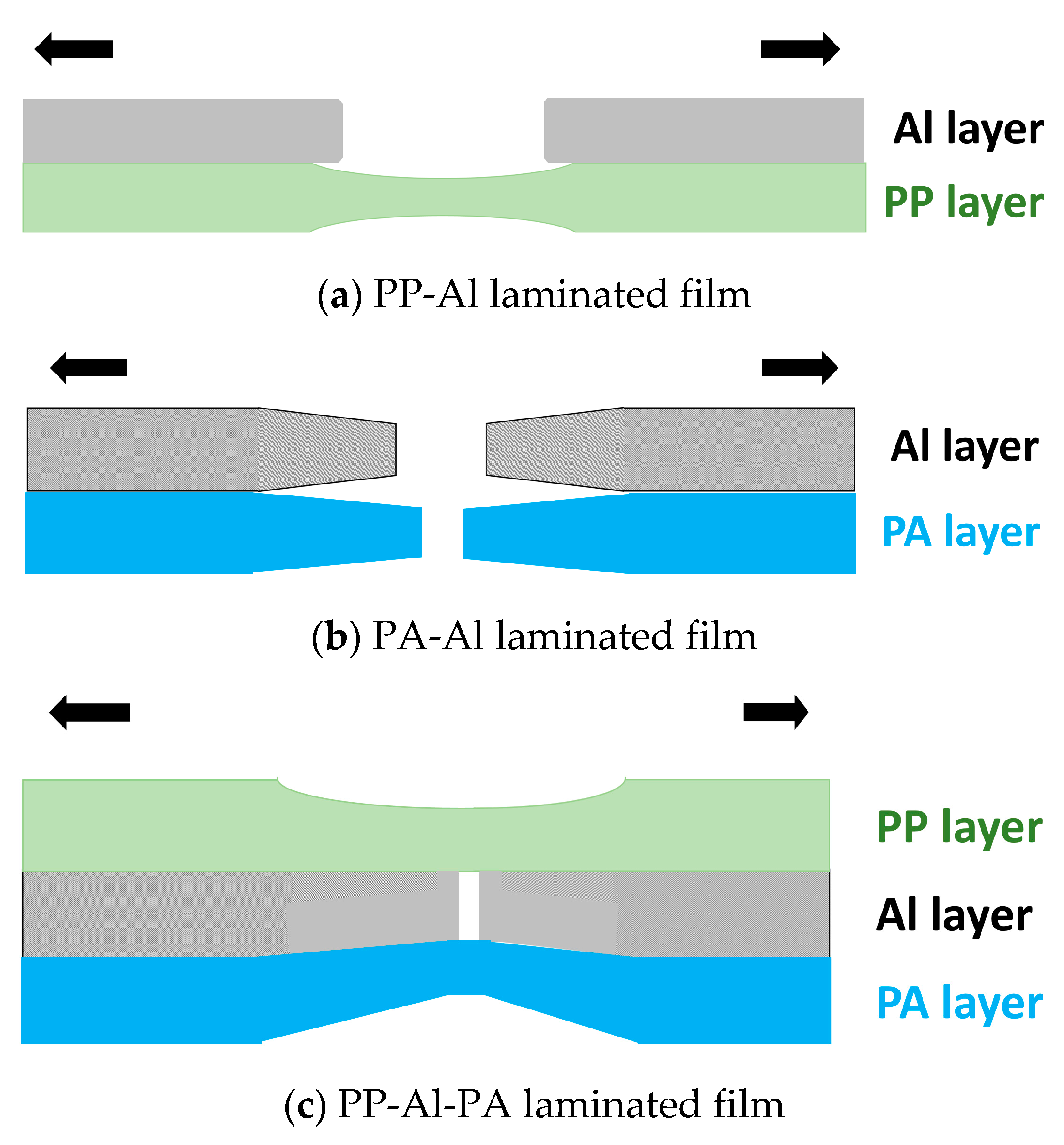
3.1.1. Lamination Structure Observation
3.1.2. DSC Analysis
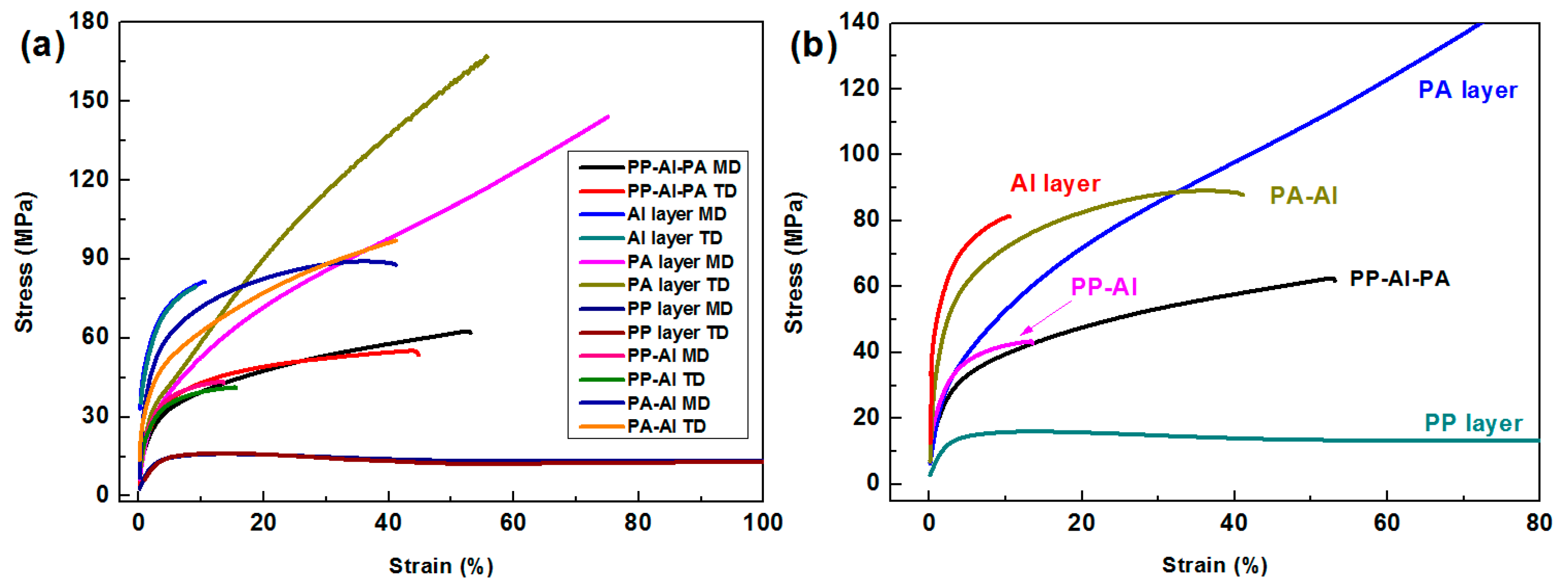
3.1.3. Tensile Properties of the Laminated Films
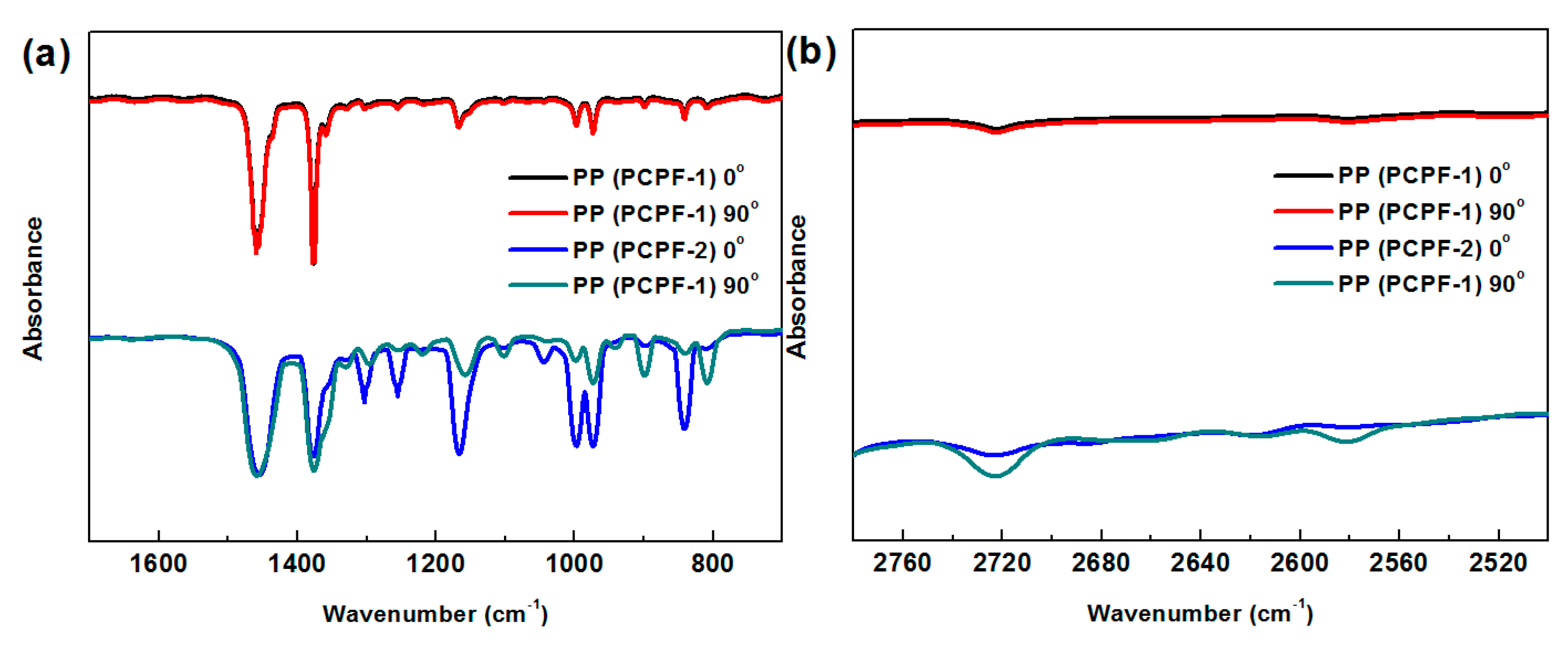
3.2. Fourier Transform Infrared Spectroscopy Analysis on PP Layer
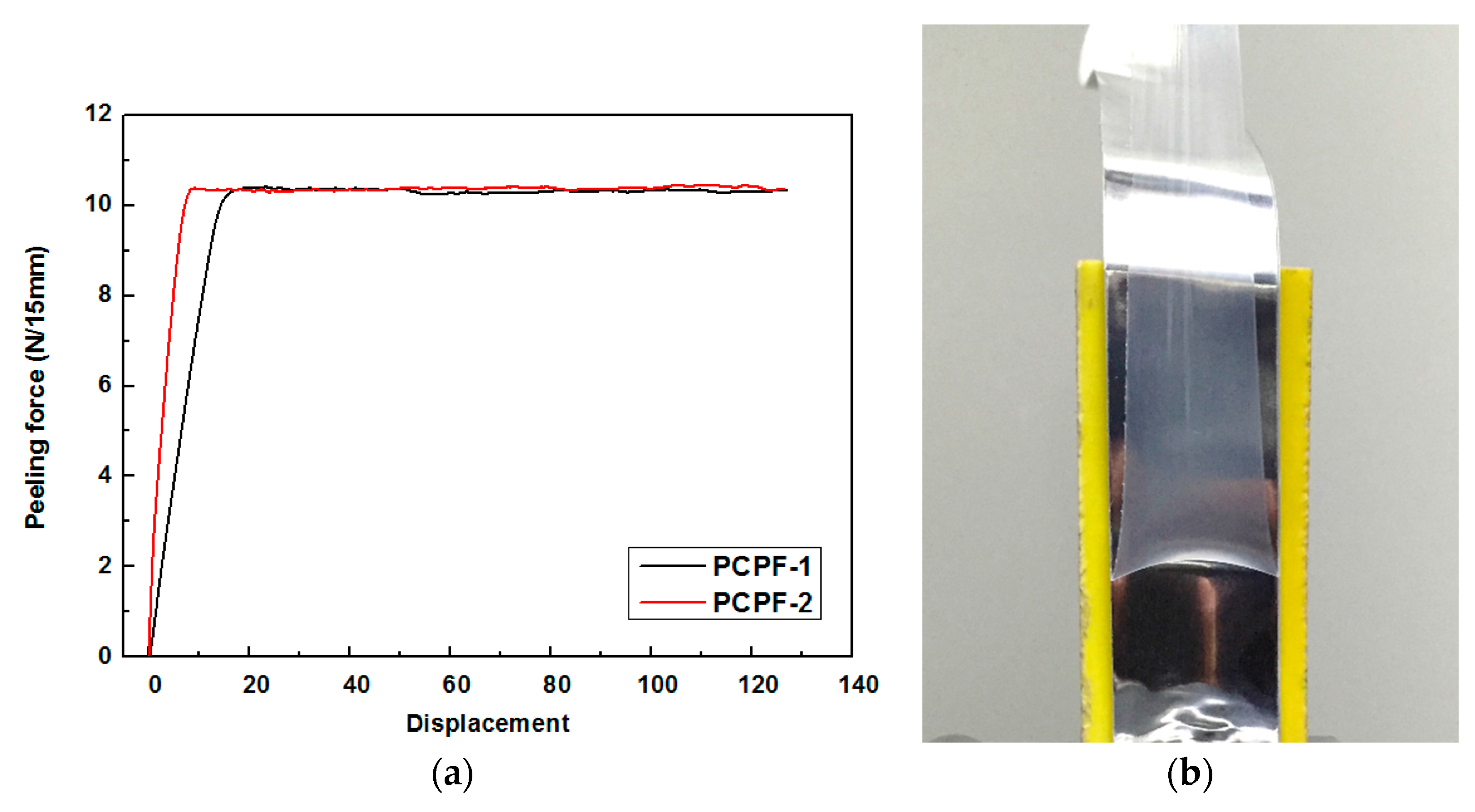
3.3. Peeling Behavior of PCPF-1 and PCPF-2
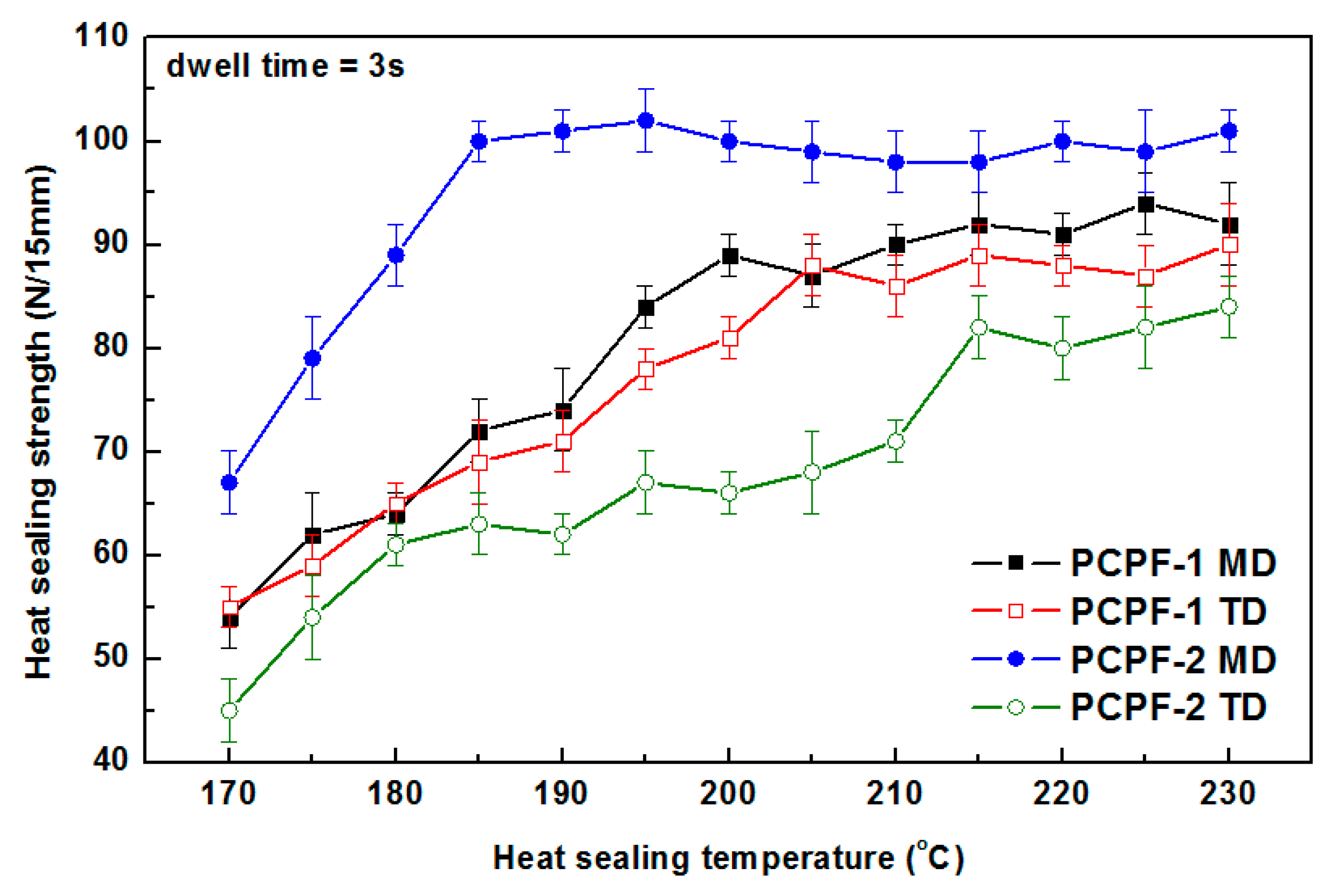
3.4. Heat Seal Properties
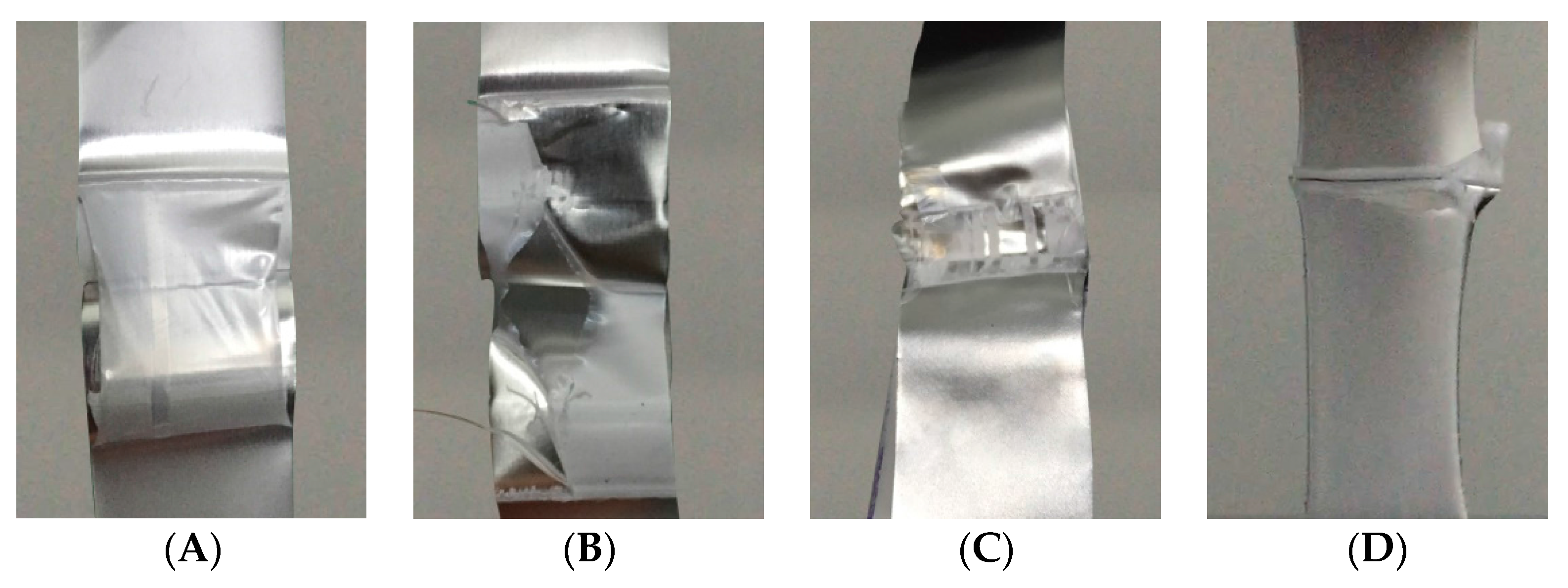
- Effects of heat seal temperature
- Effects of dwell time
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, H.; Liu, J.; Zhou, Q.; Wang, J. Novel polymer electrolyte based on PVDF/HDPE blending for lithium-ion battery. Mater. Lett. 2013, 99, 164–167. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, K.; Wu, F.; Zhang, C. A High Power Density Dual-electrolyte Lithium-Silver Battery with Celgard® 2325 Separator. Electrochim. Acta 2014, 116, 429–433. [Google Scholar] [CrossRef]
- Guo, Z.; Fan, Y. Heat seal properties of polymer–aluminum–polymer composite films for application in pouch lithium-ion battery. RSC Adv. 2016, 6, 8971–8979. [Google Scholar] [CrossRef]
- Li, T.; Suo, Z. Ductility of thin metal films on polymer substrates modulated by interfacial adhesion. Int. J. Solids Struct. 2007, 44, 1696–1705. [Google Scholar] [CrossRef]
- O’Kane, W.J.; Young, R.J. Interlaminar Bond Strength and Failure Mechanisms in Commercial Flexible Polymer-Metal Laminates. J. Adhes. 2006, 41, 203–224. [Google Scholar] [CrossRef]
- Li, T.; Suo, Z. Deformability of thin metal films on elastomer substrates. Int. J. Solids Struct. 2006, 43, 2351–2363. [Google Scholar] [CrossRef]
- Planes, E.; Marouani, S.; Flandin, L. Optimizing the heat sealing parameters of multilayers polymeric films. J. Mater. Sci. 2011, 46, 5948–5958. [Google Scholar] [CrossRef]
- Li, T.; Huang, Z.Y.; Xi, Z.C.; Lacour, S.P.; Wagner, S.; Suo, Z. Delocalizing strain in a thin metal film on a polymer substrate. Mech. Mater. 2005, 37, 261–273. [Google Scholar] [CrossRef]
- Li, Z.H.T.; Suo, Z.; Lacour, S.P.; Wagner, S. Stretchability of thin metal films on elastomer substrates. Appl. Phys. Lett. 2004, 85, 3435–3437. [Google Scholar] [CrossRef]
- Alaca, B.E.; Saif, M.T.A.; Sehitoglu, H. On the interface debond at the edge of a thin film on a thick substrate. Acta Mater. 2002, 50, 1197–1209. [Google Scholar] [CrossRef]
- Macionczyk, F.; Brückner, W. Tensile testing of AlCu thin films on polyimide foils. J. Appl. Phys. 1999, 86, 4922–4929. [Google Scholar] [CrossRef]
- Leu, J.; Ho, P.S.; Chiu, S.L. Fracture of metal-polymer line structures. II. Rigid-rodlike polyimide. J. Appl. Phys. 1994, 76, 5143–5148. [Google Scholar] [CrossRef]
- Chiu, S.L.; Leu, J.; Ho, P.S. Fracture of metal-polymer line structures. I. Semiflexible polyimide. J. Appl. Phys. 1994, 76, 5136–5142. [Google Scholar] [CrossRef]
- Andreasson, E.; Kao-Walter, S.; Ståhle, P. Micro-mechanisms of a laminated packaging material during fracture. Eng. Fract. Mech. 2014, 127, 313–326. [Google Scholar] [CrossRef]
- Stehing, F.C.; Meka, P. Heat Sealing of semicrystalline polymer films I. Calculation and measurement of interfacial temperatures: Effect of process variable on seal properties. J. Appl. Polym. Sci. 1994, 51, 89–103. [Google Scholar]
- James, P.M.; Farley, M. Heat Sealing of Semicrystalline Polymer Films. III. Effect of Corona Discharge Treatment of LLDPE. J. Appl. Polym. Sci. 1994, 51, 121–131. [Google Scholar]
- Ferdinand, P.M.; Stehlinc, C. Heat sealing of semicrystalline polymer films. II. Effect of melting distribution on heat. J. Appl. Polym. Sci. 1994, 51, 105–119. [Google Scholar]
- Simanke, A.G.; de Lemos, C.; Pires, M. Linear low density polyethylene: Microstructure and sealing properties correlation. Polym. Test. 2013, 32, 279–290. [Google Scholar] [CrossRef]
- Mazzola, N.; Cáceres, C.A.; França, M.P.; Canevarolo, S.V. Correlation between thermal behavior of a sealant and heat sealing of polyolefin films. Polym. Test. 2012, 31, 870–875. [Google Scholar] [CrossRef]
- Lin, Y.J.; Poon, B.C.; Marchand, G.R.; Hiltner, A.; Baer, E. Adhesion of propylene-ethylene copolymers to high-density polyethylene. Polym. Eng. Sci. 2010, 50, 592–605. [Google Scholar] [CrossRef]
- Funasaka, T.A.T.; Maekawa, S.; Ohno, S.; Meguro, M.; Nishino, T.; Nakamae, K. Adhesive ability and solvent solubility of propylene-butene copolymers modified with maleic anhydride. Int. J. Adhes. Adhes. 1999, 19, 367–371. [Google Scholar] [CrossRef]
- Chaffin, K.A. High-Strength Welds in Metallocene Polypropylene/Polyethylene Laminates. Science 2000, 288, 2187–2190. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.-Q.; Tervoort, T.A.; Rastogi, S.; Lemstra, P.J. Welding Behavior of Semicrystalline Polymers. 2. Effect of Cocrystallization on Autoadhesion. Macromolecules 2000, 33, 7084–7087. [Google Scholar] [CrossRef]
- Kang, J.; Yang, F.; Wu, T.; Li, H.; Cao, Y.; Xiang, M. Polymerization control and fast characterization of the stereo-defect distribution of heterogeneous Ziegler–Natta isotactic polypropylene. Eur. Polym. J. 2012, 48, 425–434. [Google Scholar] [CrossRef]
- Kang, J.; Cao, Y.; Li, H.; Li, J.; Chen, S.; Yang, F.; Xiang, M. Influence of the stereo-defect distribution on the crystallization behavior of Ziegler-Natta isotactic polypropylene. J. Polym. Res. 2012, 19, 1–11. [Google Scholar] [CrossRef]
- Kang, J.; Weng, G.; Chen, Z.; Chen, J.; Cao, Y.; Yang, F.; Xiang, M. New understanding in the influence of melt structure and β-nucleating agents on the polymorphic behavior of isotactic polypropylene. RSC Adv. 2014, 56, 29514–29526. [Google Scholar] [CrossRef]
- Tabatabaei, S.H.; Carreau, P.J.; Ajji, A. Effect of processing on the crystalline orientation, morphology, and mechanical properties of polypropylene cast films and microporous membrane formation. Polymer 2009, 50, 4228–4240. [Google Scholar] [CrossRef]
- Kang, J.; Li, X.; Xiong, B.; Liu, D.; Chen, J.; Yang, F.; Cao, Y.A.; Xiang, M. Investigation on the Tensile Behavior and Morphology Evolution of Isotactic Polypropylene Films Polymerized with Different Ziegler–Natta Catalysts. Adv. Polym. Technol. 2017, 36, 44–57. [Google Scholar] [CrossRef]
- Wu, T.; Xiang, M.; Cao, Y.; Kang, J.; Yang, F. Pore formation mechanism of β nucleated polypropylene stretched membranes. RSC Adv. 2014, 4, 36689–36701. [Google Scholar] [CrossRef]
- Kang, J.; Chen, J.; Cao, Y.; Li, H. Effects of ultrasound on the conformation and crystallization behavior of isotactic polypropylene and [beta]-isotactic polypropylene. Polymer 2010, 51, 249–256. [Google Scholar] [CrossRef]
- Kang, J.; Li, J.; Chen, S.; Zhu, S.; Li, H.; Cao, Y.; Yang, F.; Xiang, M. Hydrogenated petroleum resin effect on the crystallization of isotactic polypropylene. J. Appl. Polym. Sci. 2013, 130, 25–38. [Google Scholar] [CrossRef]
- Kang, J.; Li, J.; Chen, S.; Peng, H.; Wang, B.; Cao, Y.; Li, H.; Chen, J.; Gai, J.; Yang, F.; et al. Investigation of the crystallization behavior of isotactic polypropylene polymerized with different Ziegler-Natta catalysts. J. Appl. Polym. Sci. 2013, 129, 2663–2670. [Google Scholar] [CrossRef]





| Sample | Cooling Process | Subsequent Heating Process | |||
|---|---|---|---|---|---|
| Tc (°C) | Tconset (°C) | Tcendset (°C) | Tm (°C) | Xc (%) | |
| PP Layer | 110.2 | 116.1 | 103.0 | 159.3 | 34.2 |
| PA Layer | 180.5 | 187.4 | 174.0 | 216.2 | 36.1 |
| Polyolefin adhesive | - | - | - | 85.4 | 7.8 |
| PU adhesive | - | - | - | - | - |
| Samples | Tensile Strength (MPa) | Elongation at Break (%) | ||
|---|---|---|---|---|
| MD | TD | MD | TD | |
| PP Layer | 25.3 ± 0.1 | 23.4 ± 0.1 | 596.5 ± 24.1 | 604.7 ± 32.0 |
| PA Layer | 144.5 ± 0.4 | 167.8 ± 0.3 | 75.3 ± 5.2 | 55.5 ± 3.1 |
| Al Layer | 81.6 ± 0.2 | 80.5 ± 0.1 | 10.8 ± 1.1 | 9.4 ± 0.7 |
| PP-Al | 43.3 ± 0.1 | 40.9 ± 0.1 | 13.7 ± 1.2 | 14.7 ± 1.1 |
| PA-Al | 87.5 ± 0.1 | 97.5 ± 0.2 | 41.2 ± 2.9 | 41.4 ± 2.2 |
| PP-Al-PA | 61.5 ± 0.1 | 55.9 ± 0.1 | 53.2 ± 3.1 | 47.5 ± 2.5 |
| Samples | fc | fav | fam |
|---|---|---|---|
| PP layer (PCPF-1) | 0.000 | 0.000 | 0.000 |
| PP layer (PCPF-2) | 0.294 | 0.309 | 0.350 |
| Heat Seal Temp | 170 °C | 180 °C | 190 °C | 200 °C | 210 °C | 220 °C | 230 °C |
|---|---|---|---|---|---|---|---|
| PCPF-1 MD | AAAAB | AAAAB | BBBCC | BCCCD | BBCCD | BBDDD | DDDDD |
| PCPF-1 TD | AAAAB | AAAAB | BBBBC | BBCCC | BBCCD | BCDDD | DDDDD |
| PCPF-2 MD | AAABB | BBBCC | CDDDD | DDDDD | DDDDD | DDDDD | DDDDD |
| PCPF-2 TD | AAAAA | AAABB | AAAAB | AAABB | ABBBC | BBBCC | BBCCD |
| Heat Seal Parameters | MD | TD |
|---|---|---|
| HSS | + | − |
| Dwell time needed to obtain desired HSS | − | + |
| Critical transition heat seal temperature (Tcrit) | − | + |
| Highest HSS value on the platform | + | − |
| Difference of HSS between MD and TD | + | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, F.; Chen, J.; Yang, F.; Kang, J.; Cao, Y.; Xiang, M. Effects of Polypropylene Orientation on Mechanical and Heat Seal Properties of Polymer-Aluminum-Polymer Composite Films for Pouch Lithium-Ion Batteries. Materials 2018, 11, 144. https://doi.org/10.3390/ma11010144
Zeng F, Chen J, Yang F, Kang J, Cao Y, Xiang M. Effects of Polypropylene Orientation on Mechanical and Heat Seal Properties of Polymer-Aluminum-Polymer Composite Films for Pouch Lithium-Ion Batteries. Materials. 2018; 11(1):144. https://doi.org/10.3390/ma11010144
Chicago/Turabian StyleZeng, Fangxinyu, Jinyao Chen, Feng Yang, Jian Kang, Ya Cao, and Ming Xiang. 2018. "Effects of Polypropylene Orientation on Mechanical and Heat Seal Properties of Polymer-Aluminum-Polymer Composite Films for Pouch Lithium-Ion Batteries" Materials 11, no. 1: 144. https://doi.org/10.3390/ma11010144





