Nitrogen-Doped Carbon for Red Phosphorous Based Anode Materials for Lithium Ion Batteries
Abstract
:1. Introduction
2. Experimental
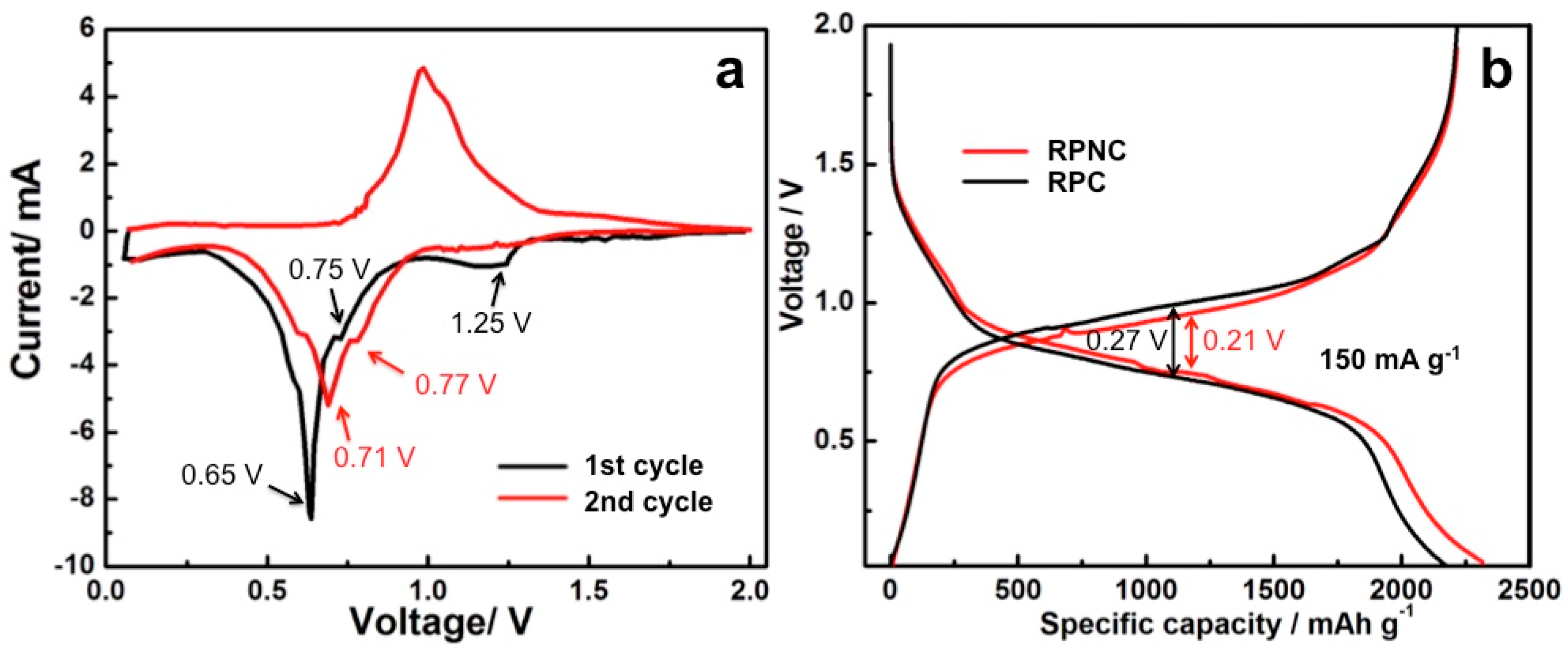
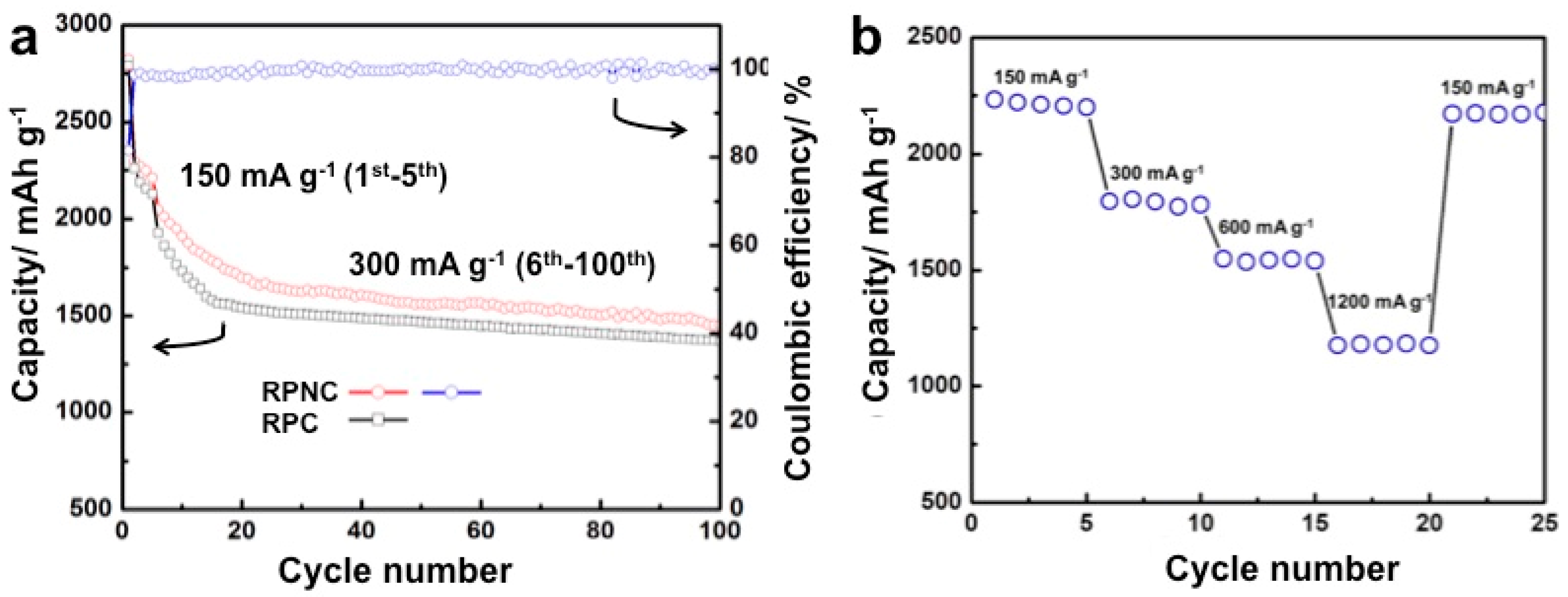
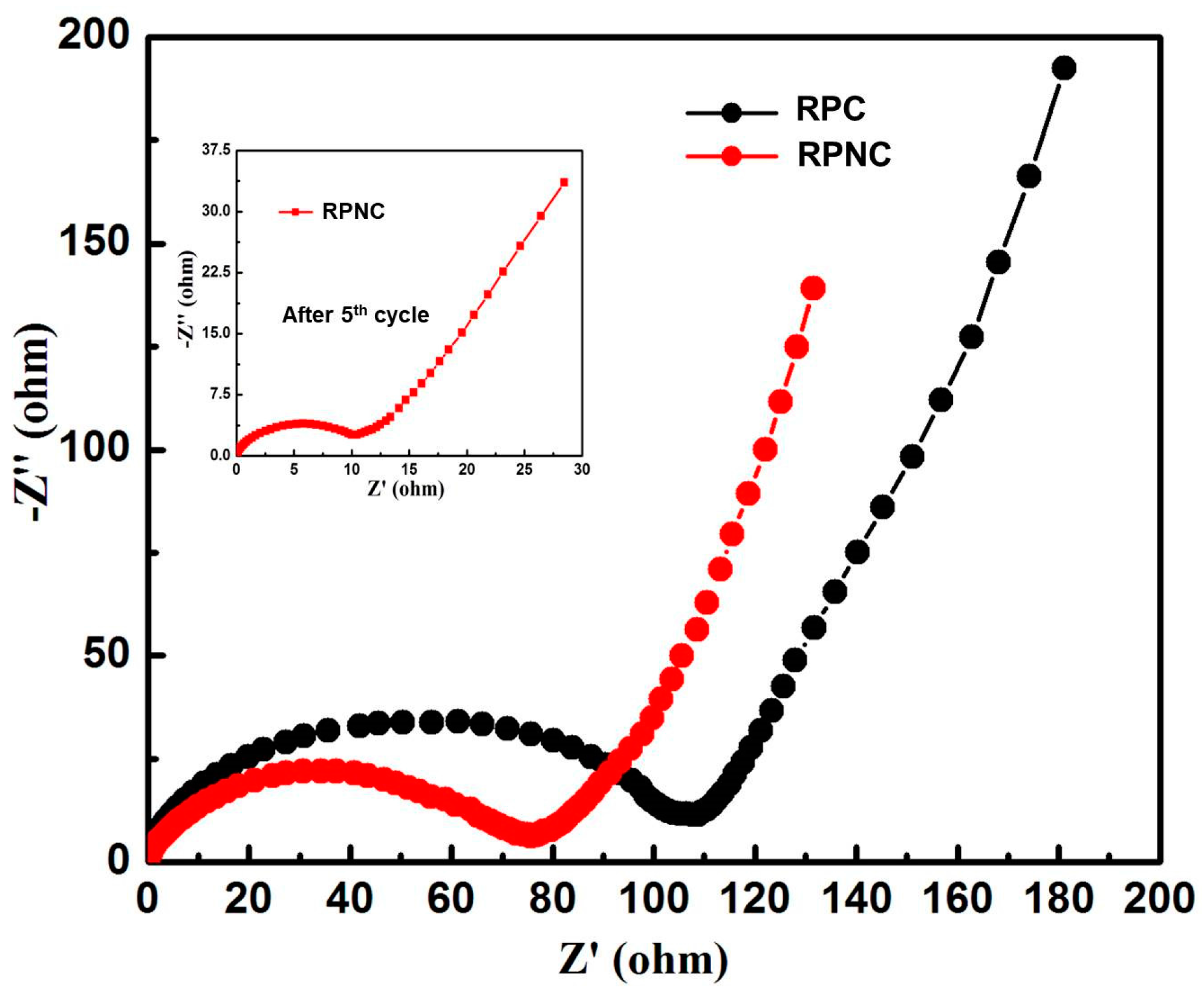
3. Results and Discussion
4. Conclusions
5. Experimental
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Piao, Y.; Kim, H.S.; Sung, Y.-E.; Hyeon, T. Facile scalable synthesis of magnetite nanocrystals embedded in carbon matrix as superior anode materials for lithium-ion batteries. Chem. Commun. 2010, 46, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, Y.; Liu, Y.; Wang, C. Electrochemical Performance of Porous Carbon/Tin Composite Anodes for Sodium-Ion and Lithium-Ion Batteries. Adv. Energy Mater. 2013, 3, 128–133. [Google Scholar] [CrossRef]
- Scrosati, B.; Hassoun, J.; Sun, Y.-K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Xu, L.; Li, F.; Cheng, H.-M. Doped Graphene Sheets As Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.-Q.; Wu, X.-Y.; Wang, K.-X.; Jiang, Y.-M.; Wang, J.-F.; Chen, J.-S. Hierarchical porous carbon spheres as an anode material for lithium ion batteries. RSC Adv. 2013, 3, 10823–10827. [Google Scholar] [CrossRef]
- Luo, Z.; Zhou, J.; Wang, L.; Fang, G.; Pan, A.; Liang, S. Two-dimensional hybrid nanosheets of few layered MoSe2 on reduced graphene oxide as anodes for long-cycle-life lithium-ion batteries. J. Mater. Chem. A 2016, 4, 15302–15308. [Google Scholar] [CrossRef]
- Etacheri, V.; Wang, C.; O’Connell, M.J.; Chan, C.K.; Pol, V.G. Porous carbon sphere anodes for enhanced lithium-ion storage. J. Mater. Chem. A 2015, 3, 9861–9868. [Google Scholar] [CrossRef]
- Campbell, B.; Ionescu, R.; Favors, Z.; Ozkan, C.S.; Ozkan, M. Bio-Derived, Binderless, Hierarchically Porous Carbon Anodes for Li-ion Batteries. Sci. Rep. 2015, 5, 14575. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, Z.; Li, M.; Jiang, Y.; Wei, X.; Zhong, X.; Gu, L.; Yu, Y. Amorphous Red Phosphorus Embedded in Highly Ordered Mesoporous Carbon with Superior Lithium and Sodium Storage Capacity. Nano Lett. 2016, 16, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-C.; Tseng, K.-W.; Tuan, H.-Y. Solution Synthesis of Iodine-Doped Red Phosphorus Nanoparticles for Lithium-Ion Battery Anodes. Nano Lett. 2017, 17, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; He, X.; Wang, J. Effect of Pore Size Distribution of Carbon Matrix on the Performance of Phosphorus@Carbon Material as Anode for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2016, 4, 4217–4223. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Li, J.; Sun, W.; Gao, J.; Guo, J.; Jiang, C. Nano-Structured Phosphorus Composite as High-Capacity Anode Materials for Lithium Batteries. Angew. Chem. Int. Ed. 2012, 51, 9034–9037. [Google Scholar] [CrossRef] [PubMed]
- Ismagilov, Z.R.; Shalagina, A.E.; Podyacheva, O.Y.; Ischenko, A.V.; Kibis, L.S.; Boronin, A.I.; Chesalov, Y.A.; Kochubey, D.I.; Romanenko, A.I.; Anikeeva, O.B.; et al. Structure and electrical conductivity of nitrogen-doped carbon nanofibers. Carbon 2009, 47, 1922–1929. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, Y.; Hu, P.A.; Yu, G.; Sun, Y.; Zhu, D. n-Type Field-Effect Transistors Made of an Individual Nitrogen-Doped Multiwalled Carbon Nanotube. J. Am. Chem. Soc. 2005, 127, 8614–8617. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Liu, Z.; Wang, L.; Han, P.; Xu, H.; Zhang, K.; Dong, S.; Yao, J.; Cui, G. Nitrogen-doped graphene nanosheets with excellent lithium storage properties. J. Mater. Chem. 2011, 21, 5430–5434. [Google Scholar] [CrossRef]
- Han, P.; Yue, Y.; Zhang, L.; Xu, H.; Liu, Z.; Zhang, K.; Zhang, C.; Dong, S.; Ma, W.; Cui, G. Nitrogen-doping of chemically reduced mesocarbon microbead oxide for the improved performance of lithium ion batteries. Carbon 2012, 50, 1355–1362. [Google Scholar] [CrossRef]
- Zaug, J.M.; Soper, A.K.; Clark, S.M. Pressure-dependent structures of amorphous red phosphorus and the origin of the first sharp diffraction peaks. Nat. Mater. 2008, 7, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; Ren, Y.; Zhang, Y.; Wang, Y.; Hu, A.; He, X. Distinctive slit-shaped porous carbon encapsulating phosphorus as a promising anode material for lithium batteries. Ionics 2016, 22, 167–172. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Okotrub, A.V.; Kurenya, A.G.; Zhang, H.; Zhang, H.; Chen, X.; Song, H. Electrochemical properties of nitrogen-doped carbon nanotube anode in Li-ion batteries. Carbon 2011, 49, 4013–4023. [Google Scholar] [CrossRef]
- Lin, X.; Lu, X.; Huang, T.; Liu, Z.; Yu, A. Binder-free nitrogen-doped carbon nanotubes electrodes for lithium-oxygen batteries. J. Power Sources 2013, 242, 855–859. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.; Liu, Z.; Tan, C.; Luo, Z.; Li, H.; Lin, J.; Sun, L.; Chen, W.; Xu, Z.; et al. Black Phosphorus Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 3653–3657. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yu, Z.; Gordin, M.L.; Hu, S.; Yi, R.; Tang, D.; Walter, T.; Regula, M.; Choi, D.; Li, X.; et al. Chemically Bonded Phosphorus/Graphene Hybrid as a High Performance Anode for Sodium-Ion Batteries. Nano Lett. 2014, 14, 6329–6335. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Hou, S.; Cao, G.; Wu, F.; Yang, Y. Sustainable nitrogen-doped porous carbon with high surface areas prepared from gelatin for supercapacitors. J. Mater. Chem. 2012, 22, 19088–19093. [Google Scholar] [CrossRef]
- Seredych, M.; Hulicova-Jurcakova, D.; Lu, G.Q.; Bandosz, T.J. Surface functional groups of carbons and the effects of their chemical character, density and accessibility to ions on electrochemical performance. Carbon 2008, 46, 1475–1488. [Google Scholar] [CrossRef]
- Wan, L.; Wang, J.; Xie, L.; Sun, Y.; Li, K. Nitrogen-Enriched Hierarchically Porous Carbons Prepared from Polybenzoxazine for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 15583–15596. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Qiao, D.; Ai, X.; Cao, Y.; Yang, H. Reversible 3-Li storage reactions of amorphous phosphorus as high capacity and cycling-stable anodes for Li-ion batteries. Chem. Commun. 2012, 48, 8931–8933. [Google Scholar] [CrossRef] [PubMed]
- Marino, C.; Boulet, L.; Gaveau, P.; Fraisse, B.; Monconduit, L. Nanoconfined phosphorus in mesoporous carbon as an electrode for Li-ion batteries: Performance and mechanism. J. Mater. Chem. 2012, 22, 22713–22720. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Ashuri, M.; Liu, C.; Shaw, L.L. Li2S encapsulated by nitrogen-doped carbon for lithium sulfur batteries. J. Mater. Chem. A 2014, 2, 18026–18032. [Google Scholar] [CrossRef]
- Bhattacharjya, D.; Park, H.-Y.; Kim, M.-S.; Choi, H.-S.; Inamdar, S.N.; Yu, J.-S. Nitrogen-Doped Carbon Nanoparticles by Flame Synthesis as Anode Material for Rechargeable Lithium-Ion Batteries. Langmuir 2014, 30, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Yermukhambetova, A.; Bakenov, Z.; Chen, P. Ternary sulfur/polyacrylonitrile/Mg0.6Ni0.4O composite cathodes for high performance lithium/sulfur batteries. J. Mater. Chem. A 2013, 1, 295–301. [Google Scholar] [CrossRef]
- Bakenov, Z.; Taniguchi, I. Physical and electrochemical properties of LiMnPO4/C composite cathode prepared with different conductive carbons. J. Power Sources 2010, 195, 7445–7451. [Google Scholar] [CrossRef]
- Chung, S.-H.; Manthiram, A. Lithium–sulfur batteries with superior cycle stability by employing porous current collectors. Electrochim. Acta 2013, 107, 569–576. [Google Scholar] [CrossRef]
- Youn, D.H.; Heller, A.; Mullins, C.B. Simple Synthesis of Nanostructured Sn/Nitrogen-Doped Carbon Composite Using Nitrilotriacetic Acid as Lithium Ion Battery Anode. Chem. Mater. 2016, 28, 1343–1347. [Google Scholar] [CrossRef]

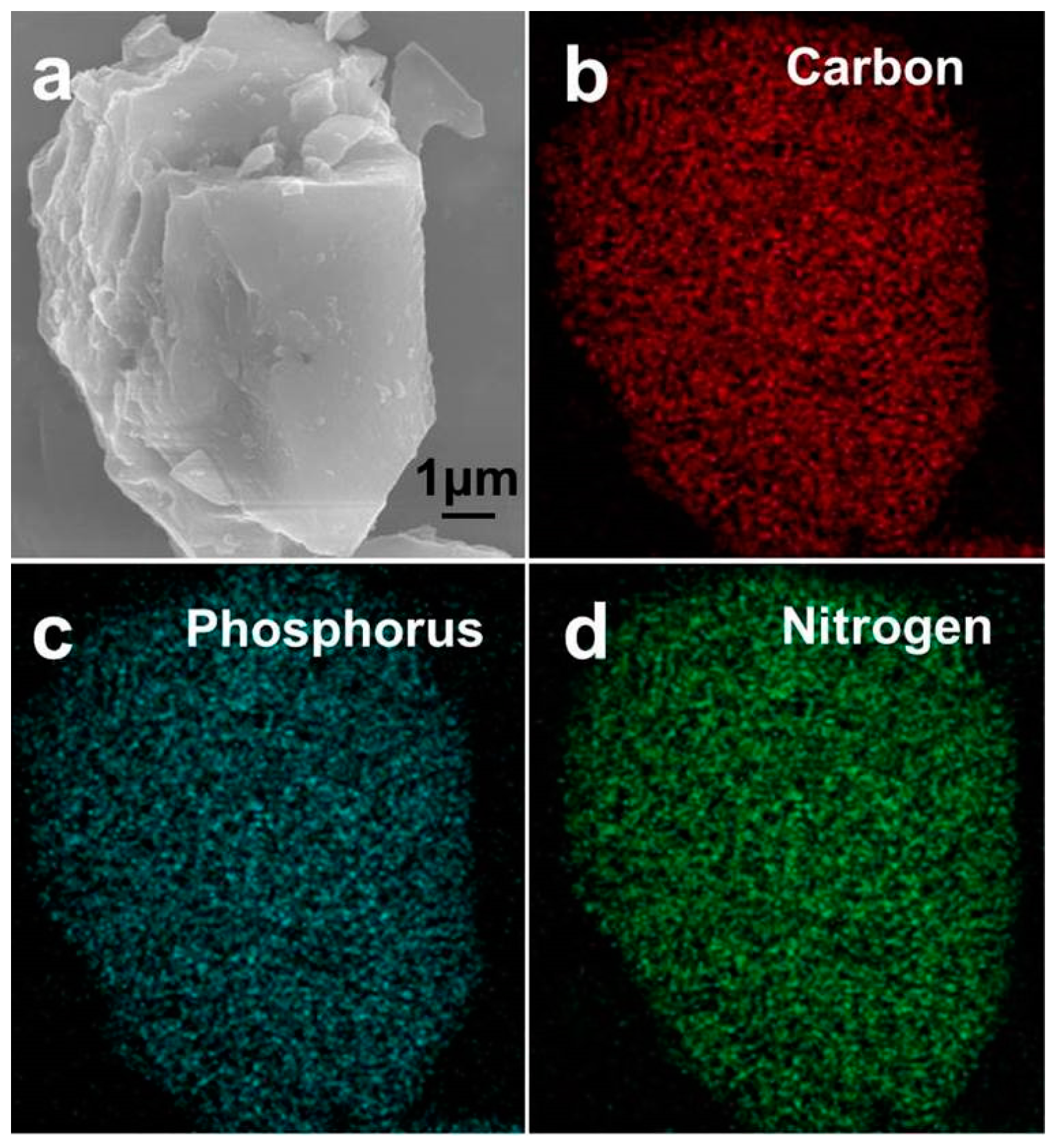
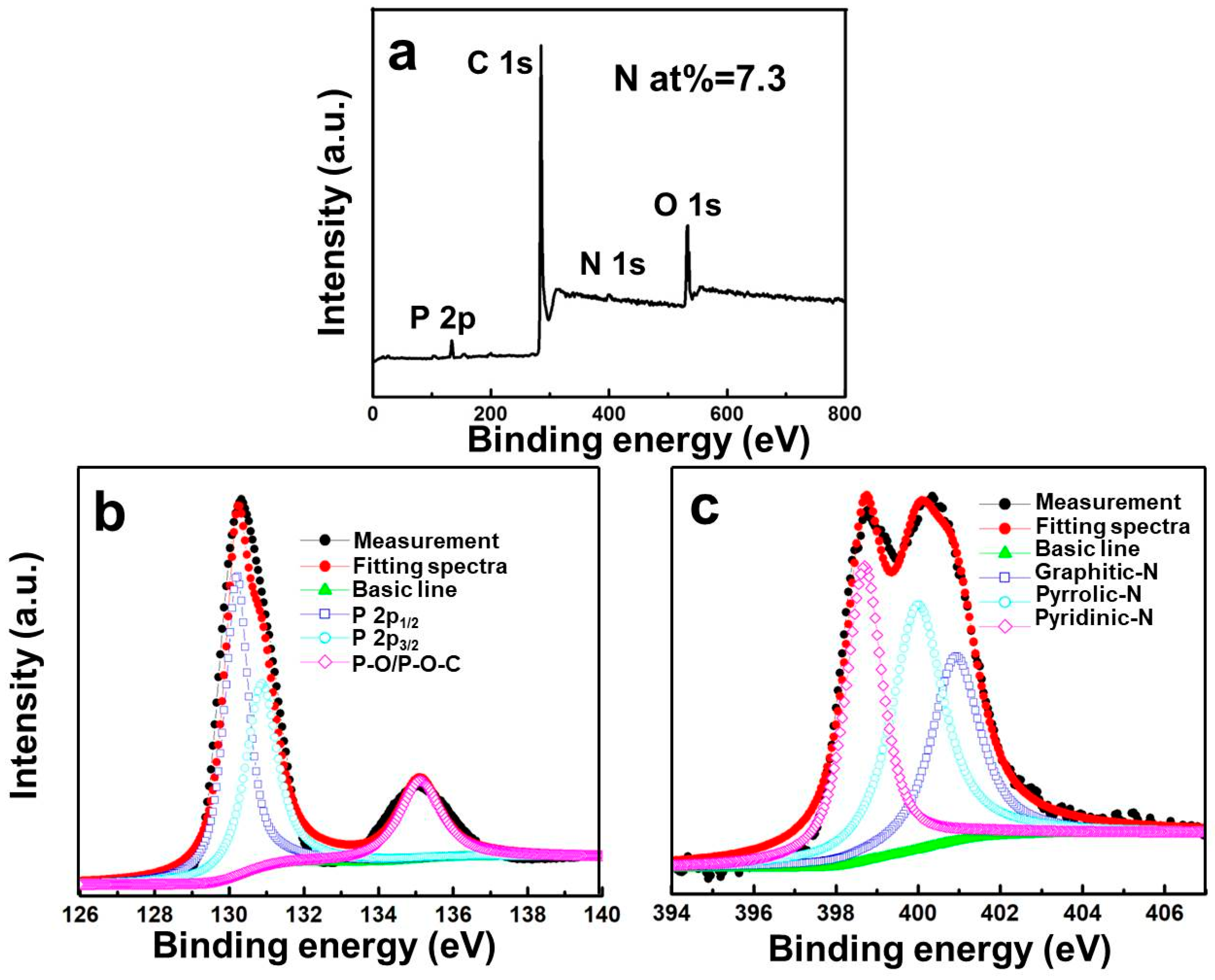

| Sample | Atomic Content (%) | ||
|---|---|---|---|
| C | O | N | |
| Nitrogen-doped carbon | 90.84 | 1.49 | 7.67 |
| Nitrogen-free carbon | 94.57 | 5.43 |
| Position | O | N | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Functional group | Pyridinic N_COOH | Pyrrolic N_COOH | Nitrogen free_COOH | Pyridinic N_C=O | Pyrrolic N_C=O | Nitrogen free_C=O | Pyridinic N_COOH | Pyrrolic N_COOH | Pyridinic N_C=O | Pyrrolic N_C=O |
| Interaction energies | −53.619 | −20.233 | −46.763 | −78.462 | −84.567 | −84.505 | −42.251 | −11.669 | −73.565 | −78.061 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Qian, Y.; Wang, L.; He, X. Nitrogen-Doped Carbon for Red Phosphorous Based Anode Materials for Lithium Ion Batteries. Materials 2018, 11, 134. https://doi.org/10.3390/ma11010134
Li J, Qian Y, Wang L, He X. Nitrogen-Doped Carbon for Red Phosphorous Based Anode Materials for Lithium Ion Batteries. Materials. 2018; 11(1):134. https://doi.org/10.3390/ma11010134
Chicago/Turabian StyleLi, Jiaoyang, Yumin Qian, Li Wang, and Xiangming He. 2018. "Nitrogen-Doped Carbon for Red Phosphorous Based Anode Materials for Lithium Ion Batteries" Materials 11, no. 1: 134. https://doi.org/10.3390/ma11010134