The Effects of a Macromolecular Charring Agent with Gas Phase and Condense Phase Synergistic Flame Retardant Capability on the Properties of PP/IFR Composites
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Synthesis of PETAT
2.3. Preparation of PP Composites
2.4. Characterization
3. Results and Discussion
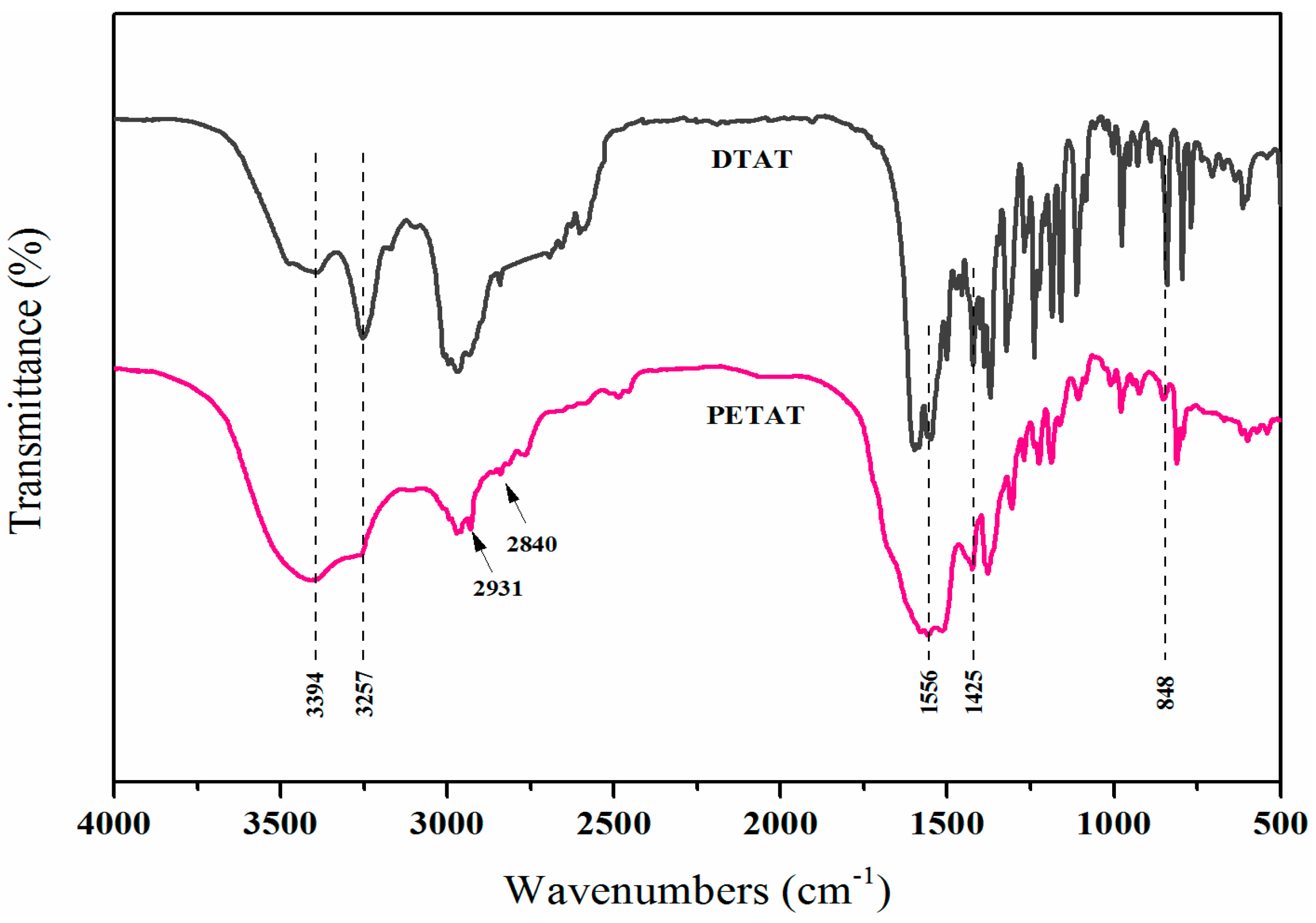
3.1. Characterization of DTAT and PETAT
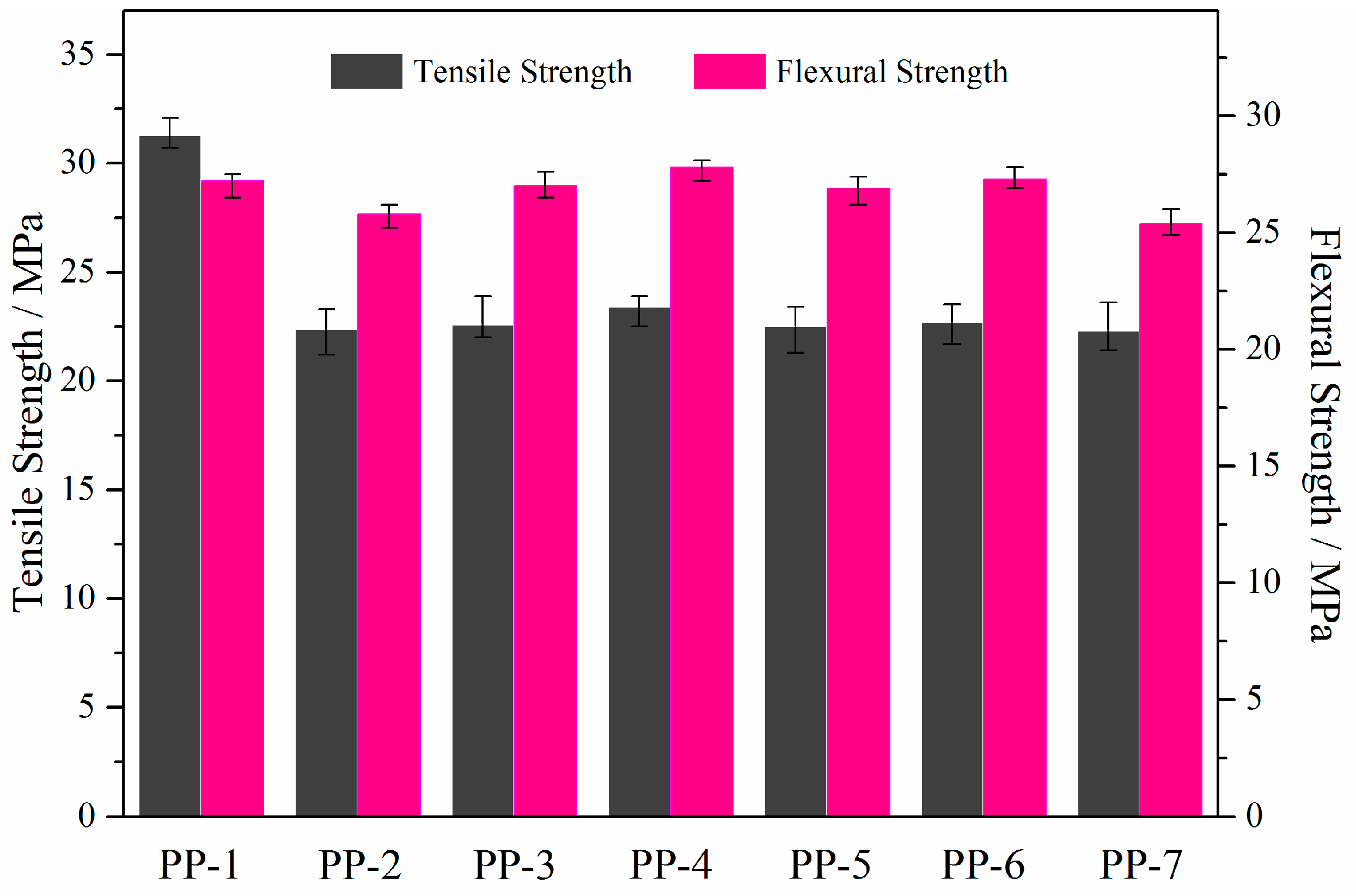
3.2. Mechanical Properties of Flame-Retardant PP Composites
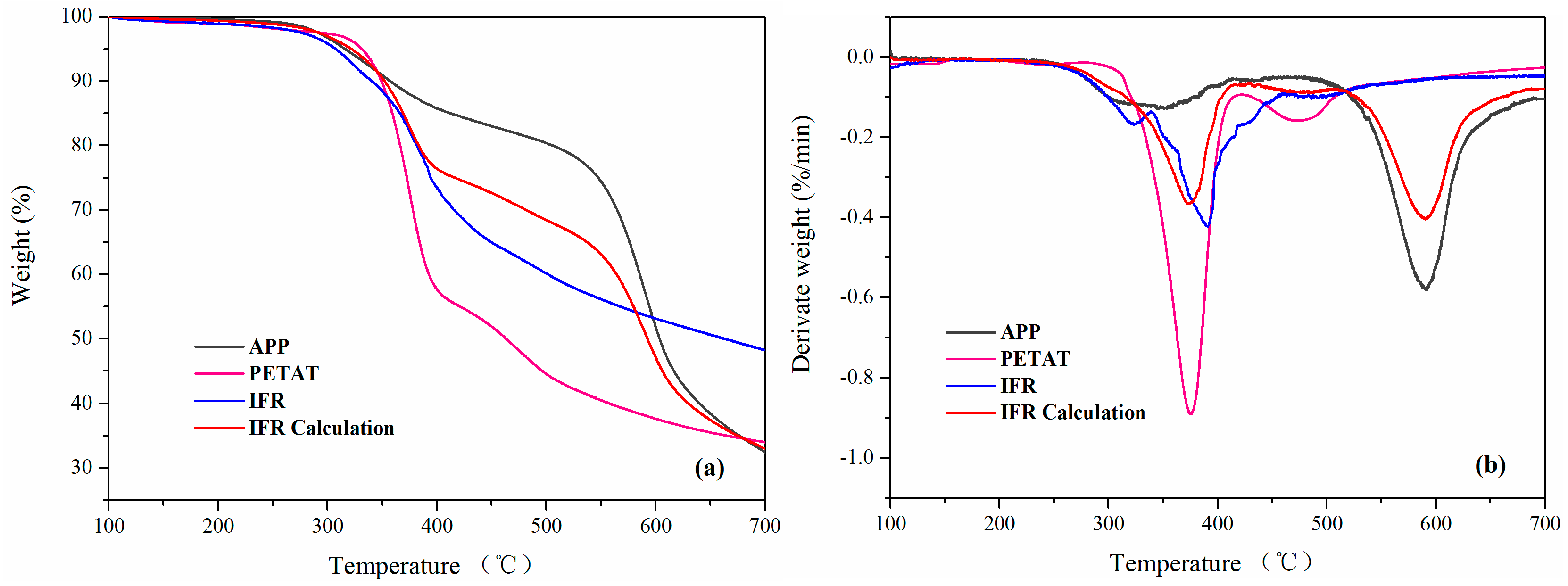
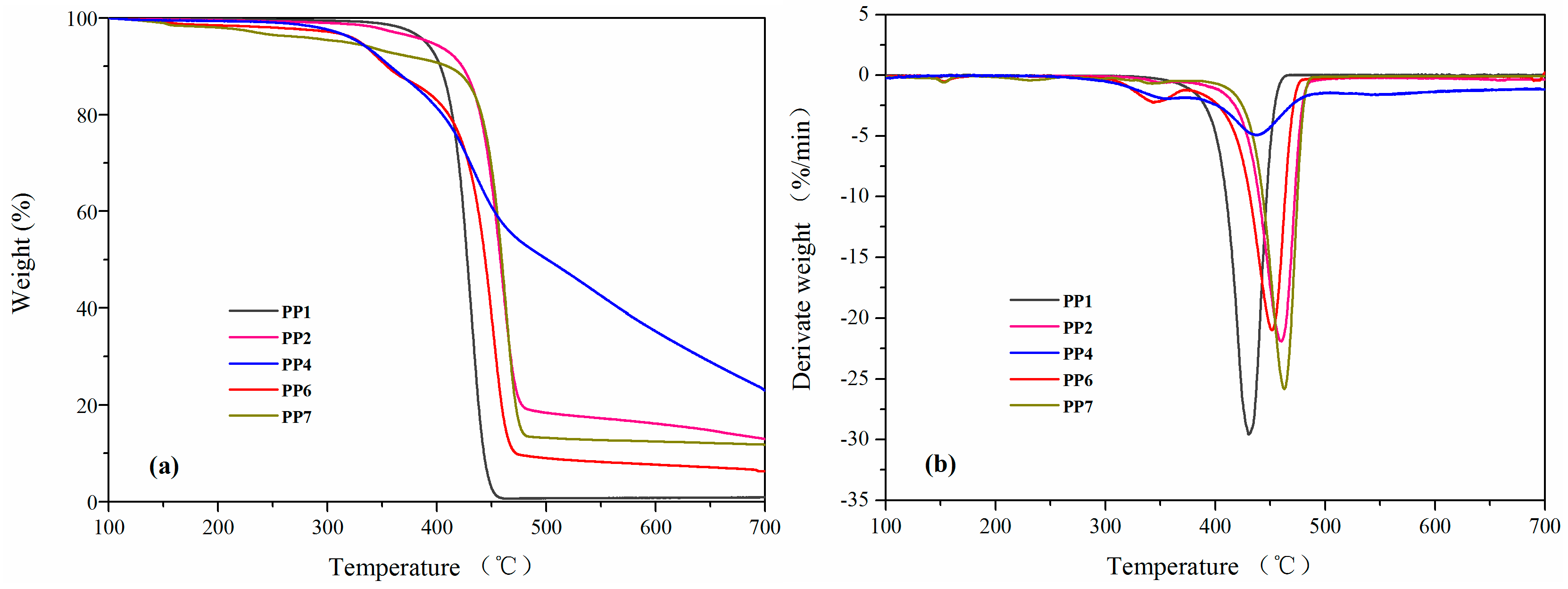
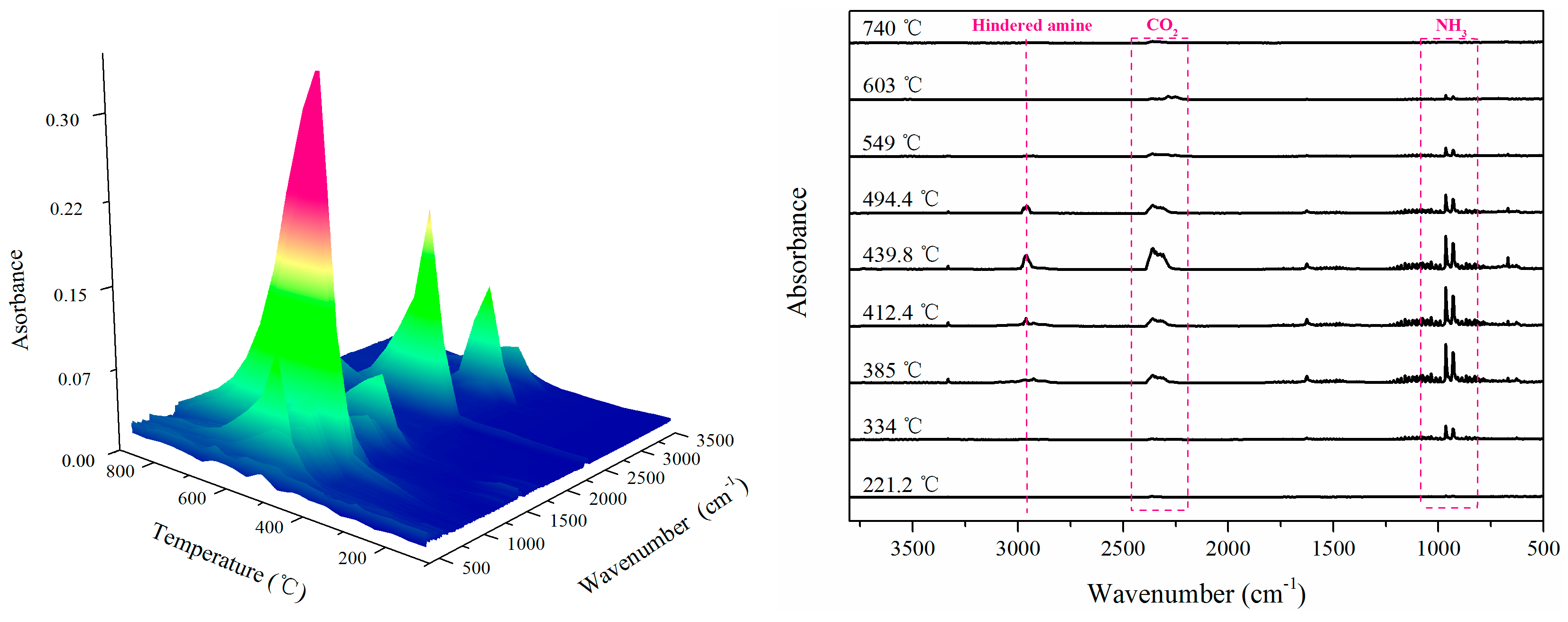
3.3. Thermal Properties and TG-FTIR Analysis
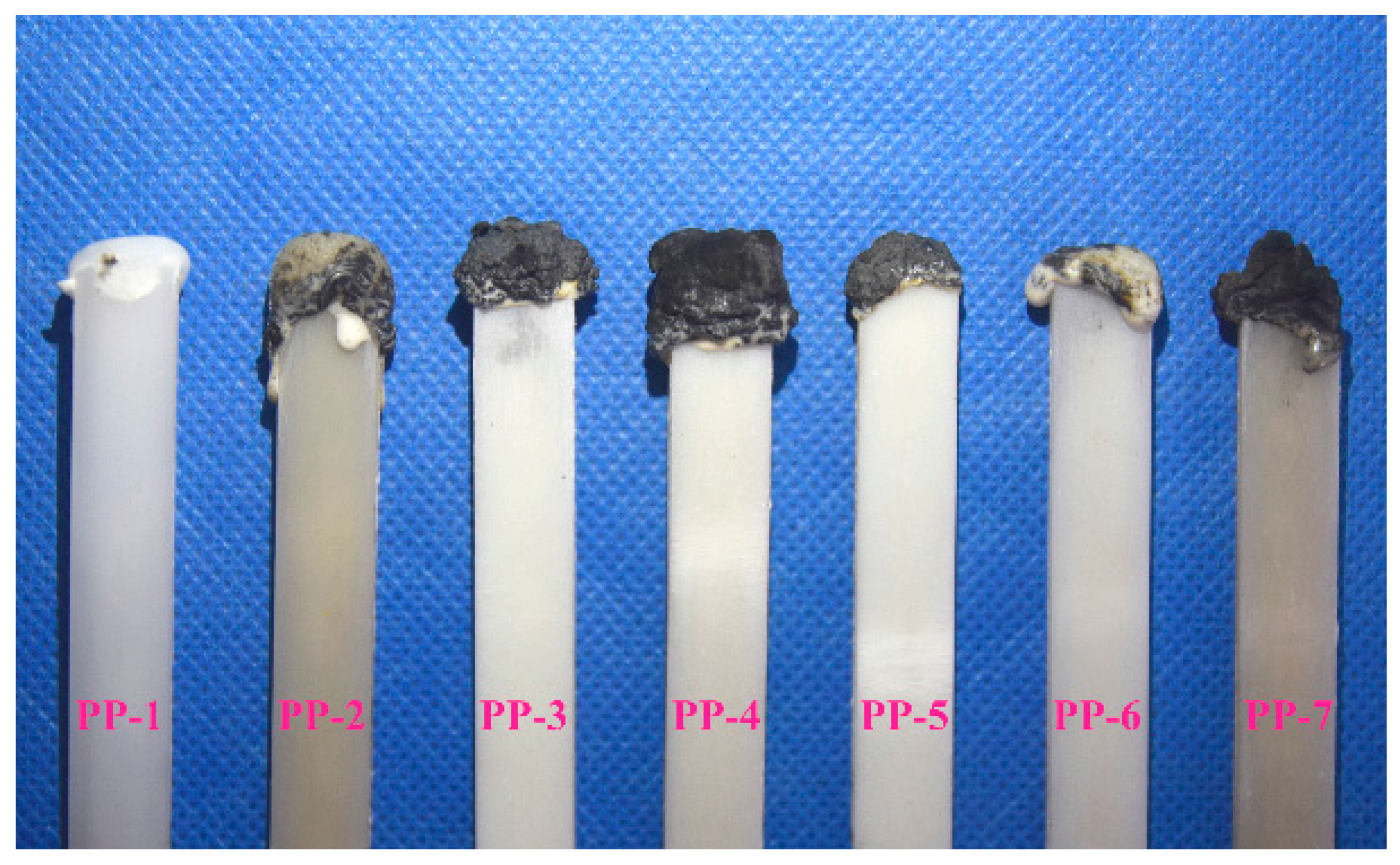
3.4. Flame Retardancy and Water Resistance of Flame-Retardant PP Composites
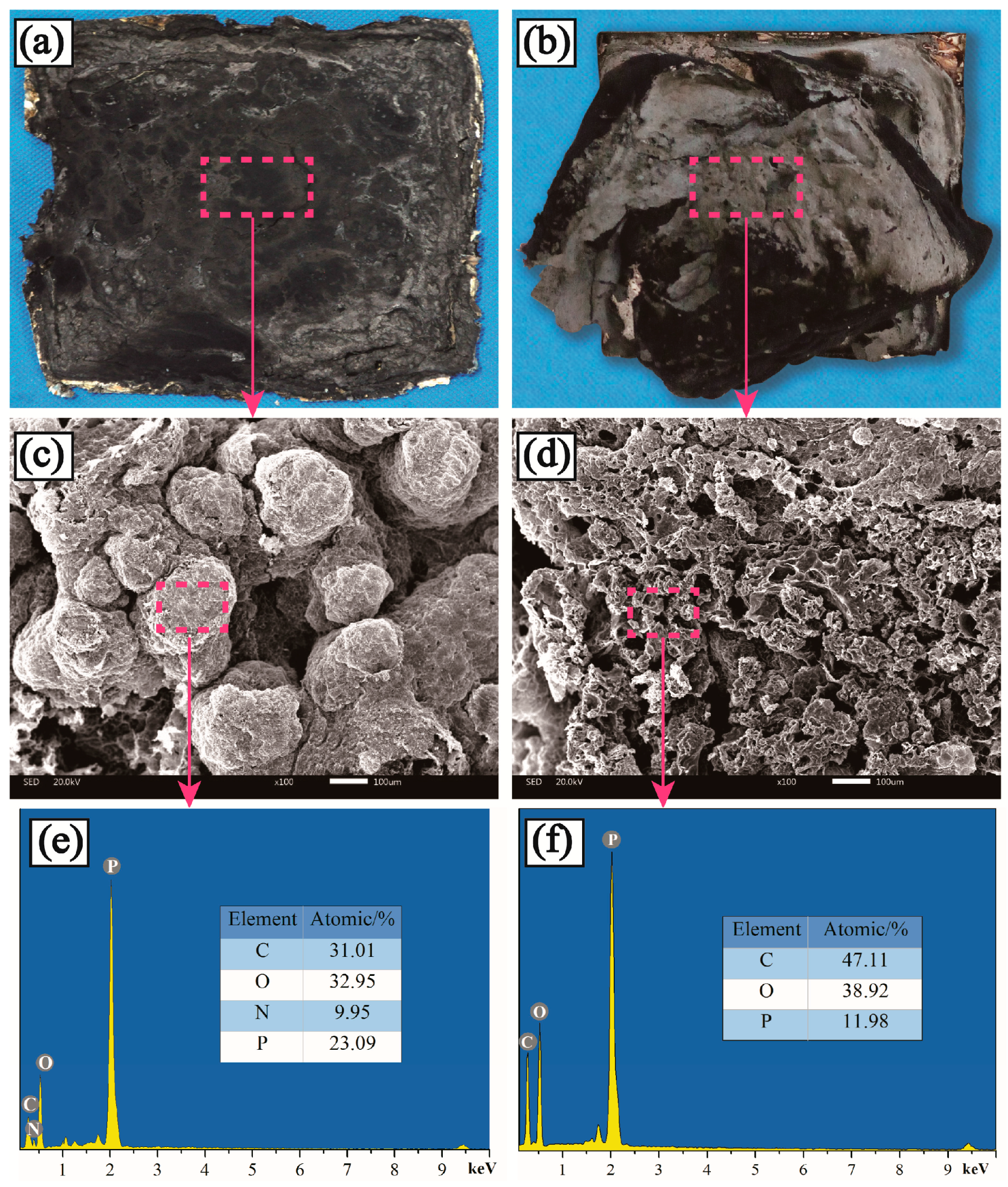
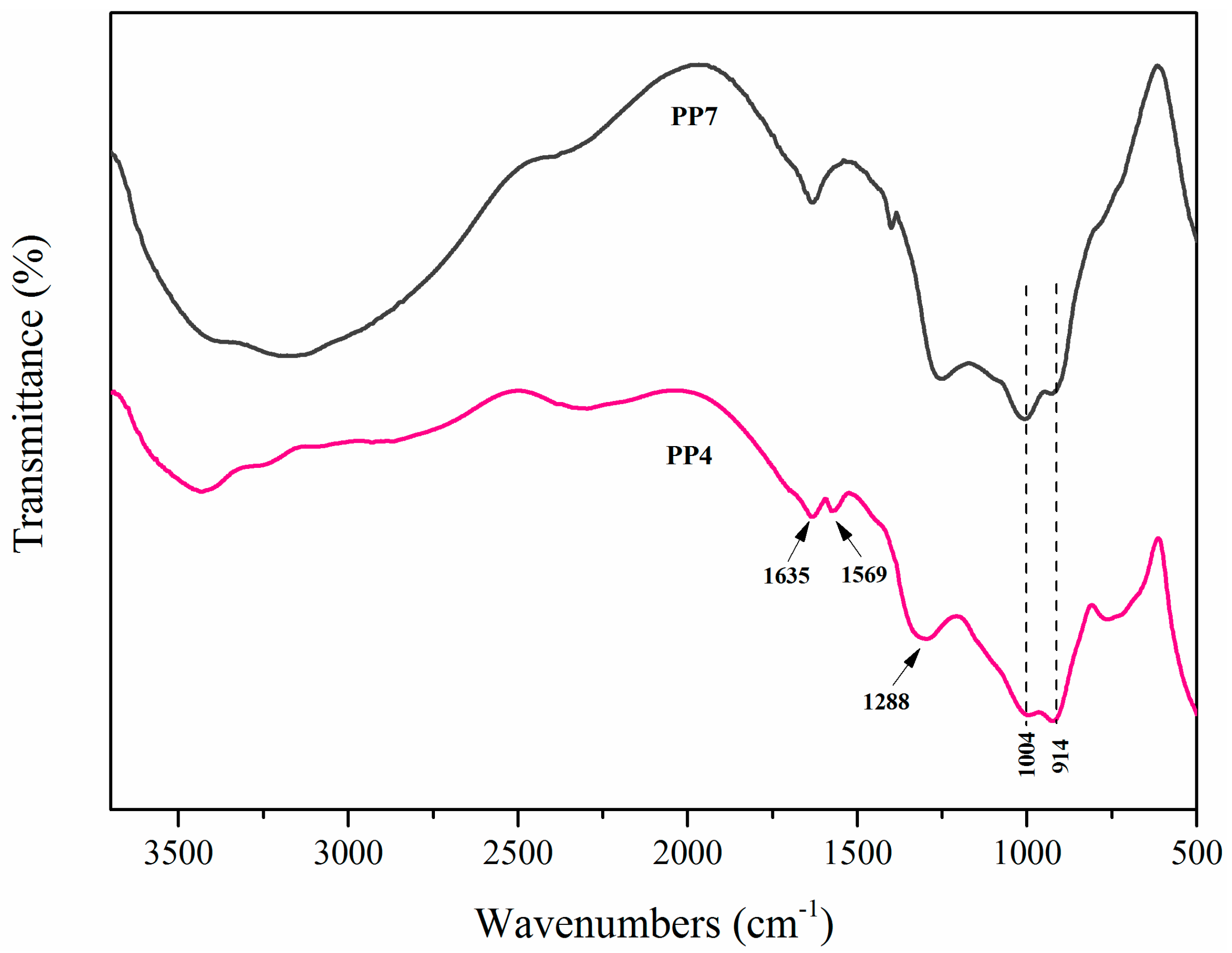
3.5. Morphological and Chemical Analysis of Residues
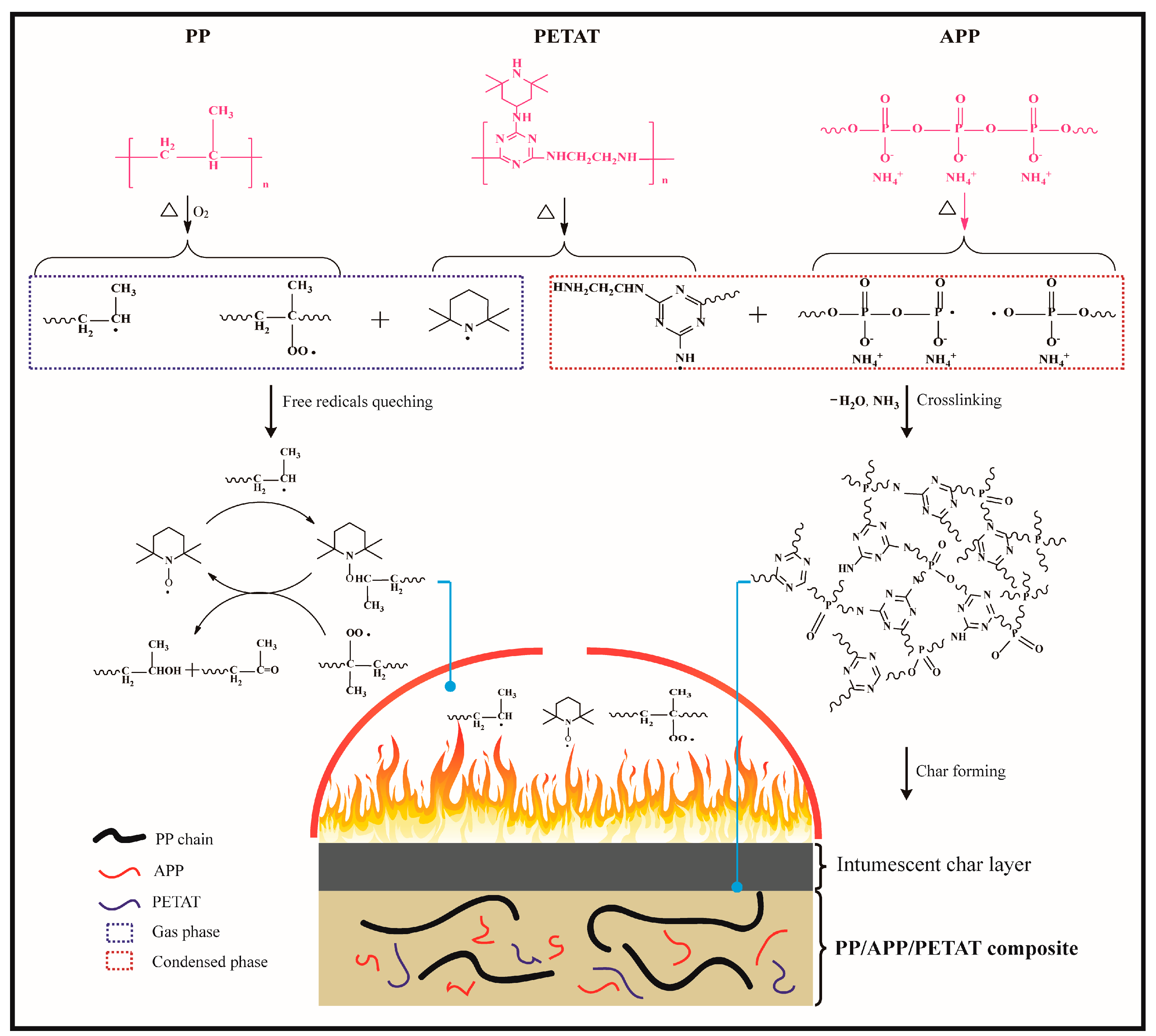
3.6. Flame-Retardant Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feng, C.; Liang, M.; Jiang, J.; Huang, J.; Liu, H. Synergistic effect of a novel triazine charring agent and ammonium polyphosphate on the flame retardant properties of halogen-free flame retardant polypropylene composites. Thermochim. Acta 2016, 627–629, 83–90. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, H.; Shen, L.; Fang, Z.; Zhang, X.; Wang, J.; Zhang, B. A phosphorus-, nitrogen- and carbon-containing polyelectrolyte complex: Preparation, characterization and its flame retardant performance on polypropylene. RSC Adv. 2014, 4, 48285–48292. [Google Scholar] [CrossRef]
- Feng, X.; Wang, B.; Wang, X.; Wen, P.; Cai, W.; Hu, Y.; Liew, K.M. Molybdenum disulfide nanosheets as barrier enhancing nanofillers in thermal decomposition of polypropylene composites. Chem. Eng. J. 2016, 295, 278–287. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, Y.; Hu, W.; Lian, J.; Hu, Y. Influence of ammonium polyphosphate microencapsulation on flame retardancy, thermal degradation and crystal structure of polypropylene composite. Compos. Sci. Technol. 2013, 81, 17–23. [Google Scholar] [CrossRef]
- Alongi, J.; Cuttica, F.; Carosio, F. DNA coatings from byproducts: A panacea for the flame retardancy of EVA, PP, ABS, PET, and PA6? ACS Sustain. Chem. Eng. 2016, 4, 3544–3551. [Google Scholar] [CrossRef]
- Wilkie, C.A.; Morgan, A.B.; Nelson, G.L. Fire and Polymers V: Materials and Concepts for Fire Retardancy; American Chemical Society: Washington, DC, USA, 2009; Volume 1013, pp. 192–204. [Google Scholar]
- Wang, Z.; Liu, Y.; Li, J. Preparation of nucleotide-based microsphere and its application in intumescent flame retardant polypropylene. J. Anal. Appl. Pyrolysis 2016, 121, 394–402. [Google Scholar] [CrossRef]
- Alexander, B.; Morgan, C.A.W. Wilkie-the Non-Halogenated Flame Retardant Handbook; Wiley-Scrivener: Hoboken, NJ, USA, 2014. [Google Scholar]
- Liu, Z.; Dai, M.; Zhang, Y.; Gao, X.; Zhang, Q. Preparation and performances of novel waterborne intumescent fire retardant coatings. Prog. Org. Coat. 2016, 95, 100–106. [Google Scholar] [CrossRef]
- Yuval, H.; Donna, M.M.; Ronald, H.N. Fire retardancy of thermoplastic materials by intumescence. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 233–238. [Google Scholar]
- Camino, G.; Costa, L.; Martinasso, G. Intumescent fire-retardant systems. Polym. Degrad. Stab. 1989, 23, 359–376. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, M.; Wang, C.; Zhang, Q.; Zhang, Y.; Jin, B.; Gao, X. Effects of the addition mode and amount of organic montmorillonite in soft-core/hard-shell emulsion on fire protection, water resistance and stability of fire retardant coating. Prog. Org. Coat. 2016, 101, 350–358. [Google Scholar] [CrossRef]
- Yang, R.; Ma, B.; Zhao, H.; Li, J. Preparation, thermal degradation, and fire behaviors of intumescent flame retardant polypropylene with a charring agent containing pentaerythritol and triazine. Ind. Eng. Chem. Res. 2016, 55, 5298–5305. [Google Scholar] [CrossRef]
- Deng, C.-L.; Du, S.-L.; Zhao, J.; Shen, Z.-Q.; Deng, C.; Wang, Y.-Z. An intumescent flame retardant polypropylene system with simultaneously improved flame retardancy and water resistance. Polym. Degrad. Stab. 2014, 108, 97–107. [Google Scholar] [CrossRef]
- Chen, X.; Jiao, C.; Li, S.; Hu, Y. Preparation and properties of a single molecule intumescent flame retardant. Fire Saf. J. 2013, 58, 208–212. [Google Scholar] [CrossRef]
- Lai, X.; Yin, C.; Li, H.; Zeng, X. Synergistic effect between silicone-containing macromolecular charring agent and ammonium polyphosphate in flame retardant polypropylene. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Su, X.; Yi, Y.; Tao, J.; Qi, H.; Li, D. Synergistic effect between a novel triazine charring agent and ammonium polyphosphate on flame retardancy and thermal behavior of polypropylene. Polym. Degrad. Stab. 2014, 105, 12–20. [Google Scholar] [CrossRef]
- Song, P.; Fang, Z.; Tong, L.; Jin, Y.; Lu, F. Effects of metal chelates on a novel oligomeric intumescent flame retardant system for polypropylene. J. Anal. Appl. Pyrolysis 2008, 82, 286–291. [Google Scholar] [CrossRef]
- Cao, K.; Wu, S.-L.; Qiu, S.-L.; Li, Y.; Yao, Z. Synthesis of N-alkoxy hindered amine containing silane as a multifunctional flame retardant synergist and its application in intumescent flame retardant polypropylene. Ind. Eng. Chem. Res. 2013, 52, 309–317. [Google Scholar] [CrossRef]
- Xie, H.; Lai, X.; Zhou, R.; Li, H.; Zhang, Y.; Zeng, X.; Guo, J. Effect and mechanism of n-alkoxy hindered amine on the flame retardancy, uv aging resistance and thermal degradation of intumescent flame retardant polypropylene. Polym. Degrad. Stab. 2015, 118, 167–177. [Google Scholar] [CrossRef]
- Reddy, K.R.; Kumar, B.; Rana, S.; Tevtia, A.K.; Singh, R.P. Synthesis and characterization of hindered amine light stabilizers based on end functionalization of polypropylene. J. Appl. Polym. Sci. 2007, 104, 1596–1602. [Google Scholar] [CrossRef]
- Xie, H.; Lai, X.; Li, H.; Zeng, X. Synthesis of a novel macromolecular charring agent with free-radical quenching capability and its synergism in flame retardant polypropylene. Polym. Degrad. Stab. 2016, 130, 68–77. [Google Scholar] [CrossRef]
- Nie, S.; Hu, Y.; Song, L.; He, Q.; Yang, D.; Chen, H. Synergistic effect between a char forming agent (CFA) and microencapsulated ammonium polyphosphate on the thermal and flame retardant properties of polypropylene. Polym. Adv. Technol. 2008, 19, 1077–1083. [Google Scholar] [CrossRef]
- Xu, Z.-Z.; Huang, J.-Q.; Chen, M.-J.; Tan, Y.; Wang, Y.-Z. Flame retardant mechanism of an efficient flame-retardant polymeric synergist with ammonium polyphosphate for polypropylene. Polym. Degrad. Stab. 2013, 98, 2011–2020. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Deng, C.-L.; Chen, L.; Wang, D.-Y.; Wang, Y.-Z. Flame-retardant effect of sepiolite on an intumescent flame-retardant polypropylene system. Ind. Eng. Chem. Res. 2011, 50, 2047–2054. [Google Scholar] [CrossRef]
- Chen, W.; Fu, X.; Ge, W.; Xu, J.; Jiang, M. Microencapsulation of bisneopentyl glycol dithiopyrophosphate and its flame retardant effect on polyvinyl alcohol. Polym. Degrad. Stab. 2014, 102, 81–87. [Google Scholar] [CrossRef]
- Du, B.; Guo, Z.; Song, P.A.; Liu, H.; Fang, Z.; Wu, Y. Flame retardant mechanism of organo-bentonite in polypropylene. Appl. Clay Sci. 2009, 45, 178–184. [Google Scholar] [CrossRef]
- Wang, X.; Pang, H.; Chen, W.; Lin, Y.; Zong, L.; Ning, G. Controllable fabrication of zinc borate hierarchical nanostructure on brucite surface for enhanced mechanical properties and flame retardant behaviors. ACS Appl. Mater. Interfaces 2014, 6, 7223–7235. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; Nai, X.; Dong, Y.; Li, W. Functional group effect on flame retardancy, thermal, and mechanical properties of organophosphorus-based magnesium oxysulfate whiskers as a flame retardant in polypropylene. RSC Adv. 2017, 7, 21655–21665. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, P.; Gui, H.; Wang, X.; Ding, Y. Effect of imidazolium phosphate and multiwalled carbon nanotubes on thermal stability and flame retardancy of polylactide. Compos. Part A Appl. Sci. Manuf. 2015, 77, 147–153. [Google Scholar] [CrossRef]
- Wen, P.; Wang, X.; Wang, B.; Yuan, B.; Zhou, K.; Song, L.; Hu, Y.; Yuen, R.K.K. One-pot synthesis of a novel s-triazine-based hyperbranched charring foaming agent and its enhancement on flame retardancy and water resistance of polypropylene. Polym. Degrad. Stab. 2014, 110, 165–174. [Google Scholar] [CrossRef]
- Tao, Z.; Yan, H.; Wang, L.; Fang, Z. Controlled formation of self-extinguishing intumescent coating on ramie fabric via layer-by-layer assembly. Ind. Eng. Chem. Res. 2013, 52, 6138–6146. [Google Scholar]
- Lai, X.; Qiu, J.; Li, H.; Zhou, R.; Xie, H.; Zeng, X. Thermal degradation and combustion behavior of novel intumescent flame retardant polypropylene with N-alkoxy hindered amine. J. Anal. Appl. Pyrolysis 2016, 120, 361–370. [Google Scholar] [CrossRef]
- Song, P.; Zhu, Y.; Tong, L.; Fang, Z. C(60) reduces the flammability of polypropylene nanocomposites by in situ forming a gelled-ball network. Nanotechnology 2008, 19, 225707. [Google Scholar] [CrossRef] [PubMed]
- Cao, K. Flame retardant based on n-alkoxyoxy hindered amines and their application in polyolefins. Prog. Chem. 2011, 23, 1189–1195. [Google Scholar]


| Samples | PP (wt %) | APP (wt %) | PETAT (wt %) | PER (wt %) |
|---|---|---|---|---|
| PP1 | 100 | 0 | 0 | 0 |
| PP2 | 75 | 25 | 0 | 0 |
| PP3 | 75 | 18.7 | 6.3 | 0 |
| PP4 | 75 | 16.6 | 8.4 | 0 |
| PP5 | 75 | 12.5 | 12.5 | 0 |
| PP6 | 75 | 0 | 25 | 0 |
| PP7 | 75 | 18.7 | 0 | 6.3 |
| Samples | c Ti (°C) | c T50% (°C) | d Tmax (°C) | e Residue (%) |
|---|---|---|---|---|
| APP | 316.3 | 603.5 | 591.6 | 32.5 |
| PETAT | 331.6 | 463.4 | 375.3 | 33.9 |
| a IFR | 307.2 | 662.7 | 389.8 | 48.2 |
| IFR Calculation b | 321.9 | 592.4 | 590.1 | 32.9 |
| Samples | a Ti (°C) | a T50% (°C) | b Tmax (°C) | c Rmax (%/min) | d Residue (%) |
|---|---|---|---|---|---|
| PP1 | 390.0 | 427.6 | 430 | 29.5 | 0.9 |
| PP2 | 394.2 | 457.9 | 459.9 | 21.9 | 13.0 |
| PP4 | 328.8 | 501.1 | 437.3 | 4.9 | 23.0 |
| PP6 | 329.8 | 444.0 | 451.2 | 21.0 | 6.3 |
| PP7 | 317.6 | 459.1 | 462.8 | 25.8 | 11.8 |
| Samples | LOI (%) | UL-94 Rating | Dropping | After Soaking LOI (%) |
|---|---|---|---|---|
| PP1 | 16.4 | No rating | Yes | 16.4 |
| PP2 | 21.2 | No rating | Yes | 19.1 |
| PP3 | 28.1 | No rating | Yes | 25.6 |
| PP4 | 30.3 | V-0 | No | 27.8 |
| PP5 | 28.4 | V-1 | No | 26.6 |
| PP6 | 17.8 | No rating | Yes | 16.9 |
| PP7 | 24.7 | V-1 | No | 19.4 |
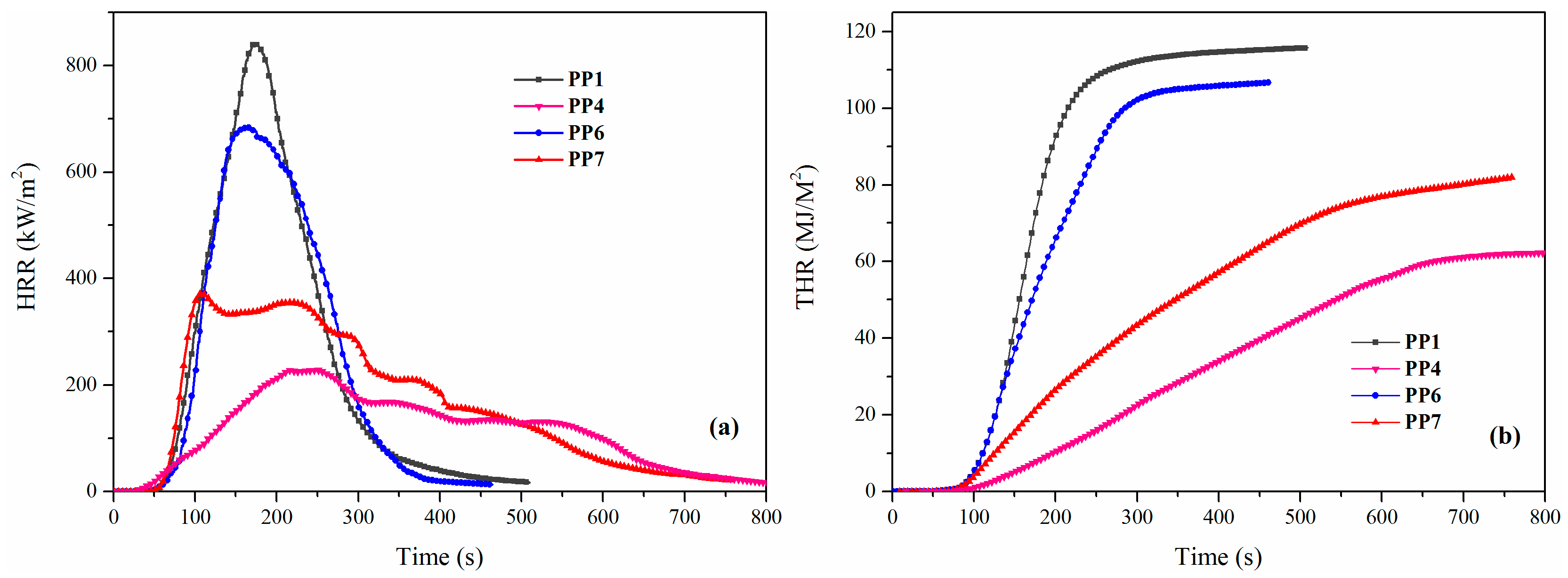
| Samples | TTI (s) | TPHRR (s) | PHRR (kW/m2) | THR (MJ/m2) | FPI (s·m2/kW) | Residue (wt %) |
|---|---|---|---|---|---|---|
| PP1 | 41 | 174 | 840.3 | 115.7 | 0.0488 | 1.4 |
| PP4 | 28 | 250 | 227.9 | 62.0 | 0.1229 | 43.5 |
| PP6 | 30 | 164 | 684.0 | 106.7 | 0.0439 | 5.8 |
| PP7 | 32 | 218 | 354.7 | 82.0 | 0.0902 | 29.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, J.; Ni, A.; Ding, A.; Han, X.; Sun, Z. The Effects of a Macromolecular Charring Agent with Gas Phase and Condense Phase Synergistic Flame Retardant Capability on the Properties of PP/IFR Composites. Materials 2018, 11, 111. https://doi.org/10.3390/ma11010111
Chen H, Wang J, Ni A, Ding A, Han X, Sun Z. The Effects of a Macromolecular Charring Agent with Gas Phase and Condense Phase Synergistic Flame Retardant Capability on the Properties of PP/IFR Composites. Materials. 2018; 11(1):111. https://doi.org/10.3390/ma11010111
Chicago/Turabian StyleChen, Hongda, Jihui Wang, Aiqing Ni, Anxin Ding, Xia Han, and Ziheng Sun. 2018. "The Effects of a Macromolecular Charring Agent with Gas Phase and Condense Phase Synergistic Flame Retardant Capability on the Properties of PP/IFR Composites" Materials 11, no. 1: 111. https://doi.org/10.3390/ma11010111





