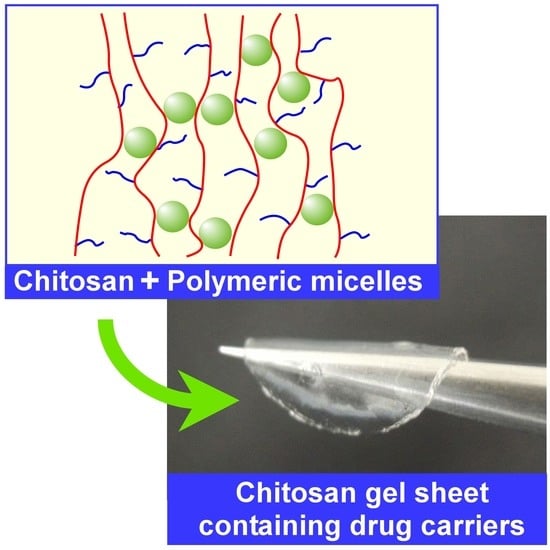
Chitosan Gel Sheet Containing Polymeric Micelles: Synthesis and Gelation Properties of PEG-Grafted Chitosan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
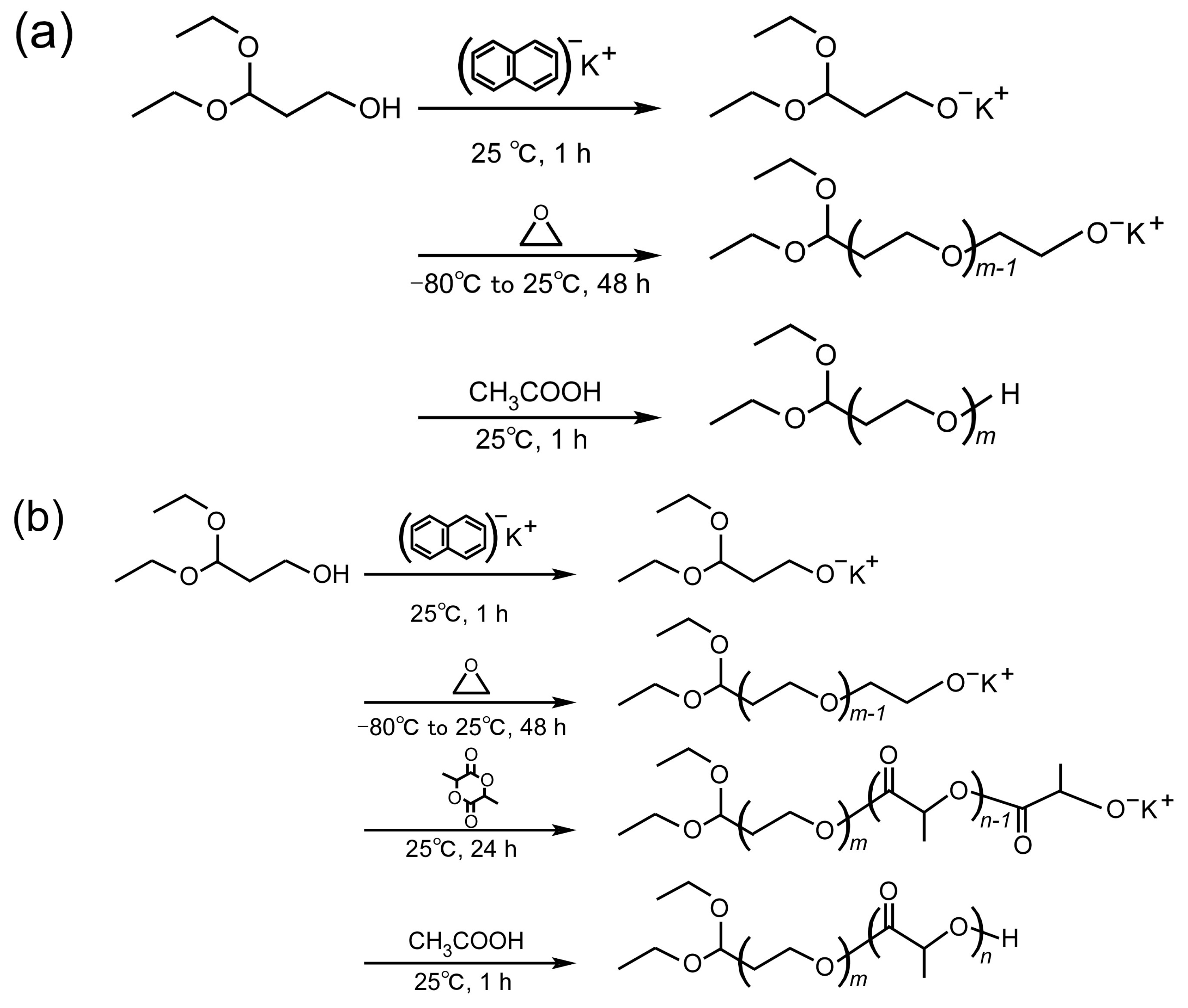
2.2. Synthesis and Characterization of PEG Derivatives
2.3. Synthesis and Characterization of PEG-Grafted Chitosans
2.4. Quantitative Analysis of PEG-Grafted Chitosans by Means of 2,4,6-Trinitrobenzene Sulfonic Acid (TNBS) Measurement
2.5. Evaluation of the Solubility of PEG-Grafted Chitosans by Visual Observation and Static Light Scattering Measurement
2.6. Preparation and Characterization of an Aldehyde-Terminated Polymeric Micelle Formed from Block Polymers
2.7. Preparation and Characterization of an Aldehyde-Terminated Polymeric Micelle Formed from Block Polymers
3. Results and Discussion
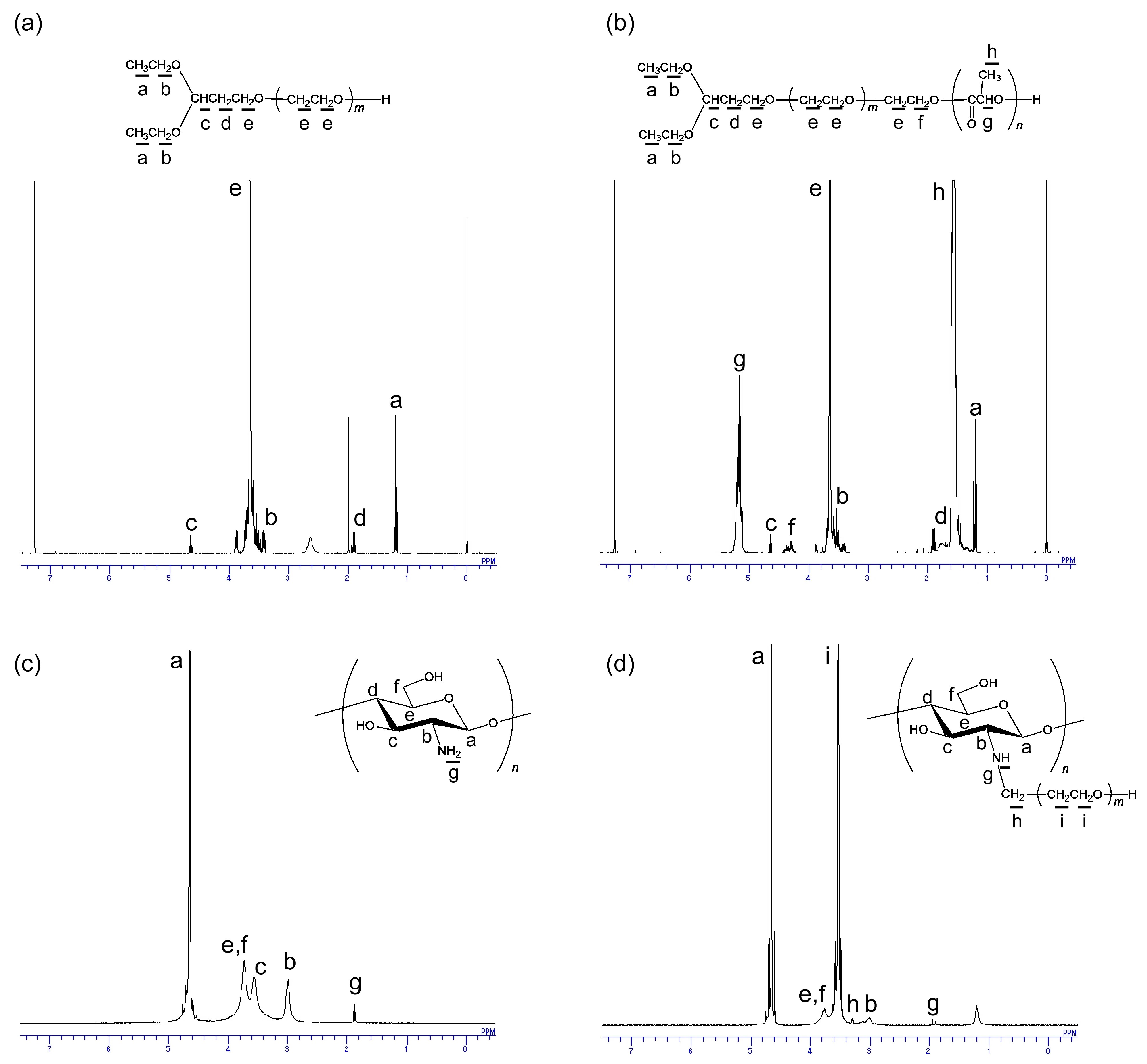
3.1. Synthesis and Characterization of PEG Derivatives
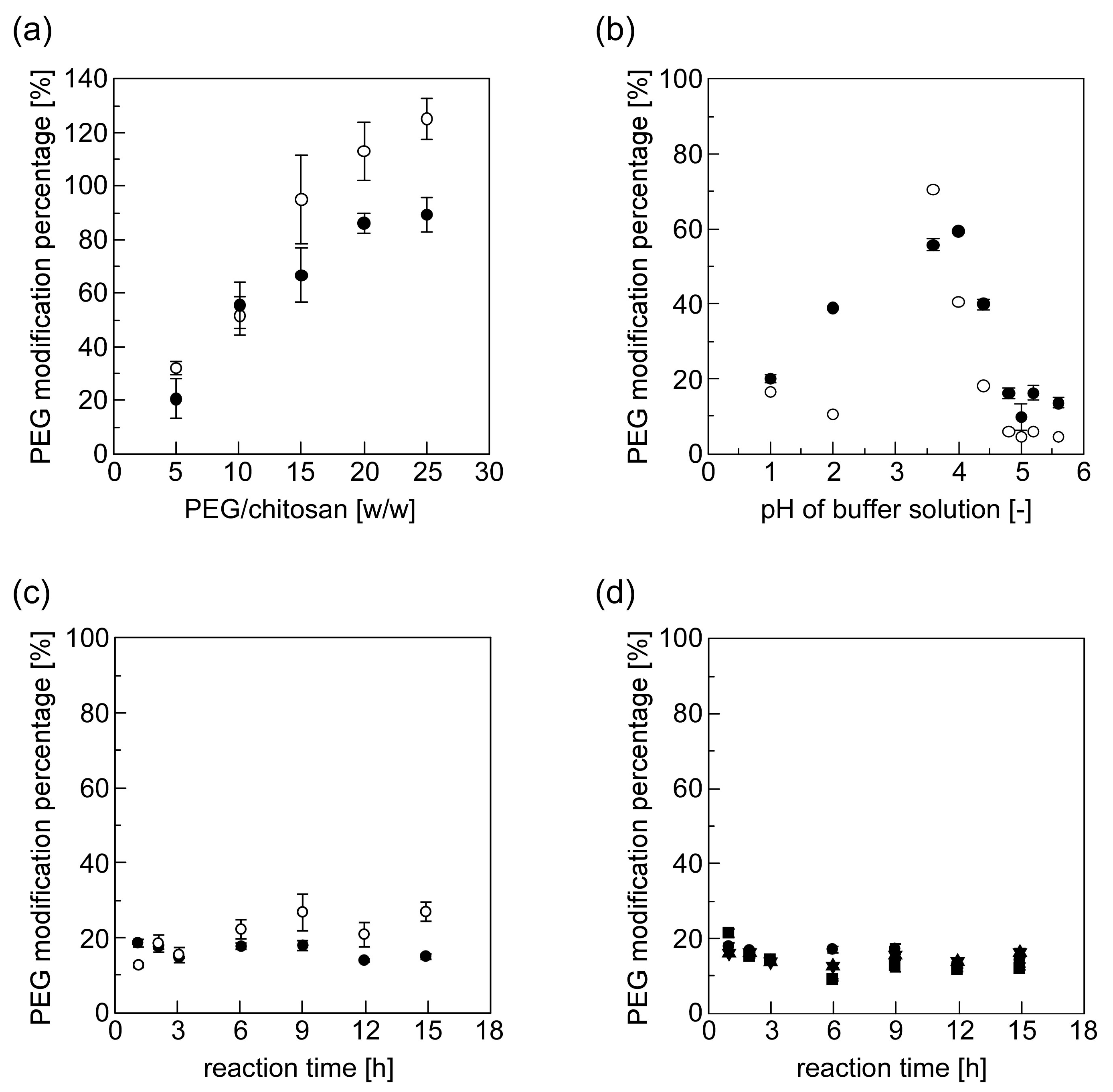
3.2. Synthesis and Characterization of PEG-Grafted Chitosans
3.3. Evaluation of the Solubility of PEG-Grafted Chitosan by Static Light Scattering Measurement
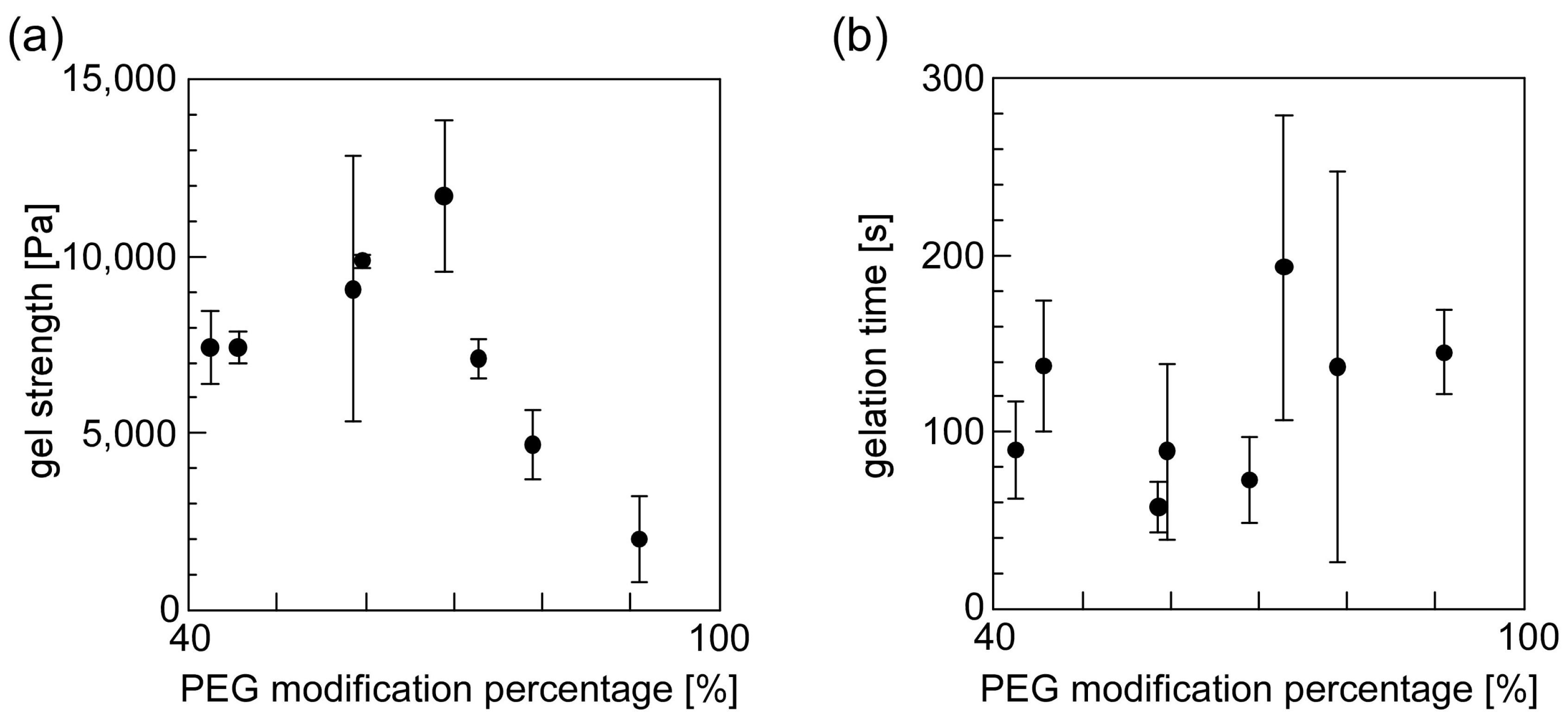
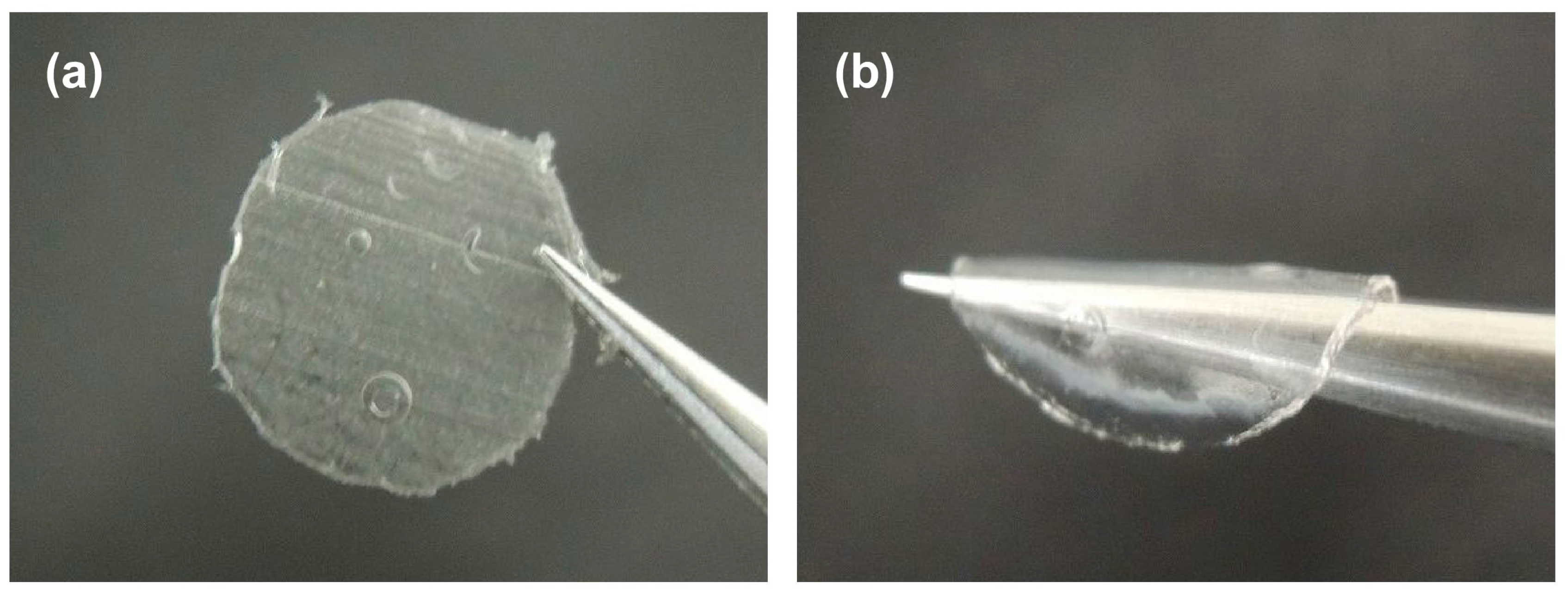
3.4. Evaluation of the Gelation Property of PEG-Grafted Chitosan and Formation of the Hydrogel Sheet
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Loo, Y.; Wong, Y.-C.; Cai, E.Z.; Ang, C.-H.; Raju, A.; Lakshmanan, A.; Koh, A.G.; Zhou, H.J.; Lim, T.-C.; Moochhala, S.M.; et al. Ultrashort peptide nanofibrous hydrogels for the acceleration of healing of burn wounds. Biomaterials 2014, 35, 4805–4814. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Hassan, W.U.; Kennedy, R.; Greiser, U.; Pandit, A.; Garcia, Y.; Wang, W. Performance of an in situ formed bioactive hydrogel dressing from a PEG-based hyperbranched multifunctional copolymer. Acta Biomater. 2014, 10, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Yokoyama, M.; Okano, T.; Nishida, H.; Tomizawa, Y.; Endo, M.; Kurosawa, H. A novel synthetic tissue-adhesive hydrogel using a crosslinkable polymeric micelle. J. Biomed. Mater. Res. 2007, 80A, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Yokoyama, M.; Nishida, H.; Tomizawa, Y.; Kurosawa, H. A simple hemostasis model for the quantitative evaluation of hydrogel-based local hemostatic biomaterials on tissue surface. Colloids Surf. B Biointerfaces 2008, 65, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Yokoyama, M.; Nishida, H.; Tomizawa, Y.; Kurosawa, H. In vivo and in vitro evaluation of gelation and hemostatic properties of a novel tissue-adhesive hydrogel containing a cross-linkable polymeric micelle. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91B, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Fukuda, K.; Murakami, Y. The hydrogel containing a novel vesicle-like soft crosslinker, a “trilayered” polymeric micelle, shows characteristic rheological properties. J. Polym. Sci. B Polym. Phys. 2013, 51, 124–131. [Google Scholar] [CrossRef]
- Murata, M.; Uchida, Y.; Takami, T.; Ito, T.; Anzai, R.; Sonotaki, S.; Murakami, Y. Dual drug release from hydrogels covalently containing polymeric micelles that possess different drug release properties. Colloids Surf. B Biointerfaces 2017, 153, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Moroishi, H.; Yoshida, C.; Murakami, Y. A free-standing, sheet-shaped, “hydrophobic” biomaterial containing polymeric micelles formed from poly(ethylene glycol)-poly(lactic acid) block copolymer for possible incorporation/release of “hydrophilic” compounds. Colloids Surf. B Biointerfaces 2013, 102, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Anzai, R.; Murakami, Y. Poly(ε-caprolactone) (PCL)-polymeric micelle hybrid sheets for the incorporation and release of hydrophilic compounds. Colloids Surf. B Biointerfaces 2015, 127, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Anzai, R.; Takami, T.; Uchida, Y.; Murakami, Y. Poly(ε-caprolactone) (PCL) hybrid sheets containing polymeric micelles: Effects of inner structures on the material properties of the sheet. Mater. Sci. Eng. C 2017, 72, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kanakubo, Y.; Ito, F.; Murakami, Y. Novel one-pot facile technique for preparing nanoparticles modified with hydrophilic polymers on the surface via block polymer-assisted emulsification/evaporation process. Colloids Surf. B Biointerfaces 2010, 78, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Takami, T.; Murakami, Y. Development of PEG-PLA/PLGA microparticles for pulmonary drug delivery prepared by a novel emulsification technique assisted with amphiphilic block copolymers. Colloids Surf. B Biointerfaces 2011, 87, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Takami, T.; Murakami, Y. Unexpected and successful “one-step” formation of porous polymeric particles only by mixing organic solvent and water under “low-energy-input” conditions. Langmuir 2014, 30, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Yoneki, N.; Takami, T.; Ito, T.; Anzai, R.; Fukuda, K.; Kinoshita, K.; Sonotaki, S.; Murakami, Y. One-pot facile preparation of PEG-modified PLGA nanoparticles: Effects of PEG and PLGA on release properties of the particles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 66–72. [Google Scholar] [CrossRef]
- Nishimura, S.; Takami, T.; Murakami, Y. Porous PLGA microparticles formed by “one-step” emulsification forpulmonary drug delivery: The surface morphology and theaerodynamic properties. Colloids Surf. B Biointerfaces 2017, 159, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, M.; Murakami, Y.; Shinoda, T.; Yamamoto, J.; Yokoyama, M. Synthesis and characterization of a temperature-responsive amphiphilic block copolymer containing a liquid crystalline unit. Chem. Lett. 2008, 37, 1214–1215. [Google Scholar] [CrossRef]
- Gupta, R.; Shea, J.; Scafe, C.; Shurlygina, A.; Rapoport, N. Polymeric micelles and nanoemulsions as drug carriers: Therapeutic efficacy, toxicity, and drug resistance. J. Control. Release 2015, 212, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Emami, J.; Rezazadeh, M.; Hasanzadeh, F.; Sadeghi, H.; Mostafavi, A.; Minaiyan, M.; Rostami, M.; Davies, N. Development and in vitro/in vivo evaluation of a novel targeted polymeric micelle for delivery of paclitaxel. Int. J. Biol. Macromol. 2015, 80, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, W.; Huang, Y.; Gao, F.; Sha, X.; Fang, X. Pluronic-based functional polymeric mixed micelles for co-delivery of doxorubicin and paclitaxel to multidrug resistant tumor. Int. J. Pharm. 2015, 488, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Murakami, Y. Trilayered polymeric micelle: A newly developed macromolecular assembly that can incorporate hydrophilic compounds. Colloids Surf. B Biointerfaces 2010, 79, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Murakami, Y. Successful preferential formation of a novel macromolecular assembly–trilayered polymeric micelle—That can incorporate hydrophilic compounds: The optimization of factors affecting the micelle formation from amphiphilic block copolymers. Colloids Surf. B Biointerfaces 2011, 84, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–649. [Google Scholar] [CrossRef]
- Boucard, N.; Viton, C.; Agay, D.; Mari, E.; Roger, T.; Chancerelle, Y.; Domard, A. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 2007, 28, 3478–3488. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.I.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Morimoto, M.; Sashiwa, H.; Saimoto, H.; Shigemasa, Y. Synthesis and bioactivities of poly (ethylene glycol)-chitosan hybrids. Carbohydr. Polym. 1998, 36, 49–59. [Google Scholar] [CrossRef]
- Shantha, K.L.; Harding, D.R.K. Synthesis and characterisation of chemically modified chitosan microspheres. Carbohydr. Polym. 2002, 48, 247–253. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.R.; Babukutty, Y.; Ohyama, T.; Kogoma, M.; Kodama, M. Biocompatibility evaluation of ePTFE membrane modified with PEG in atmospheric pressure glow discharge. J. Biomed. Mater. Res. 2002, 60, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.S.; Chansakul, T.; Yu, C.; Elisseeff, J.H.; Yu, S.M. Collagen mimetic peptide-conjugated photopolymerizable PEG hydrogel. Biomaterials 2006, 27, 5268–5276. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Hirata, A. Complex between α-chymotrypsin and poly(ethylene glycol) catalytically active in organic media. Biotechnol. Tech. 1999, 13, 545–548. [Google Scholar] [CrossRef]
- Murakami, Y.; Hirata, A. Poly(ethylene glycol)-α-chymotrypsin complex catalytically active in anhydrous isooctane. J. Biosci. Bioeng. 1999, 88, 441–443. [Google Scholar] [CrossRef]
- Murakami, Y.; Hoshi, R.; Hirata, A. Borate buffer dramatically enhances the activity of poly(ethylene glycol)-α-chymotrypsin complex catalytically active in anhydrous isooctane than conventional phosphate buffer even at low concentration. Biotechnol. Lett. 2001, 23, 125–129. [Google Scholar] [CrossRef]
- Murakami, Y.; Hoshi, R.; Hirata, A. Characterization of polymer–enzyme complex as a novel biocatalyst for nonaqueous enzymology. J. Mol. Catal. B Enzym. 2003, 22, 79–88. [Google Scholar] [CrossRef]
- Murakami, Y.; Hirata, A. Enzymatic synthesis of peptides-review. Seibutsu Kogaku Kais 1998, 76, 238–254. [Google Scholar]
- Ito, T.; Yoshida, C.; Murakami, Y. Design of novel sheet-shaped chitosan hydrogel for wound healing: A hybrid biomaterial consisting of both PEG-grafted chitosan and crosslinkable polymeric micelles acting as drug containers. Mater. Sci. Eng. C 2013, 33, 3697–3703. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Heredia-Guerrero, J.A.; Perotto, G.; Valentini, P.; Pompa, P.P.; Spanò, R.; Goldoni, L.; Bertorelli, R.; Athanassiou, A.; Bayer, I.S. Transparent ciprofloxacin-povidone antibiotic films and nanofiber mats as potential skin and wound care dressings. Eur. J. Pharm. Sci. 2017, 104, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, N.; Tripathi, A.; Kar, K.K.; Kumar, A. Synthesis and characterization of elastic and macroporous chitosan-gelatin cryogels for tissue engineering. Acta Biomater. 2009, 5, 406–418. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Smyth, H.D.C. Poly(ethylene glycol)–carboxymethyl chitosan-based pH-responsive hydrogels: Photo-induced synthesis, characterization, swelling, and in vitro evaluation as potential drug carriers. Carbohydr. Res. 2010, 345, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Ramay, H.R.; Gunn, J.; Matsen, F.A.; Zhang, M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J. Control. Release 2005, 103, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, H.; Xu, C.; Wang, Y.; Zhu, K. Preparation and characterization of poly(ethylene glycol)-g-chitosan with water- and organosolubility. Carbohydr. Polym. 2005, 61, 472–479. [Google Scholar] [CrossRef]
- Zimm, B.H. The Scattering of light and the radial distribution function of high polymer solutions. J. Chem. Phys. 1948, 16, 1093–1099. [Google Scholar] [CrossRef]
- Vekilov, P.G.; Feeling-Taylor, A.R.; Petsev, D.N.; Galkin, O.; Nagel, R.L.; Hirsch, R.E. Intermolecular interactions, nucleation, and thermodynamics of crystallization of hemoglobin C. Biophys. J. 2002, 83, 1147–1156. [Google Scholar] [CrossRef]
- Debye, P. Molecular-weight determination by light scattering. J. Phys. Colloid Chem. 1947, 51, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocoll. 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Chen, H.; Hu, X.; Chen, E.; Wu, S.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. Preparation, characterization, and properties of chitosan films with cinnamaldehyde nanoemulsions. Food Hydrocoll. 2016, 61, 662–671. [Google Scholar] [CrossRef]
- Soni, B.; Hassan, E.B.; Schilling, M.W.; Mahmound, B. Transparent bionanocomposite films based on chitosan and TEMPO-oxidized cellulose nanofibers with enhanced mechanical andbarrier properties. Carbohydr. Polym. 2016, 151, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Romano, I.; Ayadi, F.; Rizzello, L.; Summa, M.; Bertorelli, R.; Pompa, P.P.; Brandi, F.; Bayer, I.S.; Athanassiou, A. Controlled antiseptic/eosin release from chitosan-based hydrogel modified fibrous substrates. Carbohydr. Polym. 2015, 131, 306–314. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, C.; Uchida, Y.; Ito, T.; Takami, T.; Murakami, Y. Chitosan Gel Sheet Containing Polymeric Micelles: Synthesis and Gelation Properties of PEG-Grafted Chitosan. Materials 2017, 10, 1075. https://doi.org/10.3390/ma10091075
Yoshida C, Uchida Y, Ito T, Takami T, Murakami Y. Chitosan Gel Sheet Containing Polymeric Micelles: Synthesis and Gelation Properties of PEG-Grafted Chitosan. Materials. 2017; 10(9):1075. https://doi.org/10.3390/ma10091075
Chicago/Turabian StyleYoshida, Chikara, Yusuke Uchida, Tomoki Ito, Taku Takami, and Yoshihiko Murakami. 2017. "Chitosan Gel Sheet Containing Polymeric Micelles: Synthesis and Gelation Properties of PEG-Grafted Chitosan" Materials 10, no. 9: 1075. https://doi.org/10.3390/ma10091075