Pt-Co Alloys-Loaded Cubic SiC Electrode with Improved Photoelectrocatalysis Property
Abstract
:1. Introduction
2. Results and Discussion
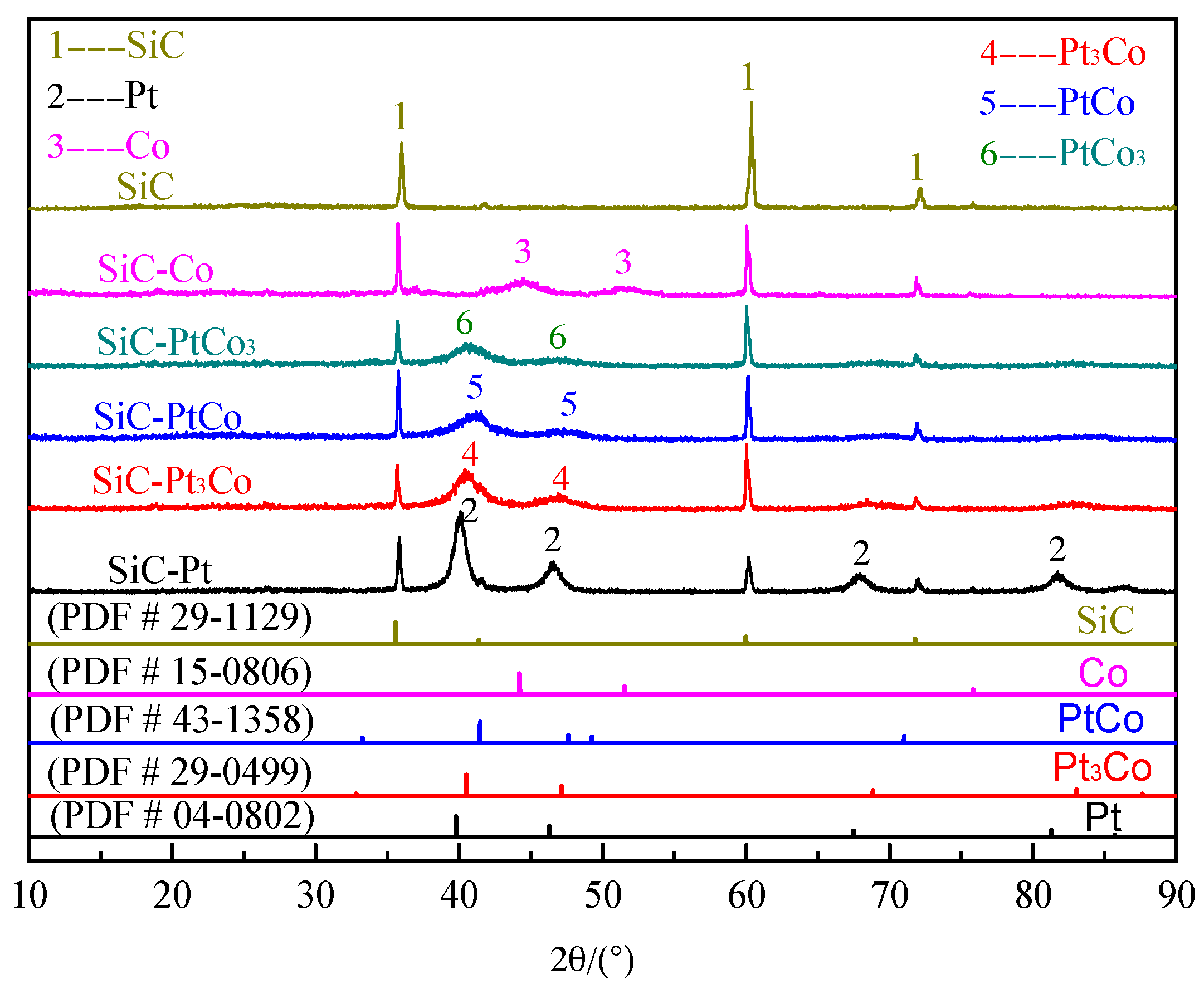
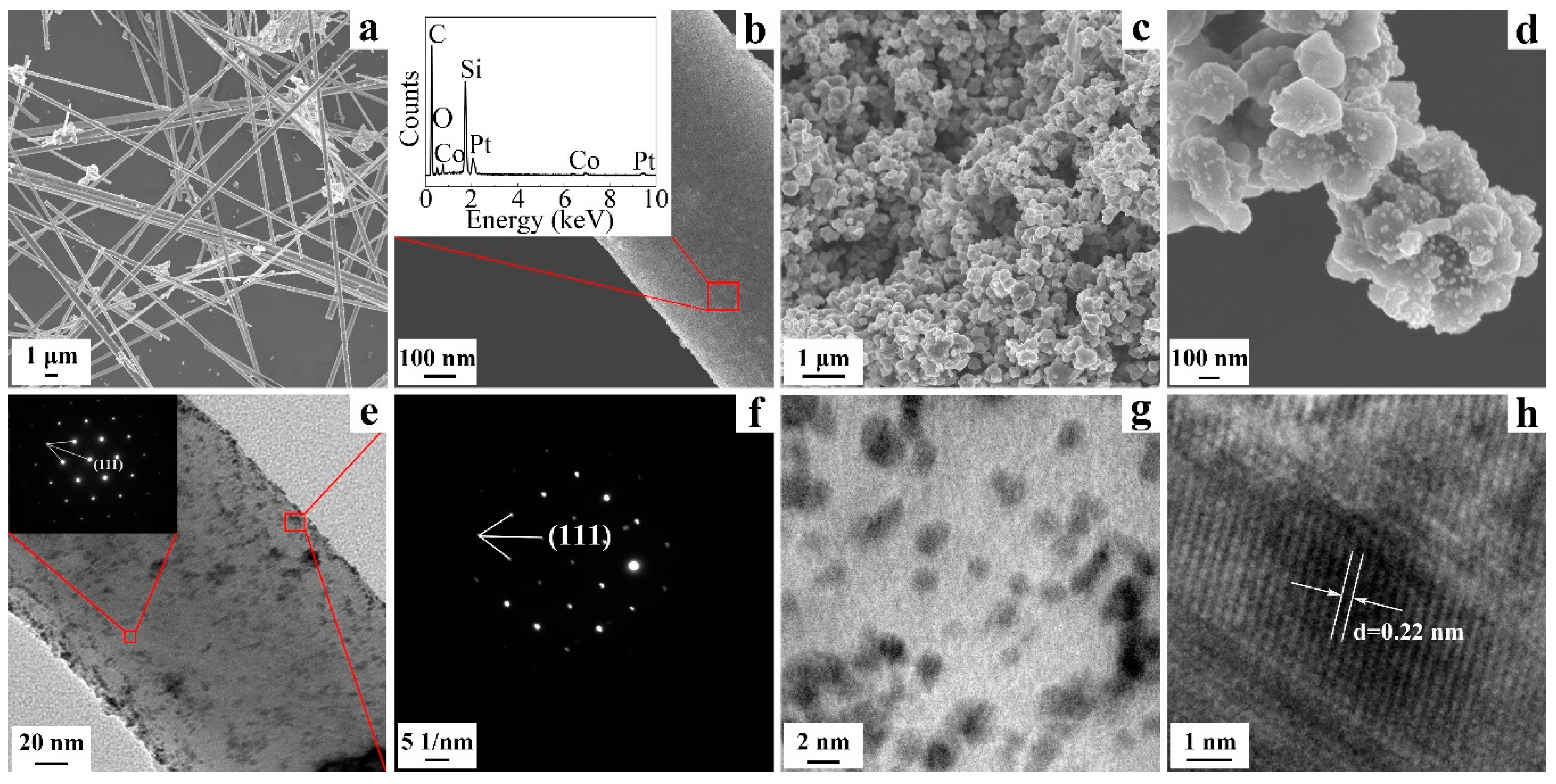
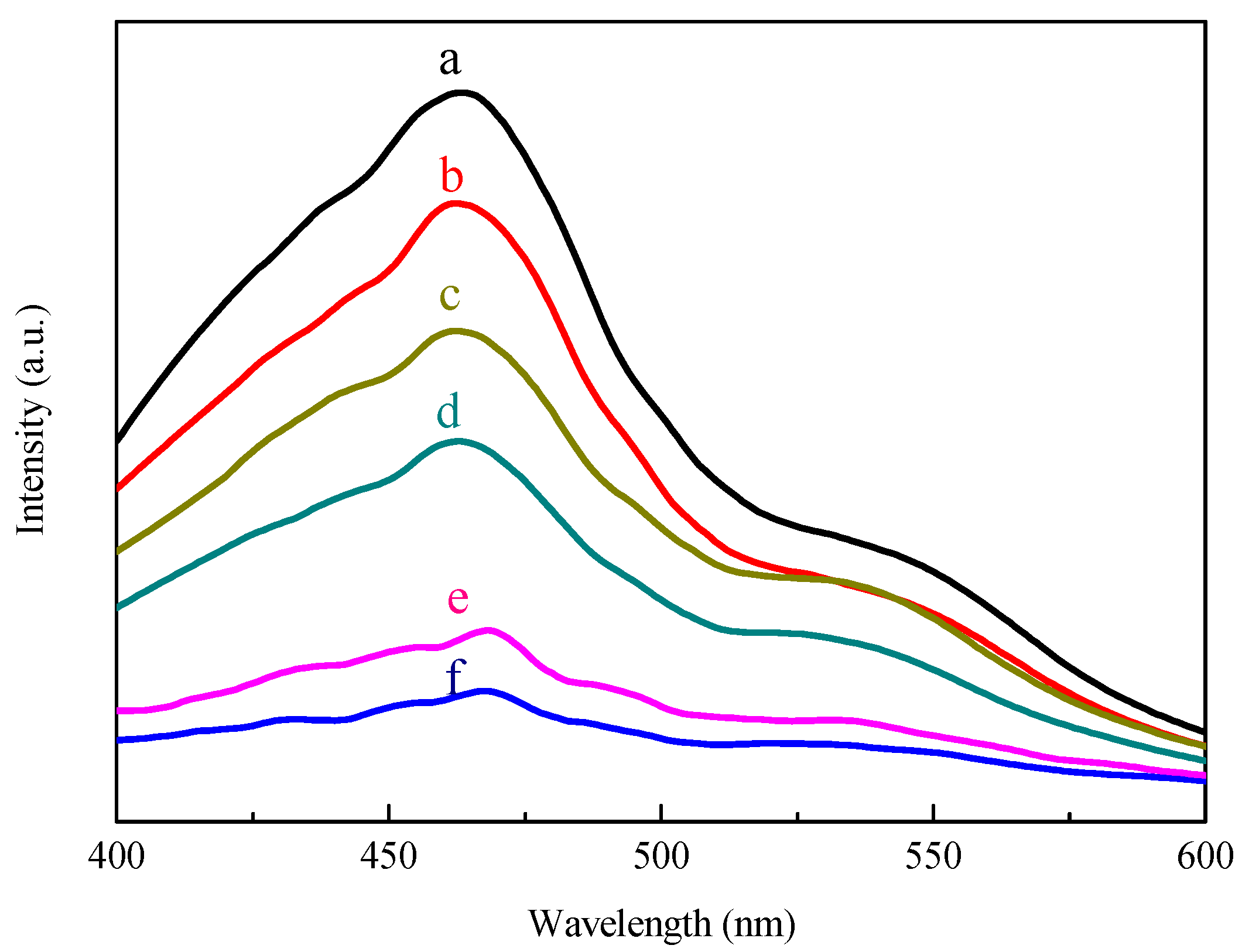
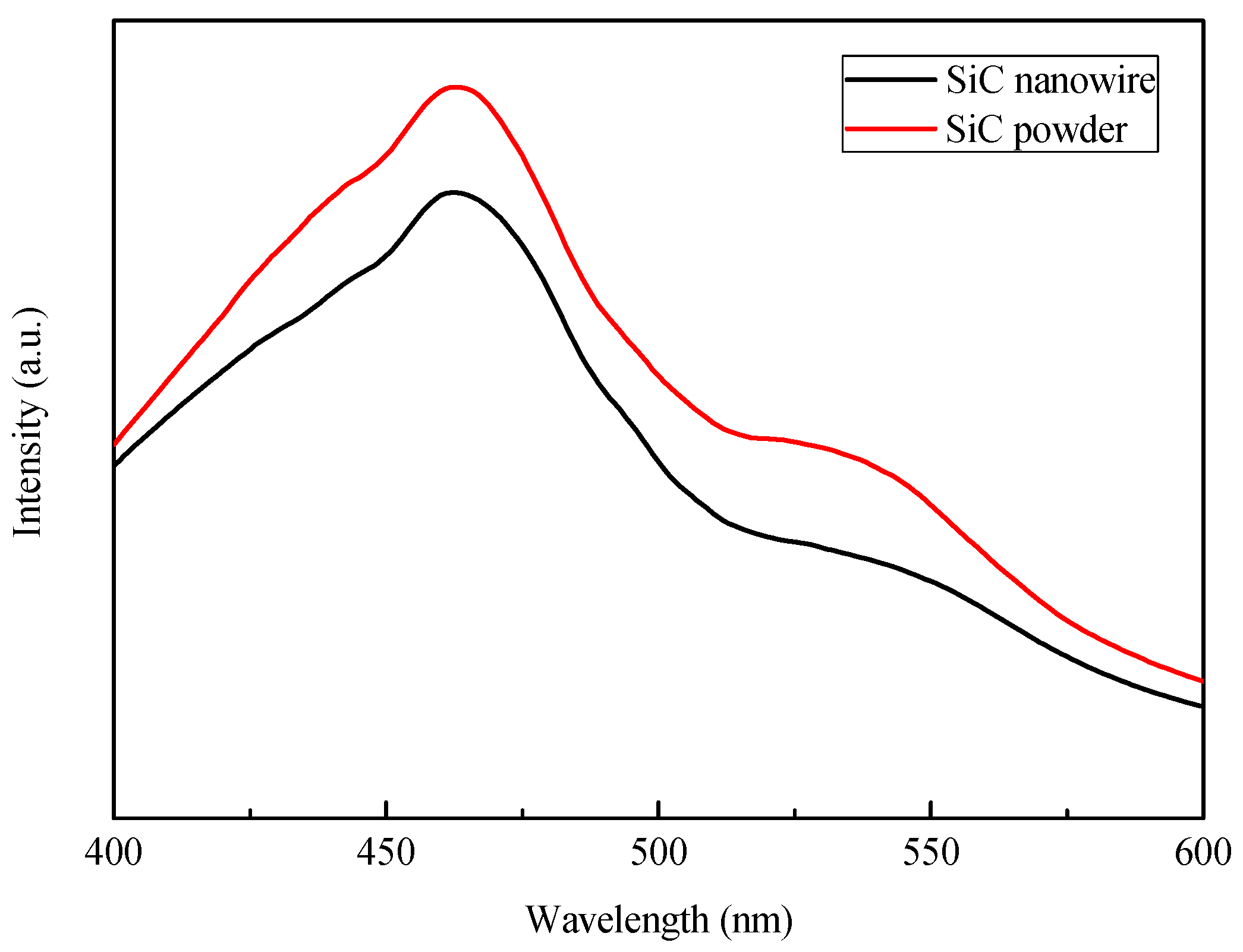
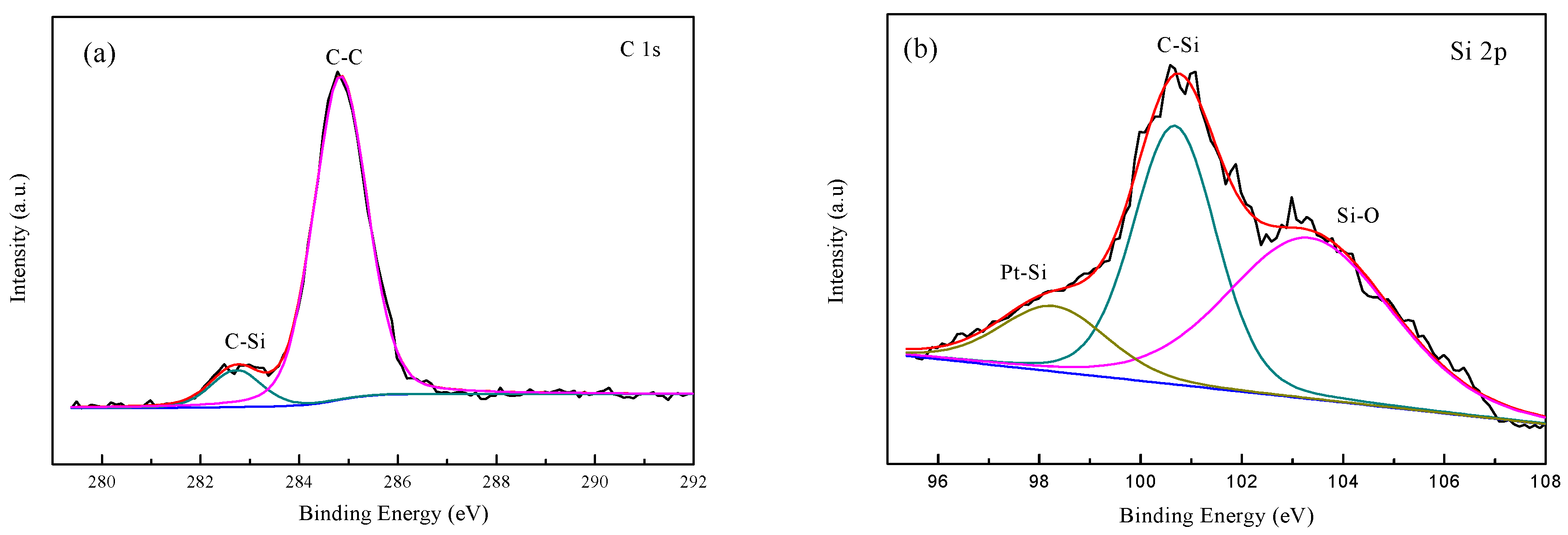
2.1. Characterization of SiC, Modified with the Pt-Co Alloy
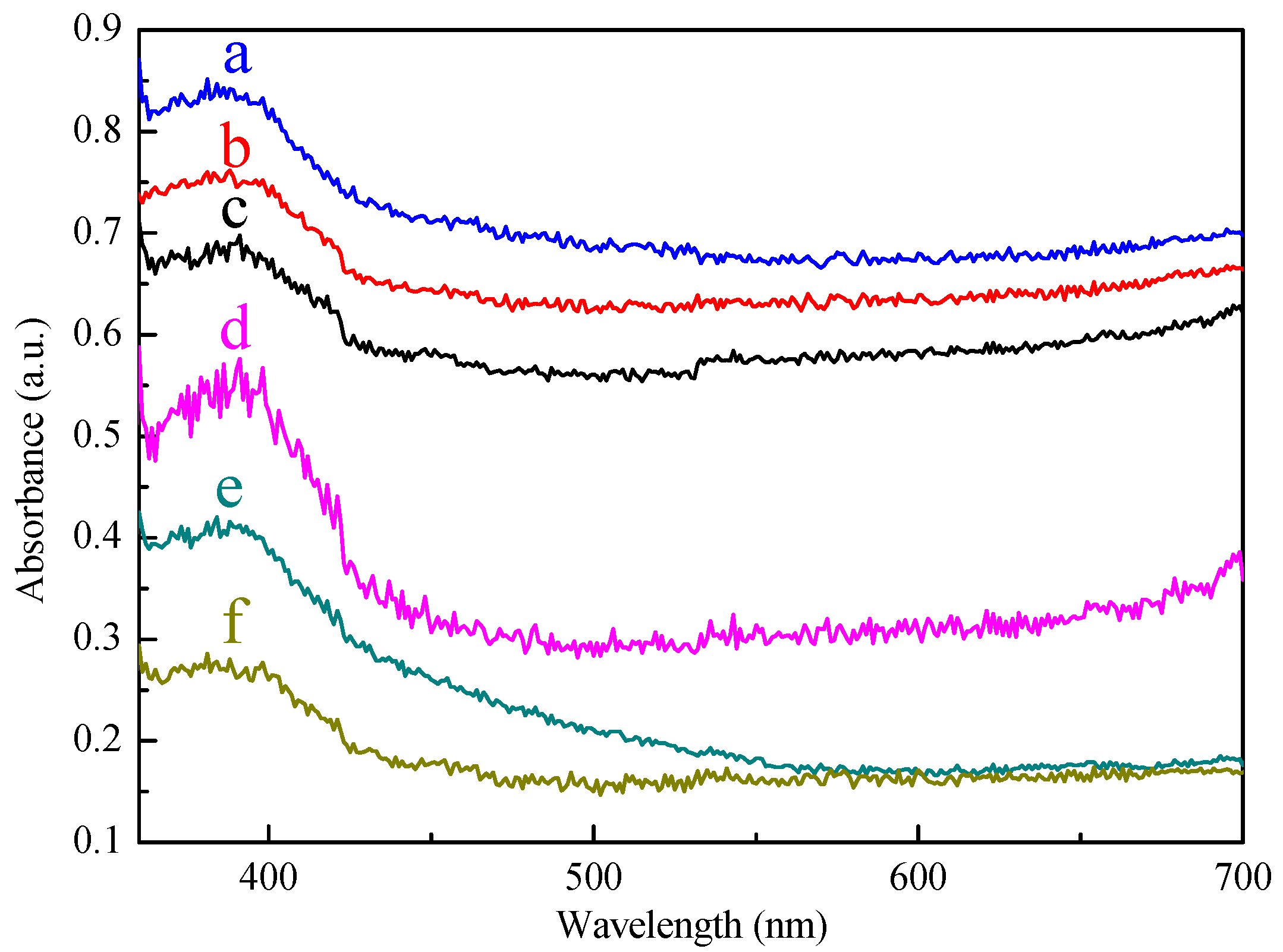
2.2. The Photoelectrochemical Property of SiC, Modified with Pt-Co Alloy
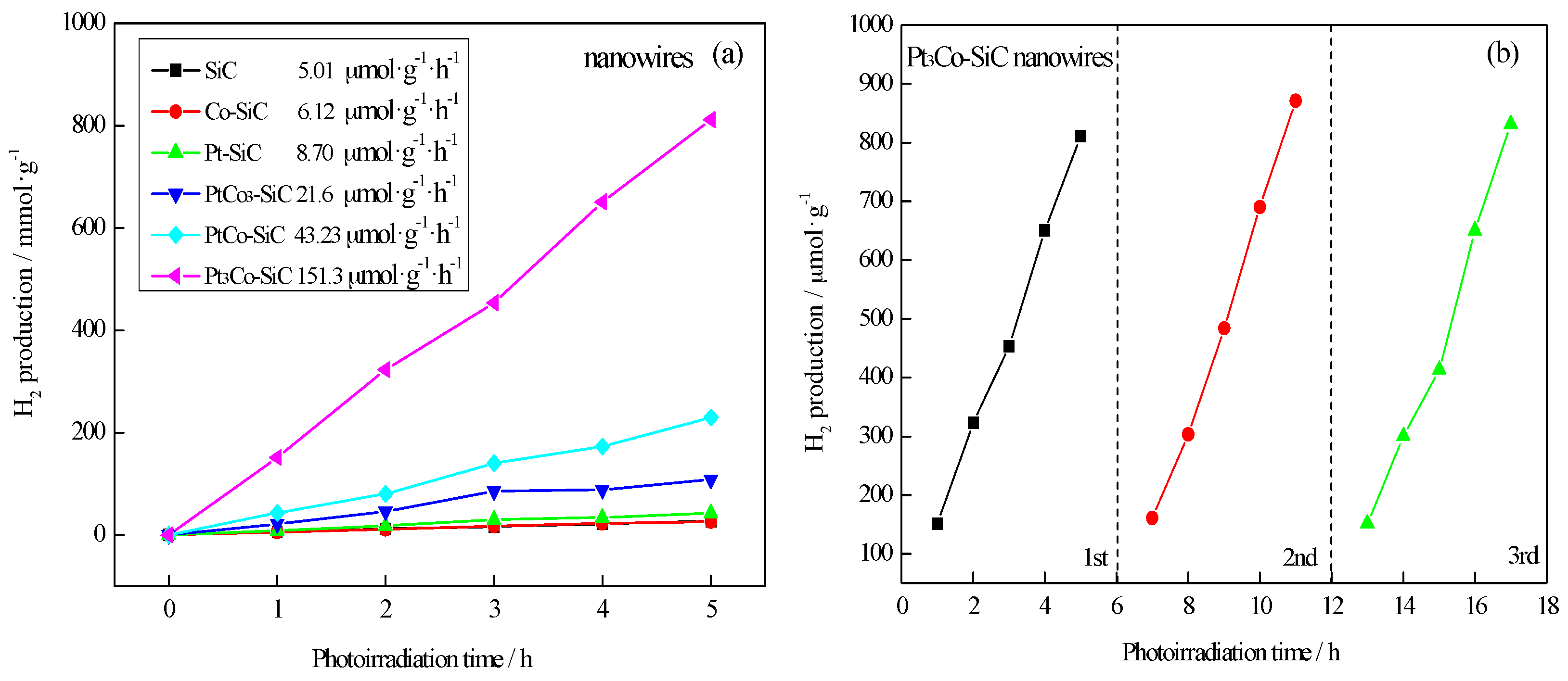
2.3. Water Splitting for Hydrogen
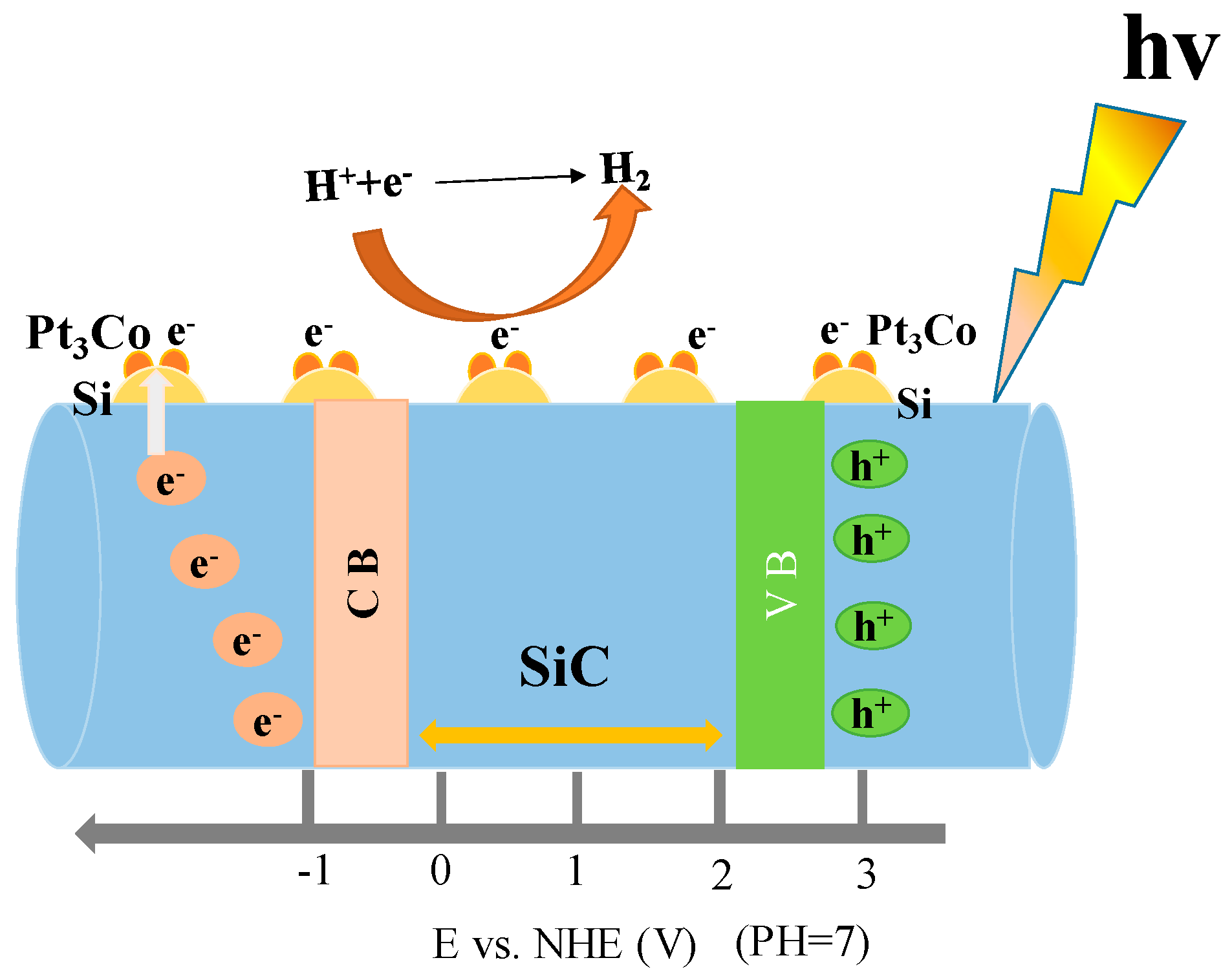
2.4. Photoresponse Mechanism
3. Experimental Section
3.1. Materials
3.2. Preparation of the SiC Nanowires and Powders
3.3. Synthesis of the Pt-Co-SiC Electrode
3.4. Characterization
3.5. Photoelectrochemical Measurements
3.6. Photocatalytic Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, Z.; Huang, B. Enhanced photocatalytic H2 production on hierarchical rutile TiO2 microspheres. RSC Adv. 2013, 3, 5156–5161. [Google Scholar] [CrossRef]
- Pop, L.C.; Tantis, I.; Lianos, P. Photoelectrocatalytic hydrogen production using nitrogen containing water soluble wastes. Int. J. Hydrogen Energy 2015, 40, 8304–8310. [Google Scholar] [CrossRef]
- Peng, R.; Zhao, D.; Baltrusaitis, J. Visible light driven photocatalytic evolution of hydrogen from water over CdS encapsulated MCM-48 materials. RSC Adv. 2012, 2, 5754–5767. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Meng, J.; Li, Y.D. Enhancing the activity of a SiC-TiO2 composite catalyst for photo-stimulated catalytic water splitting. Int. J. Hydrogen Energy 2013, 38, 3898–3904. [Google Scholar] [CrossRef]
- Zhao, C.; Luo, H.; Chen, F.; Zhang, P.; Yi, L.; You, K. A novel composite of TiO2 nanotubes with remarkably high efficiency for hydrogen production in solar-driven water splitting. Energy Environ. Sci. 2014, 7, 1700–1707. [Google Scholar] [CrossRef]
- Fujishima, A. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.B.; Kaiser, B.; Jaegermann, W. Novel photoelectrochemical behaviors of p-SiC films on Si for solar water splitting. J. Power Sources 2014, 253, 41–47. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Gao, Q.; Yuan, J.; Wen, J.; Fang, Y. Metal-free carbon nanotube-SiC nanowire heterostructures with enhanced photocatalytic H2 evolution under visible light irradiation. Catal. Sci. Technol. 2015, 5, 2798–2806. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Wang, M.; Gao, L.; Li, Y. SiC nanowire film grown on the surface of graphite paper and its electrochemical performance. J. Alloys Compd. 2014, 605, 168–172. [Google Scholar] [CrossRef]
- Hao, J.Y.; Wang, Y.Y.; Tong, X.L.; Jin, G.Q.; Guo, X.Y. Photocatalytic hydrogen production over modified SiC nanowires under visible light irradiation. Int. J. Hydrogen Energy 2012, 37, 15038–15044. [Google Scholar] [CrossRef]
- He, C.; Wu, X.; Shen, J.; Chu, P.K. High-efficiency electrochemical hydrogen evolution based on surface autocatalytic effect of ultrathin 3C-SiC nanocrystals. Nano Lett. 2012, 12, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Guo, L.; Zhao, L.; Zhang, X.; Liu, H.; Li, M. Efficient solar hydrogen production by photocatalytic water splitting: From fundamental study to pilot demonstration. Int. J. Hydrogen Energy 2010, 35, 7087–7097. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893–2939. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Liu, Z.; Ding, L.; Chen, J. Facile fabrication and efficient photoelectrochemical water-splitting activity of electrodeposited nickel/SiC nanowires composite electrode. Catal. Commun. 2017, 96, 46–49. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wang, L.; Chen, X.; Du, J.; Xiong, Y. Chemically exfoliated metallic MoS2 nanosheets: A promising supporting co-catalyst for enhancing the photocatalytic performance of TiO2 nanocrystals. Nano Res. 2015, 8, 175–183. [Google Scholar] [CrossRef]
- Tran, P.D.; Xi, L.; Batabyal, S.K.; Wong, L.H. Enhancing the photocatalytic efficiency of TiO2 nanopowders for H2 production by using non-noble transition metal co-catalysts. Phys. Chem. Chem. Phys. 2012, 14, 11596–11599. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Wang, X.; Huang, L.; Ma, G.; Yang, J.; Li, C. A hybrid photocatalytic system comprising ZnS as light harvester and an [Fe2S2] hydrogenase mimic as hydrogen evolution catalyst. ChemSusChem 2012, 5, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, N.; Kato, M.; Ichimura, M. The enhanced performance of 3C-SiC photocathodes for the generation of hydrogen through the use of cocatalysts. Appl. Phys. Lett. 2016, 109, 153904–153908. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, J.; Liao, X.; Liu, Z.; Zhang, J.; Gao, L. Highly efficient photocatalytic hydrogen production of platinum nanoparticle-decorated SiC nanowires under simulated sunlight irradiation. Int. J. Hydrogen Energy 2014, 39, 14581–14587. [Google Scholar] [CrossRef]
- Kong, C.; Min, S.; Lu, G. Robust Pt-Sn alloy decorated graphene nanohybrid cocatalyst for photocatalytic hydrogen evolution. Chem. Commun. 2014, 50, 9281–9283. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Zhu, H.; Wan, M.; Dong, L.; Zhang, M. Highly efficient and durable PtCo alloy nanoparticles encapsulated in carbon nanofibers for electrochemical hydrogen generation. Chem. Commun. 2016, 52, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Kaito, T.; Tanaka, H.; Mitsumoto, H. In situ X-ray absorption fine structure analysis of PtCo, PtCu, and PtNi alloy electrocatalysts: The correlation of enhanced oxygen reduction reaction activity and structure. J. Phys. Chem. C 2016, 120, 11519–11527. [Google Scholar] [CrossRef]
- Song, C.; Zhang, D.; Wang, B.; Cai, Z.; Yan, P.; Sun, Y.; Ye, K. Uniformly grown PtCo-modified Co3O4 nanosheets as a highly efficient catalyst for sodium borohydride electrooxidation. Nano Res. 2016, 9, 3322–3333. [Google Scholar] [CrossRef]
- Han, C.; Lu, Y.; Zhang, J. Novel PtCo alloy nanoparticle decorated 2D g-C3N4 nanosheets with enhanced photocatalytic activity for H2 evolution under visible light irradiation. J. Mater. Chem. A 2015, 3, 23274–23282. [Google Scholar] [CrossRef]
- Hu, Z.; Jimmy, C.Y. Pt3Co-loaded CdS and TiO2 for photocatalytic hydrogen evolution from water. J. Mater. Chem. A 2013, 1, 12221–12228. [Google Scholar] [CrossRef]
- Liao, X.; Liu, Z.; Ding, L.; Chen, J.; Tang, W. Photoelectrocatalytic activity of flexible PEDOT–PSS/silicon carbide nanowire films. RSC Adv. 2015, 5, 99143–99147. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Liu, L.; Hu, J.; Cui, W. Reduced graphene oxide wrapped Bi2WO6 hybrid with ultrafast charge separation and improved photoelectrocatalytic performance. Appl. Surf. Sci. 2017, 392, 51–60. [Google Scholar] [CrossRef]
- Yang, T.; Chang, X. W.; Chen, J. H.; Hou, X. M. B-doped 3C-SiC nanowires with a finned microstructure for efficient visible light-driven photocatalytic hydrogen production. Nanoscale 2015, 7, 8955–8961. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zeng, X.; Chen, L. Photocatalytic water splitting to hydrogen production of reduced graphene oxide/SiC under visible light. Appl. Phys. Lett. 2013, 102, 083101. [Google Scholar] [CrossRef]
- Qi, F.; Li, Y.; Wang, Y.; Liu, S.; Zhao, X. Ag-Doped g-C3N4 film electrode: Fabrication, characterization and photoelectrocatalysis property. RSC Adv. 2016, 6, 81378–81385. [Google Scholar] [CrossRef]
- Feng, D.H.; Jia, T.Q.; Li, X.X.; Xu, Z.Z.; Chen, J.; Deng, S.Z. Catalytic synthesis and photoluminescence of needle-shaped 3C-SiC nanowires. Solid State Commun. 2003, 128, 295–297. [Google Scholar] [CrossRef]
- Wang, D.; Wang, W.; Wang, Q. Spatial separation of Pt and IrO2 cocatalysts on SiC surface for enhanced photocatalysis. Mater. Lett. 2017, 201, 114–117. [Google Scholar] [CrossRef]
- Arpóna, R.; Narciso, J.; Rodríguez-Reinosoa, F.; Komatsu, M. Synthesis of mixed disilicides/SiC composites by displacement reaction between metal carbides and silicon. Mater. Sci. Eng. A 2004, 380, 62–66. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Y.; Wang, Q.; Pan, N.; Guo, Z. High-efficient photo-electron transport channel in SiC constructed by depositing cocatalysts selectively on specific surface sites for visible-light H2 production. Appl. Phys. Lett. 2016, 108, 161601–161606. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Ramaprabhu, S. Platinum–TM (TM = Fe, Co) alloy nanoparticles dispersed nitrogen doped (reduced graphene oxide-multiwalled carbon nanotube) hybrid structure cathode electrocatalysts for high performance PEMFC applications. Nanoscale 2013, 5, 5109–5118. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liang, Y.; Han, M. Hierarchical construction of composite hollow structures of Co@ CoO and their magnetic behavior. J. Phys. Chem. C 2008, 112, 9272–9277. [Google Scholar] [CrossRef]
- Shi, J.; Nie, R.; Zhang, M.; Zhao, M.; Hou, Z. Microwave-assisted fast fabrication of a nanosized Pt3Co alloy on reduced graphene oxides. Chin. J. Catal. 2014, 35, 2029–2037. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Han, C.; Ge, L.; Fang, S.; Qiu, P. Synthesis of novel flower-like PtCo-Bi2MoO6 photocatalysts with enhanced visible light photocatalytic performance. RSC Adv. 2016, 6, 84485–84492. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, Z.; Yang, J.; Wang, D.; Yuan, W. Enhanced photocatalytic H2 evolution over micro-SiC by coupling with CdS under visible light irradiation. J. Mater. Chem. A 2014, 2, 6296–6300. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Hou, X.; Su, L.; Fan, H.; Chou, K.C. Fabrication and characterization of ultralight SiC whiskers decorated by RuO2 nanoparticles as hybrid supercapacitors. RSC Adv. 2016, 6, 19626–19631. [Google Scholar] [CrossRef]
- Antoniadou, M.; Panagiotopoulou, P.; Kondarides, D.I. Photocatalysis and photoelectrocatalysis using nanocrystalline Titania alone or combined with Pt, RuO2 or NiO co-catalysts. J. Appl. Electrochem. 2012, 42, 737–743. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xu, Y.; Li, T.; Wang, Y.C. Topological morphology conversion towards SnO2/SiC hollow sphere nanochains with efficientphotocatalytic hydrogen evolution. Chem. Commun. 2012, 10, 46–50. [Google Scholar]
- Zhou, X.; Liu, Y.; Li, X.; Gao, Q.; Liu, X.; Fang, Y. Preparation of ternary Cr2O3–SiC–TiO2 composites for the photocatalytic production of hydrogen. Particuology 2014, 50, 1070–1073. [Google Scholar]



| Material | Morphology | H2 Production (μmol·h−1·g−1) | Year/Reference |
|---|---|---|---|
| Modified SiC | nanowires | 2.68 | 2012/[30] |
| RGO/SiC | powder | 42.4 | 2013/[31] |
| SiC | fibre | 67.5 | 2015/[33] |
| SiC-PEDOT/PSS | fibre | 100.7 | 2015/[33] |
| B-SiC | nanowires | 108.4 | 2015/[32] |
| Pt3Co-SiC | nanowires | 151.3 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Yang, T.; Chen, J.; Chou, K.-C.; Hou, X. Pt-Co Alloys-Loaded Cubic SiC Electrode with Improved Photoelectrocatalysis Property. Materials 2017, 10, 955. https://doi.org/10.3390/ma10080955
Liu D, Yang T, Chen J, Chou K-C, Hou X. Pt-Co Alloys-Loaded Cubic SiC Electrode with Improved Photoelectrocatalysis Property. Materials. 2017; 10(8):955. https://doi.org/10.3390/ma10080955
Chicago/Turabian StyleLiu, Dan, Tao Yang, Junhong Chen, Kuo-Chih Chou, and Xinmei Hou. 2017. "Pt-Co Alloys-Loaded Cubic SiC Electrode with Improved Photoelectrocatalysis Property" Materials 10, no. 8: 955. https://doi.org/10.3390/ma10080955




