Samarium Monosulfide (SmS): Reviewing Properties and Applications
Abstract
:1. Introduction
2. Properties of SmS
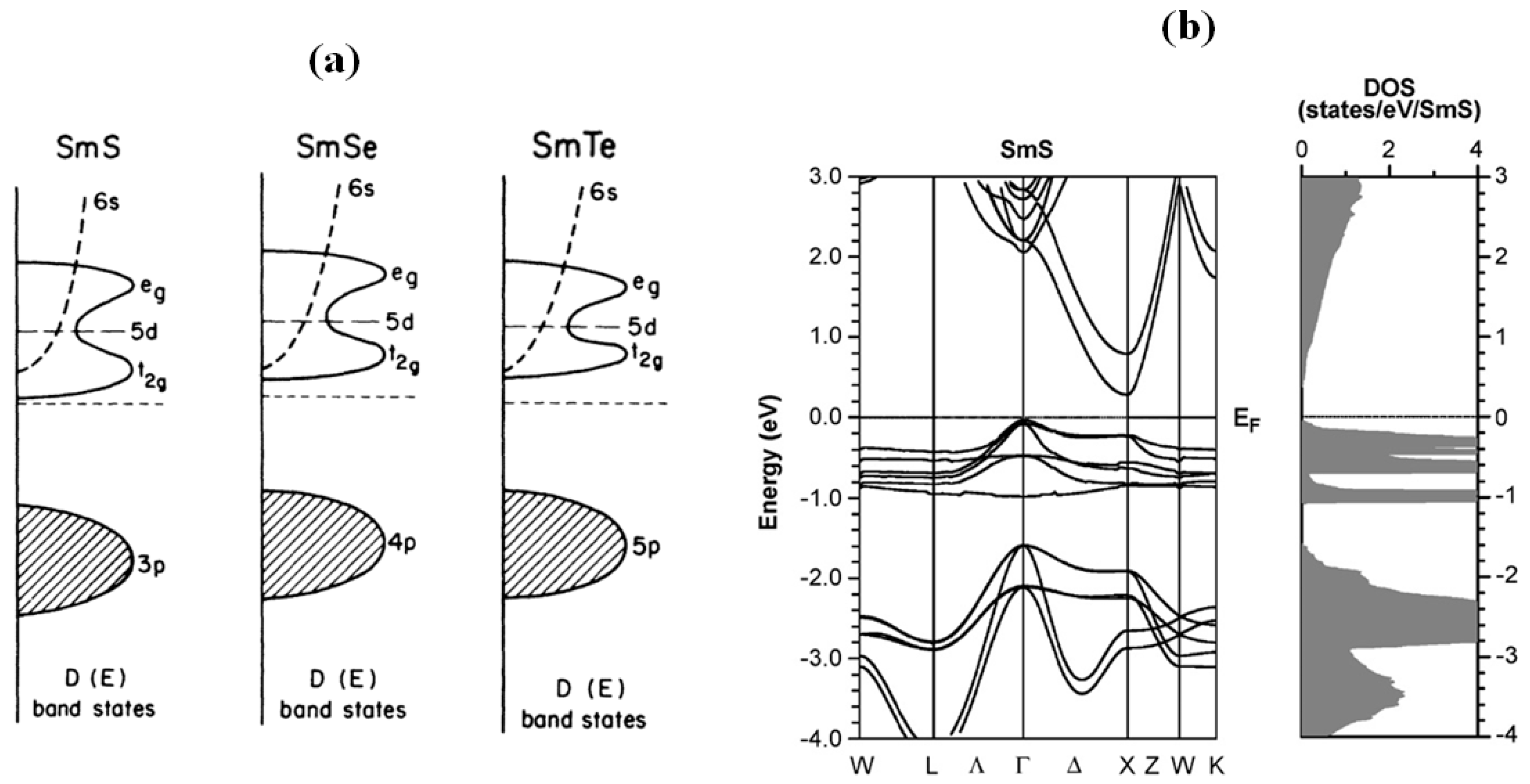
2.1. Energy Level Diagram
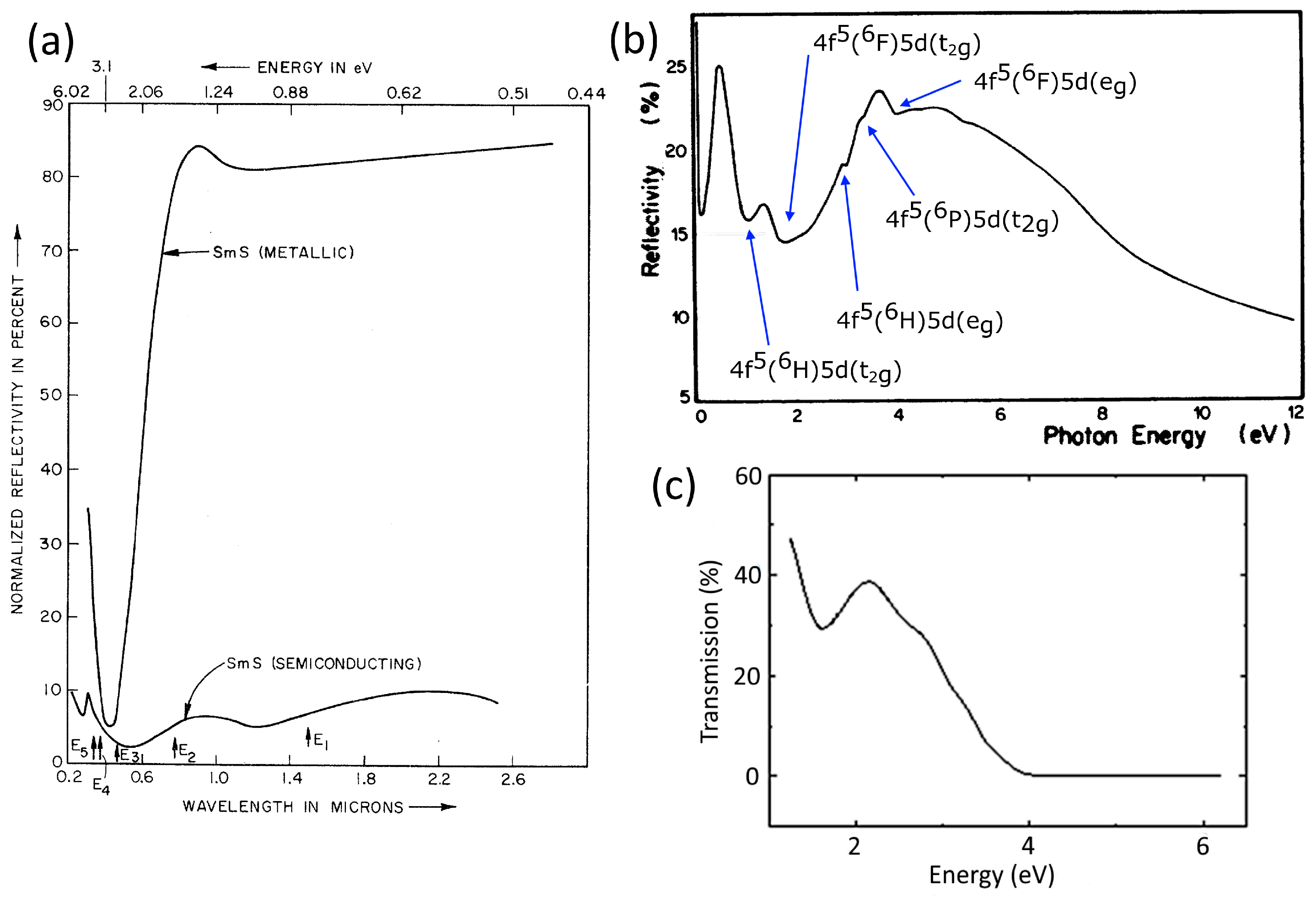
2.2. Electrical and Optical Properties
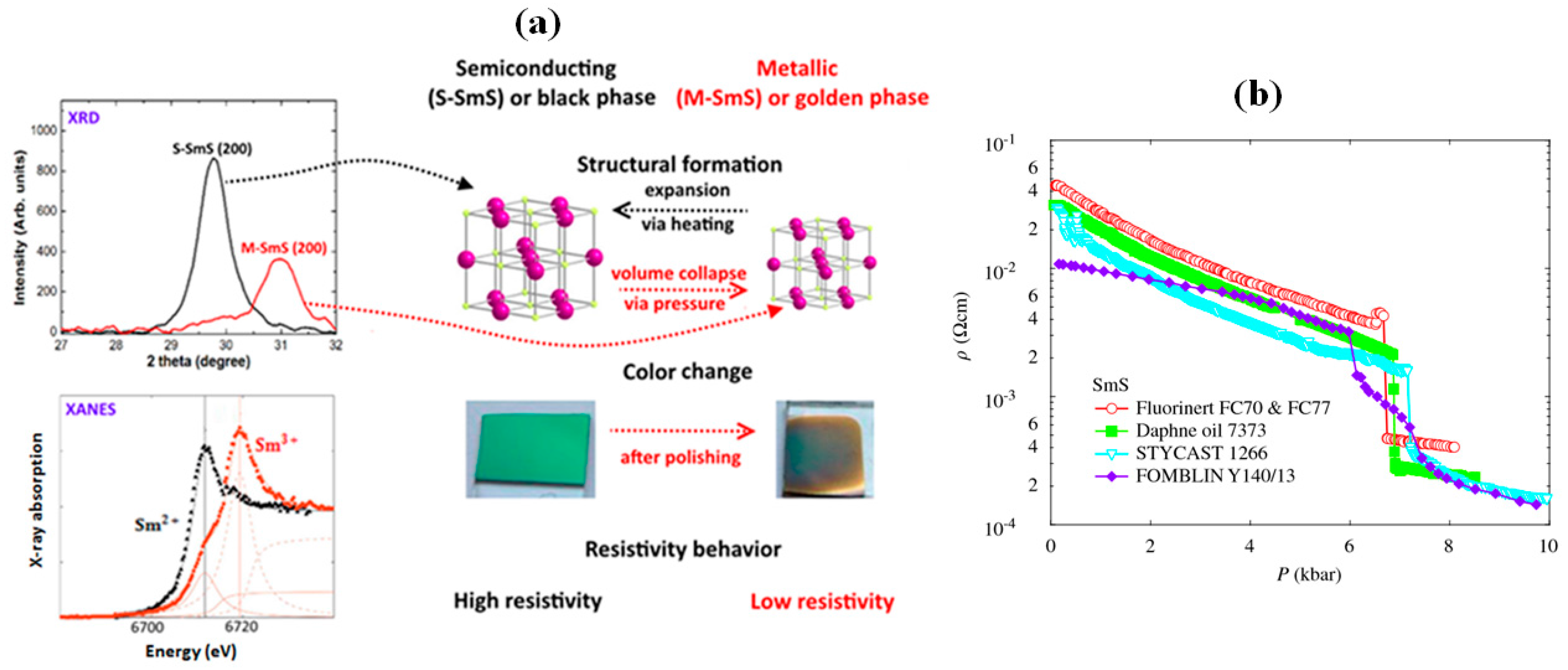
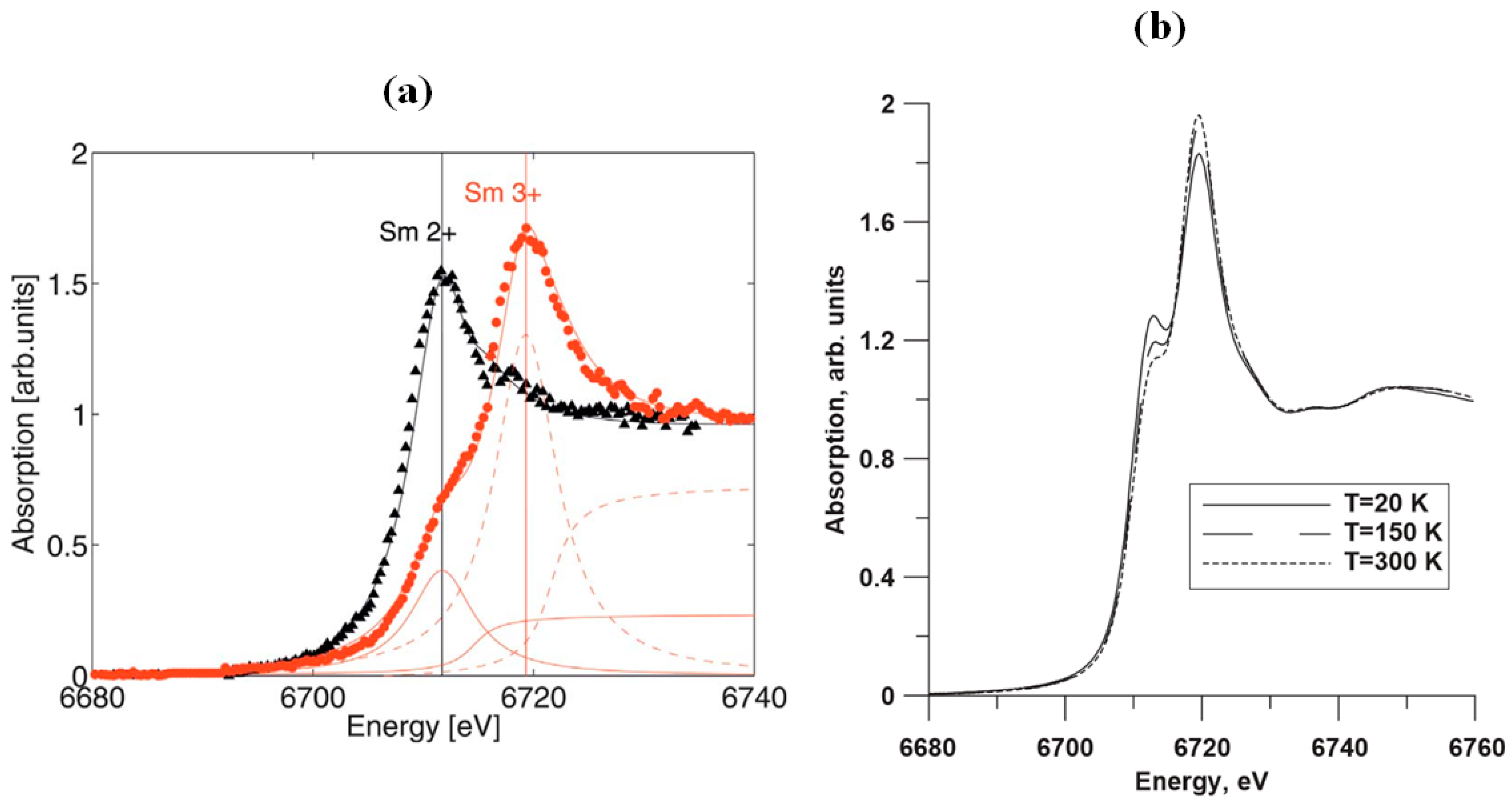
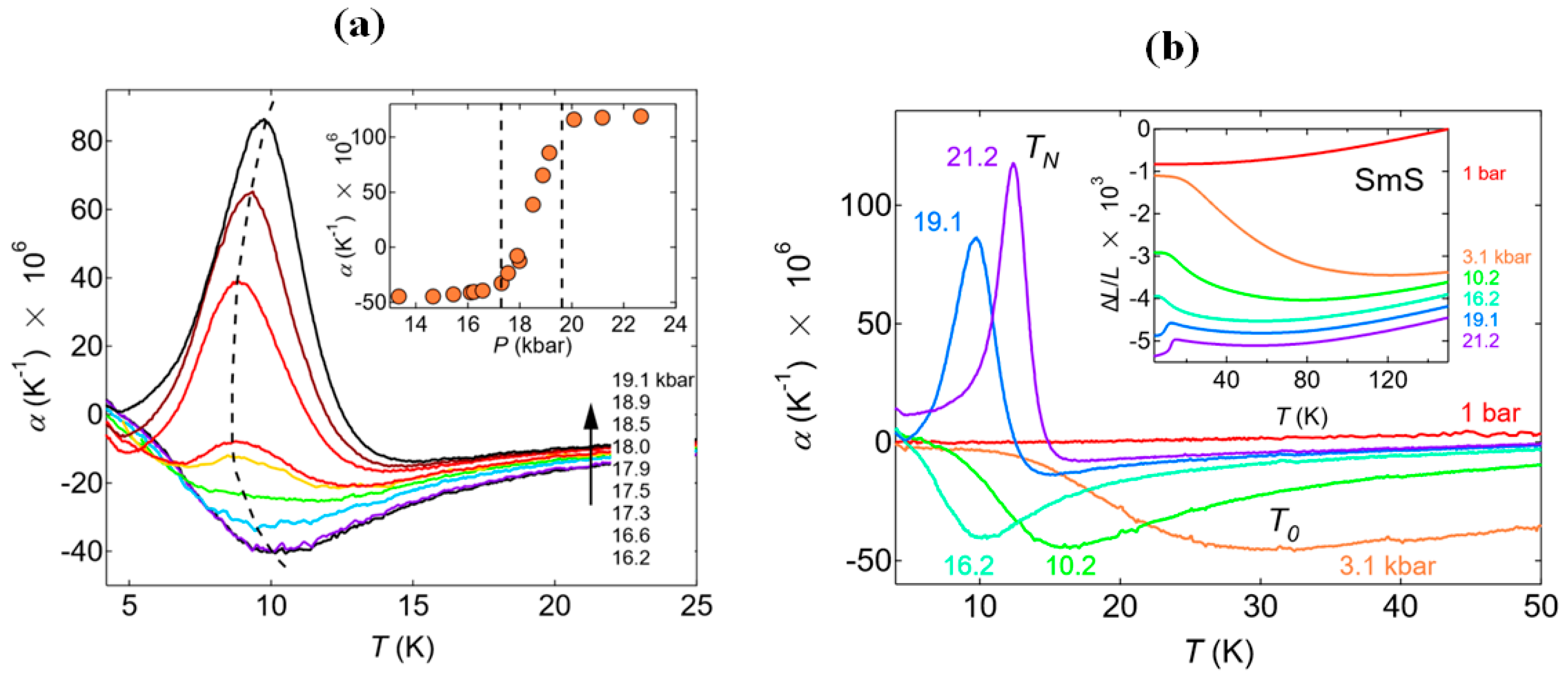
2.3. Resistivity Drop and Structural Properties
2.4. Magnetic Properties
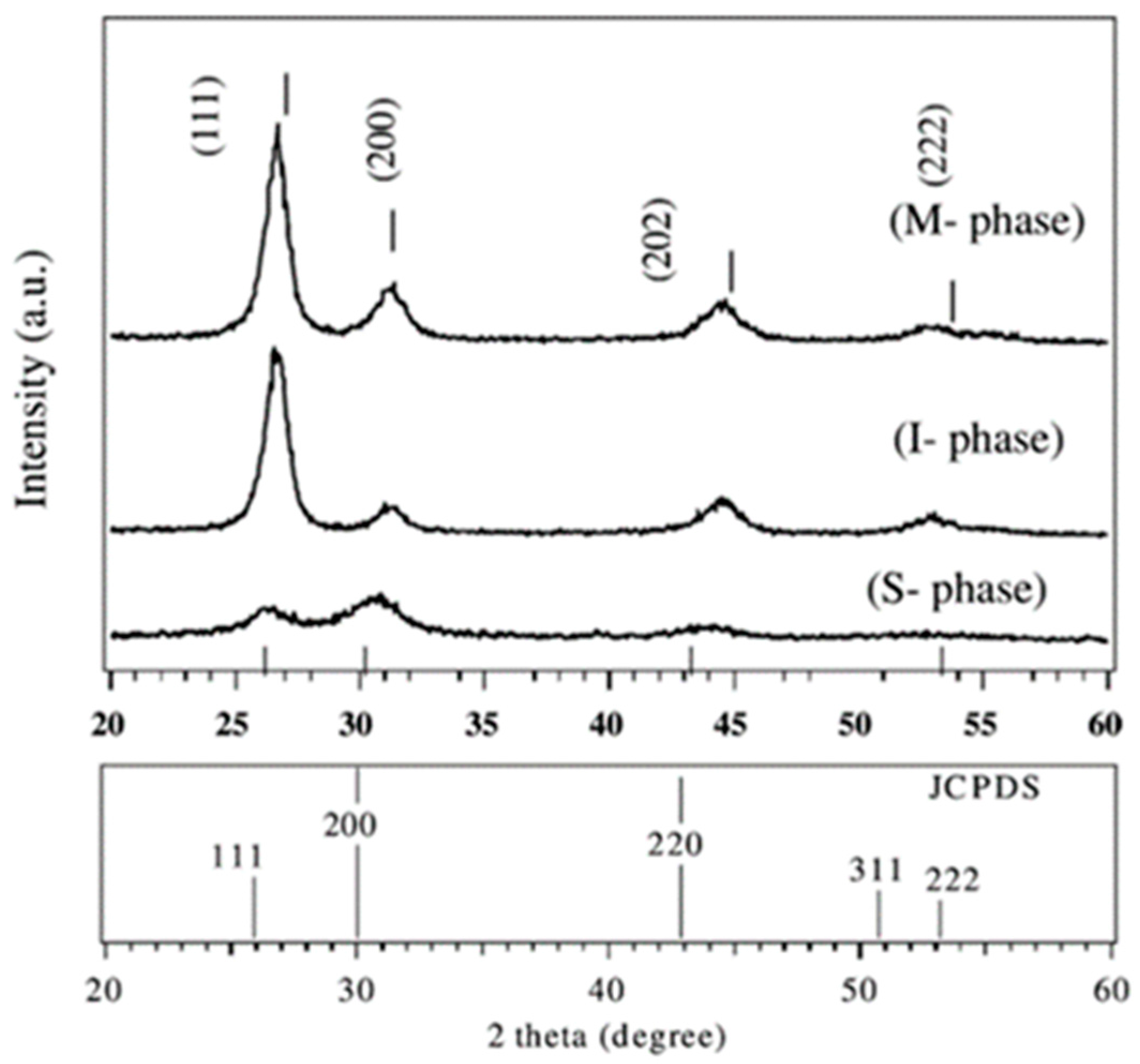
3. Thin Film and Bulk SmS Preparation Techniques
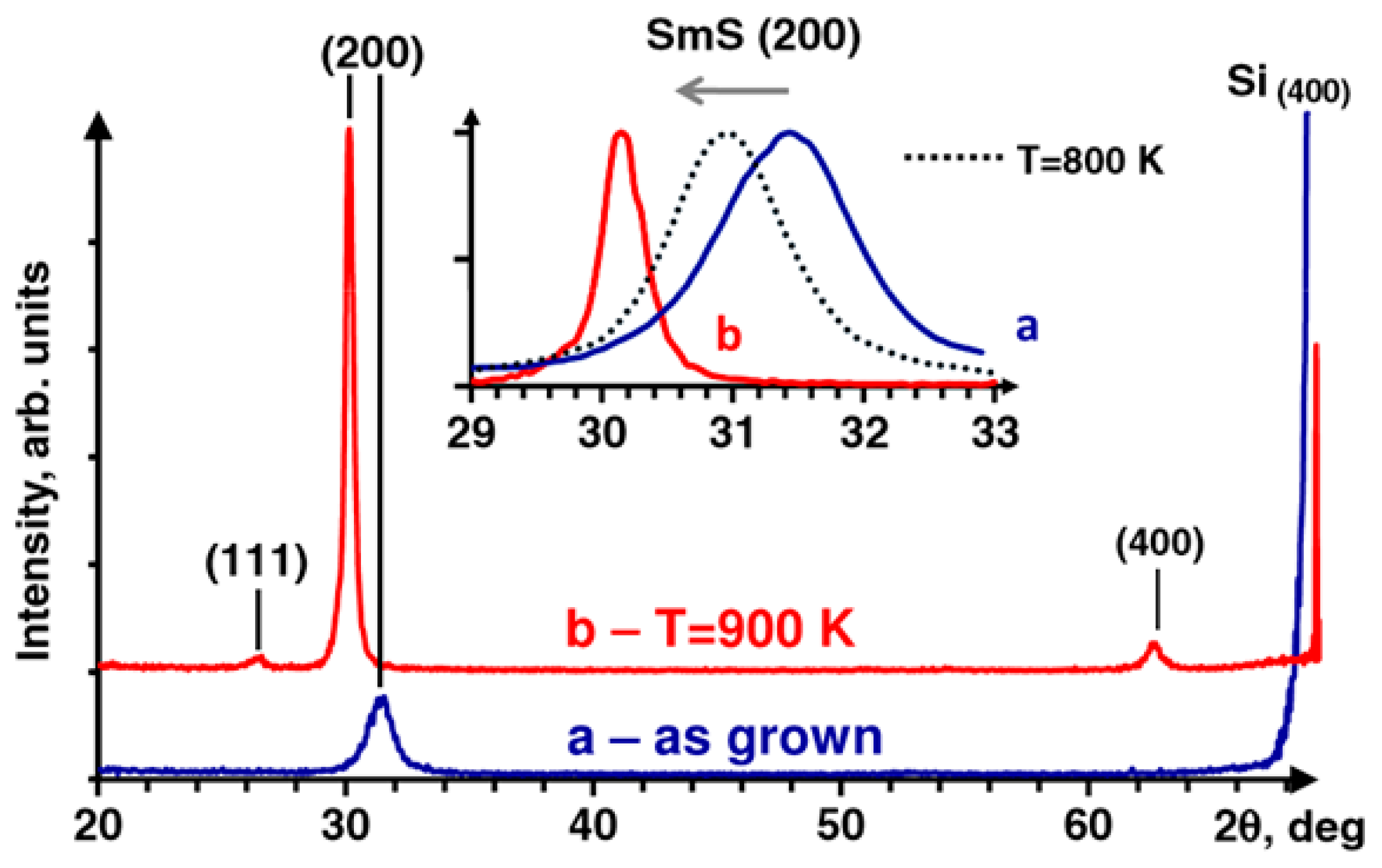
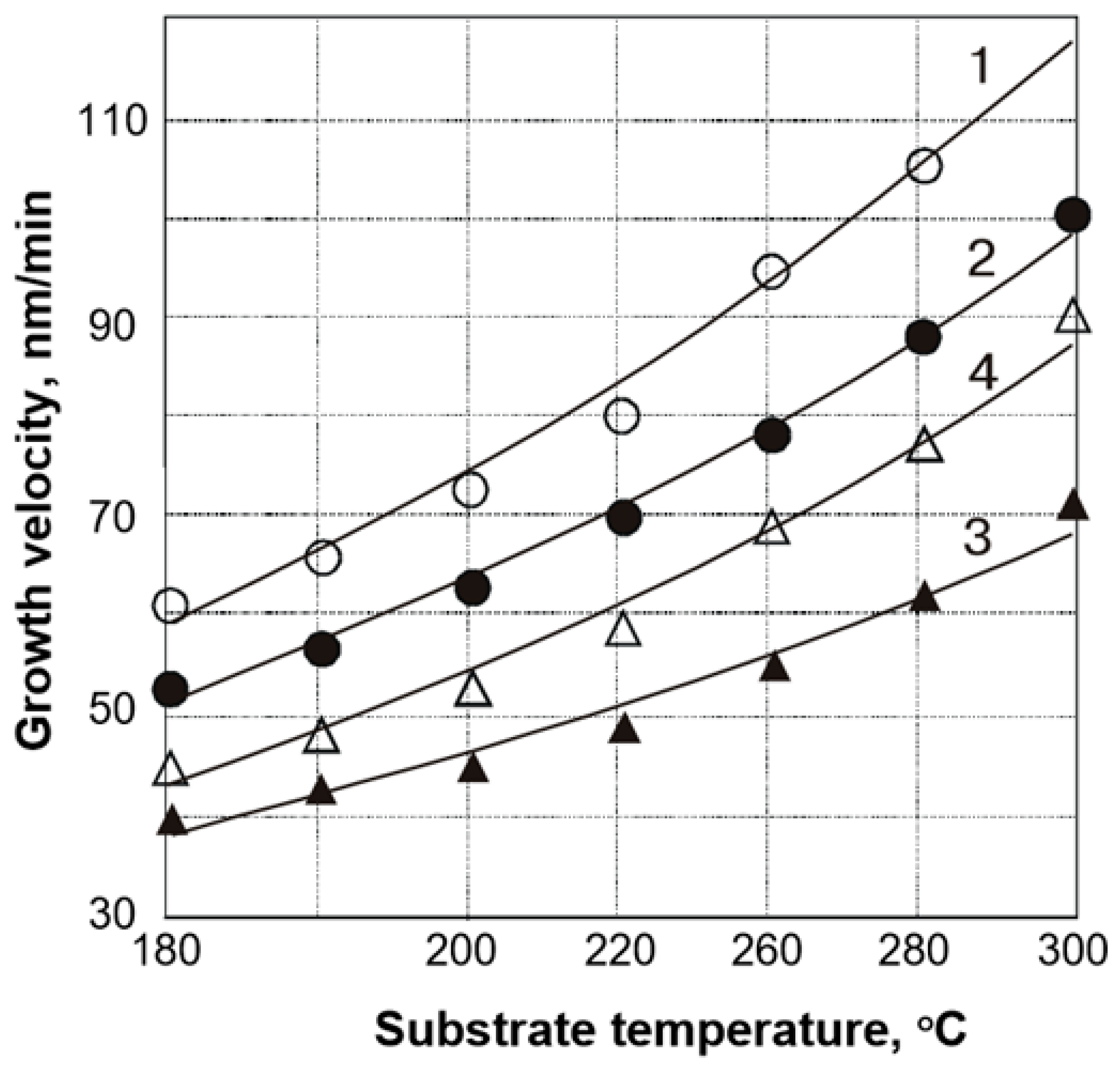
3.1. Electron Beam Evaporation—Reactive Evaporation
3.2. Sputtering
3.3. Pulsed Laser Deposition
3.4. MOCVD of SmS
3.5. Other Thin Film Deposition Techniques
3.6. Bulk—Single Crystals
4. Applications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burr, G.W.; Breitwisch, M.J.; Franceschini, M.; Garetto, D.; Gopalakrishnan, K.; Jackson, B.; Kurdi, B.; Lam, C.; Lastras, L.A.; Padilla, A.; et al. Phase change memory technology. J. Vac. Sci. Technol. B 2010, 28, 223–262. [Google Scholar] [CrossRef]
- Lencer, D.; Salinga, M.; Wuttig, M. Design rules for phase-change materials in data storage applications. Adv. Mater. 2011, 23, 2030–2058. [Google Scholar] [CrossRef] [PubMed]
- Lencer, D.; Salinga, M.; Grabowski, B.; Hickel, T.; Neugebauer, J.; Wuttig, M. A map for phase-change materials. Nat. Mater. 2008, 7, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Chernova, N.A.; Roppolo, M.; Dillon, A.C.; Whittingham, M.S. Layered vanadium and molybdenum oxides: Batteries and electrochromics. J. Mater. Chem. 2009, 19, 2526–2552. [Google Scholar] [CrossRef]
- Fortier, J.P.; Baloukas, B.; Zabeida, O.; Klemberg-Sapieha, J.E.; Martinu, L. Thermochromic VO2 thin films deposited by HiPIMS. Sol. Energy Mater. Sol. Cells 2014, 125, 291–296. [Google Scholar] [CrossRef]
- Li, S.Y.; Niklasson, G.A.; Granqvist, C.G. Thermochromic fenestration with VO2-based materials: Three challenges and how they can be met. Thin Solid Films 2012, 520, 3823–3828. [Google Scholar] [CrossRef]
- Maaza, M.; Nemraoui, O.; Sella, C.; Beye, A.C.; Baruch-Barak, B. Thermal induced tunability of surface plasmon resonance in Au-VO2 nano-photonics. Opt. Commun. 2005, 254, 188–195. [Google Scholar] [CrossRef]
- Jarrige, I.; Yamaoka, H.; Rueff, J.P.; Lin, J.F.; Taguchi, M.; Hiraoka, N.; Ishii, H.; Tsuei, K.D.; Imura, K.; Matsumura, T.; et al. Unified understanding of the valence transition in the rare-earth monochalcogenides under pressure. Phys. Rev. B 2013, 87, 115107. [Google Scholar] [CrossRef]
- Sousanis, A.; Smet, P.F.; Detavernier, C.; Poelman, D. Stability of switchable SmS for piezoresistive applications. In Proceedings of the IEEE Nanotechnol Materials and Devices Conference (NMDC), Toulouse, France, 9–12 October 2016. [Google Scholar]
- Kitagawa, R.; Takebe, H.; Morinaga, K. Photoinduced phase transition of metallic SmS thin films by a femtosecond laser. Appl. Phys. Lett. 2003, 82, 3641. [Google Scholar] [CrossRef]
- Suryanarayanan, R.; Smirnov, I.A.; Brun, G.; Shul’Man, S.G. Optical absorption of semiconducting and metallic SmS nd Sm1-xLnxS films. J. Phys. Colloq. 1976, 37, 271–274. [Google Scholar] [CrossRef]
- Hickey, C.F. Optical, chemical, and structural properties of thin films of samarium-sulfide and zinc-sulfide. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 1987. [Google Scholar]
- Imura, K.; Matsubayashi, K.; Suzuki, H.S.; Kabeya, N.; Deguchi, K.; Sato, N.K. Pressure-temperature phase diagram of golden SmS. J. Phys. Soc. Jpn. 2009, 78, 104602. [Google Scholar] [CrossRef]
- Jayaraman, A.; Bucher, E.; Dernier, P.D.; Longinotti, L.D. Temperature-induced explosive first-order electronic phase-transition in Gd-doped Sms. Phys. Rev. Lett. 1973, 31, 700–703. [Google Scholar] [CrossRef]
- Rogers, E.; Smet, P.F.; Dorenbos, P.; Poelman, D.; van der Kolk, E. The thermally induced metal-semiconducting phase transition of samarium monosulfide (SmS) thin films. J. Phys. Condens. Matter 2010, 22, 015005. [Google Scholar] [CrossRef] [PubMed]
- Maple, M.B.; Wohllebe, D. Nonmagnetic 4f shell in high pressure phase of Sms. Phys. Rev. Lett. 1971, 27, 511–515. [Google Scholar] [CrossRef]
- Ariponnammal, S.; Chandrasekaran, S. Theoretical study on the electrical properties of some semiconducting rare earth chalcogenides Sm1−xEuxS and Sm1−xYbxS under pressure. J. Nano- Electron. Phys. 2011, 3, 529–535. [Google Scholar]
- Menushenkov, A.P.; Chernikov, R.V.; Sidorov, V.V.; Klementiev, K.V.; Alekseev, P.A.; Rybina, A.V. Relationship between the local electronic and local crystal structures of intermediate-valence Sm1−xYxS. JETP Lett. 2006, 84, 119–123. [Google Scholar] [CrossRef]
- Pergament, A. Metal-insulator transition: The Mott criterion and coherence length. J. Phys. Condens. Matter 2003, 15, 3217–3223. [Google Scholar] [CrossRef]
- Pergament, A.; Kazakova, E.; Morak, A. The photoinduced Mott transition from metal to insulator: The problem of critical concentration. J. Phys. Condens. Matter 2005, 17, 1151–1156. [Google Scholar] [CrossRef]
- Kampfer, T. Die dynamik des laserinduzierten metall-halbleiter-phasenubergangs von samariumsulfid (SmS). Ph.D. Thesis, University of Jena, Jena, Germany, 2009. [Google Scholar]
- Barla, A.; Sanchez, J.P.; Haga, Y.; Lapertot, G.; Doyle, B.P.; Leupold, O.; Ruffer, R.; Abd-Elmeguid, M.M.; Lengsdorf, R.; Flouquet, J. Pressure-induced magnetic order in golden SmS. Phys. Rev. Lett. 2004, 92, 066401. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Xia, J.; Fisk, Z. Topological surface state in the Kondo insulator samarium hexaboride. Nat. Mater. 2014, 13, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Imura, K.; Matsubayashi, K.; Suzuki, H.S.; Deguchi, K.; Sato, N.K. Thermodynamic and transport properties of SmS under high pressure. Physica B 2009, 404, 3028–3031. [Google Scholar] [CrossRef]
- Jayaraman, A.; Maines, R.G. Study of the valence transition in Eu-, Yb-, and Ca-substituted SmS under high pressure and some comments on other substitutions. Phys. Rev. B 1979, 19, 4154–4161. [Google Scholar] [CrossRef]
- Deen, P.P.; Braithwaite, D.; Kernavanois, N.; Paolasini, L.; Raymond, S.; Barla, A.; Lapertot, G.; Sanchez, J.P. Structural and electronic transitions in the low-temperature, high-pressure phase of SmS. Phys. Rev. B 2005, 71, 245118. [Google Scholar] [CrossRef]
- Wang, L.; Marin, C.M.; Mei, W.-N.; Li Cheung, C. Electronic structures of lanthanum, samarium, and gadolinium sulfides. AIMS Mater. Sci. 2015, 2, 97–105. [Google Scholar] [CrossRef]
- Wachter, P. Handbook of the Physical and Chemistry of Rare Earths. In Handbook of the Physical and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 1993; Volume 19. [Google Scholar]
- Rogers, E.; Dorenbos, P.; Haas, J.T.M.D.; Kolk, E.V.d. Experimental study of the 4fn→4fn and 4fn→4fn−15d1 transitions of the lanthanide diiodides LnI2 (Ln = Nd, Sm, Eu, Dy, Tm, Yb). J. Phys. Condens. Matter 2012, 24, 275502. [Google Scholar] [CrossRef] [PubMed]
- Batlogg, B.; Kaldis, E.; Schlegel, A.; Wachter, P. Electronic structure of Sm monochalcogenides. Phys. Rev. B 1976, 14, 5503. [Google Scholar] [CrossRef]
- Van der Kolk, E.; Dorenbos, P. Systematic and material independent variation of electrical, optical, and chemical properties of Ln-materials over the Ln-series (Ln = La, Ce, Pr, ..., Lu). Chem. Mater. 2006, 18, 3458–3462. [Google Scholar] [CrossRef]
- Imura, K.; Hajiri, T.; Matsunami, M.; Kimura, S.; Kaneko, M.; Ito, T.; Nishi, Y.; Sato, N.K.; Suzuki, H.S. Angle-resolved photoemission spectroscopy on mixed-valent Sm1−xYxS. J. Korean Phys. Soc. 2013, 62, 2028–2031. [Google Scholar] [CrossRef]
- Binnemans, K.; Van Deun, R.; Görller-Walrand, C.; Adam, J.L. Spectroscopic properties of trivalent lanthanide ions in fluorophosphate glasses. J. Non-Cryst. Solids 1998, 238, 11–29. [Google Scholar] [CrossRef]
- Kimura, S.I.; Mizuno, T.; Matsubayashi, K.; Imura, K.; Suzuki, H.S.; Sato, N.K. Infrared study on the electronic structure of SmS in the black phase. Physica B 2008, 403, 805–807. [Google Scholar] [CrossRef]
- Van Deun, R.; Binnemans, K.; Gorller-Walrand, C.; Adam, J.L. Spectroscopic properties of trivalent samarium ions in glasses. Rare-Earth-Doped Mater. Devices III 1999, 3622, 175–181. [Google Scholar] [CrossRef]
- Matsubayashi, K.; Imura, K.; Suzuki, H.S.; Ban, S.; Chen, G.F.; Deguchi, K.; Sato, N.K. Magnetic properties of golden SmS. J. Magn. Magn. Mater. 2007, 310, 408–410. [Google Scholar] [CrossRef]
- Kikoin, K.A. Nature of the “golden” phase of samarium monosulfide. Zh Eksp Teor Fiz+ 1983, 85, 1000–1016. [Google Scholar]
- Shlimak, I.; Kaveh, M.; Ussyshkin, R.; Ginodman, V.; Resnick, L.; Gantmakher, V.F. Quantitative analysis of delocalization in the vicinity of the metal-insulator transition in doped semiconductors. J. Phys.Condens. Matter 1997, 9, 9873–9880. [Google Scholar] [CrossRef]
- Kirk, J.L.; Vedam, K.; Narayanamurti, V.; Jayaraman, A.E.B. Direct optical observation of the semiconductor to metal transition in SmS under pressure. Phys. Rev. B 1972, 6, 3023. [Google Scholar] [CrossRef]
- Mizuno, T.; Iizuka, T.; Kimura, S.; Matsubayashi, K.; Imura, K.; Suzuki, H.S.; Sato, N.K. Excitonic instability in the transition from the black phase to the golden phase of SmS under pressure investigated by infrared spectroscopy. J. Phys. Soc. Jpn. 2008, 77, 113704. [Google Scholar] [CrossRef]
- Hickey, C.F.; Gibson, U.J. Optical response of switching SmS in thin films prepared by reactive evaporation. J. Appl. Phys. 1987, 62, 3912. [Google Scholar] [CrossRef]
- Imura, K.; Saito, M.; Kaneko, M.; Ito, T.; Hajiri, T.; Matsunami, M.; Kimura, S.; Deguchi, K.; Suzuki, H.S.; Sato, N.K. Origin of the black-golden transition in Sm1−xYxS. J. Phys. Conf. Ser. 2015, 592. [Google Scholar] [CrossRef]
- Sharenkova, N.V.; Kaminskii, V.V.; Golubkov, A.V.; Romanova, M.V.; Stepanov, N.N. Mechanism of stabilization of the Sm1−xGdxS metallic modification at the pressure-induced semiconductor-metal phase transition. Phys. Solid State 2009, 51, 1700–1702. [Google Scholar] [CrossRef]
- Chaboy, J.; Garcia, J.; Marcelli, A. Correlation between mixed valence behavior of cerium and the magnetic and superconducting phenomena of CeFe2 and CeRu2. J. Magn. Magn. Mater. 1992, 104, 661–662. [Google Scholar] [CrossRef]
- Alekseev, P.A.; Chernikov, R.V.; Golubkov, A.V.; Klementiev, K.V.; Menushenkov, A.P.; Nemkovsky, K.S. XAFS spectroscopy of the mixed valent Sm1−xYxS. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2005, 543, 205–207. [Google Scholar] [CrossRef]
- Mori, Y.; Tanemura, S. Chemical analysis of semiconducting and metallic SmS thin films by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2007, 253, 3856–3859. [Google Scholar] [CrossRef]
- Mori, Y.; Tanemura, S.; Koide, S.; Senzaki, Y.; Jin, P.; Kaneko, K.; Terai, A.; Nabotova-Gabin, N. Thermo-coloration of SmS thin flims by XPS in situ observation. Appl. Surf. Sci. 2003, 212, 38–42. [Google Scholar] [CrossRef]
- De Tomasi, F.; Perrone, M.R.; Protopapa, M.L.; Leo, G. Laser irradiation effects on the resistance of SmS films. Thin Solid Films 2002, 413, 171–176. [Google Scholar] [CrossRef]
- Miodushevsky, P.; Protopapa, M.L.; De Tomasi, F.; Perrone, M.R.; Tundo, S.; Vasanelli, L. Fine trimming of SmS film resistance by XeCl laser ablation. Thin Solid Films 2000, 359, 251–254. [Google Scholar] [CrossRef]
- Imura, K.; Kanematsu, S.; Matsubayashi, K.; Suzuki, H.S.; Deguchi, K.; Sato, N.K. Discontinuous transition from a real bound state to virtual bound state in a mixed-valence state of SmS. J. Phys. Soc. Jpn. 2011, 80, 113704. [Google Scholar] [CrossRef]
- Kang, C.J.; Choi, H.C.; Kim, K.; Min, B.I. Topological properties and the dynamical crossover from mixed-valence to Kondo lattice behavior in the golden phase of SmS. Phys. Rev. Lett. 2015, 114, 166404. [Google Scholar] [CrossRef] [PubMed]
- Barla, A.; Sanchez, J.P.; Derr, J.; Salce, B.; Lapertot, G.; Flouquet, J.; Doyle, B.P.; Leupold, O.; Ruffer, R.; Abd-Elmeguid, M.M.; et al. Valence and magnetic instabilities in Sm compounds at high pressures. J. Phys. Condens. Matter 2005, 17, 837–848. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Porsch, F.; Holzapfel, W.B. Intermediate 4f bonding structure for samarium under pressure. Phys. Rev. B 1994, 50, 6603–6608. [Google Scholar] [CrossRef]
- Haga, Y.; Derr, J.; Barla, A.; Salce, B.; Lapertot, G.; Sheikin, I.; Matsubayashi, K.; Sato, N.K.; Flouquet, J. Pressure-induced magnetic phase transition in gold-phase SmS. Phys. Rev. B 2004, 70, 220406. [Google Scholar] [CrossRef]
- Takahashi, H.; Okazaki, R.; Taniguchi, H.; Terasaki, I.; Saito, M.; Imura, K.; Deguchi, K.; Sato, N.K.; Suzuki, H.S. Electrical oscillation in SmS induced by a constant external voltage. Phys. Rev. B 2014, 89, 195103. [Google Scholar] [CrossRef]
- Witczak-Krempa, W.; Chen, G.; Kim, Y.B.; Balents, L. Correlated Quantum Phenomena in the Strong Spin-Orbit Regime. Ann. Rev. Condens. Matter Phys. 2014, 5, 57–82. [Google Scholar] [CrossRef]
- Shukla, N.; Parihar, A.; Freeman, E.; Paik, H.; Stone, G.; Narayanan, V.; Wen, H.; Cai, Z.; Gopalan, V.; Engel-Herbert, R.; et al. Synchronized charge oscillations in correlated electron systems. Sci. Rep. 2014, 4, 4964. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Blaha, P.; Kioussis, N. Predicted topological phase transition in the SmS Kondo insulator under pressure. Phys. Rev. B 2014, 89, 121117. [Google Scholar] [CrossRef]
- Wachter, P.; Jung, A.; Steiner, P. Pressure-driven metal-insulator transition in La-doped SmS—Excitonic Condensation. Phys. Rev. B 1995, 51, 5542–5545. [Google Scholar] [CrossRef]
- Stern, A.; Dzero, M.; Galitski, V.M.; Fisk, Z.; Xia, J. Surface-dominated conduction up to 240 K in the Kondo insulator SmB6 under strain. Nat. Mater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E. Engineering the Electronic Structure of Lanthanide Based Materials. Ph.D. Thesis, TUDelft, Delft, The Netherland, 2012. [Google Scholar]
- Jin, P.; Tazawa, M.; Huang, J.F.; Tanemura, S. Growth of samarium monosulfide thin films by co-sputtering deposition. J. Cryst. Growth 1998, 191, 285–289. [Google Scholar] [CrossRef]
- Tanemura, S.; Koide, S.; Senzaki, Y.; Miao, L.; Hirai, H.; Mori, Y.; Jin, P.; Kaneko, K.; Terai, A.; Nabatova-Gabain, N. Fabrication and characterization of metal and semiconductor SmS thin films by rf/dc dual magnetron sputtering. Appl. Surf. Sci. 2003, 212, 279–286. [Google Scholar] [CrossRef]
- Zenkevich, A.V.; Parfenov, O.E.; Storchak, V.G.; Teterin, P.E.; Lebedinskii, Y.Y. Highly oriented metallic SmS films on Si(100) grown by pulsed laser deposition. Thin Solid Films 2011, 519, 6323–6325. [Google Scholar] [CrossRef]
- Volodin, N.M.; Zavyalova, L.V.; Kirillov, A.I.; Svechnikov, S.V.; Prokopenko, I.V.; Khanova, A.V. Investigation of growth conditions, crystal structure and surface morphology of SmS films fabricated by MOCVD technique. Semicond. Phys. Quantum Electron. Optoelectron. 1999, 2, 78–83. [Google Scholar]
- Suryanarayanan, R.; Brun, G. A compact multi-source multi-substrate evaporator for thin film studies of rare earth sulphides. Thin Solid Films 1976, 35, 263–271. [Google Scholar] [CrossRef]
- Huang, J.F.; Ma, X.B.; Cao, L.Y.; Wu, J.P.; He, H.Y. Electrodeposition of SmS optical thin films on ITO glass substrate. Mater. Lett. 2007, 61, 3920–3922. [Google Scholar] [CrossRef]
- Matsubayashi, K.; Suzuki, H.S.; Imura, K.; Nishioka, T.; Sato, N.K. Single crystal growth of “pure” SmS. Physica B 2005, 359, 151–153. [Google Scholar] [CrossRef]
- Kaminskii, V.V.; Solov’ev, S.M. Thermal effects in homogeneously heated samarium sulfide single crystals. Tech. Phys. Lett. 2005, 31, 603–604. [Google Scholar] [CrossRef]
- Imura, K.; Matsubayashi, K.; Suzuki, H.S.; Nishioka, T.; Mori, N.; Sato, N.K. Thermal expansion study on high-pressure phases of SmS. Physica B 2006, 378–380, 728–729. [Google Scholar] [CrossRef]
- Imura, K.; Matsubayashi, K.; Suzuki, H.S.; Deguchi, K.; Sato, N.K. Transport properties of golden SmS. Physica B 2008, 403, 895–897. [Google Scholar] [CrossRef]
- Volodin, N.M.; Mishin, Y.N.; Kaminskii, V.V.; Zakharov, Y.V. Samarium monosulfide-based semiconductor strain gages for spacecraft-strain transformation. Sol. Syst. Res. 2013, 47, 601–604. [Google Scholar] [CrossRef]
- Petrov, M.P.; Grachev, A.I.; Kukharskii, A.A.; Smirnov, I.A.; Shulman, S.G. Holographic storage in Sms thin films. Opt. Commun. 1977, 22, 293–296. [Google Scholar] [CrossRef]
- Welnic, W.; Pamungkas, A.; Detemple, R.; Steimer, C.; Blugel, S.; Wuttig, M. Unravelling the interplay of local structure and physical properties in phase-change materials. Nat. Mater. 2006, 5, 56–62. [Google Scholar] [CrossRef]
- Wuttig, M.; Raoux, S. The Science and Technology of Phase Change Materials. Z. Anorg. Allg. Chem. 2012, 638, 2455–2465. [Google Scholar] [CrossRef]
- Meena, J.S.; Sze, S.M.; Chand, U.; Tseng, T.Y. Overview of emerging nonvolatile memory technologies. Nanoscale Res. Lett. 2014, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Kaminskii, V.V.; Kazanin, M.M. Thermovoltaic effect in thin-film samarium-sulfide-based structures. Tech. Phys. Lett. 2008, 34, 361–362. [Google Scholar] [CrossRef]
- Kaminskii, V.V.; Didik, V.A.; Kazanin, M.M.; Romanova, M.V.; Solov’ev, S.M. Thermovoltaic effect in polycrystalline samarium sulfide. Tech. Phys. Lett. 2009, 35, 981–984. [Google Scholar] [CrossRef]
- Ohta, M.; Kuzuya, T.; Sasaki, H.; Kawasaki, T.; Hirai, S. Synthesis of multinary rare-earth sulfides PrGdS3, NdGdS3, and SmEuGdS4, and investigation of their thermoelectric properties. J. Alloys Compd. 2009, 484, 268–272. [Google Scholar] [CrossRef]
- Copel, M.; Kuroda, M.A.; Gordon, M.S.; Liu, X.H.; Mahajan, S.S.; Martyna, G.J.; Moumen, N.; Armstrong, C.; Rossnagel, S.M.; Shaw, T.M.; et al. Giant piezoresistive On/Off ratios in rare-earth chalcogenide thin films enabling nanomechanical switching. Nano Lett. 2013, 13, 4650–4653. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousanis, A.; Smet, P.F.; Poelman, D. Samarium Monosulfide (SmS): Reviewing Properties and Applications. Materials 2017, 10, 953. https://doi.org/10.3390/ma10080953
Sousanis A, Smet PF, Poelman D. Samarium Monosulfide (SmS): Reviewing Properties and Applications. Materials. 2017; 10(8):953. https://doi.org/10.3390/ma10080953
Chicago/Turabian StyleSousanis, Andreas, Philippe F. Smet, and Dirk Poelman. 2017. "Samarium Monosulfide (SmS): Reviewing Properties and Applications" Materials 10, no. 8: 953. https://doi.org/10.3390/ma10080953






