Smart Carriers and Nanohealers: A Nanomedical Insight on Natural Polymers
Abstract
:1. Introduction
2. Polysaccharides
2.1. Chitosan
2.1.1. Nanotechnology Applications of CS and Its Composites
2.1.2. CS Nanocomposites in Tissue Engineering and Regeneration
2.2. Carrageenan
2.2.1. Nanotechnology Applications of CG and Its Composites
2.2.2. CG Nanocomposites in Tissue Engineering and Regeneration
2.3. Alginates
2.3.1. Nanotechnology Applications of AG and Its Composites
2.3.2. AG Nanocomposites in Tissue Engineering and Regeneration
2.4. Starch
2.4.1. Nanotechnology Applications of Starch and Its Composites
2.4.2. Starch in Tissue Engineering and Regeneration
2.5. Cellulose
2.5.1. Nanotechnology Applications of Cellulose and Its Composites
2.5.2. Cellulose Nanoconstructs in Tissue Engineering and Regeneration
2.6. Heparin
2.6.1. Nanotechnology Applications of HP and Its Composites
2.6.2. HP Nanoconstructs in Tissue Engineering and Regeneration
2.7. Hyaluronic Acid
2.8. Chondroitin Sulfate
2.9. Microbial Polysaccharides
3. Proteins
3.1. Collagen
3.2. Gelatin
3.2.1. Nanotechnology Applications of Gelatin and its Composites
3.2.2. Gelatin Nanoconstructs in Tissue Engineering and Regeneration
3.3. Casein
3.3.1. Nanotechnology Applications of Casein and Its Composites
3.3.2. Casein Nanoconstruct in Tissue Engineering and Regeneration
3.4. Silk Fibroin
Silk Fibroin (SF) Nanoconstruct in Tissue Engineering and Regeneration
3.5. Keratin
Keratin in Tissue Engineering and Regeneration
3.6. Laminins
3.7. Fibronectin
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trindade, T.; Daniel-Da-Silva, A.L. Biofunctional composites of polysaccharides containing inorganic nanoparticles. In Advances in Nanocomposite Technology; Hashim, A., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Sushmitha, S.; Joydip, K.; Subhas, C.K. Biopolymeric nanoparticles. Sci. Technol. Adv. Mater. 2010, 11, 014104. [Google Scholar]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.M.; Opdenaker, L.M.; Flynn, D.; Sims-Mourtada, J. Wound healing and cancer stem cells: Inflammation as a driver of treatment resistance in breast cancer. Cancer Growth Metastasis 2015, 8, 1–13. [Google Scholar] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Anwunobi, A.P.; Emeje, M.O. Recent applications of natural polymers in nanodrug delivery. J. Nanomed. Nanotechnol. 2011, 4, 002. [Google Scholar] [CrossRef]
- Sasaki, T.; Takagi, J.; Giudici, C.; Yamada, Y.; Arikawa-Hirasawa, E.; Deutzmann, R.; Timpl, R.; Sonnenberg, A.; Bachinger, H.P.; Tonge, D. Laminin-121—Recombinant expression and interactions with integrins. Matrix Biol. 2010, 29, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Reddy, B.H.; Ashok Kumar, C.K.; Jayraj Kumar, K. Application of chitosan microsphere as drug carriers: A review. J. Pharm. Sci. Res. 2009, 1, 1–12. [Google Scholar]
- Chen, Y.; Mohanraj, V.J.; Parkin, J.E. Chitosan-dextran sulfate nanoparticles for delivery of an anti-angiogenesis peptide. Lett. Pept. Sci. 2003, 10, 621–629. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Bodmeier, R.; Chen, H.G.; Paeratakul, O. A novel approach to the oral delivery of micro- or nanoparticles. Pharm. Res. 1989, 6, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm. Res. 1997, 14, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Mao, H.Q.; Huang, S.K.; Leong, K.W. Oral gene delivery with chitosan—DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat. Med. 1999, 5, 387–391. [Google Scholar] [PubMed]
- Fernandez-Urrusuno, R.; Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Enhancement of nasal absorption of insulin using chitosan nanoparticles. Pharm. Res. 1999, 16, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Tokumitsu, H.; Hiratsuka, J.; Sakurai, Y.; Kobayashi, T.; Ichikawa, H.; Fukumori, Y. Gadolinium neutron-capture therapy using novel gadopentetic acid-chitosan complex nanoparticles: In vivo growth suppression of experimental melanoma solid tumor. Cancer Lett. 2000, 150, 177–182. [Google Scholar] [CrossRef]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.N.; Davis, S.S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 2001, 51, 81–96. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Kulkarni, A.R.; Aminabhavi, M. Crosslinked chitosan microspheres for encapsulation of diclofenac sodium: Effect of crosslinking agent. J. Microencapsul. 2002, 19, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Binulal, N.S.; Anitha, A.; Nair, S.V.; Furuike, T.; Tamura, H.; Jayakumar, R. Preparation of poly(lactic acid)/chitosan nanoparticles for anti-hiv drug delivery applications. Carbohydr. Polym. 2010, 80, 833–838. [Google Scholar] [CrossRef] [Green Version]
- Zhi, J.; Wang, Y.; Lu, Y.; Ma, J.; Luo, G. In situ preparation of magnetic chitosan/Fe3O4 composite nanoparticles in tiny pools of water-in-oil microemulsion. React. Funct. Polym. 2006, 66, 1552–1558. [Google Scholar] [CrossRef]
- Hall, L.D.; Yalpani, M. Formation of branched-chain, soluble polysaccharides from chitosan. J. Chem. Soc. Chem. Commun. 1980, 23, 1153–1154. [Google Scholar] [CrossRef]
- Yalpani, M.; Hall, L.D.; Tung, M.A.; Brooks, D.E. Unusual rheology of a branched, water-soluble chitosan derivative. Nature 1983, 302, 812–814. [Google Scholar] [CrossRef]
- Hawary, D.L.; Motaleb, M.A.; Farag, H.; Guirguis, O.W.; Elsabee, M.Z. Water-soluble derivatives of chitosan as a target delivery system of 99mtc to some organs in vivo for nuclear imaging and biodistribution. J. Radioanal. Nucl. Chem. 2011, 290, 557–567. [Google Scholar] [CrossRef]
- Wu, Q.X.; Lin, D.Q.; Yao, S.J. Design of chitosan and its water soluble derivatives-based drug carriers with polyelectrolyte complexes. Mar. Drugs 2014, 12, 6236–6253. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ikram, S. Chitosan based scaffolds and their applications in wound healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef]
- Sarvaiya, J.; Agrawal, Y.K. Chitosan as a suitable nanocarrier material for anti-alzheimer drug delivery. Int. J. Biol. Macromol. 2015, 72, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Wu, S.J.; Mi, F.L.; Chiu, Y.L.; Yu, S.H.; Panda, N.; Sung, H.W. Thiol-modified chitosan sulfate nanoparticles for protection and release of basic fibroblast growth factor. Bioconjug. Chem. 2010, 21, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, L.; Zeng, R.; Li, C.; Qiao, R.; Hu, L.; Li, Z. Synthesis, nanosizing and in vitro drug release of a novel anti-HIV polymeric prodrug: Chitosan-o-isopropyl-5′-o-d4t monophosphate conjugate. Bioorg. Med. Chem. 2010, 18, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chang, S.-W.; Chen, D.-H. Magnetic chitosan nanoparticles: Studies on chitosan binding and adsorption of co(II) ions. React. Funct. Polym. 2006, 66, 335–341. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, D.H. Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of cu(ii) ions. J. Colloid Interface Sci. 2005, 283, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Sargin, I.; Arslan, G. Chitosan/sporopollenin microcapsules: Preparation, characterisation and application in heavy metal removal. Int. J. Biol. Macromol. 2015, 75, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, H.; Sun, Y.; Qiu, L.; Zhang, J.; Guan, G.; Zhao, X.; Qiao, M.; Cheng, L.; Cheng, L.; et al. A pH-sensitive gene delivery system based on folic acid-PEG-chitosan—PAMAM-plasmid DNA complexes for cancer cell targeting. Biomaterials 2013, 34, 10120–10132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Ma, D.; Tang, H.; Tan, L.; Xie, Q.; Yao, S. Biocompatible multi-walled carbon nanotube-chitosan-folic acid nanoparticle hybrids as gfp gene delivery materials. Colloids Surf. B Biointerfaces 2013, 111, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Khan, T.H.; Sultana, N. Fabrication and in vitro evaluation of nanosized hydroxyapatite/chitosan-based tissue engineering scaffolds. J. Nanomater. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Kuo, S.M.; Chang, S.J.; Cheng-Chie Niu, G.; Lan, C.-W.; Cheng, W.T.; Yang, C.Z. Guided tissue regeneration with use of β-TCP/chitosan composite membrane. J. Appl. Polym. Sci. 2009, 112, 3127–3134. [Google Scholar] [CrossRef]
- Ahmadi, R.; Burns, A.J.; de Bruijn, J.D. Chitosan-based hydrogels do not induce angiogenesis. J. Tissue Eng. Regen. Med. 2010, 4, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, P.; Vulesevic, B.; Kuraitis, D.; Li, F.; Yang, A.F.; Griffith, M.; Ruel, M.; Suuronen, E.J. A collagen-chitosan hydrogel for endothelial differentiation and angiogenesis. Tissue Eng. Part A 2010, 16, 3099–3109. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.K.; Kim, K.Y.; Yoo, Y.J.; Oh, S.J.; Choi, J.H.; Kim, C.Y. In vitro antimicrobial activity of a chitooligosaccharide mixture against actinobacillus actinomycetemcomitans and streptococcus mutans. Int. J. Antimicrob. Agents 2001, 18, 553–557. [Google Scholar] [CrossRef]
- Jiang, F.; Deng, Y.; Yeh, C.K.; Sun, Y. Quaternized chitosans bind onto preexisting biofilms and eradicate pre-attached microorganisms. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 8518–8527. [Google Scholar] [CrossRef] [PubMed]
- Mei, N.; Xuguang, L.; Jinming, D.; Husheng, J.; Liqiao, W.; Bingshe, X. Antibacterial activity of chitosan coated Ag-loaded nano-SiO2 composites. Carbohydr. Polym. 2009, 78, 54–59. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, Y.; Yang, J.; Wang, X.; Shi, X.; Hu, Y. Preparation, characterization and antimicrobial activity of chitosan/layered silicate nanocomposites. Polymer 2006, 47, 6738–6744. [Google Scholar] [CrossRef]
- Han, Y.-S.; Lee, S.-H.; Choi, K.H.; Park, I. Preparation and characterization of chitosan–clay nanocomposites with antimicrobial activity. J. Phys. Chem. Solids 2010, 71, 464–467. [Google Scholar] [CrossRef]
- Sall, K.N.; Kreter, J.K.; Keates, R.H. The effect of chitosan on corneal wound healing. Ann. Ophthalmol. 1987, 19, 31–33. [Google Scholar] [PubMed]
- Cui, R.; Lu, Q.; Teng, Y.; Li, K.; Li, N. Chitosan promoted the corneal epithelial wound healing via activation of ERK pathway. Curr. Eye Res. 2017, 42, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Fischak, C.; Klaus, R.; Werkmeister, R.M.; Hohenadl, C.; Prinz, M.; Schmetterer, L.; Garhofer, G. Effect of topically administered chitosan-N-acetylcysteine on corneal wound healing in a rabbit model. J. Ophthalmol. 2017, 2017, 5192924. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar]
- Raveendran, S.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Pharmaceutically versatile sulfated polysaccharide based bionano platforms. Nanomedicine 2013, 9, 605–626. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.A.; Quemener, B. Measurement of Carrageenans in Food: Challenges, Progress, and Trends in Analysis. Trends Food Sci. Technol. 1999, 10, 169–181. [Google Scholar] [CrossRef]
- Jones, F.; Colfen, H.; Antonietti, M. Interaction of kappa-carrageenan with nickel, cobalt, and iron hydroxides. Biomacromolecules 2000, 1, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.G.; Dong, L.C.; Song, Y.Q. Nanosizing of a drug/carrageenan complex to increase solubility and dissolution rate. Int. J. Pharm. 2007, 342, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, C.; Margaritis, A.; Xenocostas, A. Encapsulation and controlled release of recombinant human erythropoietin from chitosan-carrageenan nanoparticles. Curr. Drug Deliv. 2012, 9, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Grassauer, A.; Weinmuellner, R.; Meier, C.; Pretsch, A.; Prieschl-Grassauer, E.; Unger, H. Iota-carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.; Guptill-Yoran, L.; Moore, G.E.; Pogranichniy, R.M. Effects of lambda-carrageenan on in vitro replication of feline herpesvirus and on experimentally induced herpetic conjunctivitis in cats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- McGowan, I. Microbicides: A new frontier in hiv prevention. Biologicals 2006, 34, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Girod, S.; Boissière, M.; Longchambon, K.; Begu, S.; Tourne-Pétheil, C.; Devoisselle, J.M. Polyelectrolyte complex formation between iota-carrageenan and poly (l-lysine) in dilute aqueous solutions: A spectroscopic and conformational study. Carbohydr. Polym. 2004, 55, 37–45. [Google Scholar] [CrossRef]
- Daniel-da-Silva, A.L.; Trindade, T.; Goodfellow, B.J.; Costa, B.F.; Correia, R.N.; Gil, A.M. In situ synthesis of magnetite nanoparticles in carrageenan gels. Biomacromolecules 2007, 8, 2350–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjoberg, H.; Persson, S.; Caram-Lelham, N. How interactions between drugs and agarose-carrageenan hydrogels influence the simultaneous transport of drugs. J. Control. Release 1999, 59, 391–400. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Barzegar, S.; Zeidabadi, F. Synthesis and properties of biodegradable hydrogels of κ-carrageenan grafted acrylic acid-co-2-acrylamido-2-methylpropanesulfonic acid as candidates for drug delivery systems. React. Funct. Polym. 2007, 67, 644–654. [Google Scholar] [CrossRef]
- Siepmann, F.; Muschert, S.; Zach, S.; Leclercq, B.; Carlin, B.; Siepmann, J. Carrageenan as an efficient drug release modifier for ethylcellulose-coated pharmaceutical dosage forms. Biomacromolecules 2007, 8, 3984–3991. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.Y.; Wei, G.; Lu, W.Y. Effect of carrageenan on poloxamer-based in situ gel for vaginal use: Improved in vitro and in vivo sustained-release properties. Eur. J. Pharm. Sci. 2009, 37, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.L.; Feng, Q.L. Preparation and characterization of a new injectable bone substitute-carrageenan/nano-hydroxyapatite/collagen. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2006, 28, 710–713. [Google Scholar] [PubMed]
- Thakur, A.; Jaiswal, M.K.; Peak, C.W.; Carrow, J.K.; Gentry, J.; Dolatshahi-Pirouz, A.; Gaharwar, A.K. Injectable shear-thinning nanoengineered hydrogels for stem cell delivery. Nanoscale 2016, 8, 12362–12372. [Google Scholar] [CrossRef] [PubMed]
- Daniel-da-Silva, A.L.; Ferreira, L.; Gil, A.M.; Trindade, T. Synthesis and swelling behavior of temperature responsive kappa-carrageenan nanogels. J. Colloid Interface Sci. 2011, 355, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Pawar, H.V.; Tetteh, J. Polyox and carrageenan based composite film dressing containing anti-microbial and anti-inflammatory drugs for effective wound healing. Int. J. Pharm. 2013, 441, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Yeh, L.K.; Chen, Y.H.; Chiu, C.S.; Hu, F.R.; Young, T.H.; Wang, I.J. The phenotype of bovine corneal epithelial cells on chitosan membrane. J. Biomed. Mater. Res. A 2009, 90, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.L.; Hsu, C.C.; Hung, K.H.; Chang, C.W.; Cheng, Y.H. Applications of biomaterials in corneal wound healing. J. Chin. Med. Assoc. 2015, 78, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.; Cave, R.A.; Cook, J.P.; Khutoryanskiy, V.V.; Mi, S.; Chen, B.; Leyland, M.; Connon, C.J. Enhanced viability of corneal epithelial cells for efficient transport/storage using a structurally modified calcium alginate hydrogel. Regen. Med. 2012, 7, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.; Mi, S.; Connon, C.J. Towards the use of hydrogels in the treatment of limbal stem cell deficiency. Drug Discov. Today 2013, 18, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Tummala, G.K.; Joffre, T.; Lopes, V.R.; Liszka, A.; Buznyk, O.; Ferraz, N.; Persson, C.; Griffith, M.; Mihranyan, A. Hyperelastic nanocellulose-reinforced hydrogel of high water content for ophthalmic applications. ACS Biomater. Sci. Eng. 2016, 2, 2072–2079. [Google Scholar] [CrossRef]
- Wang, J.; Gao, C.; Zhang, Y.; Wan, Y. Preparation and in vitro characterization of BC/PVA hydrogel composite for its potential use as artificial cornea biomaterial. Mater. Sci. Eng. C 2010, 30, 214–218. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Tonsomboon, K.; Oyen, M.L. Composite electrospun gelatin fiber-alginate gel scaffolds for mechanically robust tissue engineered cornea. J. Mech. Behav. Biomed. Mater. 2013, 21, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Qiang, L.; Gao, Y.; Cui, X.; Zhou, H.; Zhong, S.; Wang, Q.; Wang, H. Effect of fiber alignment in electrospun scaffolds on keratocytes and corneal epithelial cells behavior. J. Biomed. Mater. Res. A 2012, 100, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wen, J.; Yan, J.; Kao, Y.; Ni, Z.; Cui, X.; Wang, H. In situ growth induction of the corneal stroma cells using uniaxially aligned composite fibrous scaffolds. RSC Adv. 2015, 5, 12123–12130. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Biazar, E.; Heidari-Keshel, S. Cellular response of limbal stem cells on phbv/gelatin nanofibrous scaffold for ocular epithelial regeneration. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 879–887. [Google Scholar] [CrossRef]
- Hazra, S.; Nandi, S.; Naskar, D.; Guha, R.; Chowdhury, S.; Pradhan, N.; Kundu, S.C.; Konar, A. Non-mulberry silk fibroin biomaterial for corneal regeneration. Sci. Rep. 2016, 6, 21840. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Pongjanyakul, T.; Rongthong, T. Enhanced entrapment efficiency and modulated drug release of alginate beads loaded with drug–clay intercalated complexes as microreservoirs. Carbohydr. Polym. 2010, 81, 409–419. [Google Scholar] [CrossRef]
- Sinha, V.R.; Kumria, R. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar] [CrossRef]
- Murata, Y.; Miyamoto, E.; Kawashima, S. Additive effect of chondroitin sulfate and chitosan on drug release from calcium-induced alginate gel beads. J. Control. Release 1996, 38, 101–108. [Google Scholar] [CrossRef]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. Ph-sensitive sodium alginate/poly(vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze-thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Lim, S.M.; Han, D.K.; Yuk, S.H.; Im, G.I.; Lee, J.H. Time-dependent alginate/polyvinyl alcohol hydrogels as injectable cell carriers. J. Biomater. Sci. Polym. Ed. 2009, 20, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Hsueh, H.J.; Jiang, Y.L. Light-addressable electrodeposition of cell-encapsulated alginate hydrogels for a cellular microarray using a digital micromirror device. Biomicrofluidics 2011, 5, 34109. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, S.J.; Leung, M.; Li, Z.; Huang, J.I.; Hopper, R.A.; Zhang, M. Evaluation of three-dimensional porous chitosan-alginate scaffolds in rat calvarial defects for bone regeneration applications. J. Biomed. Mater. Res. A 2013, 101, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.R.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Gilmartin, D.J.; Alexaline, M.M.; Thrasivoulou, C.; Phillips, A.R.; Jayasinghe, S.N.; Becker, D.L. Integration of scaffolds into full-thickness skin wounds: The connexin response. Adv. Healthc. Mater. 2013, 2, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Madaghiele, M.; Demitri, C.; Sannino, A.; Ambrosio, L. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burns Trauma 2014, 2, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Coskun, G.; Karaca, E.; Ozyurtlu, M.; Ozbek, S.; Yermezler, A.; Cavusoglu, I. Histological evaluation of wound healing performance of electrospun poly(vinyl alcohol)/sodium alginate as wound dressing in vivo. Biomed. Mater. Eng. 2014, 24, 1527–1536. [Google Scholar] [PubMed]
- Kataria, K.; Gupta, A.; Rath, G.; Mathur, R.B.; Dhakate, S.R. In vivo wound healing performance of drug loaded electrospun composite nanofibers transdermal patch. Int. J. Pharm. 2014, 469, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, W.; Han, B.; Yang, C.; Ma, Q.; Song, F.; Bi, Q. An in situ formed biodegradable hydrogel for reconstruction of the corneal endothelium. Colloids Surf. B Biointerfaces 2011, 82, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cyprych, K.; Sznitko, L.; Mysliwiec, J. Starch: Application of biopolymer in random lasing. Org. Electron. 2014, 15, 2218–2222. [Google Scholar] [CrossRef]
- Bel Haaj, S.; Magnin, A.; Petrier, C.; Boufi, S. Starch nanoparticles formation via high power ultrasonication. Carbohydr. Polym. 2013, 92, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Luo, Z.; Fu, X. Preparation and characterization of starch nanoparticles in ionic liquid-in-oil microemulsions system. Ind. Crops Prod. 2014, 52, 105–110. [Google Scholar] [CrossRef]
- Shi, A.M.; Wang, L.J.; Li, D.; Adhikari, B. Characterization of starch films containing starch nanoparticles: Part 1: Physical and mechanical properties. Carbohydr. Polym. 2013, 96, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Qiu, C.; Xiong, L.; Sun, Q. Characterisation of corn starch-based films reinforced with taro starch nanoparticles. Food Chem. 2015, 174, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.S.; Grossmann, M.V.E.; Shirai, M.A.; Lazaretti, M.M.; Yamashita, F.; Muller, C.M.O.; Mali, S. Improving action of citric acid as compatibiliser in starch/polyester blown films. Ind. Crops Prod. 2014, 52, 305–312. [Google Scholar] [CrossRef]
- Dufresne, A. Crystalline starch based nanoparticles. Curr. Opin. Colloid Interface Sci. 2014, 19, 397–408. [Google Scholar] [CrossRef]
- Rutkaite, R.; Bendoraitiene, J.; Klimaviciute, R.; Zemaitaitis, A. Cationic starch nanoparticles based on polyelectrolyte complexes. Int. J. Biol. Macromol. 2012, 50, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, N.; Nachane, R.P.; Balasubramanya, R.H.; Varadarajan, P.V. A novel one-pot ‘green’ synthesis of stable silver nanoparticles using soluble starch. Carbohydr. Res. 2006, 341, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-Z.; Wang, L.-J.; Li, D.; Adhikari, B.; Mao, Z.-H. Preparation and characterization of crosslinked starch microspheres using a two-stage water-in-water emulsion method. Carbohydr. Polym. 2012, 88, 912–916. [Google Scholar] [CrossRef]
- Fang, Y.-Y.; Wang, L.-J.; Li, D.; Li, B.-Z.; Bhandari, B.; Chen, X.D.; Mao, Z.-H. Preparation of crosslinked starch microspheres and their drug loading and releasing properties. Carbohydr. Polym. 2008, 74, 379–384. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Gao, C.; Liu, M.; Zhang, X. Fabrication and evaluation of the novel reduction-sensitive starch nanoparticles for controlled drug release. Colloids Surf. B Biointerfaces 2014, 115, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Tan, Y.; Xu, K.; Lu, C.; Wang, P. In situ hydrogel constructed by starch-based nanoparticles via a schiff base reaction. Carbohydr. Polym. 2014, 110, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Chung, S.; Pedro, A.J.; Sousa, R.A.; Marques, A.P.; Reis, R.L.; Neves, N.M. Hierarchical starch-based fibrous scaffold for bone tissue engineering applications. J. Tissue Eng. Regen. Med. 2009, 3, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, M.E.; Godinho, J.S.; Tchalamov, D.; Cunha, A.M.; Reis, R.L. Alternative tissue engineering scaffolds based on starch: Processing methodologies, morphology, degradation and mechanical properties. Mater. Sci. Eng. C 2002, 20, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Kuniak, L.; Marchessault, R.H. Study of the crosslinking reaction between epichlorohydrin and starch. Starch Stärke 1972, 24, 110–116. [Google Scholar] [CrossRef]
- Lamme, E.N.; Gustafsson, T.O.; Middelkoop, E. Cadexomer-iodine ointment shows stimulation of epidermal regeneration in experimental full-thickness wounds. Arch. Dermatol. Res. 1998, 290, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Starch-based biomaterials for wound-dressing applications. Starch Stärke 2013, 65, 543–551. [Google Scholar] [CrossRef]
- Nourmohammadi, J.; Ghaee, A.; Liavali, S.H. Preparation and characterization of bioactive composite scaffolds from polycaprolactone nanofibers-chitosan-oxidized starch for bone regeneration. Carbohydr. Polym. 2016, 138, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Iwata, H.; Ikada, Y. A tissue adhesives evaluated in vitro and in vivo analysis. J. Biomed. Mater. Res. A 2010, 94, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Nairetti, D.; Mironescu, M.; Tita, O. Antimicrobial activity of active biodegradable starch films on pathogenic microorganisms. Ann. RSCB 2014, 19, 73–78. [Google Scholar]
- Mehdizadeh, T.; Tajik, H.; Razavi Rohani, S.M.; Oromiehie, A.R. Antibacterial, antioxidant and optical properties of edible starch-chitosan composite film containing thymus kotschyanus essential oil. Vet. Res. Forum 2012, 3, 167–173. [Google Scholar] [PubMed]
- Villanueva, M.E.; Diez, A.M.R.; González, J.A.; Pérez, C.J.; Orrego, M.; Piehl, L.; Teves, S.; Copello, G.J. Antimicrobial activity of starch hydrogel incorporated with copper nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 16280–16288. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.-R.S. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Liu, G.; Gu, Z.; Hong, Y.; Cheng, L.; Li, C. Electrospun starch nanofibers: Recent advances, challenges, and strategies for potential pharmaceutical applications. J. Control. Release 2017, 252, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhang, J.; Jiang, A.; Lv, W.; Wang, Y.; Geng, H.; Wang, J.; Qin, M. Fabrication of cellulose self-assemblies and high-strength ordered cellulose films. Carbohydr. Polym. 2015, 117, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Nguyen, S.T.; Fan, Z.; Duong, H.M. Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chem. Eng. J. 2015, 270, 168–175. [Google Scholar] [CrossRef]
- Tehrani, A.D.; Basiryan, A. Dendronization of cellulose nanowhisker with cationic hyperbranched dendritic polyamidoamine. Carbohydr. Polym. 2015, 120, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Devarayan, K.; Kim, H.Y.; Kim, B.S. Facile fabrication of hierarchical cellulose nanospicules via hydrolytic hydrogenation. Carbohydr. Polym. 2015, 117, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Novotna, K.; Parizek, M. Polysaccharides as cell carriers for tissue engineering: The use of cellulose in vascular wall reconstruction. Physiol. Res. 2014, 63, S29–S47. [Google Scholar] [PubMed]
- Bhattacharya, M.; Malinen, M.M.; Lauren, P.; Lou, Y.R.; Kuisma, S.W.; Kanninen, L.; Lille, M.; Corlu, A.; GuGuen-Guillouzo, C.; Ikkala, O.; et al. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 2012, 164, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Poirier, J.-M.; Girandon, L.; Frohlich, M.; Oksman, K.; Mathew, A.P. 3-dimensional porous nanocomposite scaffolds based on cellulose nanofibers for cartilage tissue engineering: Tailoring of porosity and mechanical performance. RSC Adv. 2016, 6, 5999–6007. [Google Scholar] [CrossRef]
- Kang, B.S.; Na, Y.C.; Jin, Y.W. Comparison of the wound healing effect of cellulose and gelatin: An in vivo study. Arch. Plast. Surg. 2012, 39, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Makitie, A.; Luukko, K.; et al. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release 2016, 244, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.; Watt, P.W.; Lundqvist, C.; Silcock, D.; Schmidt, R.J.; Bogan, D.; Light, N.D. The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int. J. Biochem. Cell Biol. 2002, 34, 1544–1556. [Google Scholar] [CrossRef]
- Liu, X.; Lin, T.; Gao, Y.; Xu, Z.; Huang, C.; Yao, G.; Jiang, L.; Tang, Y.; Wang, X. Antimicrobial electrospun nanofibers of cellulose acetate and polyester urethane composite for wound dressing. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Boehnke, M. Influences of methylcellulose on corneal epithelial wound healing. J. Ocul. Pharmacol. Ther. 1999, 15, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Garrett, Q.; Simmons, P.A.; Xu, S.; Vehige, J.; Zhao, Z.; Ehrmann, K.; Willcox, M. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.M.; Linhardt, R.J. Heparin-based nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, H.; Liu, J.; Zhai, G. Advanced nanocarriers based on heparin and its derivatives for cancer management. Biomacromolecules 2015, 16, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Passirani, C.; Barratt, G.; Devissaguet, J.P.; Labarre, D. Long-circulating nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate). Pharm. Res. 1998, 15, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Debergh, I.; Van Damme, N.; Pattyn, P.; Peeters, M.; Ceelen, W.P. The low-molecular-weight heparin, nadroparin, inhibits tumour angiogenesis in a rodent dorsal skinfold chamber model. Br. J. Cancer 2010, 102, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Hoffart, V.; Lamprecht, A.; Maincent, P.; Lecompte, T.; Vigneron, C.; Ubrich, N. Oral bioavailability of a low molecular weight heparin using a polymeric delivery system. J. Control. Release 2006, 113, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Ubrich, N.; Marchand-Arvier, M.; Vigneron, C.; Hoffman, M.; Lecompte, T.; Maincent, P. In vitro and in vivo evaluation of oral heparin-loaded polymeric nanoparticles in rabbits. Circulation 2002, 105, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nakamura, S.; Kishimoto, S.; Kawakami, M.; Suzuki, S.; Matsui, T.; Ishihara, M. Preparation and characterization of low-molecular-weight heparin/protamine nanoparticles (LMW-H/P NPs) as FGF-2 carrier. Int. J. Nanomed. 2010, 5, 147–155. [Google Scholar] [CrossRef]
- Park, I.-K.; Kim, Y.J.; Tran, T.H.; Huh, K.M.; Lee, Y. Water-soluble heparin–PTX conjugates for cancer targeting. Polymer 2010, 51, 3387–3393. [Google Scholar] [CrossRef]
- Hou, L.; Yao, J.; Zhou, J.; Zhang, Q. Pharmacokinetics of a paclitaxel-loaded low molecular weight heparin-all-trans-retinoid acid conjugate ternary nanoparticulate drug delivery system. Biomaterials 2012, 33, 5431–5440. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kim, J.K.; Huh, K.M.; Lee, Y.-k.; Kim, S.Y. Targeted delivery of paclitaxel using folate-conjugated heparin-poly(β-benzyl-l-aspartate) self-assembled nanoparticles. Carbohydr. Polym. 2012, 87, 2120–2128. [Google Scholar] [CrossRef]
- Liang, P.; Zhao, D.; Wang, C.Q.; Zong, J.Y.; Zhuo, R.X.; Cheng, S.X. Facile preparation of heparin/CaCO3/CaP hybrid nano-carriers with controllable size for anticancer drug delivery. Colloids Surf. B Biointerfaces 2013, 102, 783–788. [Google Scholar] [CrossRef] [PubMed]
- She, W.; Li, N.; Luo, K.; Guo, C.; Wang, G.; Geng, Y.; Gu, Z. Dendronized heparin-doxorubicin conjugate based nanoparticle as pH-responsive drug delivery system for cancer therapy. Biomaterials 2013, 34, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Nurunnabi, M.; Nafiujjaman, M.; Lee, Y.K.; Huh, K.M. GSH-mediated photoactivity of pheophorbide a-conjugated heparin/gold nanoparticle for photodynamic therapy. J. Control. Release 2013, 171, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Ting, S.R.; Whitelock, J.M.; Tomic, R.; Gunawan, C.; Teoh, W.Y.; Amal, R.; Lord, M.S. Cellular uptake and activity of heparin functionalised cerium oxide nanoparticles in monocytes. Biomaterials 2013, 34, 4377–4386. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Kim, S.; Park, K.; Kim, K.; Woo, D.G.; Kwon, I.C.; Chung, H.M.; Park, K.H. Heparin/poly (l-lysine) nanoparticle-coated polymeric microspheres for stem-cell therapy. J. Am. Chem. Soc. 2007, 129, 5788–5789. [Google Scholar] [CrossRef] [PubMed]
- Sakiyama-Elbert, S.E. Incorporation of heparin into biomaterials. Acta Biomater. 2014, 10, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kiick, K.L. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014, 10, 1588–1600. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Mammadov, R.; Mammadov, B.; Toksoz, S.; Aydin, B.; Yagci, R.; Tekinay, A.B.; Guler, M.O. Heparin mimetic peptide nanofibers promote angiogenesis. Biomacromolecules 2011, 12, 3508–3519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senturk, B.; Mercan, S.; Delibasi, T.; Guler, M.O.; Tekinay, A.B. Angiogenic peptide nanofibers improve wound healing in STZ-induced diabetic rats. ACS Biomater. Sci. Eng. 2016, 2, 1180–1189. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef] [PubMed]
- Kurpinski, K.T.; Stephenson, J.T.; Janairo, R.R.; Lee, H.; Li, S. The effect of fiber alignment and heparin coating on cell infiltration into nanofibrous PLLA scaffolds. Biomaterials 2010, 31, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, Y.; Fang, Z.; Xiao, R.; Yuan, W.; Khan, M. Fabrication and characterization of electrospun gelatin-heparin nanofibers as vascular tissue engineering. Macromol. Res. 2013, 21, 860–869. [Google Scholar] [CrossRef]
- Jiao, Y.; Pang, X.; Zhai, G. Advances in hyaluronic acid-based drug delivery systems. Curr. Drug Targets 2016, 17, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Tripodo, G.; Trapani, A.; Torre, M.L.; Giammona, G.; Trapani, G.; Mandracchia, D. Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. Eur. J. Pharm. Biopharm. 2015, 97, 400–416. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Mishra, P.; Agrawal, G.P. An insight on hyaluronic acid in drug targeting and drug delivery. J. Drug Target 2008, 16, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Aya, K.L.; Stern, R. Hyaluronan in wound healing: Rediscovering a major player. Wound Repair Regen. 2014, 22, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Uppal, R.; Ramaswamy, G.N.; Arnold, C.; Goodband, R.; Wang, Y. Hyaluronic acid nanofiber wound dressing—Production, characterization, and in vivo behavior. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Tan, Y.; Xu, T.; Yin, D.; Wang, M.; Shen, M.; Chen, X.; Shi, X.; Zhu, X. Hyaluronic acid-functionalized electrospun PLGA nanofibers embedded in a microfluidic chip for cancer cell capture and culture. Biomater. Sci. 2017, 5, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Robertson, D.D.; van Reenen, A.J.; Dicks, L.M. Polyethylene oxide (PEO)-hyaluronic acid (HA) nanofibers with kanamycin inhibits the growth of listeria monocytogenes. Biomed. Pharmacother. 2017, 86, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Figueira, D.R.; Miguel, S.P.; de Sa, K.D.; Correia, I.J. Production and characterization of polycaprolactone- hyaluronic acid/chitosan- zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Meng, J.; Ezoulin, M.J.; Youm, I.; Dim, D.C.; Molteni, A.; Hung, W.T.; Christenson, L.K.; Youan, B.C. Stimuli-sensitive thiolated hyaluronic acid based nanofibers: Synthesis, preclinical safety and in vitro anti-hiv activity. Nanomedicine 2016, 11, 2935–2958. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lu, Q.; Sommerfeld, S.D.; Chan, A.; Menon, N.G.; Schmidt, T.A.; Elisseeff, J.H.; Singh, A. Targeted delivery of hyaluronic acid to the ocular surface by a polymer-peptide conjugate system for dry eye disease. Acta Biomater. 2017, 55, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.T.; Chiang, T.H.; Chang, S.W.; Chen, Y.H.; Hu, F.R.; Wang, I.J. Enhanced corneal wound healing with hyaluronic acid and high-potassium artificial tears. Clin. Exp. Optom. 2013, 96, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Haylock-Jacobs, S.; Keough, M.B.; Lau, L.; Yong, V.W. Chondroitin sulphate proteoglycans: Extracellular matrix proteins that regulate immunity of the central nervous system. Autoimmun. Rev. 2011, 10, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.H.; Foong, W.C.; Cao, T.; Bay, B.H.; Ouyang, H.W.; Yip, G.W. Chondroitin sulfate in palatal wound healing. J. Dent. Res. 2004, 83, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.E.; Kirker, K.R.; Gray, S.D.; Ward, P.D.; Szakacs, J.G.; Prestwich, G.D.; Orlandi, R.R. Chondroitin sulfate hydrogel and wound healing in rabbit maxillary sinus mucosa. Laryngoscope 2004, 114, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Coburn, J.M.; Gibson, M.; Monagle, S.; Patterson, Z.; Elisseeff, J.H. Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proc. Natl. Acad. Sci. USA 2012, 109, 10012–10017. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, H.; Akram, M.Y.; Mu, X.; Nie, J.; Ma, G. Characterization and application of chondroitin sulfate/polyvinyl alcohol nanofibres prepared by electrospinning. Carbohydr. Polym. 2016, 143, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Tran, K.; Chang, W.; Shelke, N.B.; Kumbar, S.G.; Yu, X. Influence of chondroitin sulfate and hyaluronic acid presence in nanofibers and its alignment on the bone marrow stromal cells: Cartilage regeneration. J. Biomed. Nanotechnol. 2014, 10, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, T.A.; Ibrahim, N.J.; Warsi, M.H. Chondroitin sulfate-chitosan nanoparticles for ocular delivery of bromfenac sodium: Improved permeation, retention, and penetration. Int. J. Pharm. Investig. 2016, 6, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Nakayasu, K.; Kanai, A. Corneal wound healing: Immunohistological features of extracellular matrix following penetrating keratoplasty in rabbits. Jpn. J. Ophthalmol. 2000, 44, 334–341. [Google Scholar] [CrossRef]
- Lai, J.Y.; Li, Y.T.; Cho, C.H.; Yu, T.C. Nanoscale modification of porous gelatin scaffolds with chondroitin sulfate for corneal stromal tissue engineering. Int. J. Nanomed. 2012, 7, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Mourao, P.A. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Amornru, C.; Toshihiko, T.; Robert, J.L. Structure and bioactivity of sulfated polysaccharides. Trends Glycosci. Glycotechnol. 2003, 15, 29–46. [Google Scholar]
- Reddy, C.S.K.; Ghai, R.; Rashmi; Kalia, V.C. Polyhydroxyalkanoates: An overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Lopez, J.; Kenny, J.M. Bionanocomposite films based on plasticized PLA-PHB/cellulose nanocrystal blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Michalak, M.; Marek, A.A.; Zawadiak, J.; Kawalec, M.; Kurcok, P. Synthesis of phb-based carrier for drug delivery systems with pH-controlled release. Eur. Polym. J. 2013, 49, 4149–4156. [Google Scholar] [CrossRef]
- Rodríguez-Contreras, A.; Canal, C.; Calafell-Monfort, M.; Ginebra, M.-P.; Julio-Moran, G.; Marqués-Calvo, M.-S. Methods for the preparation of doxycycline-loaded phb micro- and nano-spheres. Eur. Polym. J. 2013, 49, 3501–3511. [Google Scholar] [CrossRef]
- Ramier, J.; Bouderlique, T.; Stoilova, O.; Manolova, N.; Rashkov, I.; Langlois, V.; Renard, E.; Albanese, P.; Grande, D. Biocomposite scaffolds based on electrospun poly(3-hydroxybutyrate) nanofibers and electrosprayed hydroxyapatite nanoparticles for bone tissue engineering applications. Mater. Sci. Eng. C 2014, 38, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Gredes, T.; Gedrange, T.; Hinuber, C.; Gelinsky, M.; Kunert-Keil, C. Histological and molecular-biological analyses of poly(3-hydroxybutyrate) (PHB) patches for enhancement of bone regeneration. Ann. Anat. 2015, 199, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Arias, S.; del Moral, A.; Ferrer, M.R.; Tallon, R.; Quesada, E.; Bejar, V. Mauran, an exopolysaccharide produced by the halophilic bacterium halomonas maura, with a novel composition and interesting properties for biotechnology. Extremophiles 2003, 7, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Bouchotroch, S.; Quesada, E.; del Moral, A.; Llamas, I.; Bejar, V. Halomonas maura sp. Nov., a novel moderately halophilic, exopolysaccharide-producing bacterium. Int. J. Syst. Evol. Microbiol. 2001, 51, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Poulose, A.C.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Bacterial exopolysaccharide based nanoparticles for sustained drug delivery, cancer chemotherapy and bioimaging. Carbohydr. Polym. 2013, 91, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Dhandayuthapani, B.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Sakthi Kumar, D. Biocompatible nanofibers based on extremophilic bacterial polysaccharide, mauran from halomonas maura. Carbohydr. Polym. 2013, 92, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Girija, A.R.; Balasubramanian, S.; Ukai, T.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Green approach for augmenting biocompatibility to quantum dots by extremophilic polysaccharide conjugation and nontoxic bioimaging. ACS Sustain. Chem. Eng. 2014, 2, 1551–1558. [Google Scholar] [CrossRef]
- Raveendran, S.; Chauhan, N.; Palaninathan, V.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Extremophilic polysaccharide for biosynthesis and passivation of gold nanoparticles and photothermal ablation of cancer cells. Part. Part. Syst. Charact. 2015, 32, 54–64. [Google Scholar] [CrossRef]
- Raveendran, S.; Palaninathan, V.; Chauhan, N.; Sakamoto, Y.; Yoshida, Y.; Maekawa, T.; Mohanan, P.V.; Kumar, D.S. In vitro evaluation of antioxidant defense mechanism and hemocompatibility of mauran. Carbohydr. Polym. 2013, 98, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, S.; Palaninathan, V.; Nagaoka, Y.; Fukuda, T.; Iwai, S.; Higashi, T.; Mizuki, T.; Sakamoto, Y.; Mohanan, P.V.; Maekawa, T.; et al. Extremophilic polysaccharide nanoparticles for cancer nanotherapy and evaluation of antioxidant properties. Int. J. Biol. Macromol. 2015, 76, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, B.; Aswathy, R.G.; Sreejith, R.; Nagaoka, Y.; Iwai, S.; Suzuki, M.; Fukuda, T.; Hasumura, T.; Yoshida, Y.; Maekawa, T.; et al. Bacterial exopolysaccharide based magnetic nanoparticles: A versatile nanotool for cancer cell imaging, targeted drug delivery and synergistic effect of drug and hyperthermia mediated cancer therapy. J. Biomed. Nanotechnol. 2014, 10, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, H.S.; Gattani, S.G. Gellan gum based microparticles of metoclopromide hydrochloride for intranasal delivery: Development and evaluation. Chem. Pharm. Bull. 2009, 57, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Sharma, M.; Parmar, V.; Upadhyay, U. Formulation of sidenafil citrate loaded nasal microspheres: An in vitro, ex vivo characterisation. Int. J. Drug Deliv. 2010, 2, 213–220. [Google Scholar] [CrossRef]
- Racovita, S.; Vasiliu, S.; Popa, M.; Luca, C. Polysaccharides based on micro-and nanoparticles obtained by ionic gleation and their applications as drug delivery systems. Rev. Roum. Chim. 2009, 54, 709–718. [Google Scholar]
- Verma, A.; Pandit, J.K. Rifabutin loaded floating gellan gum beads: Effect of calcium and polymer concentration on incorporation efficiency and drug release. Trop. J. Pharm. Res. 2011, 10, 61–67. [Google Scholar] [CrossRef]
- Lázaro, N.; Sevilla, A.L.; Morales, S.; Marqués, A.M. Heavy metal biosorption by gellan gum gel beads. Water Res. 2003, 37, 2118–2126. [Google Scholar] [CrossRef]
- Rodriguez-Carmona, E.; Villaverde, A. Nanostructured bacterial materials for innovative medicines. Trends Microbiol. 2010, 18, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Mali, V.; Bodhankar, S.; Shiras, A.; Prasad, B.L.; Pokharkar, V. Biocompatible gellan gum-reduced gold nanoparticles: Cellular uptake and subacute oral toxicity studies. J. Appl. Toxicol. 2011, 31, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Reddy, E.M.; Shiras, A.; Pokharkar, V.; Prasad, B.L. Natural gum reduced/stabilized gold nanoparticles for drug delivery formulations. Chemistry 2008, 14, 10244–10250. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, W. Large-scale aligned carbon nanotubes from their purified, highly concentrated suspension. ACS Nano 2010, 4, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Rast, J.; Johnston, J.M.; Allen, J.E.; Drum, C. Effects of nutritional and mechanical properties of food on ruminative behavior. J. Exp. Anal. Behav. 1985, 44, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.T.; Martins, L.; Picciochi, R.; Malafaya, P.B.; Sousa, R.A.; Neves, N.M.; Mano, J.F.; Reis, R.L. Gellan gum: A new biomaterial for cartilage tissue engineering applications. J. Biomed. Mater. Res. A 2010, 93, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Vitta, S.; Thiruvengadam, V. Multifunctional bacterial cellulose and nanoparticle-embedded composites. Curr. Sci. 2012, 102, 1398–1405. [Google Scholar]
- Palaninathan, V.; Chauhan, N.; Poulose, A.C.; Raveendran, S.; Mizuki, T.; Hasumura, T.; Fukuda, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; et al. Acetosulfation of bacterial cellulose: An unexplored promising incipient candidate for highly transparent thin film. Mater. Express 2014, 4, 415–421. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J.; Fan, J.; Sun, D.; Tang, W.; Yang, X. Biotemplated preparation of cds nanoparticles/bacterial cellulose hybrid nanofibers for photocatalysis application. J. Hazard. Mater. 2011, 189, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, S.; Li, X.; Shi, S.; Shen, W.; Zhang, X.; Wang, H. In situ synthesis of silver chloride nanoparticles into bacterial cellulose membranes. Mater. Sci. Eng. C 2009, 29, 1216–1219. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Lamme, E.N.; van Leeuwen, R.T.; Jonker, A.; van Marle, J.; Middelkoop, E. Living skin substitutes: Survival and function of fibroblasts seeded in a dermal substitute in experimental wounds. J. Investig. Dermatol. 1998, 111, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Fleck, C.A.; Simman, R. Modern collagen wound dressings: Function and purpose. J. Am. Coll. Certif. Wound Spec. 2010, 2, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.M.; Park, Y.J.; Hong, S.D.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B.M. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, N.; Xue, Y.; Ding, T.; Liu, X.; Mo, X.; Sun, J. Electrospun tilapia collagen nanofibers accelerating wound healing via inducing keratinocytes proliferation and differentiation. Colloids Surf. B Biointerfaces 2016, 143, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Aquavella, J.V.; del Cerro, M.; Musco, P.S.; Ueda, S.; DePaolis, M.D. The effect of a collagen bandage lens on corneal wound healing: A preliminary report. Ophthalmic Surg. 1987, 18, 570–573. [Google Scholar] [PubMed]
- Babaei, Z.; Jahanshahi, M. Protein nanoparticle: A unique system as drug delivery vehicles. Afr. J. Biotechnol. 2008, 7, 4926–4934. [Google Scholar]
- Djagny, V.B.; Wang, Z.; Xu, S. Gelatin: A valuable protein for food and pharmaceutical industries: Review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Li, M.; Liu, X.; Gao, H.; Wu, Y. Construction of amphiphilic copolymer nanoparticles based on gelatin as drug carriers for doxorubicin delivery. Colloids Surf. B Biointerfaces 2013, 102, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Li, W.M.; Chen, S.Y.; Liu, D.M. In situ doxorubicin–cap shell formation on amphiphilic gelatin–iron oxide core as a multifunctional drug delivery system with improved cytocompatibility, ph-responsive drug release and mr imaging. Acta Biomater. 2013, 9, 5360–5368. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, G.K.; Parraga, J.E.; Seijo, B.; Sanchez, A. Hybrid nanoparticle design based on cationized gelatin and the polyanions dextran sulfate and chondroitin sulfate for ocular gene therapy. Macromol. Biosci. 2011, 11, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Gaowa, A.; Horibe, T.; Kohno, M.; Sato, K.; Harada, H.; Hiraoka, M.; Tabata, Y.; Kawakami, K. Combination of hybrid peptide with biodegradable gelatin hydrogel for controlled release and enhancement of anti-tumor activity in vivo. J. Control. Release 2014, 176, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mimi, H.; Ho, K.M.; Siu, Y.S.; Wu, A.; Li, P. Polyethyleneimine-based core-shell nanogels: A promising sirna carrier for argininosuccinate synthetase mrna knockdown in hela cells. J. Control. Release 2012, 158, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Truong-Le, V.L.; Walsh, S.M.; Schweibert, E.; Mao, H.Q.; Guggino, W.B.; August, J.T.; Leong, K.W. Gene transfer by DNA-gelatin nanospheres. Arch. Biochem. Biophys. 1999, 361, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Nakamura, Y.; Jo, J.; Tabata, Y. Gelatin nanospheres incorporating sirna for controlled intracellular release. Biomaterials 2012, 33, 9097–9104. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yhee, J.Y.; Kim, S.H.; Kwon, I.C.; Kim, K. Biocompatible gelatin nanoparticles for tumor-targeted delivery of polymerized sirna in tumor-bearing mice. J. Control. Release 2013, 172, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Kido, Y.; Jo, J.; Tabata, Y. A gene transfection for rat mesenchymal stromal cells in biodegradable gelatin scaffolds containing cationized polysaccharides. Biomaterials 2011, 32, 919–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zou, Q.; Boerman, O.C.; Nijhuis, A.W.; Jansen, J.A.; Li, Y.; Leeuwenburgh, S.C. Combined delivery of BMP-2 and bfgf from nanostructured colloidal gelatin gels and its effect on bone regeneration in vivo. J. Control. Release 2013, 166, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Boerman, O.C.; Sariibrahimoglu, K.; Li, Y.; Jansen, J.A.; Leeuwenburgh, S.C. Comparison of micro- vs. Nanostructured colloidal gelatin gels for sustained delivery of osteogenic proteins: Bone morphogenetic protein-2 and alkaline phosphatase. Biomaterials 2012, 33, 8695–8703. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bongio, M.; Farbod, K.; Nijhuis, A.W.; van den Beucken, J.; Boerman, O.C.; van Hest, J.C.; Li, Y.; Jansen, J.A.; Leeuwenburgh, S.C. Development of injectable organic/inorganic colloidal composite gels made of self-assembling gelatin nanospheres and calcium phosphate nanocrystals. Acta Biomater. 2014, 10, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Lin, C.C. Accelerated nerve regeneration using induced pluripotent stem cells in chitin-chitosan-gelatin scaffolds with inverted colloidal crystal geometry. Colloids Surf. B Biointerfaces 2013, 103, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, M.; Eshak, N.; Zorlutuna, P.; Annabi, N.; Castello, M.; Kim, K.; Dolatshahi-Pirouz, A.; Edalat, F.; Bae, H.; Yang, Y.; et al. Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials 2012, 33, 9009–9018. [Google Scholar] [CrossRef] [PubMed]
- Koul, V.; Mohamed, R.; Kuckling, D.; Adler, H.J.; Choudhary, V. Interpenetrating polymer network (IPN) nanogels based on gelatin and poly(acrylic acid) by inverse miniemulsion technique: Synthesis and characterization. Colloids Surf. B Biointerfaces 2011, 83, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Gamez Sazo, R.E.; Maenaka, K.; Gu, W.; Wood, P.M.; Bunge, M.B. Fabrication of growth factor- and extracellular matrix-loaded, gelatin-based scaffolds and their biocompatibility with schwann cells and dorsal root ganglia. Biomaterials 2012, 33, 8529–8539. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Tonda-Turo, C.; De Pasquale, D.; Ruini, F.; Genchi, G.; Nitti, S.; Cappello, V.; Gemmi, M.; Mattoli, V.; Ciardelli, G.; et al. Gelatin/nanoceria nanocomposite fibers as antioxidant scaffolds for neuronal regeneration. Biochim. Biophys. Acta 2017, 1861, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Maji, K.; Dasgupta, S.; Pramanik, K.; Bissoyi, A. Preparation and evaluation of gelatin-chitosan-nanobioglass 3D porous scaffold for bone tissue engineering. Int. J. Biomater. 2016, 2016, 9825659. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nagate, T.; Matsuda, H. Acceleration of wound healing by gelatin film dressings with epidermal growth factor. J. Vet. Med. Sci. 2005, 67, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.Z.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.T.; Zhang, Y.; Lim, R.; Samsonraj, R.; Masilamani, J.; Phan, T.H.; Ramakrishna, S.; Lim, I.; Kee, I.; Fahamy, M.; et al. Preclinical evaluation of tegaderm supported nanofibrous wound matrix dressing on porcine wound healing model. Adv. Wound Care 2015, 4, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Hosseinzadeh, B.; Pedram, M.; Hatamian-Zarmi, A.; Salahshour-Kordestani, S.; Rasti, M.; Mokhtari-Hosseini, Z.B.; Mir-Derikvand, M. In vivo evaluation of gelatin/hyaluronic acid nanofiber as burn-wound healing and its comparison with chitoheal gel. Fibers Polym. 2016, 17, 820–826. [Google Scholar] [CrossRef]
- Hori, K.; Sotozono, C.; Hamuro, J.; Yamasaki, K.; Kimura, Y.; Ozeki, M.; Tabata, Y.; Kinoshita, S. Controlled-release of epidermal growth factor from cationized gelatin hydrogel enhances corneal epithelial wound healing. J. Control. Release 2007, 118, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Carvalho, E.; Banthia, A.K.; Banerjee, R. Development of polyvinyl alcohol-gelatin membranes for antibiotic delivery in the eye. Drug Dev. Ind. Pharm. 2011, 37, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed. Res. Int. 2014, 2014, 180549. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D. Calcium intake, calcium bioavailability and bone health. Br. J. Nutr. 2002, 87, S169–S177. [Google Scholar] [CrossRef] [PubMed]
- Neha, A.; Tarun, G.; Ajay, B. Review on casein production and casein based nano-formulations. Drug Deliv. 2012, 3, 41–45. [Google Scholar]
- Elzoghby, A.O.; Helmy, M.W.; Samy, W.M.; Elgindy, N.A. Novel ionically crosslinked casein nanoparticles for flutamide delivery: Formulation, characterization, and in vivo pharmacokinetics. Int. J. Nanomed. 2013, 8, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Novel spray-dried genipin-crosslinked casein nanoparticles for prolonged release of alfuzosin hydrochloride. Pharm. Res. 2013, 30, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Pavithran, M.; Viswanath, A.; Narayanan, D.; Mohan, C.C.; Manzoor, K.; Menon, D. Sequentially releasing dual-drug-loaded plga-casein core/shell nanomedicine: Design, synthesis, biocompatibility and pharmacokinetics. Acta Biomater. 2014, 10, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, L.; Lin, R.; Wang, A.Y.; Yang, L.; Kuang, M.; Qian, W.; Mao, H. Casein-coated iron oxide nanoparticles for high mri contrast enhancement and efficient cell targeting. ACS Appl. Mater. Interfaces 2013, 5, 4632–4639. [Google Scholar] [CrossRef] [PubMed]
- Khadka, D.B.; Haynie, D.T. Protein- and peptide-based electrospun nanofibers in medical biomaterials. Nanomedicine 2012, 8, 1242–1262. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wang, J.; Wang, Z.; Li, Z.; Qiu, Y.; Wang, H.; Xu, Y.; Niu, L.; Gong, P.; Yang, S. Casein phosphopeptide-biofunctionalized graphene biocomposite for hydroxyapatite biomimetic mineralization. J. Phys. Chem. C 2013, 117, 10375–10382. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; Gomes, A.C.; Cavaco-Paulo, A.M. Developing scaffolds for tissue engineering using the Ca2+-induced cold gelation by an experimental design approach. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2269–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonuccelli, G.; Castello-Cros, R.; Capozza, F.; Martinez-Outschoorn, U.E.; Lin, Z.; Tsirigos, A.; Xuanmao, J.; Whitaker-Menezes, D.; Howell, A.; Lisanti, M.P.; et al. The milk protein alpha-casein functions as a tumor suppressor via activation of stat1 signaling, effectively preventing breast cancer tumor growth and metastasis. Cell Cycle 2012, 11, 3972–3982. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lin, J.H.; Tsai, C.C.; Chuang, H.C.; Ho, C.Y.; Yao, C.H.; Chen, Y.S. Biodegradable glutaraldehyde-crosslinked casein conduit promotes regeneration after peripheral nerve injury in adult rats. Macromol. Biosci. 2011, 11, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- He, Y.X.; Zhang, N.N.; Li, W.F.; Jia, N.; Chen, B.Y.; Zhou, K.; Zhang, J.; Chen, Y.; Zhou, C.Z. N-terminal domain of bombyx mori fibroin mediates the assembly of silk in response to ph decrease. J. Mol. Biol. 2012, 418, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rudym, D.D.; Walsh, A.; Abrahamsen, L.; Kim, H.J.; Kim, H.S.; Kirker-Head, C.; Kaplan, D.L. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008, 29, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Park, S.H.; Seok, G.E.; Patel, A.; Numata, K.; Lu, C.L.; Kaplan, D.L. Quantifying osteogenic cell degradation of silk biomaterials. Biomacromolecules 2010, 11, 3592–3599. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Mora, C.; Mrowiec, A.; Garcia-Vizcaino, E.M.; Alcaraz, A.; Cenis, J.L.; Nicolas, F.J. Fibroin and sericin from bombyx mori silk stimulate cell migration through upregulation and phosphorylation of c-jun. PLoS ONE 2012, 7, e42271. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naby, W.; Cole, B.; Liu, A.; Liu, J.; Wan, P.; Guaiquil, V.H.; Schreiner, R.; Infanger, D.; Lawrence, B.D.; Rosenblatt, M.I. Silk-derived protein enhances corneal epithelial migration, adhesion, and proliferation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Wang, X.Y.; Kaplan, D.L.; Garlick, J.A.; Egles, C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009, 5, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Minoura, N.; Aiba, S.; Higuchi, M.; Gotoh, Y.; Tsukada, M.; Imai, Y. Attachment and growth of fibroblast cells on silk fibroin. Biochem. Biophys. Res. Commun. 1995, 208, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Sim, B.R.; Khang, G. Nature-derived aloe vera gel blended silk fibroin film scaffolds for cornea endothelial cell regeneration and transplantation. ACS Appl. Mater. Interfaces 2016, 8, 15160–15168. [Google Scholar] [CrossRef] [PubMed]
- Jao, D.; Mou, X.; Hu, X. Tissue regeneration: A silk road. J. Funct. Biomater. 2016, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Xiang, P.; Lu, J.; Yuan, J.; Shen, J. Electrospun polyurethane/keratin/agnp biocomposite mats for biocompatible and antibacterial wound dressings. J. Mater. Chem. B 2016, 4, 635–648. [Google Scholar] [CrossRef]
- Paladini, R.D.; Takahashi, K.; Bravo, N.S.; Coulombe, P.A. Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: Defining a potential role for keratin 16. J. Cell Biol. 1996, 132, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, O.; Brem, H.; Vouthounis, C.; Lee, B.; Fallon, J.; Stallcup, M.; Merchant, A.; Galiano, R.D.; Tomic-Canic, M. Molecular pathogenesis of chronic wounds: The role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am. J. Pathol. 2005, 167, 59–69. [Google Scholar] [CrossRef]
- Wojcik, S.M.; Bundman, D.S.; Roop, D.R. Delayed wound healing in keratin 6a knockout mice. Mol. Cell. Biol. 2000, 20, 5248–5255. [Google Scholar] [CrossRef] [PubMed]
- Deek, J.; Hecht, F.; Rossetti, L.; Wissmiller, K.; Bausch, A.R. Mechanics of soft epithelial keratin networks depend on modular filament assembly kinetics. Acta Biomater. 2016, 43, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Schermer, A.; Jester, J.V.; Hardy, C.; Milano, D.; Sun, T.T. Transient synthesis of K6 and K16 keratins in regenerating rabbit corneal epithelium: Keratin markers for an alternative pathway of keratinocyte differentiation. Differentiation 1989, 42, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zoccola, M.; Aluigi, A.; Vineis, C.; Tonin, C.; Ferrero, F.; Piacentino, M.G. Study on cast membranes and electrospun nanofibers made from keratin/fibroin blends. Biomacromolecules 2008, 9, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Jarvis, D.; Hopkins, T.; Pixley, S.; Bhattarai, N. Poly(epsilon-caprolactone)/keratin-based composite nanofibers for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Thompson, Z.S.; Rijal, N.P.; Jarvis, D.; Edwards, A.; Bhattarai, N. Synthesis of keratin-based nanofiber for biomedical engineering. J. Vis. Exp. 2016, 108, e53381. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.-C.; Yuan, J.; Chae, W.-P.; Kang, I.-K. Keratin nanofibers as a biomaterial. In International Conference on Nanotechnology and Biosensors (2010); IACSIT Press: Singapore, 2011. [Google Scholar]
- Domogatskaya, A.; Rodin, S.; Tryggvason, K. Functional diversity of laminins. Annu. Rev. Cell Dev. Biol. 2012, 28, 523–553. [Google Scholar] [CrossRef] [PubMed]
- Iorio, V.; Troughton, L.D.; Hamill, K.J. Laminins: Roles and utility in wound repair. Adv. Wound Care 2015, 4, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.A.; McClugage, S.G.; Link, M.C.; Sefcik, L.S.; Ogle, R.C.; Botchwey, E.A. Laminin nanofiber meshes that mimic morphological properties and bioactivity of basement membranes. Tissue Eng. Part C Methods 2009, 15, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.A.; Lenz, S.M.; Wang, T.; Abebayehu, D.; Brooks, B.P.; Ogle, R.C.; Botchwey, E.A. Laminin- and basement membrane-polycaprolactone blend nanofibers as a scaffold for regenerative medicine. Nanomater. Environ. 2014, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Ebihara, N.; Shima, N.; Kimoto, M.; Funaki, T.; Yokoo, S.; Murakami, A.; Yamagami, S. Adhesion, migration, and proliferation of cultured human corneal endothelial cells by laminin-5. Investig. Ophthalmol. Vis. Sci. 2011, 52, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Uzunalli, G.; Soran, Z.; Erkal, T.S.; Dagdas, Y.S.; Dinc, E.; Hondur, A.M.; Bilgihan, K.; Aydin, B.; Guler, M.O.; Tekinay, A.B. Bioactive self-assembled peptide nanofibers for corneal stroma regeneration. Acta Biomater. 2014, 10, 1156–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, M.; Langer, R.; Jia, X. Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 241–268. [Google Scholar] [CrossRef] [PubMed]
- Lenselink, E.A. Role of fibronectin in normal wound healing. Int. Wound J. 2015, 12, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Mencner, L.; Komosinska-Vassev, K. The role of the extracellular matrix components in cutaneous wound healing. Biomed. Res. Int. 2014, 2014, 747584. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, L.S.; Foster, C.S.; Harrist, T.J.; Lanigan, J.M.; Colvin, R.B. Fibronectin in healing rabbit corneal wounds. Lab. Investig. 1981, 45, 120–129. [Google Scholar] [PubMed]
- Nishida, T. The role of fibronectin in corneal wound healing explored by a physician-scientist. Jpn. J. Ophthalmol. 2012, 56, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Nakagawa, S.; Nishibayashi, C.; Tanaka, H.; Manabe, R. Fibronectin enhancement of corneal epithelial wound healing of rabbits in vivo. Arch. Ophthalmol. 1984, 102, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Rexeisen, E.L.; Fan, W.; Pangburn, T.O.; Taribagil, R.R.; Bates, F.S.; Lodge, T.P.; Tsapatsis, M.; Kokkoli, E. Self-assembly of fibronectin mimetic peptide-amphiphile nanofibers. Langmuir 2010, 26, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, X.; Zhou, Y.; Xiang, Q. Core-shell PLGA/collagen nanofibers loaded with recombinant FN/CDHs as bone tissue engineering scaffolds. Connect. Tissue Res. 2014, 55, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.M.; Gritsch, K.; Salles, V.; Attik, G.N.; Grosgogeat, B. Surface entrapment of fibronectin on electrospun plga scaffolds for periodontal tissue engineering. BioRes. Open Access 2014, 3, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef] [PubMed]
- Markets, M.A. Biomaterials Market by Type of Materials (Metallic, Ceramic, Polymers, Natural) & Application (Cardiovascular, Orthopedic, Dental, Plastic Surgery, Wound Healing, Neurology, Tissue Engineering, Ophthalmology)—Global Forecast to 2021. Available online: http://www.marketsandmarkets.com/Market-Reports/biomaterials-393.html?gclid=Cj0KEQjwyN7JBRCZn7LKgb3ki8kBEiQAaLEsqhcn-mO6CKD5VU23slatls1ddshVTxtneknO1ZZJzRcaAmsx8P8HAQ (accessed on 10 June 2017).
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]

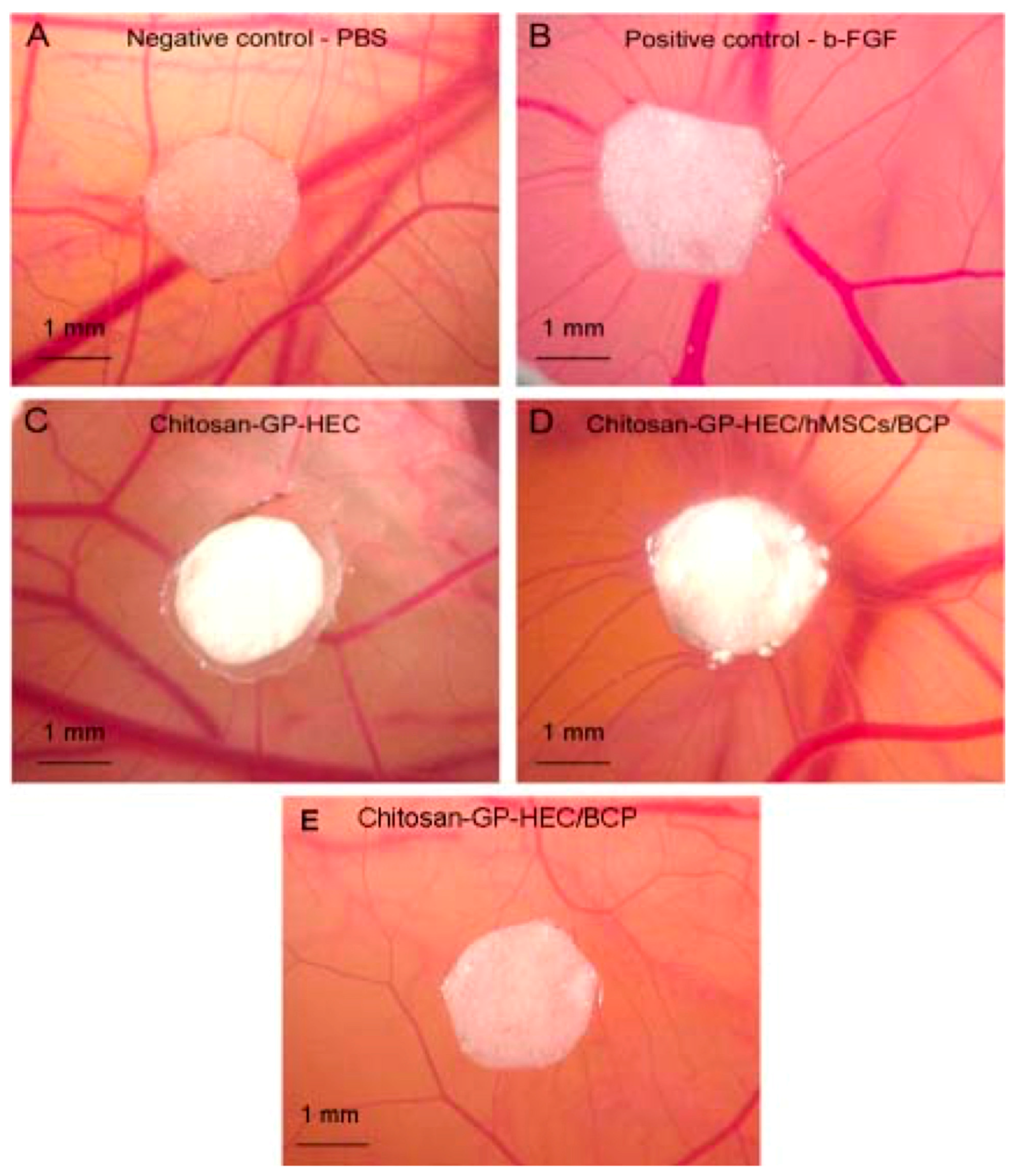
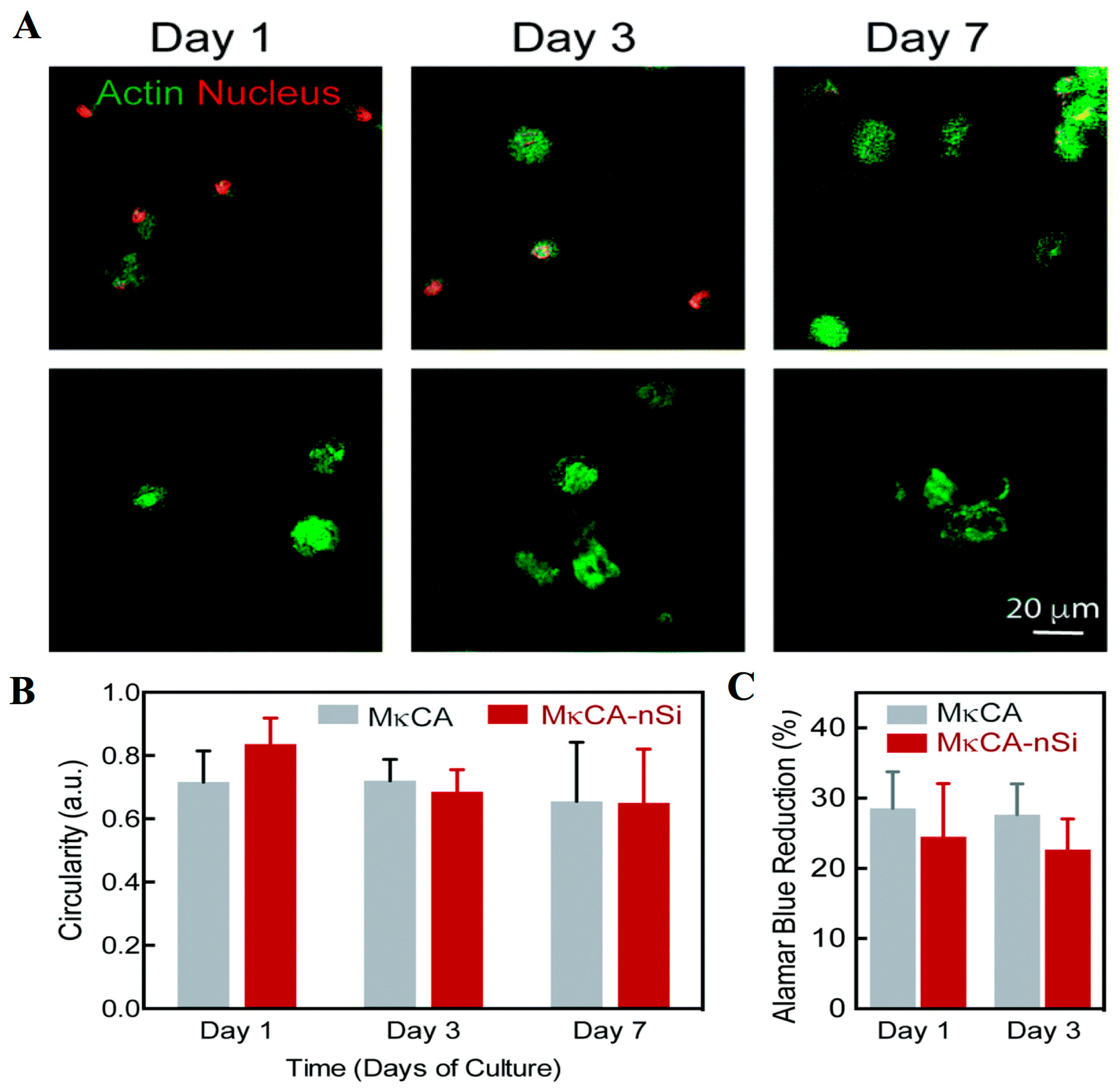
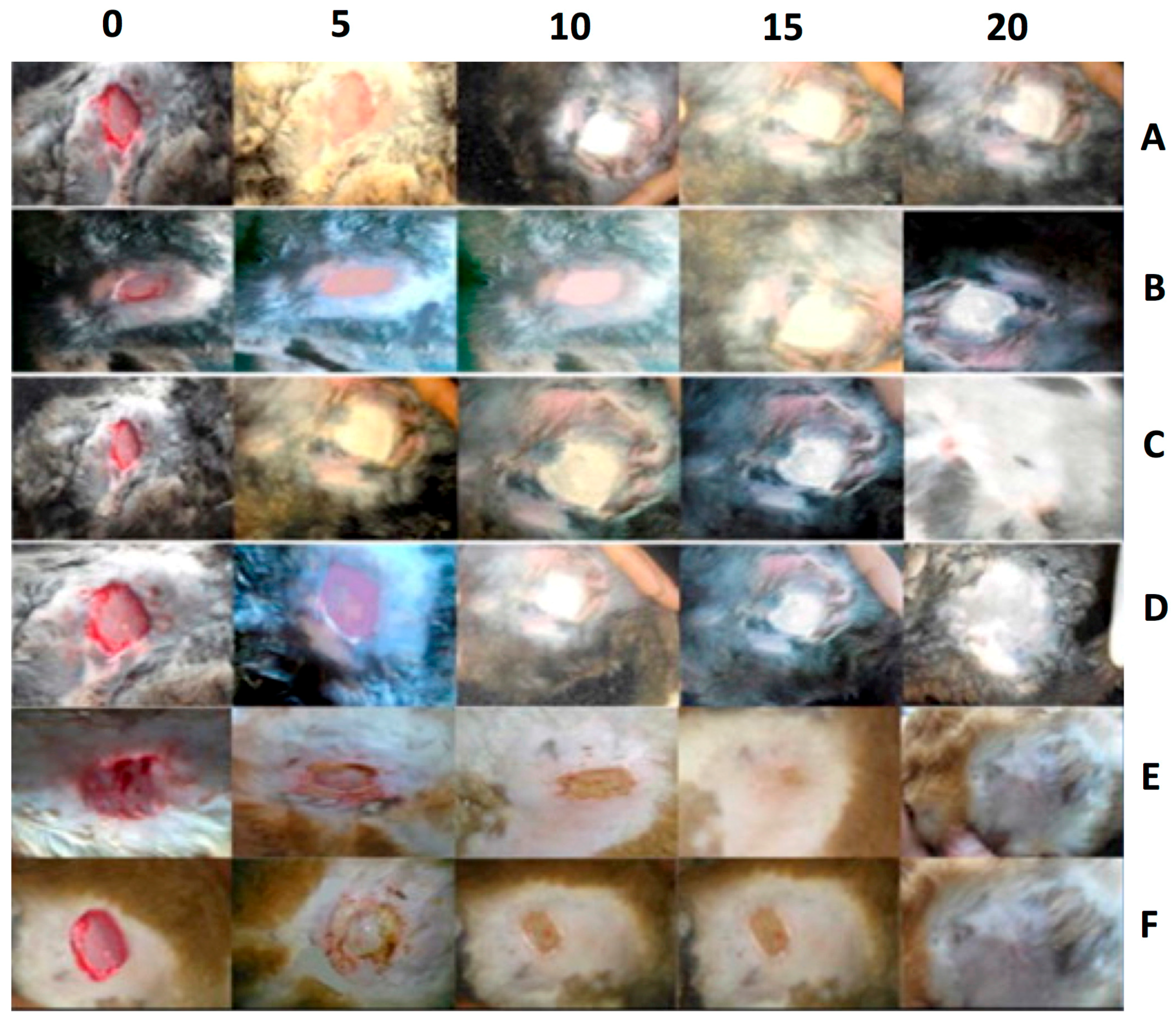
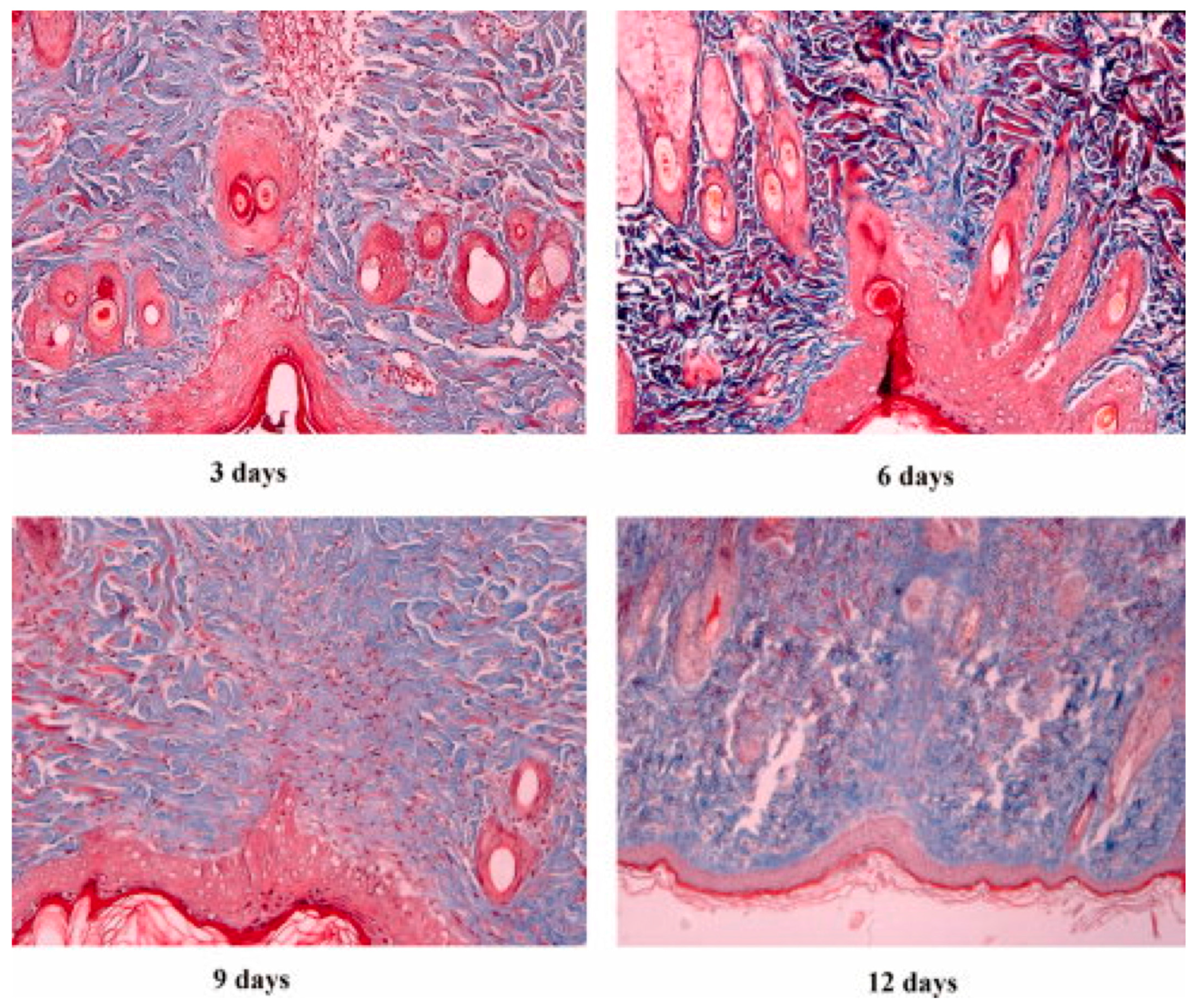
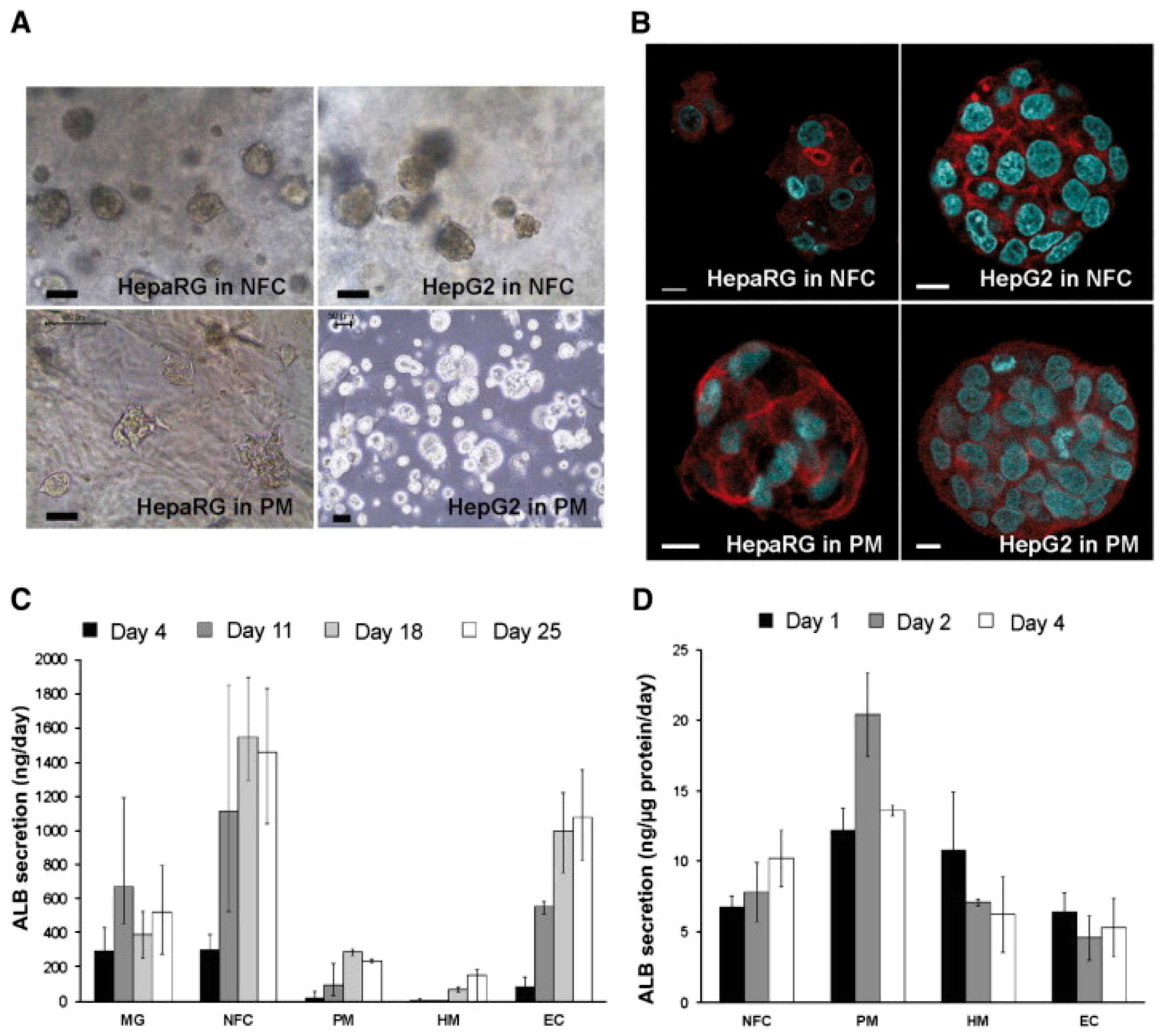

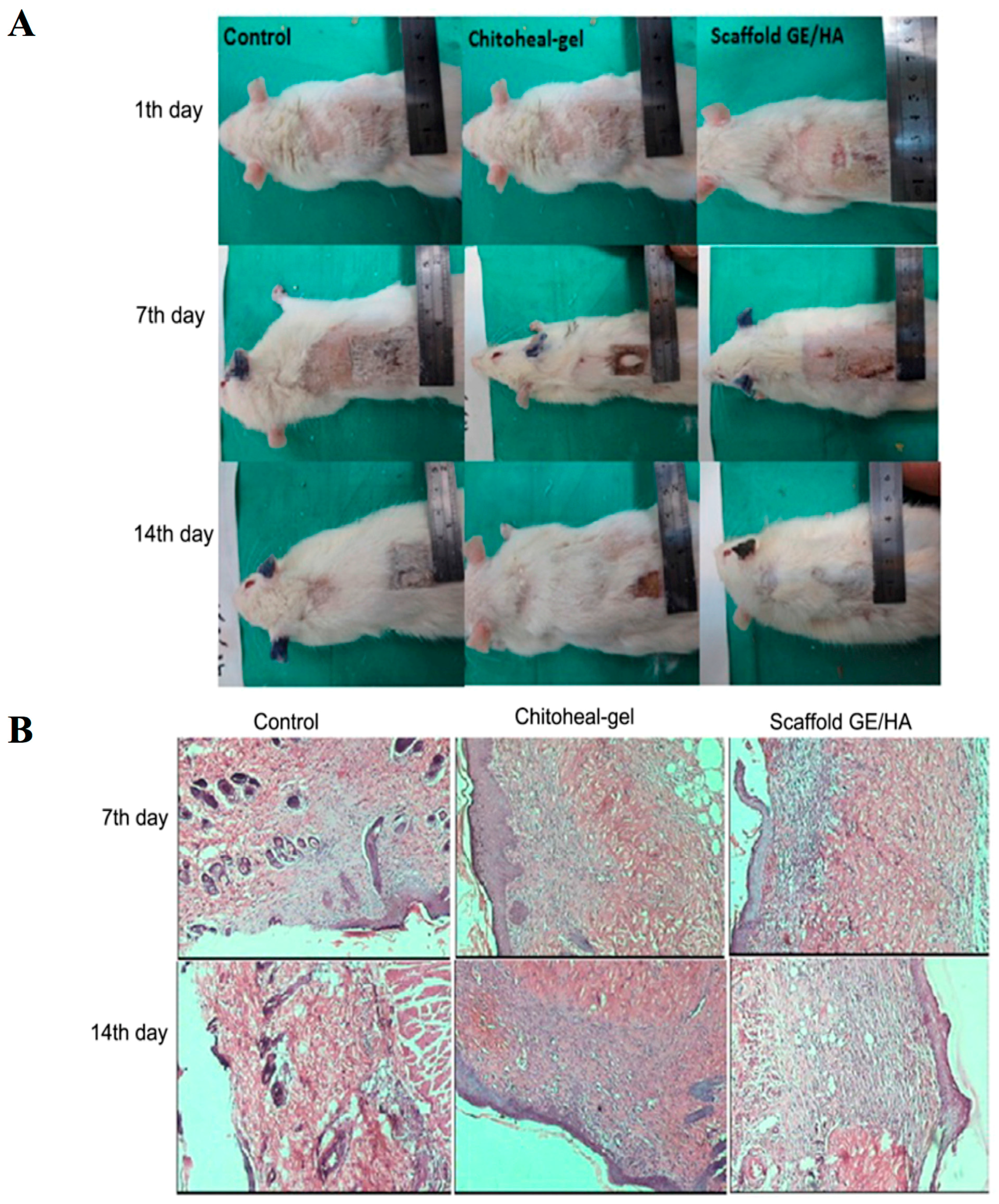
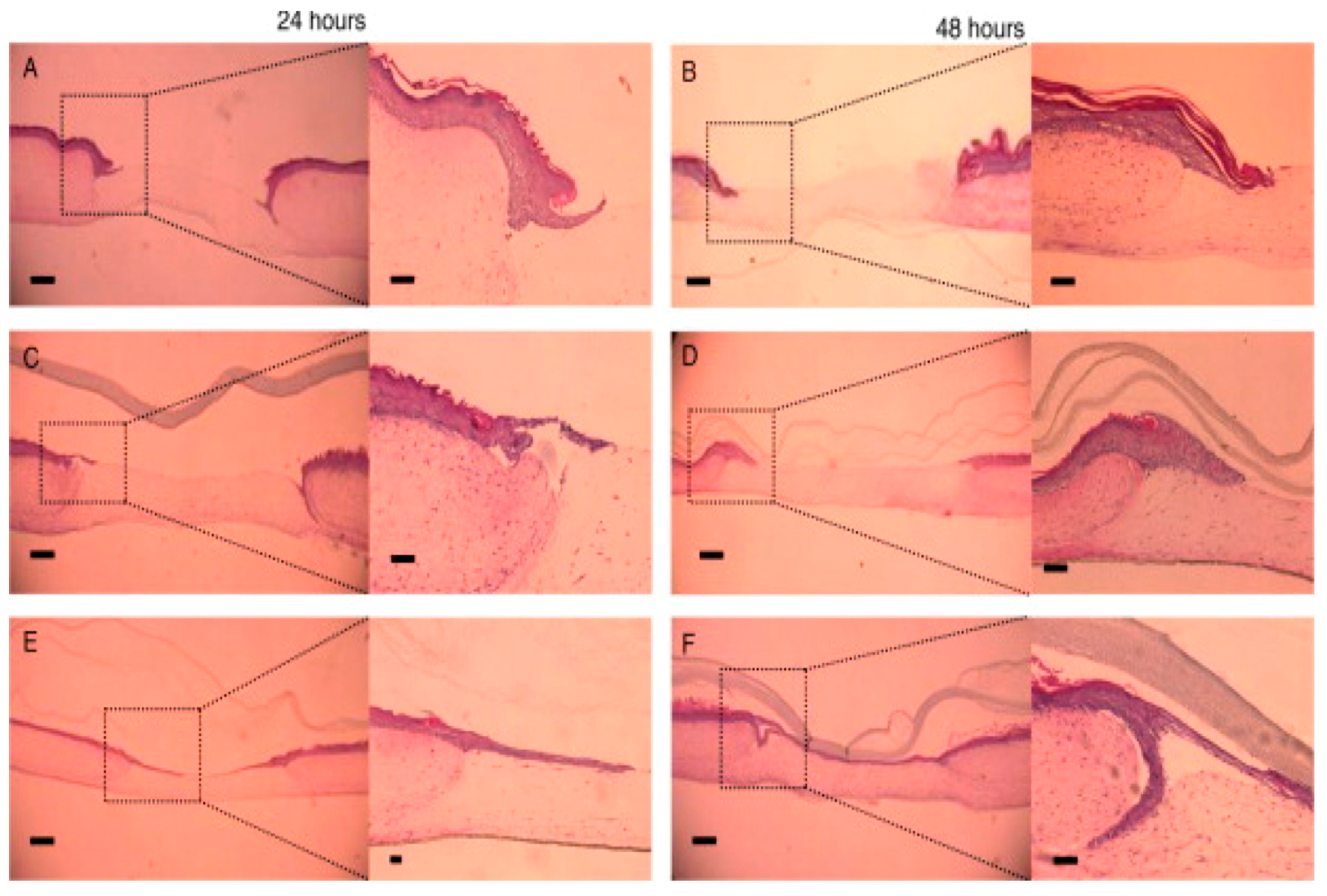
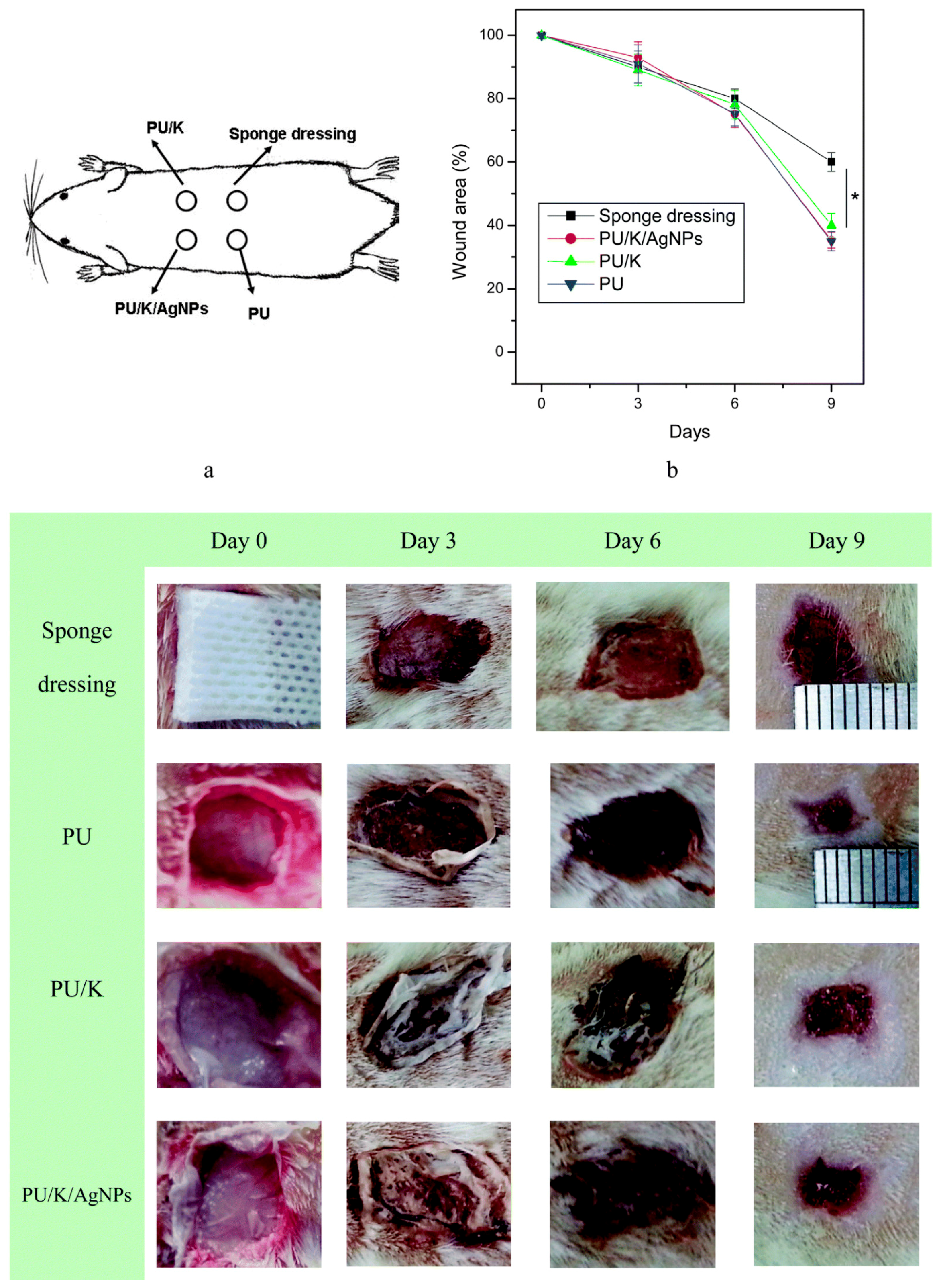
| SN. | Cells | Proteins Secreted for Cellular Regeneration |
|---|---|---|
| 1 | Platelets | PDGF, TGFβ, EGF |
| 2 | Neutrophils | IL-1, IL-6, TNFα, VEGF, PDGFR, TGFβR |
| 3 | Fibroblast and myofibroblast | KGF, TNFβ, FGFs, IGF, VEGF, EGF, HGF, PDGFR, TGFβR, FGFRs, IL-1R, TNFαR, SDF-1R |
| 4 | Keratinocytes | EGF, IL-1, TGFβ, TNFα, MCP-1, SDF1, HGF, EGFR, R-1R, TGFβR, KGFR, SDF1R |
| 5 | Endothelial Cells | FGF, VEGF, PDGF, FGF-2, FGFR, VEGFR, HGFR |
| 6 | Macrophages | IL-1, IL-6, TNFα, VEGF, PDGF, EGF, PDGFR, TGFβR, VEGFR |
| SN. | Significant Development | Year | Reference |
|---|---|---|---|
| 1 | Concept of using charged polysaccharides like CS for drug delivery applications | 1989 | [12] |
| 2 | Development of CS-ethylene oxide-propylene block polymer for protein and vaccine delivery | 1997 | [13] |
| 3 | Oral gene delivery by CS-DNA nanoparticles | 1999 | [14] |
| 4 | Enhancement of insulin by nasal absorption of CS NPs | 1999 | [15] |
| 5 | CS nanocarriers for anticancer drugs | 2000 | [16] |
| 6 | CS nanocarriers for nasal delivery of vaccine | 2001 | [17] |
| SN. | Nanocomposite | Antibacterial Type | Reference |
|---|---|---|---|
| 1 | CS coated Ag loaded SiO2 | E. coli and S. aureus | [40] |
| 2 | CS-Ag NPs loaded nanofiber mats | E. coli | [41] |
| 3 | CS layered silicate composites | S. aureus and B. subtilis | [42] |
| 4 | Clay chitosan nanocomposite | E. coli and S. aureus | [43] |
| SN. | Polymer Type | In Composite State with | Significance | Reference |
|---|---|---|---|---|
| 1 | Chitosan | Native hydrogel and membrane | Incorporation of graphene may provide antibacterial properties to nanofibrous scaffold of CS and help in wound healing process. | [66,67] |
| 2 | Alginate | Native hydrogel | Improved viability for corneal epithelial cells. | [68,69] |
| 3 | Cellulose | PVA hydrogel | Highly transparent, elastic, lubricated and biocompatible material with human corneal epithelial cells (HCE-2) | [70] |
| Bacterial Cellulose | PVA composite | Improved mechanical and chemical properties proposed for ophthalmic application | [71] | |
| 4 | Heparin | Native biomaterial | Established to have corneal wound healing potential. However, nanoscaffold evaluations remain unknown. | [72] |
| 5 | Gelatin | Native nanofibers | Improved mechanical properties. The AG-gel consisting of gelatin nanofibers provided improved transparency to formulation. | [73] |
| PLLA Nanofibers | Improved compatibility for epithelial cells and keratinocytes and regeneration of corneal stroma. | [74,75] | ||
| PHBV Nanofibers | Helps in epithelial cell proliferation and corneal formation. | [76] | ||
| 6 | Silk fibroin | Native film | Compatible with human limbal stem cell and were helpful in promotion of epithelium formation. | [77] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raveendran, S.; Rochani, A.K.; Maekawa, T.; Kumar, D.S. Smart Carriers and Nanohealers: A Nanomedical Insight on Natural Polymers. Materials 2017, 10, 929. https://doi.org/10.3390/ma10080929
Raveendran S, Rochani AK, Maekawa T, Kumar DS. Smart Carriers and Nanohealers: A Nanomedical Insight on Natural Polymers. Materials. 2017; 10(8):929. https://doi.org/10.3390/ma10080929
Chicago/Turabian StyleRaveendran, Sreejith, Ankit K. Rochani, Toru Maekawa, and D. Sakthi Kumar. 2017. "Smart Carriers and Nanohealers: A Nanomedical Insight on Natural Polymers" Materials 10, no. 8: 929. https://doi.org/10.3390/ma10080929




