Histological and Immunohistochemical Analyses of Repair of the Disc in the Rabbit Temporomandibular Joint Using a Collagen Template
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rabbits
2.2. Reconstituted Collagen Templates
2.3. Experimental Design
2.4. Surgical Techniques
2.5. Histology Preparation
2.6. Immunohistochemistry and Relative Quantification
2.7. Statistical Analysis
3. Results
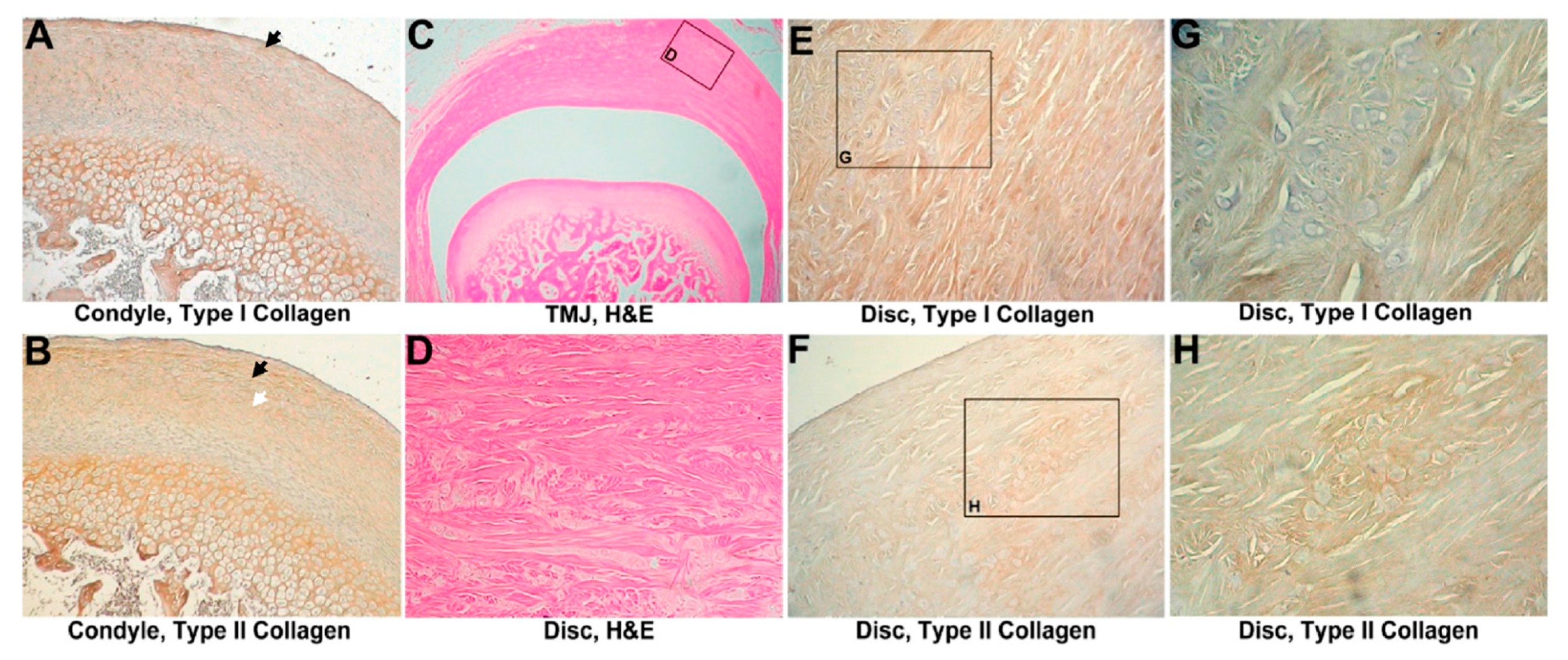
3.1. Sham-Operated Group
3.1.1. Histological Evaluation
3.1.2. IHC Evaluation
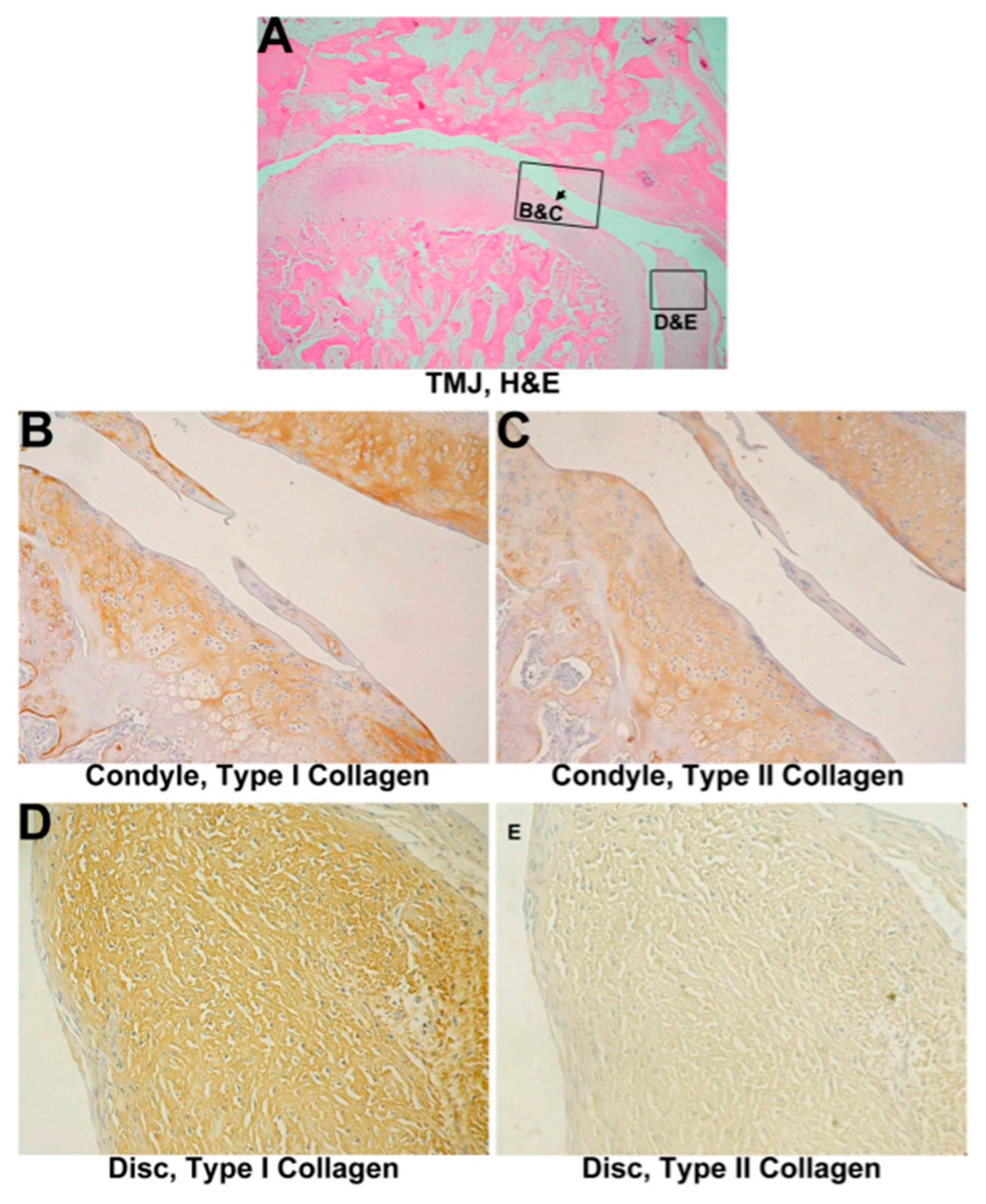
3.2. Untreated Group
3.2.1. Histological Evaluation
3.2.2. IHC Evaluation
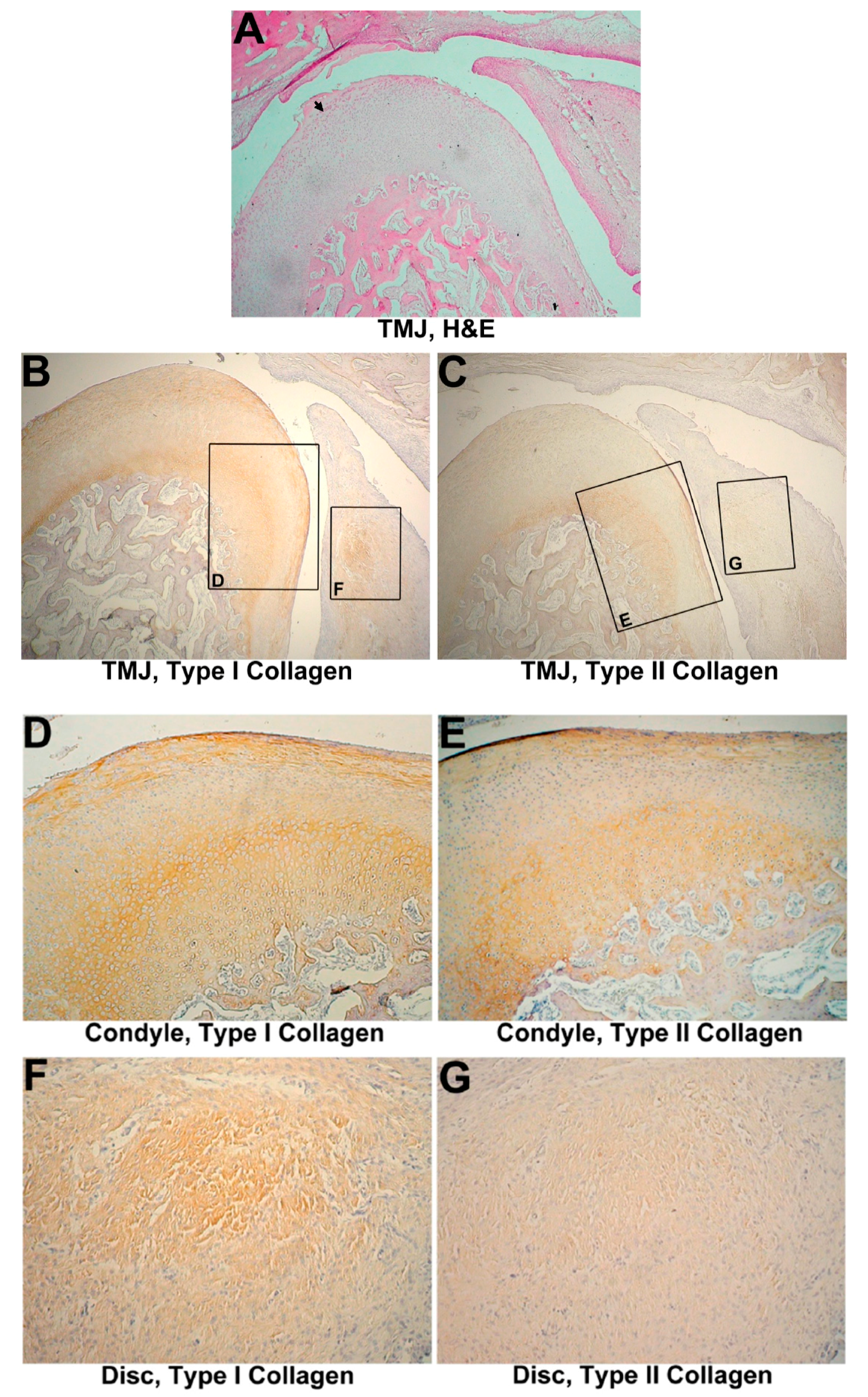
3.3. One-Month Group
3.3.1. Histological Evaluation
3.3.2. IHC Evaluation
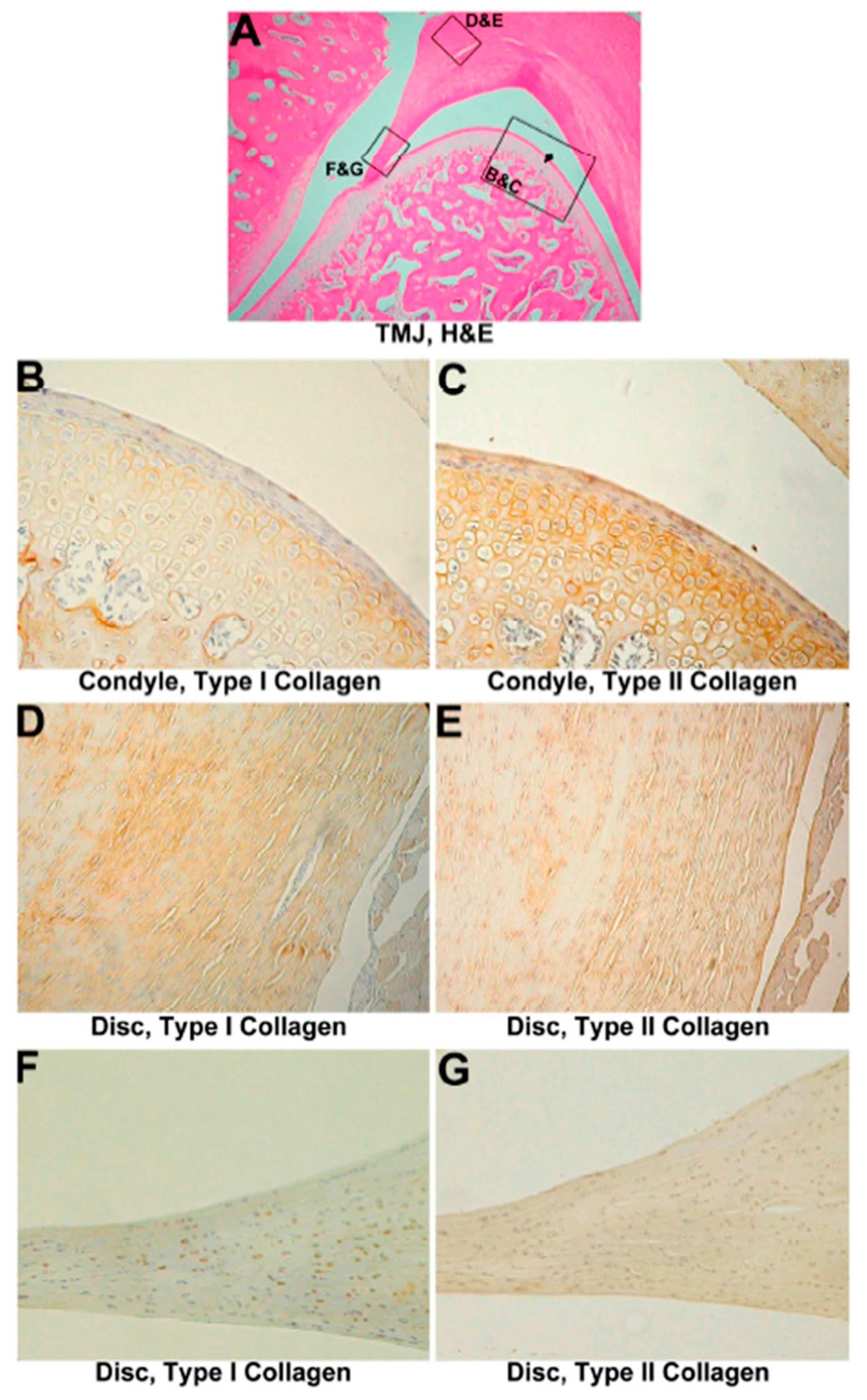
3.4. Two-Month Group
3.4.1. Histological Evaluation
3.4.2. IHC Evaluation
3.5. Three-Month Group
3.5.1. Histological Evaluation
3.5.2. IHC Evaluation
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guarda-Nardini, L.; Tito, R.; Staffieri, A.; Beltrame, A. Treatment of patients with arthrosis of the temporomandibular joint by infiltration of sodium hyaluronate: A preliminary study. Eur. Arch. Otorhinolaryngol. 2002, 259, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Fricton, J.R.; Look, J.O.; Schiffman, E.; Swift, J. Long-term study of temporomandibular joint surgery with alloplastic implants compared with nonimplant surgery and nonsurgical rehabilitation for painful temporomandibular joint disc displacement. J. Oral Maxillofac. Surg. 2002, 60, 1400–1411; discussion 1411–1412. [Google Scholar] [CrossRef] [PubMed]
- Scrivani, S.J.; Keith, D.A.; Kaban, L.B. Temporomandibular disorders. N. Engl. J. Med. 2008, 359, 2693–2705. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.L. Arthroscopic abrasion arthroplasty: A review. Clin. Orthop. Relat. Res. 2001, 391 (Suppl. 391), S306–S317. [Google Scholar] [CrossRef]
- Fujisato, T.; Sajiki, T.; Liu, Q.; Ikada, Y. Effect of basic fibroblast growth factor on cartilage regeneration in chondrocyte-seeded collagen sponge scaffold. Biomaterials 1996, 17, 155–162. [Google Scholar] [CrossRef]
- Lai, W.-F. Reconstituted Collagen Template and the Process to Prepare the Same. U.S. Patent 5,876,444 A, 2 March 1999. [Google Scholar]
- Lai, W.F.; Tsai, Y.H.; Su, S.J.; Su, C.Y.; Stockstill, J.W.; Burch, J.G. Histological analysis of regeneration of temporomandibular joint discs in rabbits by using a reconstituted collagen template. Int. J. Oral Maxillofac. Surg. 2005, 34, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.H.; Chen, S.C.; Wu, K.C.; Yang, C.B.; Fang, C.L.; Lai, W.F.; Tsai, Y.H. Differential effect of ECM molecules on re-expression of cartilaginous markers in near quiescent human chondrocytes. J. Cell. Physiol. 2011, 226, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Chiu, L.H.; Lai, W.F.; Tsai, T.T.; Fang, C.L.; Chen, S.C.; Tsai, Y.H. Phenotypic re-expression of near quiescent chondrocytes: The effects of type II collagen and growth factors. J. Biomater. Appl. 2010, 25, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.T., Jr.; Chen, C.T. Fabrication of a Cartilage Implant. U.S. Patent 6,852,331 B2, 8 Feburary 2005. [Google Scholar]
- Arqués, O.; Chicote, I.; Tenbaum, S.; Puig, I.; Palmer, H.G. Standardized Relative Quantification of Immunofluorescence Tissue Staining. Protocol. Exch. 2012. [Google Scholar] [CrossRef]
- Georgiade, N.G. The surgical correction of temporomandibular joint dysfunction by means of autogenous dermal grafts. Plast. Reconstr. Surg. Transplant. Bull. 1962, 30, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Dimitroulis, G.; Trost, N.; Morrison, W. The radiological fate of dermis-fat grafts in the human temporomandibular joint using magnetic resonance imaging. Int. J. Oral Maxillofac. Surg. 2008, 37, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Dimitroulis, G. The use of dermis grafts after discectomy for internal derangement of the temporomandibular joint. J. Oral Maxillofac. Surg. 2005, 63, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, S.E.; Larsen, P.E. The use of a pedicled temporalis muscle-pericranial flap for replacement of the TMJ disc: Preliminary report. J. Oral Maxillofac. Surg. 1989, 47, 142–146. [Google Scholar] [CrossRef]
- Guruprasad, Y.; Chauhan, D.S.; Cariappa, K.M. A Retrospective Study of Temporalis Muscle and Fascia Flap in Treatment of TMJ Ankylosis. J. Maxillofac. Oral Surg. 2010, 9, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Christianson, D.J.; Hansbrough, J.F. Structure of a collagen-GAG dermal skin substitute optimized for cultured human epidermal keratinocytes. J. Biomed. Mater. Res. 1988, 22, 939–957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yap, A.U.; Toh, W.S. Stem Cells for Temporomandibular Joint Repair and Regeneration. Stem Cell Rev. 2015, 11, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Man, C.; Zhang, B.; Hu, J.; Zhu, S.S. Effect of in vitro chondrogenic differentiation of autologous mesenchymal stem cells on cartilage and subchondral cancellous bone repair in osteoarthritis of temporomandibular joint. Int. J. Oral Maxillofac. Surg. 2013, 42, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, Y.; He, D.; Wang, L. Effect of Concentrated Growth Factors on the Repair of the Goat Temporomandibular Joint. J. Oral Maxillofac. Surg. 2017, 75, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hu, J.; Man, C.; Zhu, S. Effect of intra-articular administration of interleukin 1 receptor antagonist on cartilage repair in temporomandibular joint. J. Craniofac. Surg. 2011, 22, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Hu, J.; Man, C.; Zhang, B.; Ma, Y.Q.; Zhu, S.S. Insulin-like growth factor-1 suspended in hyaluronan improves cartilage and subchondral cancellous bone repair in osteoarthritis of temporomandibular joint. Int. J. Oral Maxillofac. Surg. 2011, 40, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Kutuk, N.; Bas, B.; Soylu, E.; Gonen, Z.B.; Yilmaz, C.; Balcioglu, E.; Ozdamar, S.; Alkan, A. Effect of platelet-rich plasma on fibrocartilage, cartilage, and bone repair in temporomandibular joint. J. Oral Maxillofac. Surg. 2014, 72, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Chung, W.L.; Pavlick, M.; Reppas, S.; Ochs, M.W.; Russell, A.J.; Badylak, S.F. Extracellular matrix as an inductive template for temporomandibular joint meniscus reconstruction: A pilot study. J. Oral Maxillofac. Surg. 2011, 69, e488–e505. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Chung, W.L.; Almarza, A.J.; Pavlick, M.D.; Reppas, S.N.; Ochs, M.W.; Russell, A.J.; Badylak, S.F. Inductive, scaffold-based, regenerative medicine approach to reconstruction of the temporomandibular joint disk. J. Oral Maxillofac. Surg. 2012, 70, 2656–2668. [Google Scholar] [CrossRef] [PubMed]
- Aitken, K.J.; Bagli, D.J. The bladder extracellular matrix. Part II: Regenerative applications. Nat. Rev. Urol. 2009, 6, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Aitken, K.J.; Bagli, D.J. The bladder extracellular matrix. Part I: Architecture, development and disease. Nat. Rev. Urol. 2009, 6, 596–611. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.P.; Feinberg, S.E. The temporalis muscle flap in contemporary oral and maxillofacial surgery. Oral Maxillofac. Surg. Clin. N. Am. 2003, 15, 513–535. [Google Scholar] [CrossRef]
- Matukas, V.J.; Lachner, J. The use of autologous auricular cartilage for temporomandibular joint disc replacement: A preliminary report. J. Oral Maxillofac. Surg. 1990, 48, 348–353. [Google Scholar] [CrossRef]
- Takatsuka, S.; Narinobou, M.; Nakagawa, K.; Yamamoto, E. Histologic evaluation of auricular cartilage grafts after discectomy in the rabbit craniomandibular joint. J. Oral Maxillofac. Surg. 1996, 54, 1216–1225. [Google Scholar] [CrossRef]
- Dimitroulis, G. The interpositional dermis-fat graft in the management of temporomandibular joint ankylosis. Int. J. Oral Maxillofac. Surg. 2004, 33, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Dolwick, M.F.; Aufdemorte, T.B. Silicone-induced foreign body reaction and lymphadenopathy after temporomandibular joint arthroplasty. Oral. Surg. Oral Med. Oral Pathol. 1985, 59, 449–452. [Google Scholar] [CrossRef]
- Heffez, L.; Mafee, M.F.; Rosenberg, H.; Langer, B. CT evaluation of TMJ disc replacement with a Proplast-Teflon laminate. J. Oral Maxillofac. Surg. 1987, 45, 657–665. [Google Scholar] [CrossRef]
- Chuong, R.; Piper, M.A. Cerebrospinal fluid leak associated with proplast implant removal from the temporomandibular joint. Oral Surg. Oral Med. Oral Pathol. 1992, 74, 422–425. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.-H.; Chan, W.P.; Chiu, L.-H.; Tsai, Y.-H.; Fang, C.-L.; Yang, C.-B.; Chen, K.-C.; Tsai, H.-L.; Lai, W.-F. Histological and Immunohistochemical Analyses of Repair of the Disc in the Rabbit Temporomandibular Joint Using a Collagen Template. Materials 2017, 10, 924. https://doi.org/10.3390/ma10080924
Wang K-H, Chan WP, Chiu L-H, Tsai Y-H, Fang C-L, Yang C-B, Chen K-C, Tsai H-L, Lai W-F. Histological and Immunohistochemical Analyses of Repair of the Disc in the Rabbit Temporomandibular Joint Using a Collagen Template. Materials. 2017; 10(8):924. https://doi.org/10.3390/ma10080924
Chicago/Turabian StyleWang, Kuo-Hwa, Wing P. Chan, Li-Hsuan Chiu, Yu-Hui Tsai, Chia-Lang Fang, Charn-Bing Yang, Kuan-Chou Chen, Hung-Li Tsai, and Wen-Fu Lai. 2017. "Histological and Immunohistochemical Analyses of Repair of the Disc in the Rabbit Temporomandibular Joint Using a Collagen Template" Materials 10, no. 8: 924. https://doi.org/10.3390/ma10080924



