1. Introduction
Over one million patients with skeletal issues require orthopedic surgery each year. Bone grafts are widely utilized in orthopedic surgery to augment bone union and regeneration [
1]. The use of calcium phosphate cements (CPCs) as a synthetic bone material has been studied since the early 1980s [
2]. CPCs are suitable bone-graft material because of their similarity in composition to the mineral phase of bone, as well as their high biocompatibility and osteoconductivity [
3]. In our previous study, we evaluated the effectiveness of three calcium phosphate bone-graft substitutes in a rat model of posterolateral lumbar fusion and found that a dicalcium phosphate (DCP)-rich CPC, which has a Ca/P ratio as low as 1.5, had the best spinal fusion effectiveness [
4].
Various strategies have been developed and evaluated for promoting the conversion of CPC to new bone, including increasing its porosity and using water-soluble polymers and platelet-rich plasma (PRP) as additives in apatite-forming CPCs [
5,
6,
7]. A previous study showed that the incorporation of mineralized collagen into bone cement improved osteogenic ability, marked by increased differentiation of human marrow mesenchymal stem cells (MSCs) into osteoclasts and then osteoblasts [
8]. Moreover, the addition of collagen to apatitic CPCs allows enhancement of several functional properties, such as significantly enhanced alkaline phosphatase (ALP) activity with collagen-containing nanosized hydroxyapatite [
9]. In a study by R.A. Perez et al., collagen incorporation in the liquid phase of the CPC resulted in improved osteoinductive ability [
10]. In addition, the adhesion and proliferation of osteoblastic cells increased in the calcium-deficient hydroxyapatite-collagen composite. These findings may be attributed to the reduced stiffness of the CPC as compared to collagen-CPCs composite in solid form. Great care must be taken when collagen-CPC grafts are applied during orthopedic surgery because the non-strengthened composite can easily be washed out by blood. We hypothesize that the incorporation of collagen into dicalcium phosphate (DCP)-rich CPC, a non-dispersive cement [
11], may have several advantages, such as increased cell adhesion, proliferation, ALP activity, resistance to wash-out, and importantly, increased ability of MSCs to undergo osteogenic differentiation.
The aim of this study was to investigate the effect of supplementing DCP-rich CPC with type I collagen on cellular activities and its performance as a bone graft material. Collagen solution and the DCP-rich CPC were mixed to create a moldable bone graft, which was then evaluated for cell adhesion, morphology, viability, and ALP activity using bone cell progenitors. The use of the bone graft in posterolateral lumbar fusion surgery in a rat model was assessed with radiology using three-dimensional micro-computed tomography (micro-CT). Finally, the optimal ratio of collagen to DCP-rich CPC was determined.
2. Materials and Methods
2.1. Preparation of the DCP-Rich CPC
Tetracalcium phosphate (TTCP; Ca
4(PO
4)
2O) powder was prepared via the sintering of dicalcium pyrophosphate (Ca
2P
2O
7; Alfa Aesar, MA, USA) and calcium carbonate (CaCO
3; Shimakyu’s Pure Chemicals, Osaka, Japan). The DCP-rich CPC was prepared by mixing TTCP and dicalcium phosphate anhydrous (DCPA; CaHPO
4; Acros Organics, Geel, Belgium) powders at a DCPA-to-TTCP molar ratio of two. The detailed procedures for preparing DCP-rich CPC have been described previously [
12].
2.2. Solubilized Type I Collagen
Concentrated type I collagen, derived from rat tail tendon (9.9 mg/mL), was purchased from BD Biosciences (Franklin Lakes, NJ, USA). Type I collagen was used because it is the main component of the organic part of bone.
2.3. Collagen-Containing DCP-Rich CPC
Collagen-containing DCP-rich CPC was prepared by mixing 0.8 g DCP-rich CPC powder (P) with 0.1, 0.2, and 0.4 mL type I collagen (S) solution to create mixtures with S/P ratios of 0.125, 0.25, and 0.5 mL/g, respectively. DCP-rich CPC with no collagen served as the control. The formation rate of collagen-containing DCP-rich CPC is not affected with the addition of collagen solution [
4]. The samples were loaded into a 1 mL syringe with the needle removed for injection as per previous experiments on D1 cell attachment and L4–L5 posterolateral fusion in lumbar vertebrae models [
12].
2.4. In Vitro Study
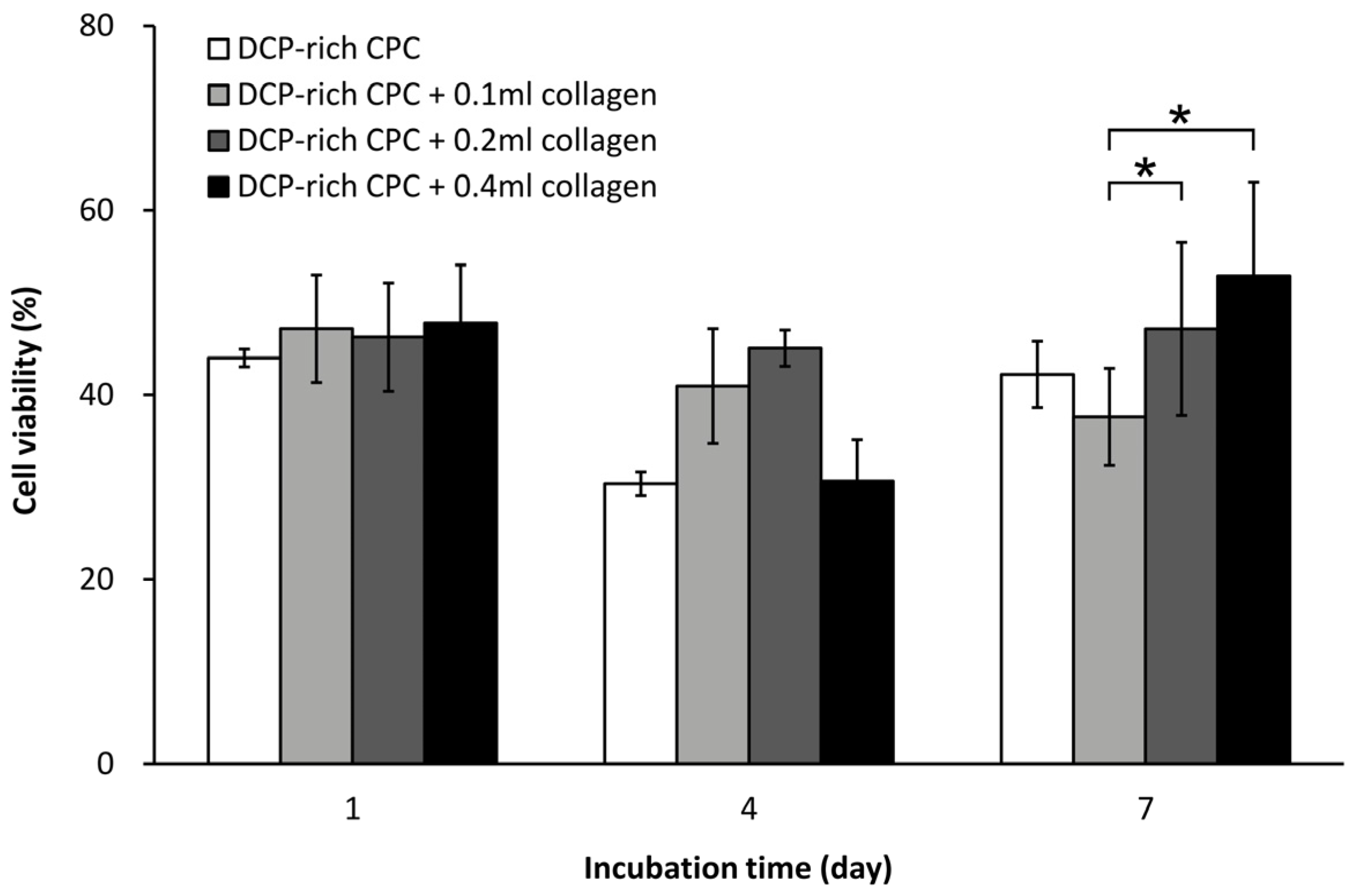
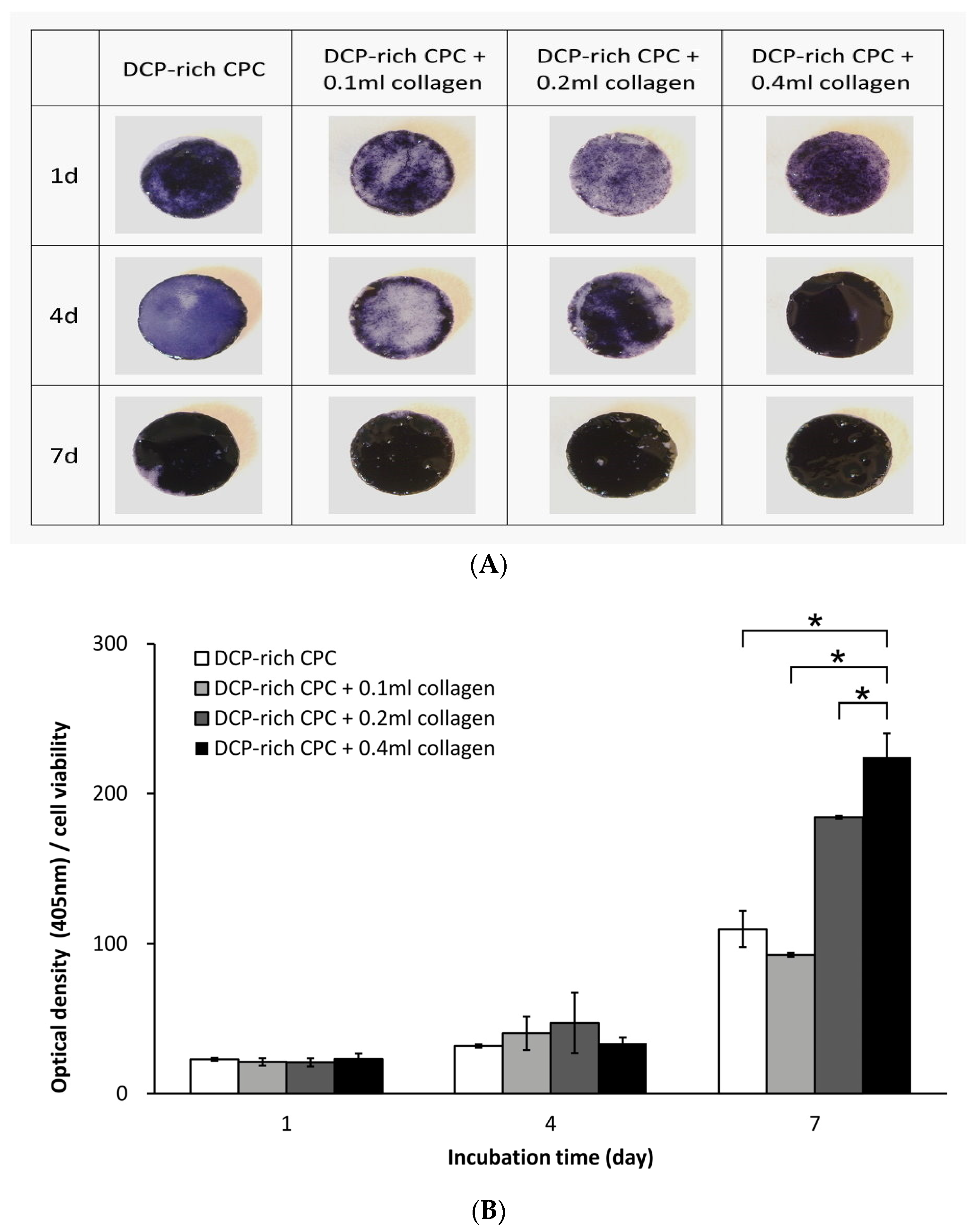
The D1 cells (bone-marrow derived MSCs from Balb/C mice) were purchased from the American Type Culture Collection. The cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum at 37 °C under a humidified 5% CO2 atmosphere, and were used before their eighth passage. The expression of ALP is a widely known osteogenic biomarker and has been commonly used in measuring the differentiation ability of progenitor bone cells. Cell proliferation and ALP activity were examined at one, four, and seven days after initial seeding of 1 × 105 D1 cells on the surface of the specimen. The Alamar Blue VR assay (Bio-Rad Laboratories, Hercules, CA, USA) and p-nitrophenyl phosphate assay (Sigma-Aldrich, Saint Louis, MO, USA) were used to determine cell viability and production of ALP, respectively. These assays were performed according to instructions from the manufacturer. After the set culturing time, blank references for the Alamar Blue VR assay were measured to coincide with a cell viability of 0%. Cell optical density was read at a wavelength of 575 and 595 nm using a microplate enzyme-linked immunosorbent assay (ELISA) reader system (Spec384; Molecular Device, Sunnyvale, CA, USA). The ALP optical density at 405 nm was also determined. Each experiment was performed in triplicate (n = 3). Production of ALP was further verified by ALP staining with serum tartrate-resistant acid phosphatase and ALP double-stain kit (Takara Bio, Shiga, Japan). ALP-stained samples were washed prior to gross examination using light microscopy.
2.5. Morphology of Cell Attachment
D1 cells (1 × 105 cells) were seeded onto the surface of the control sample and the DCP-rich CPC supplemented with type I collagen samples in 48-well plates. Morphology of the D1 cells in the different groups was observed using a scanning electron microscope (SEM; Hitachi S-3000N; Hitachi High-Technologies, Tokyo, Japan) equipped with energy dispersive spectroscopy (Horiba EX220; Horiba, Kyoto, Japan). After one hour, one day, and two days, the D1 cells were washed with 1 × PBS to remove all residual solution. The cells were then fixed with glutaraldehyde and the samples were gold coated and analyzed using SEM.
2.6. In Vivo Study
2.6.1. L4–L5 Lumbar Vertebrae Posterolateral Fusion Surgical Procedure
Forty Sprague Dawley (SD) rats (male, eight weeks old, 300–330 g) were used in this study. The rats were randomly divided into the control group and the group of 0.8 g DCP-rich CPC powder with 0.4 mL type I collagen according to the bone graft substitute to be implanted. The L4–L5 posterolateral fusion surgical procedure was performed after the rats were placed under general anesthesia by administration of tiletamine/zolazepam (Zoletil, 40 mg/kg bodyweight) and xylazine (5 mg/kg bodyweight) via intraperitoneal injection. The posterolateral lumbar fusion was performed following well-established procedures [
13]. Briefly, the rats were placed in the prone position, and the surgical site was shaved and disinfected. A posterior midline incision of approximately 30 mm was made on the skin over the lumbar spine in the L4–L5 area. Subsequently, two linear incisions were made 3–5 mm laterally from the midline on the lumbar fascia. The soft tissue was carefully dissected, and the transverse processes of the L4 and L5 vertebrae were exposed. Bilateral transverse process decortication was performed using a low-speed burr until bleeding from the bone marrow could be verified. A block of properly-shaped DCP-rich CPC (0.1 mL) with or without type I collagen was placed between the transverse processes of the L4 and L5 vertebrae on each side. Finally, the fascia and skin incisions were closed with a 3-0 nylon suture. Postoperative pain was treated with ketoprofen (3 mg/kg body weight). The animals were monitored daily until they were sacrificed one to four weeks after surgery via a cardiac injection of ethanol. All procedures in this animal study were approved by the Institutional Animal Care and Use Committee of Show Chwan Memorial Hospital (Approval No. 102031). The National Institutes of Health guidelines for the care and use of laboratory animals were observed. Furthermore, we calculated the residual graft ratio between the L4–L5 transverse processes in order to evaluate CPC resorption in both groups.
2.6.2. Radiographic Analysis
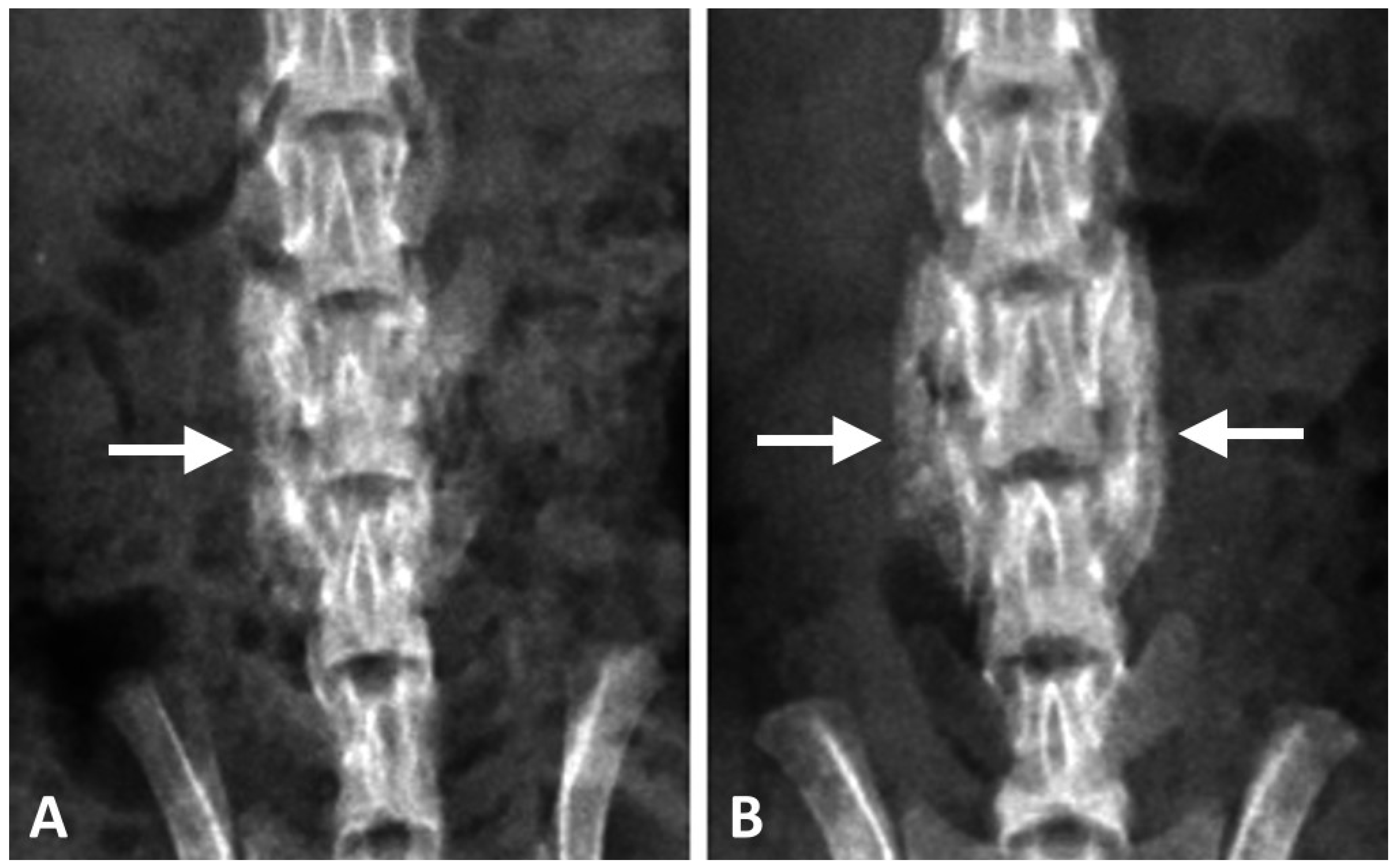
Posteroanterior radiographs (In-Vivo Xtreme; Bruker Corp., Billerica, MA, USA) of the spine were taken one to four weeks post-operation to assess spinal fusion. Bone fusion between the transverse processes of the L4 and L5 vertebrae was independently evaluated by two orthopedic surgeons. The rats were sacrificed after radiographic examination. The lower part of the spine (L1–L6) was harvested for the following analyses.
2.6.3. Microcomputed Tomography
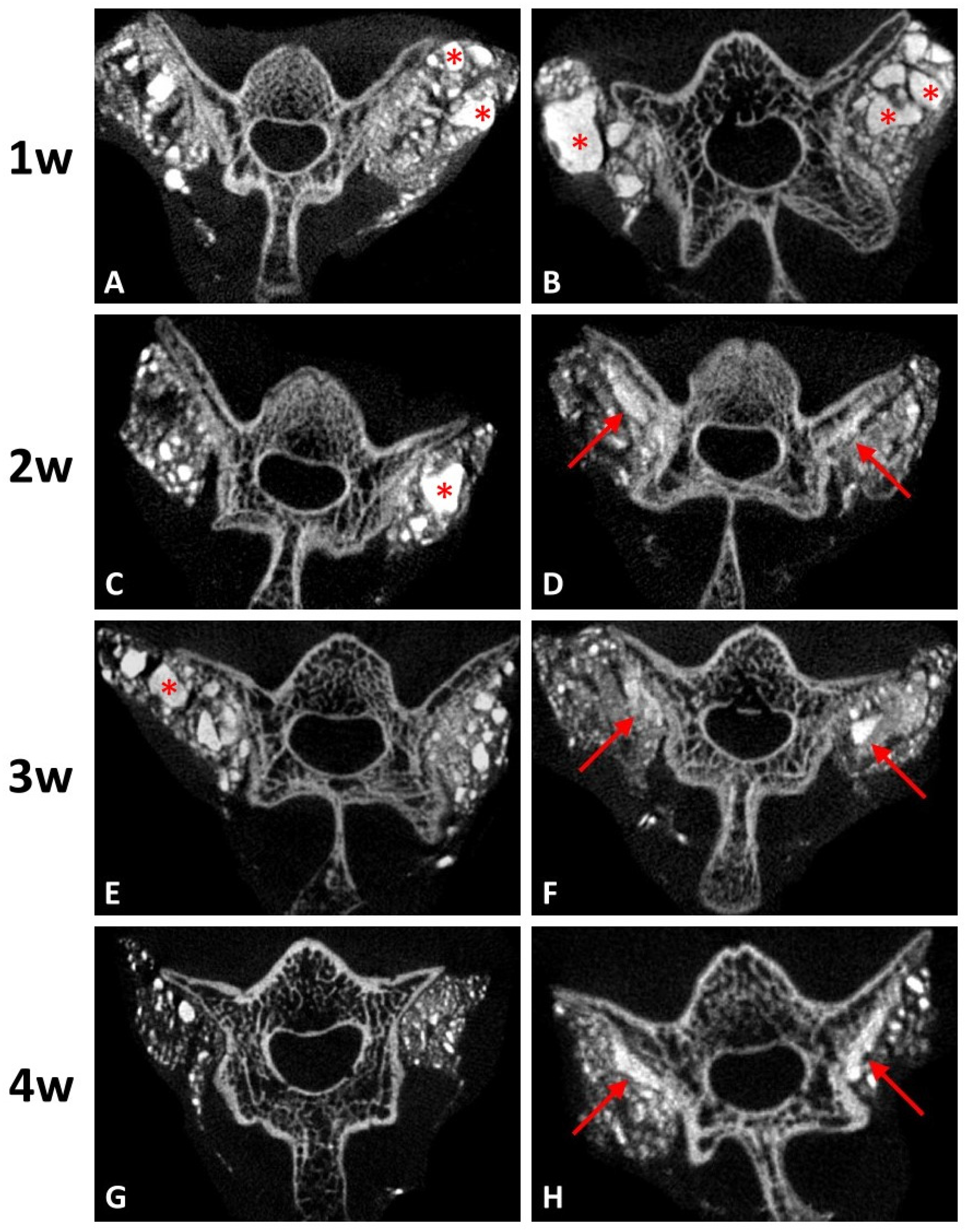
Micro-CT (SkyScan 1076, Bruker, Billerica, MA, USA) was performed on all specimens harvested. The residual ratio of graft-to-defect was calculated using image analysis software (Mimics; Materialise, Leuven, Belgium). The volume of residual implant on each side was calculated postoperatively. Residual graft ratio was calculated using the following equation: Residual graft ratio, % = [total volume of residual implant/total volume of original implant (0.1 mL)] × 100%.
2.6.4. Histological Analysis
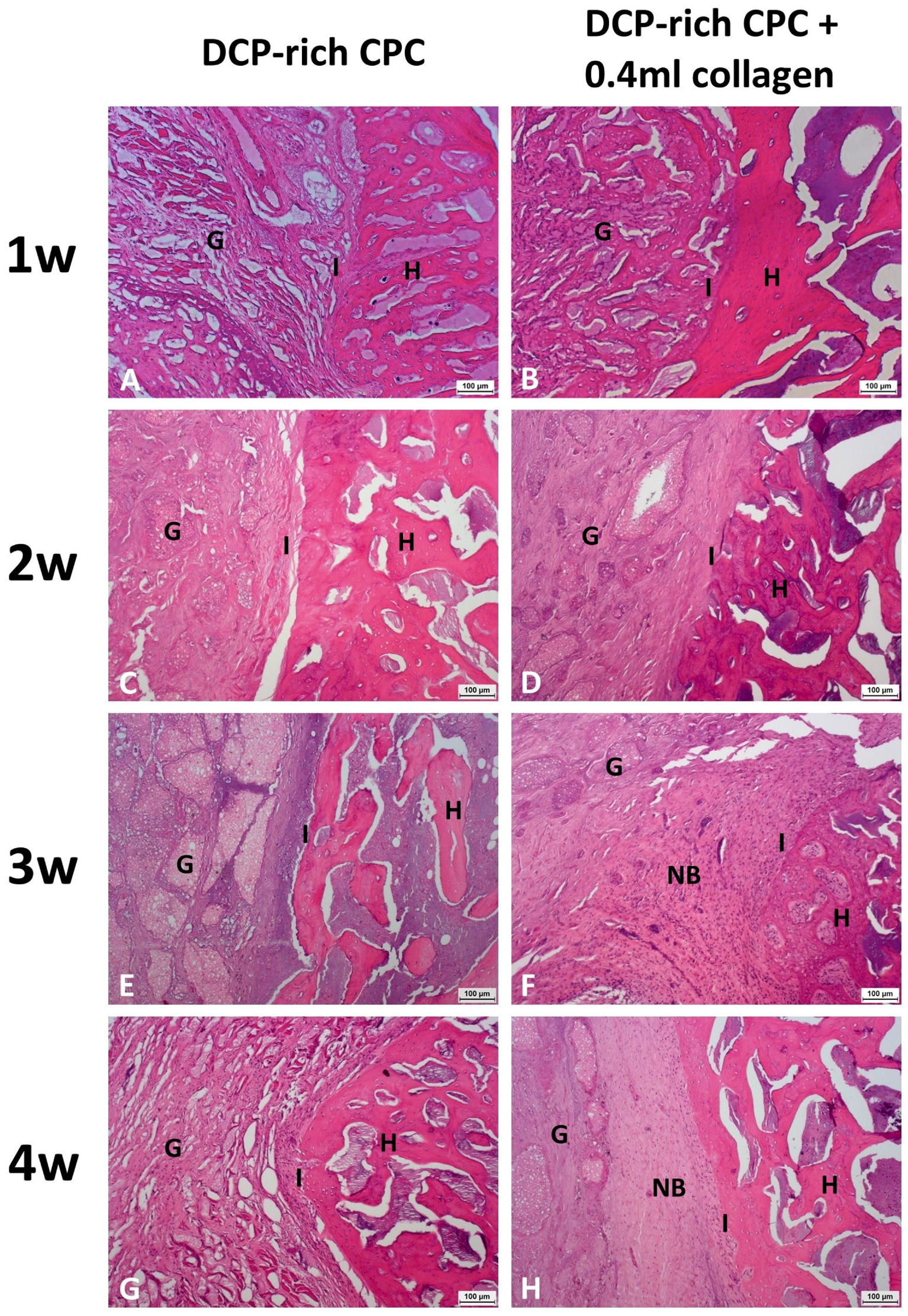
Specimens were fixed for 24 h in 10% formalin for histological analysis. The tissue was dehydrated with 50, 70, and 95% ethanol for one hour each, and with 100% ethanol for two hours. After dehydration, the tissue was left overnight at room temperature in the resin mixture, consisting of a 3:1 solution of methyl methacrylate and dibutyl phthalate. The tissue was then embedded in a polyethylene molding cup. Coronal sections were obtained from both sides of each rat (n = 3). Four-micron sections were cut and stained with hematoxylin and eosin (H&E) for analysis of general morphology and identification of the slides. Images were acquired with a light microscope (Leica DMI 3000B; Leica Biosystems, Wetzlar, Germany) equipped with a digital camera (Leica DFC295; Leica Microsystems, Wetzlar, Germany). Histological analysis was performed every week after surgery for both groups.
2.6.5. Statistical Analysis
One-way analysis of variance (ANOVA) in conjunction with Holm-Sidak post hoc testing was used to compare the results. In all cases, results were considered significantly different when p < 0.05.
4. Discussion
Autologous bone grafting has been the gold standard treatment in posterolateral lumbar fusion for many decades; however, issues associated with the use of autografts include limited availability and variability of graft quality, increased risk of hematoma, infection, and bleeding, increased operative time, chronic donor site pain, and high cost [
16]. Subsequently, much effort has been devoted to the development of novel bone graft substitutes [
17]. Similar composition to the mineral phase of bone as well as good biodegradability, bioactivity, and osteoconductivity support the rationale behind the use of calcium phosphate as a bone substitute. Among the different CPCs, DCP-rich CPC with a Ca/P ratio of 1.50 shows great potential for success in spinal fusion [
4]. While a previous study showed an increase in osteogenic ability of bone repair with incorporation of collagen into bone cement [
6], the optimal amount of collagen to maximize cell viability has not yet been determined, especially for DCP-rich CPC. The incorporation of solubilized collagen increases the collagen concentration of CPCs without reducing workability. CPC containing 1% solubilized collagen has been shown to have the highest adhesion while promoting proliferation and differentiation of human osteoblast-like SAOS-2 cells [
10]. Study results support that the effect on mechanical properties of CPC from the addition of collagen is dependent on collagen concentration.
Previous studies have revealed that mouse MSCs show higher ALP activity and higher mRNA expression of type I collagen during osteogenic differentiation, and that type I collagen can induce MSC differentiation in vitro [
18,
19,
20]. Our study found that the addition of collagen promoted attachment of D1 cells onto the surface of DCP-rich CPC and increased cell viability. The D1 cells had greater attachment on the DCP-rich CPC with 0.4 mL collagen of bone graft substitute than on DCP-rich CPC only bone graft substitute on day 2; the phenomenon was not apparently observed with 0.1 or 0.2 mL collagen added. In a cell model, we observed that D1 cell viability initially decreased with the DCP-rich CPC supplemented with 0.4 mL collagen by day 4, followed by an increase from day 4 to day 7. Upon further observation, ALP activity and viability ratio of D1 cells significantly increased from day 4 to day 7. Our results show that DCP-rich CPC supplemented with collagen better elicited the early stage of progenitor D1 cell differentiation on surfaces than DCP-rich CPC without collagen. The results may be attributed to weaker initial adherence ability of D1 cells during the first four days.
Our study demonstrated that the addition of 0.4 mL of 0.1% type I collagen to 0.8 g DCP-rich CPC enhanced osteointegration in the space between host bone and graft in a posterolateral lumbar fusion model. Histologically, a larger quantity of osteoblast-deposited lamellae of new bone tissue and a greater number of osteocytes were observed in the DCP-rich CPC supplemented with the collagen group than in the DCP-rich CPC group. It has been noted that the ions released from CPCs could cause ion–dipole interaction, cell adhesion, proliferation, and differentiation [
12]. The biochemical signaling molecules secreted by osteocytes and osteoblasts to stimulate osteogenic differentiation of MSCs has also been well-recognized [
21]. Therefore, the results might suggest that rapid CPC resorption could be achieved with a low Ca/P formula of DCP in CPC at an early stage. Our study found that regenerated bone volume at the implantation site was considerably greater in the DCP-rich CPC with 0.4 mL collagen group than in the control group, suggesting that the implanted DCP-rich CPC with 0.4 mL collagen largely transformed into bridging callus. Bone healing versus graft resorption was detectable and several calcium phosphate remnants were surrounded by provisional connective tissue, which would be eventually resorbed and transformed into new alveolar bone in situ.
There were several limitations in this study. Firstly, the in vitro study only analyzed three concentrations of collagen-containing DCP-rich CPC. The most effective concentration of DCP-rich CPC supplemented with collagen might not be included. However, we hope this study could be an anchor for following research. Secondly, only the DCP-rich CPC supplemented with 0.4 mL collagen was tested in the in vivo study because it resulted in the highest levels of cell viability, cell attachment, and ALP staining. However, if the in vivo study also included the group of the DCP-rich CPC supplemented with 0.1 mL or 0.2 mL collagen, we might obtain a better understanding of the effect of osteogenesis of type I collagen.
In this study, we systematically evaluated the effect of added collagen to the biological performance of the DCP-rich CPC and its ability to affect osteogenic differentiation in vitro and in vivo. Our results show that collagen not only promoted D1 cell attachment and proliferation, but that it also elicited the early stage of progenitor cell differentiation in terms of morphological characteristics and ALP activity. The DCP-rich CPC supplemented with collagen was also associated with a significantly increased bone turnover rate of L4–L5 transverse processes as shown via micro-CT. Overall, our results suggest that the incorporation of collagen promotes absorption of the DCP-rich CPC by osteoclasts as well as the laying down of a new osteoid matrix by osteoblasts, and that it has potential to be an effective treatment for use in bone defect restoration in spinal diseases.