Thermal Performance Study of Composite Phase Change Material with Polyacrylicand Conformal Coating
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the Composite PCMs
2.3. Characterization
3. Results and Discussion
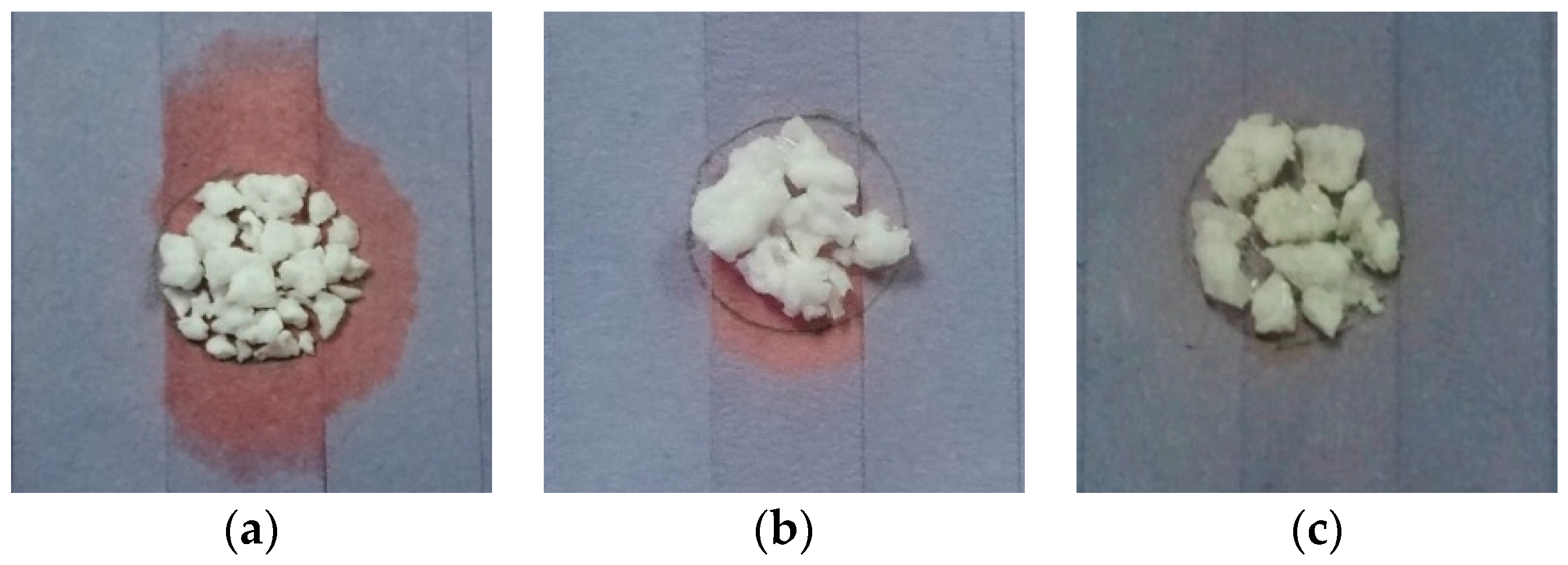
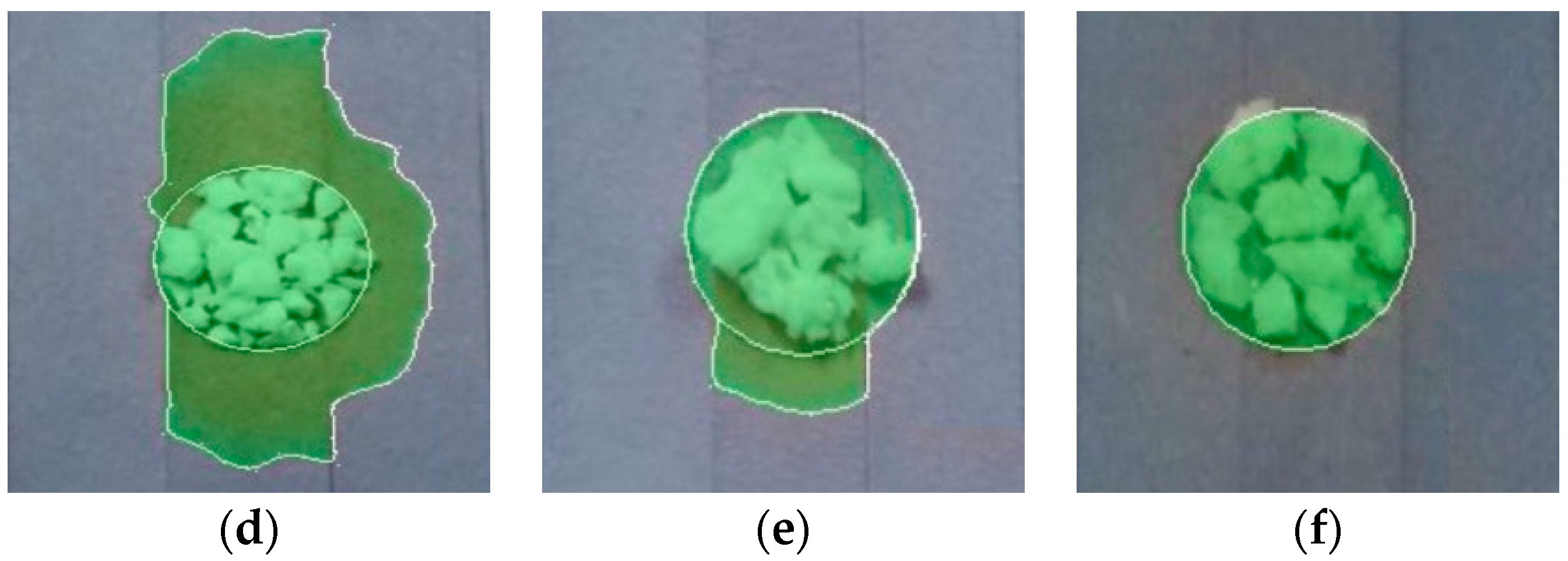
3.1. Leakage Analysis
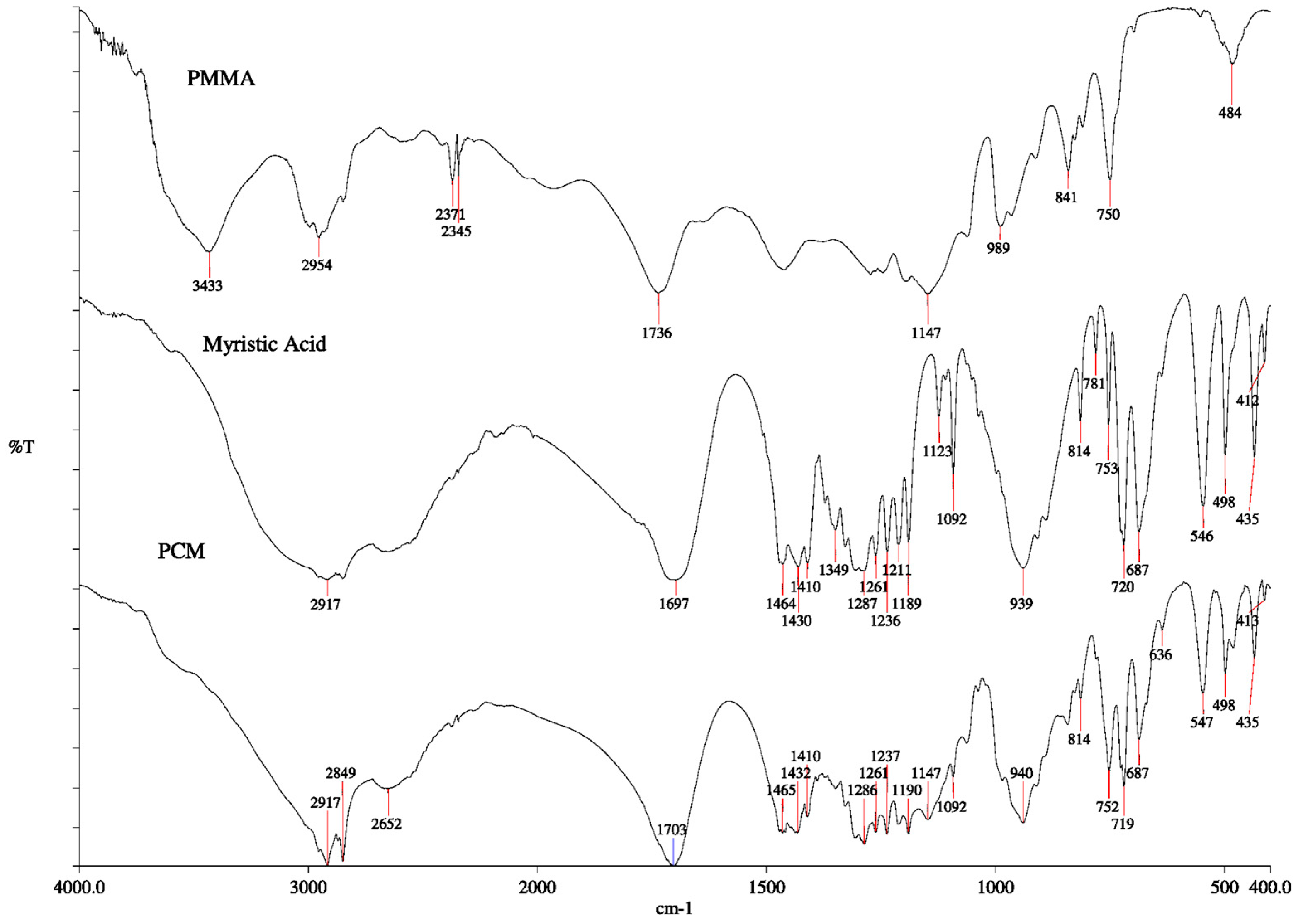
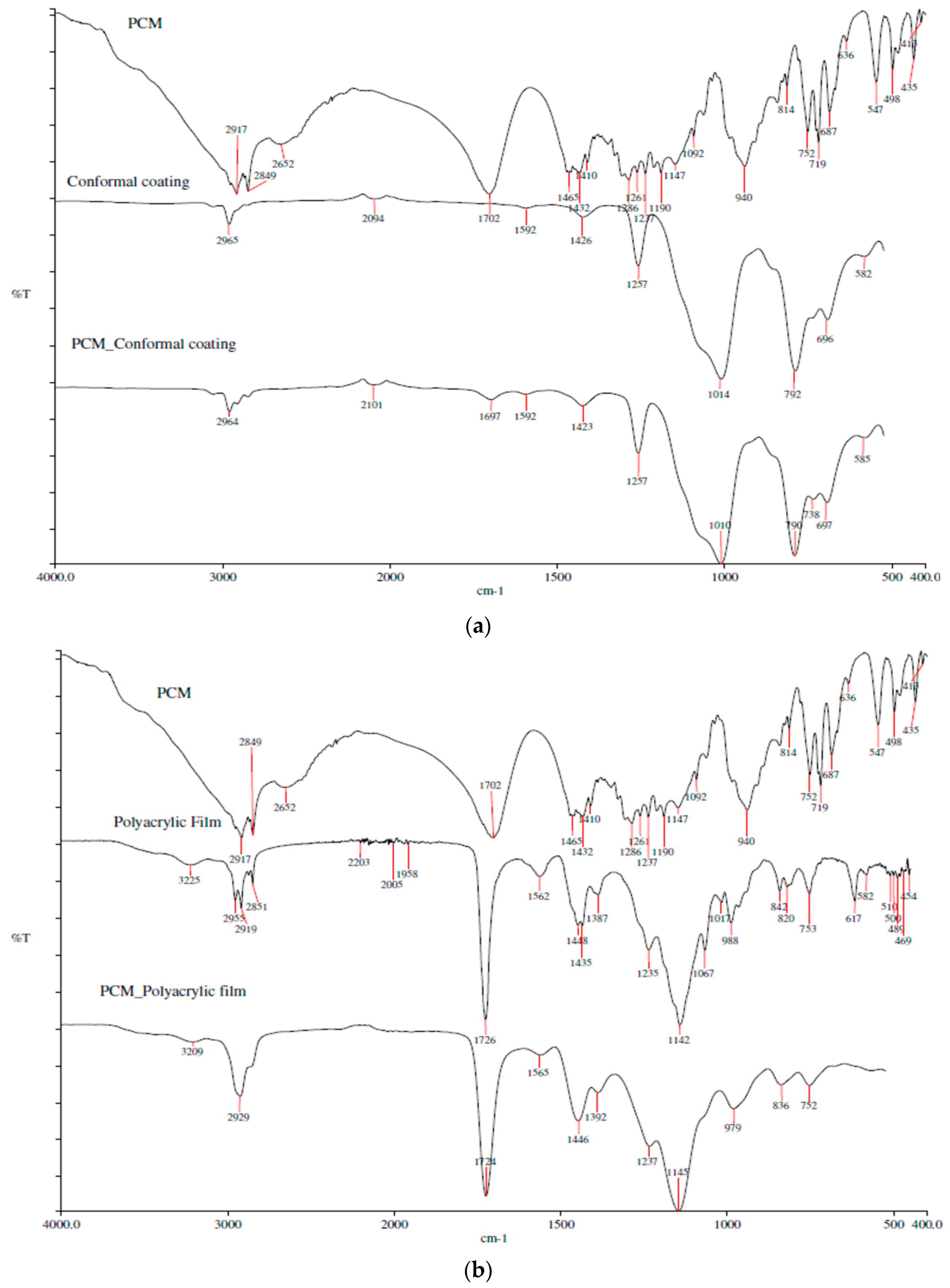
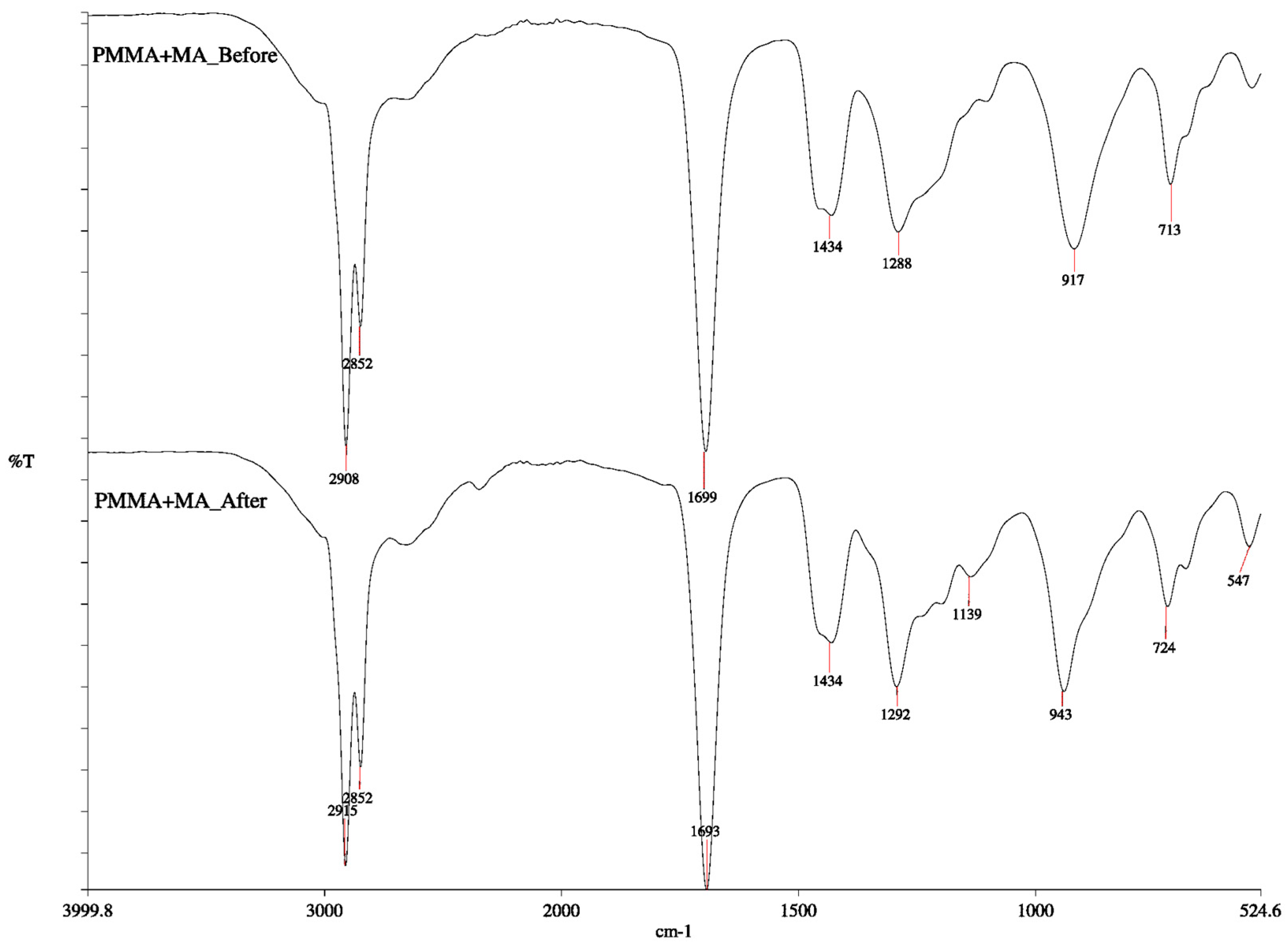
3.2. FTIR Analysis
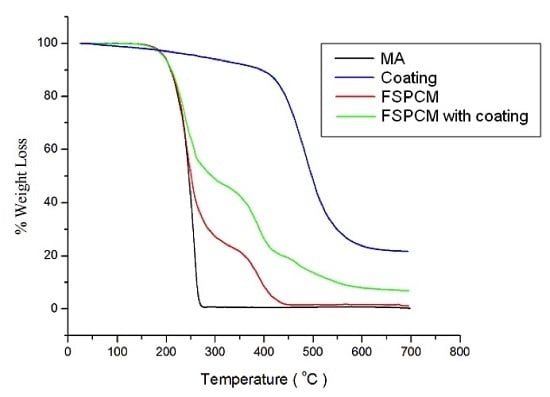
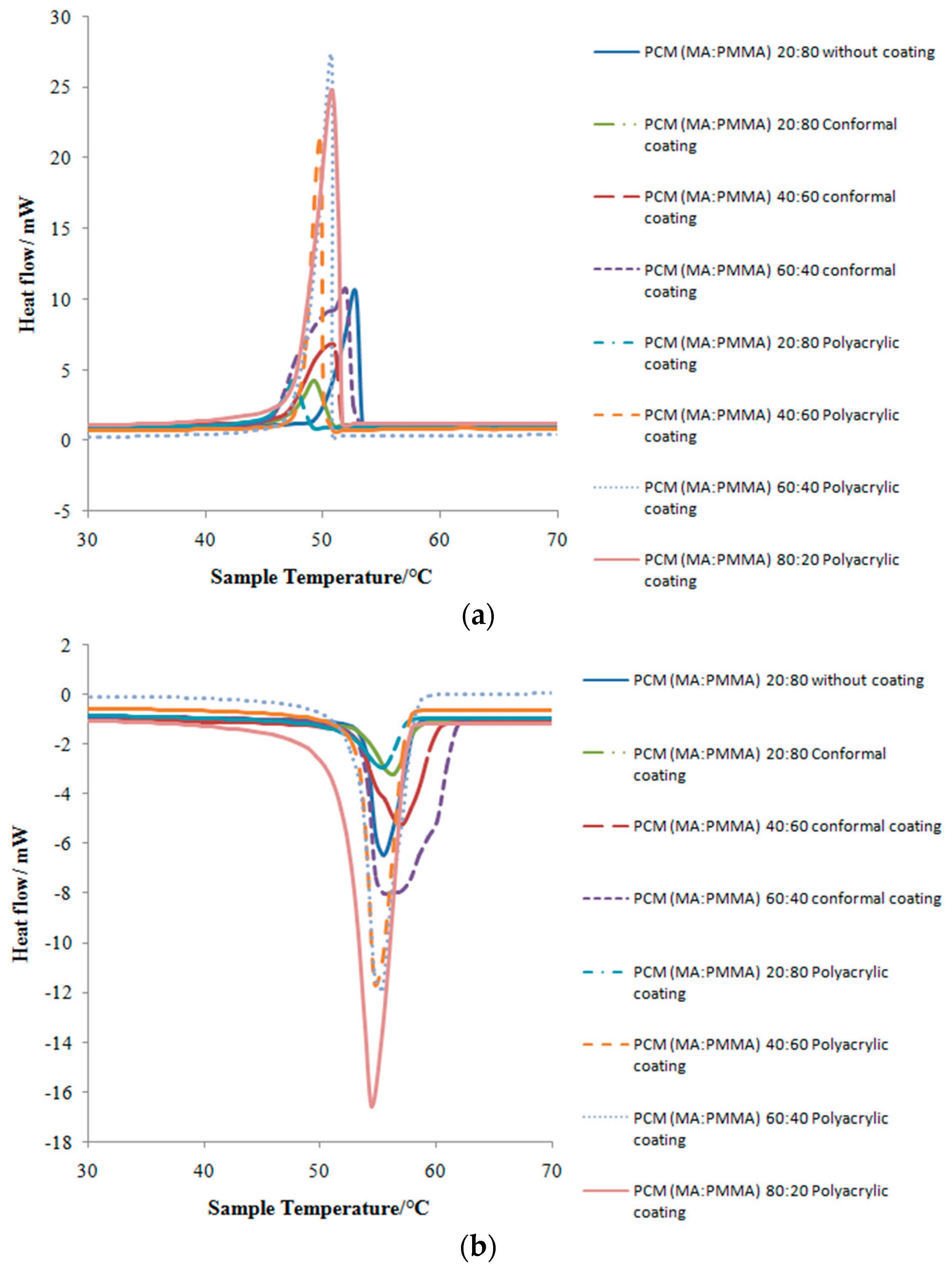
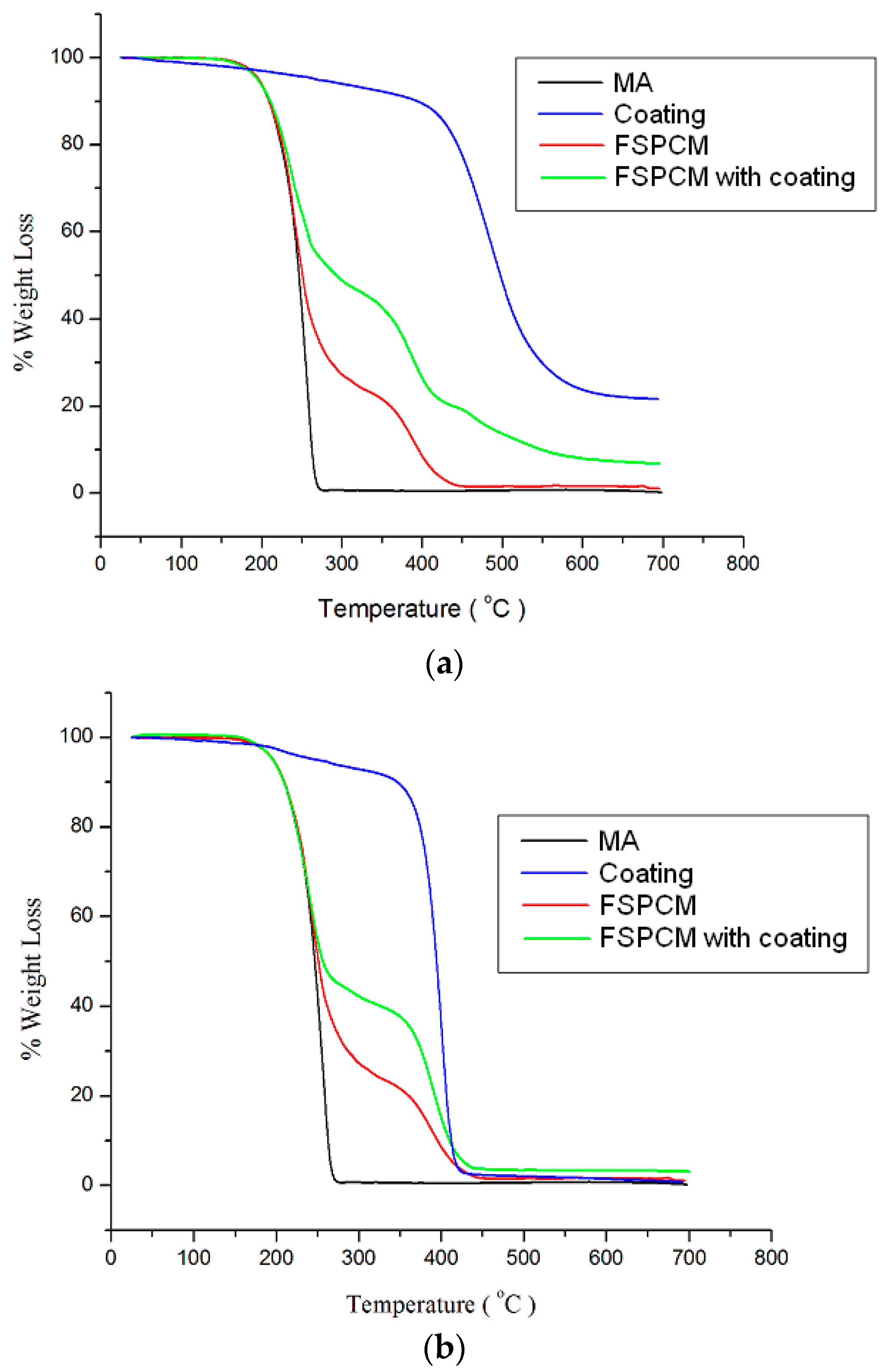
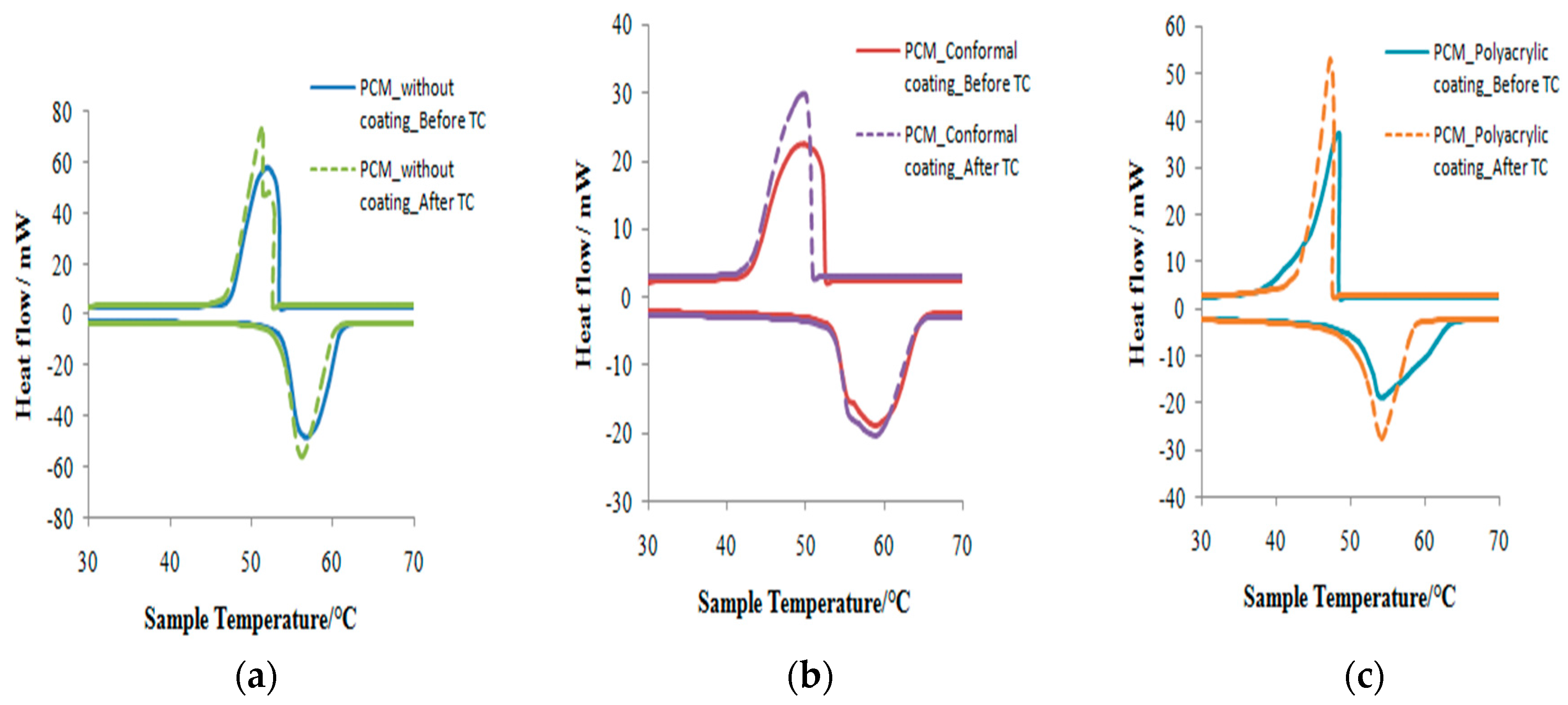
3.3. Thermal Properties and Thermal Stability Analyses
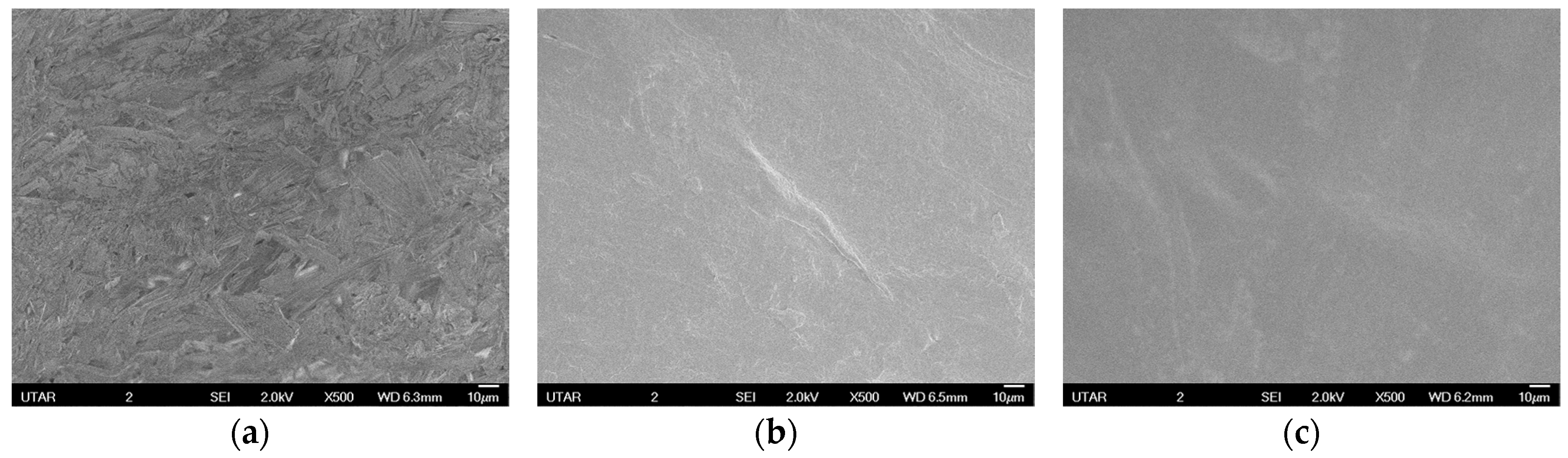
3.4. Morphology Analysis
3.5. Reliability Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mekhilef, S.; Saidur, R.; Safari, A. A review on solar energy use in industries. Renew. Sustain. Energy Rev. 2011, 15, 1777–1790. [Google Scholar] [CrossRef]
- Liu, X.; Lin, C.; Rao, Z. Diffusion and thermal conductivity of the mixture of paraffin and polystyrene for thermal energy storage: A molecular dynamics study. J. Energy Inst. 2016. [Google Scholar] [CrossRef]
- Al-Hinti, I.; Al-Ghandoor, A.; Maaly, A.; Abu Naqeera, I.; Al-Khateeb, Z.; Al-Sheikh, O. Experimental investigation on the use of water-phase change material storage in conventional solar water heating systems. Energy Convers. Manag. 2010, 51, 1735–1740. [Google Scholar] [CrossRef]
- Sivakumar, R.; Sivaramakrishnan, V.; Vivekanandan, M. Experimental study on depth of paraffin wax over floating absorber plate in built-in storage solar water heater. Adv. Mech. Eng. 2015, 7. [Google Scholar] [CrossRef]
- Lee, K.O.; Medina, M.A.; Raith, E.; Sun, X. Assessing the integration of a thin phase change material (PCM) layer in a residential building wall for heat transfer reduction and management. Appl. Energy 2015, 137, 699–706. [Google Scholar] [CrossRef]
- Tong, W.; Tong, A. Solar-absorbing metamaterial microencapsulation of phase change materials for thermo-regulating textiles. Int. J. Smart Nano Mater. 2015, 6, 105–112. [Google Scholar] [CrossRef]
- Fenollera, M.; Míguez, J.L.; Goicoechea, I.; Lorenzo, J.; ÁngelÁlvarez, M. The influence of phase change materials on the properties of self-compacting concrete. Materials 2013, 6, 3530–3546. [Google Scholar] [CrossRef]
- Memon, S.A.; Liao, W.; Yang, S.; Cui, H.; Shah, S.F.A. Development of composite PCMs by incorporation of paraffin into various building. Materials 2015, 8, 499–518. [Google Scholar] [CrossRef]
- Shukla, A.; Buddhi, D.; Sawhney, R.L. Solar water heaters with phase change material thermal energy storage medium: A review. Renew. Sustain. Energy Rev. 2009, 13, 2119–2125. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of art. Renew. Sustain. Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Solar energy storage using phase change materials. Renew. Sustain. Energy Rev. 2007, 11, 1913–1965. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Dinker, A.; Agarwal, M.; Agarwal, G.D. Heat storage materials, geometry and applications: A review. J. Energy Inst. 2017, 90, 1–11. [Google Scholar] [CrossRef]
- Rozanna, D.; Chuah, T.G.; Salmiah, A.; Choong, T.S.Y.; Sa’ari, M. Fatty acids as phase change materials (PCMs) for thermal energy storage: A Review. Int. J. Green Energy 2005, 1, 495–513. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Huang, J.; Lu, S.; Kong, X.; Liu, S.; Li, Y. Form-stable phase change materials based on eutectic mixture of tetradecanol and fatty acids for building energy storage: Preparation and performance analysis. Materials 2013, 6, 4758–4775. [Google Scholar] [CrossRef]
- Wang, L.; Meng, D. Fatty acid eutectic/polymethyl methacrylate composite as form-stable phase change material for thermal energy storage. Appl. Energy 2010, 87, 2660–2665. [Google Scholar] [CrossRef]
- Song, S.; Dong, L.; Chen, S.; Xie, H.; Xiong, C. Stearic–Capric acid eutectic/activated-attapulgiate composite as form-stable phase change material for thermal energy storage. Energy Convers. Manag. 2014, 81, 306–311. [Google Scholar] [CrossRef]
- Alkan, C.; Sari, A. Fatty acid/poly(methyl methacrylate) (PMMA) blends as form-stable phase change materials for latent heat thermal energy storage. Sol. Energy 2008, 82, 118–124. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Huang, Y. Morphology and thermal properties of electrospun fatty acids/polyethylene terephthalate composite fibers as novel form-stable phase change materials. Sol. Energy Mater. Sol. Cells 2008, 92, 1382–1387. [Google Scholar] [CrossRef]
- Gasia, J.; Miró, L.; de Gracia, A.; Barreneche, C.; Cabeza, L.F. Experimental evaluation of a paraffin as phase change material for thermal energy storage in laboratory equipment and in a shell-and-tube heat exchanger. Appl. Sci. 2016, 6, 112. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chen, H.; Li, X.; Sanjayan, J.G. Development of thermal energy storage composites and prevention of PCM leakage. Appl. Energy 2014, 135, 225–233. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Sanjayan, J.; Wang, X.; Alam, M.; Wilson, J. A novel paraffin/expanded perlite composite phase change material for prevention of PCM leakage in cementitious composites. Appl. Energy 2015, 157, 85–94. [Google Scholar] [CrossRef]
- Kheradmand, M.; Castro-Gomes, J.; Azenha, M.; Silva, P.D.; de Aguiar, J.L.B.; Zoorob, S.E. Assessing the feasibility of impregnating phase change materials in lightweight aggregate for development of thermal energy storage systems. Constr. Build. Mater. 2015, 89, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Zhong, Y.; Rong, X.; Min, C.; Qi, C. Building energy storage panel based on paraffin/expanded perlite: Preparation and thermal performance study. Materials 2016, 9, 70. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, T.D.; Feng, H.X.; Zhang, H. Stearic acid/polymethylmethacrylate composite as form-stable phase change materials for latent heat thermal energy storage. Renew. Energy 2011, 36, 1814–1820. [Google Scholar] [CrossRef]
- Moghbelli, E.; Banyay, R.; Sue, H.-J. Effect of moisture exposure on scratch resistance of PMMA. Tribol. Int. 2014, 69, 46–51. [Google Scholar] [CrossRef]
- Ma, B.; Adhikari, S.; Chang, Y.; Ren, J.; Liu, J.; You, Z. Preparation of composite shape-stabilized phase change materials for highway pavements. Constr. Build. Mater. 2013, 42, 114–121. [Google Scholar] [CrossRef]
- Silakhori, M.; Naghavi, M.S.; Metselaar, H.S.C.; Mahlia, T.M.I.; Fauzi, H.; Mehrali, M. Accelerated thermal cycling test of microencapsulated paraffin wax/polyaniline made by simple preparation method for solar thermal energy storage. Materials 2013, 6, 1608–1620. [Google Scholar] [CrossRef]
- Sun, J.; Wu, Z. Study on evaluation method of exudation of phase transition working substance for building materials. New Build. Mater. 2004, 7, 43–46. [Google Scholar]

| Sample | MA:PMMA | η (%) | ||
|---|---|---|---|---|
| - | (wt %) | Without Coating | Conformal Coating | Polyacrylic Coating |
| 1 | 20:80 | 112.59 | 100.00 | 100.00 |
| 2 | 40:60 | 280.67 | 100.00 | 100.00 |
| 3 | 60:40 | 296.29 | 121.02 | 100.00 |
| 4 | 80:20 | 616.77 | 129.40 | 113.95 |
| Coating | MA:PMMA (wt %) | Melting Point (°C) | Latent Heat of Melting (J/g) | Freezing Point (°C) | Latent Heat of Freezing (J/g) |
|---|---|---|---|---|---|
| No coating | 20:80 | 55.30 | 32.08 | 52.74 | 31.85 |
| Conformal coating | 20:80 | 56.15 | 18.13 | 49.63 | 18.44 |
| 40:60 | 57.09 | 42.94 | 51.20 | 43.13 | |
| 60:40 | 55.34 | 83.73 | 52.46 | 84.07 | |
| Polyacrylic coating | 20:80 | 55.41 | 19.34 | 48.12 | 17.41 |
| 40:60 | 54.93 | 54.49 | 51.47 | 53.68 | |
| 60:40 | 55.02 | 83.20 | 50.72 | 82.08 | |
| 80:20 | 54.19 | 103.14 | 48.47 | 103.27 |
| PCM | Melting Point (°C) | Latent Heat of Melting (J/g) | Freezing Point (°C) | Latent Heat of Freezing (J/g) | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Without coating | 56.62 | 56.23 | 170.65 | 165.79 | 51.86 | 51.51 | 171.89 | 165.82 |
| With conformal coating | 58.84 | 58.79 | 106.12 | 105.95 | 49.73 | 49.64 | 106.75 | 106.40 |
| With polyacrylic coating | 54.19 | 54.18 | 103.14 | 102.09 | 48.47 | 47.79 | 103.27 | 102.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kee, S.Y.; Munusamy, Y.; Ong, K.S.; Cornelis Metselaar, H.S.; Chee, S.Y.; Lai, K.C. Thermal Performance Study of Composite Phase Change Material with Polyacrylicand Conformal Coating. Materials 2017, 10, 873. https://doi.org/10.3390/ma10080873
Kee SY, Munusamy Y, Ong KS, Cornelis Metselaar HS, Chee SY, Lai KC. Thermal Performance Study of Composite Phase Change Material with Polyacrylicand Conformal Coating. Materials. 2017; 10(8):873. https://doi.org/10.3390/ma10080873
Chicago/Turabian StyleKee, Shin Yiing, Yamuna Munusamy, Kok Seng Ong, Hendrik Simon Cornelis Metselaar, Swee Yong Chee, and Koon Chun Lai. 2017. "Thermal Performance Study of Composite Phase Change Material with Polyacrylicand Conformal Coating" Materials 10, no. 8: 873. https://doi.org/10.3390/ma10080873