Evaluation of Toluene Adsorption Performance of Mortar Adhesives Using Porous Carbon Material as Adsorbent
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Test Method
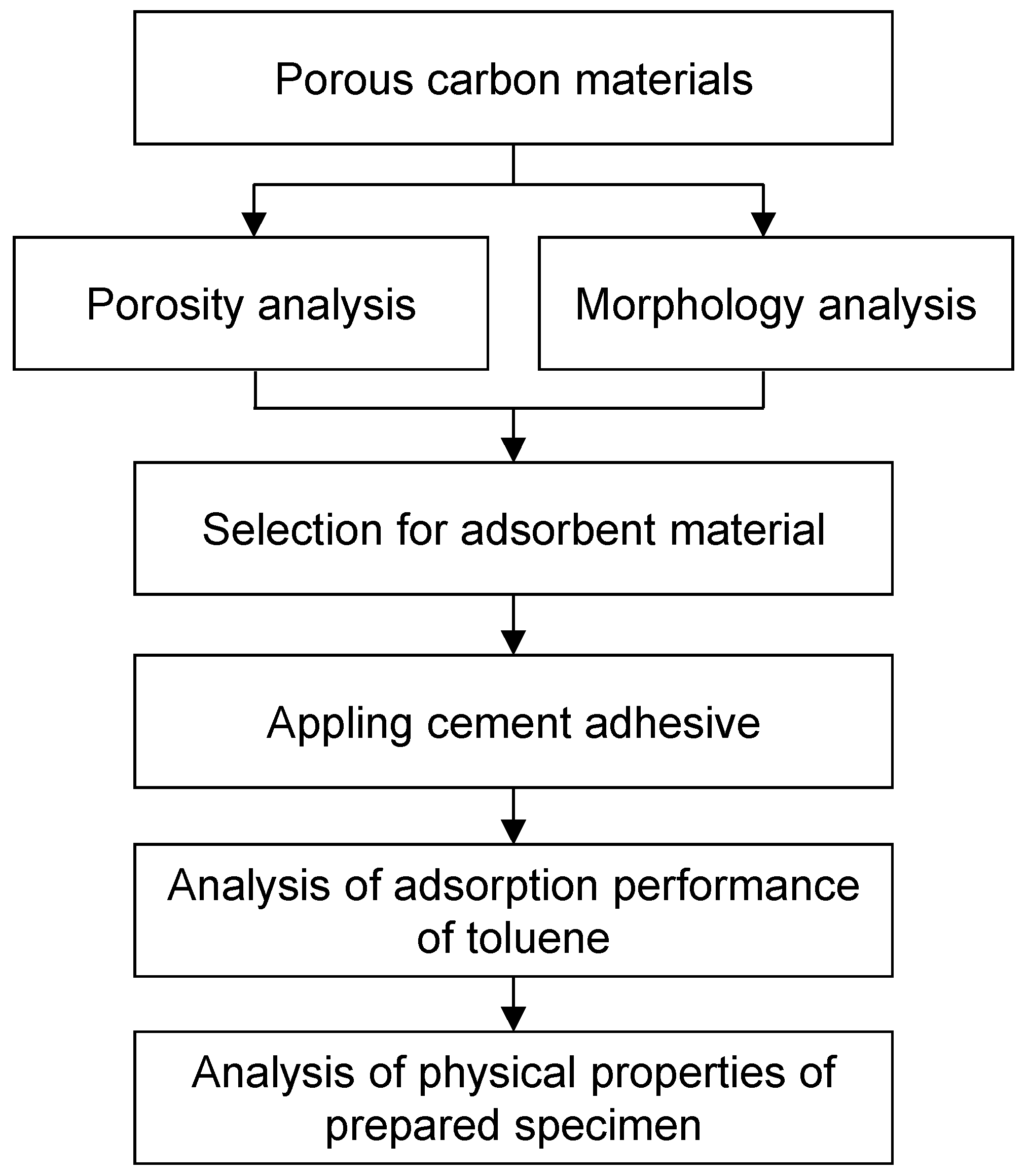
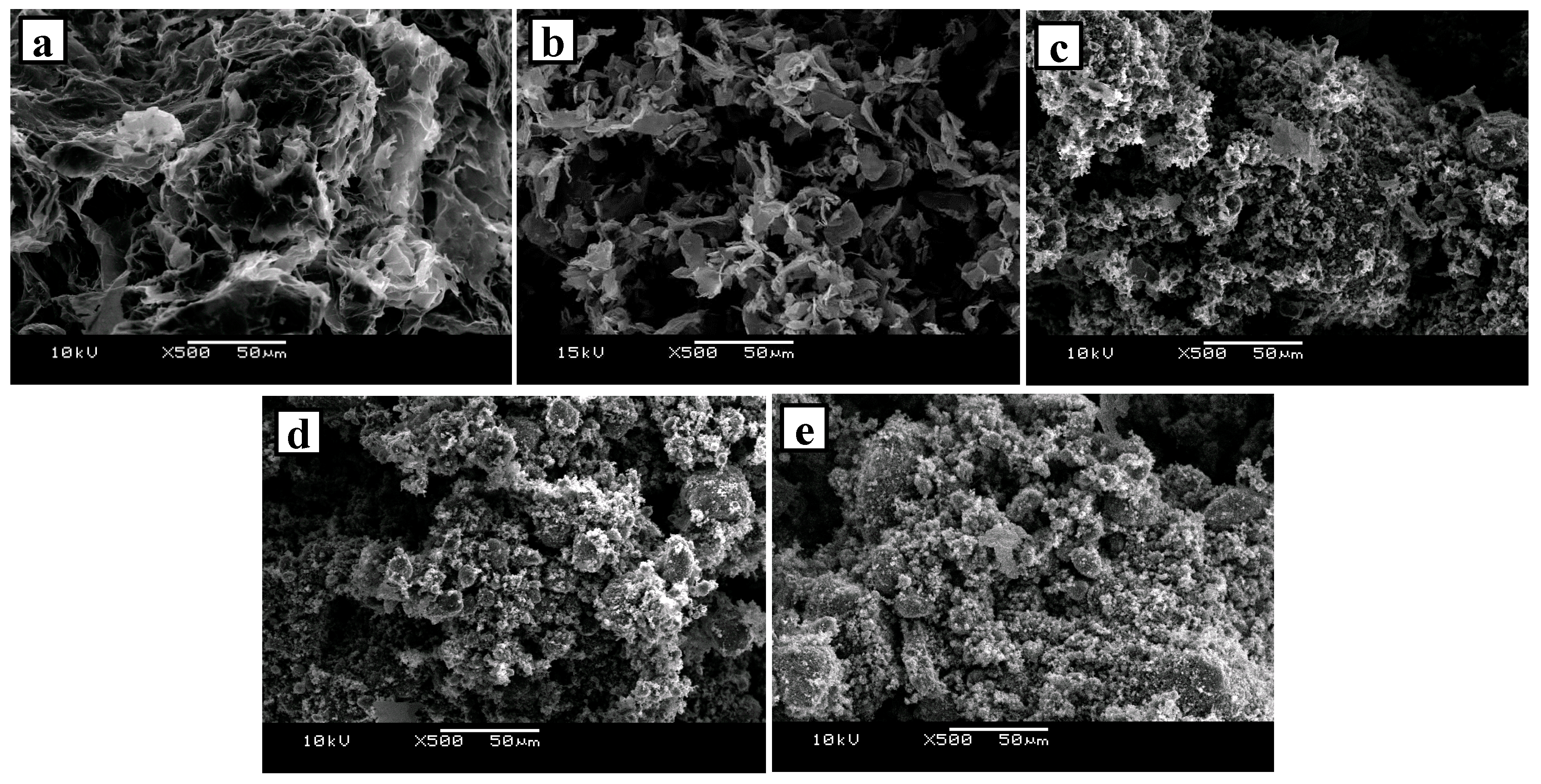
2.2.1. Porosity and Morphology Analysis for Adsorbent Material
2.2.2. Evaluation of Toluene Adsorption Performance Using a 20 L Small Chamber
2.2.3. Evaluation of Physical Properties of Adsorption Mortar Adhesive
3. Results and Discussion
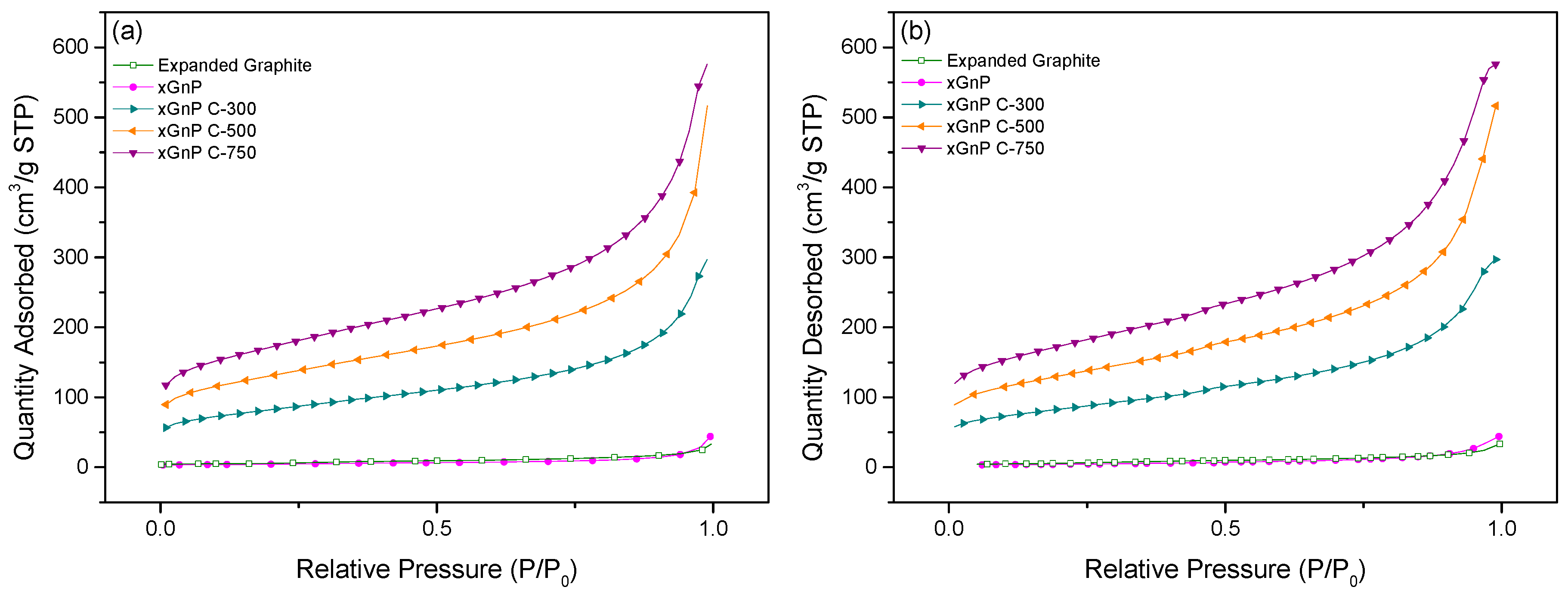
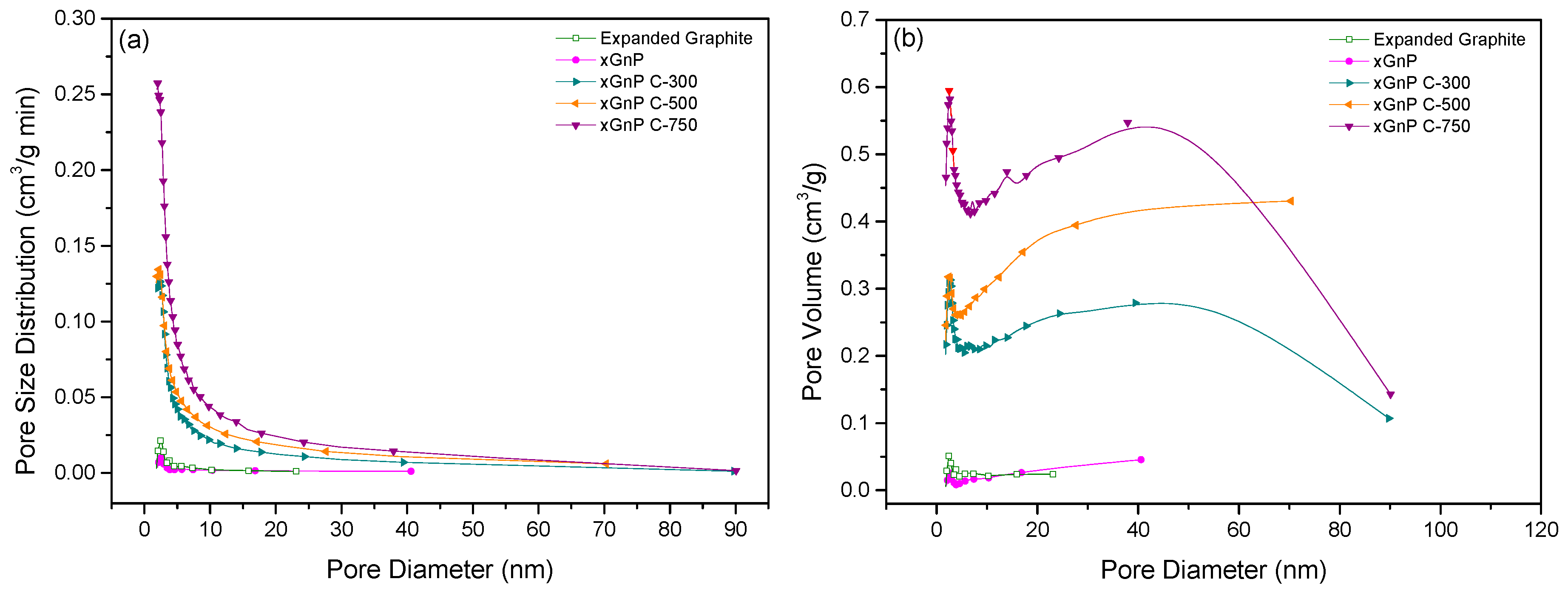
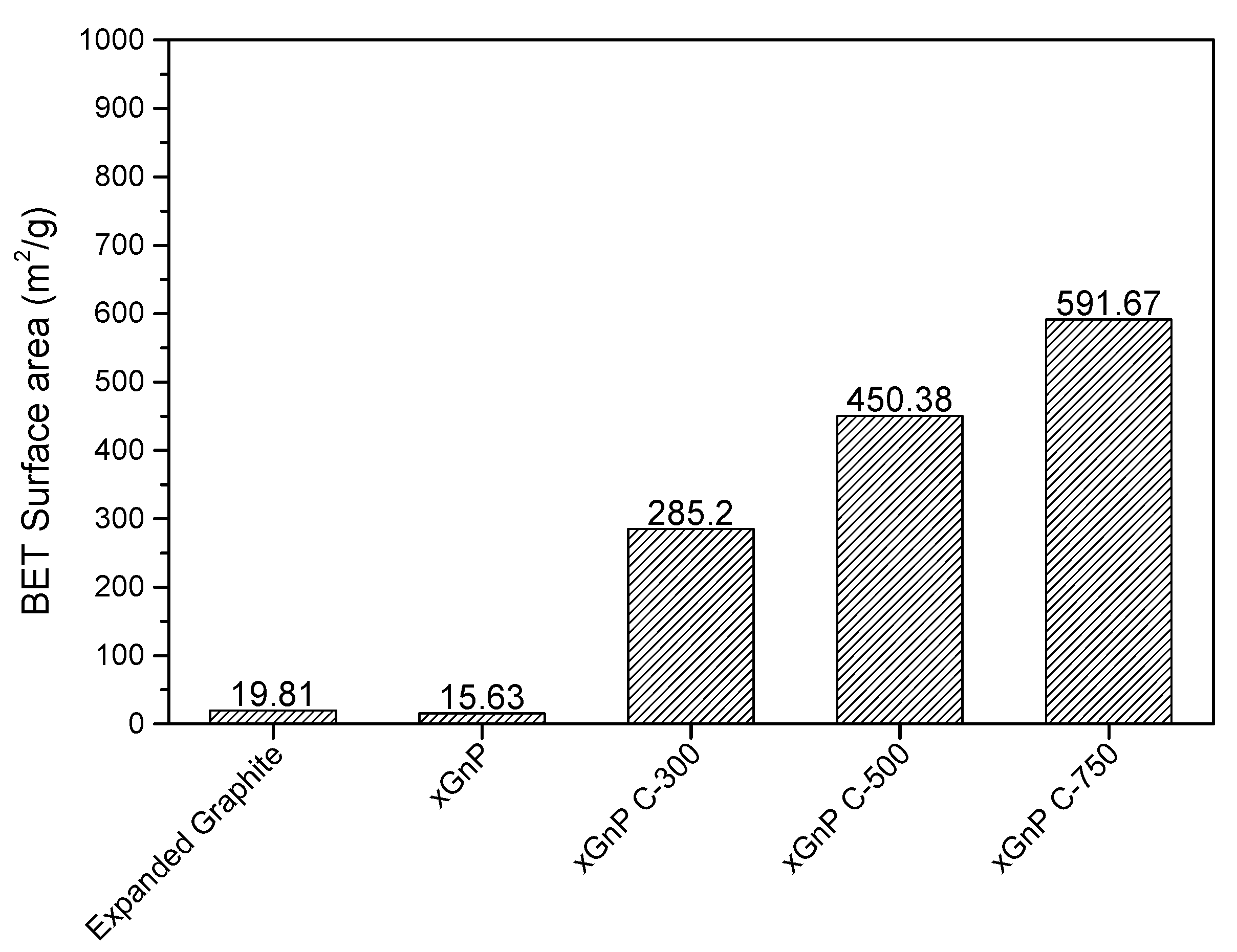
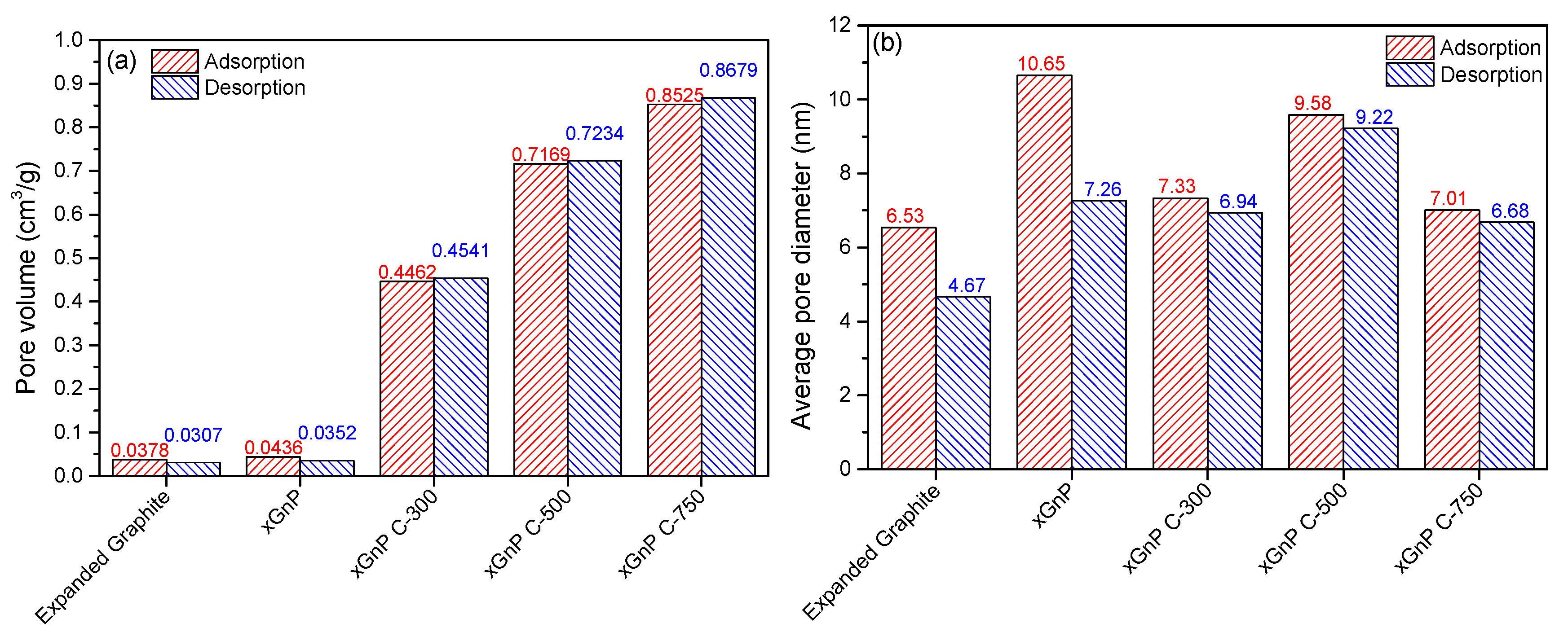
3.1. Porosity and Morphology Analysis of Porous Carbon Material for Adsorbent Material
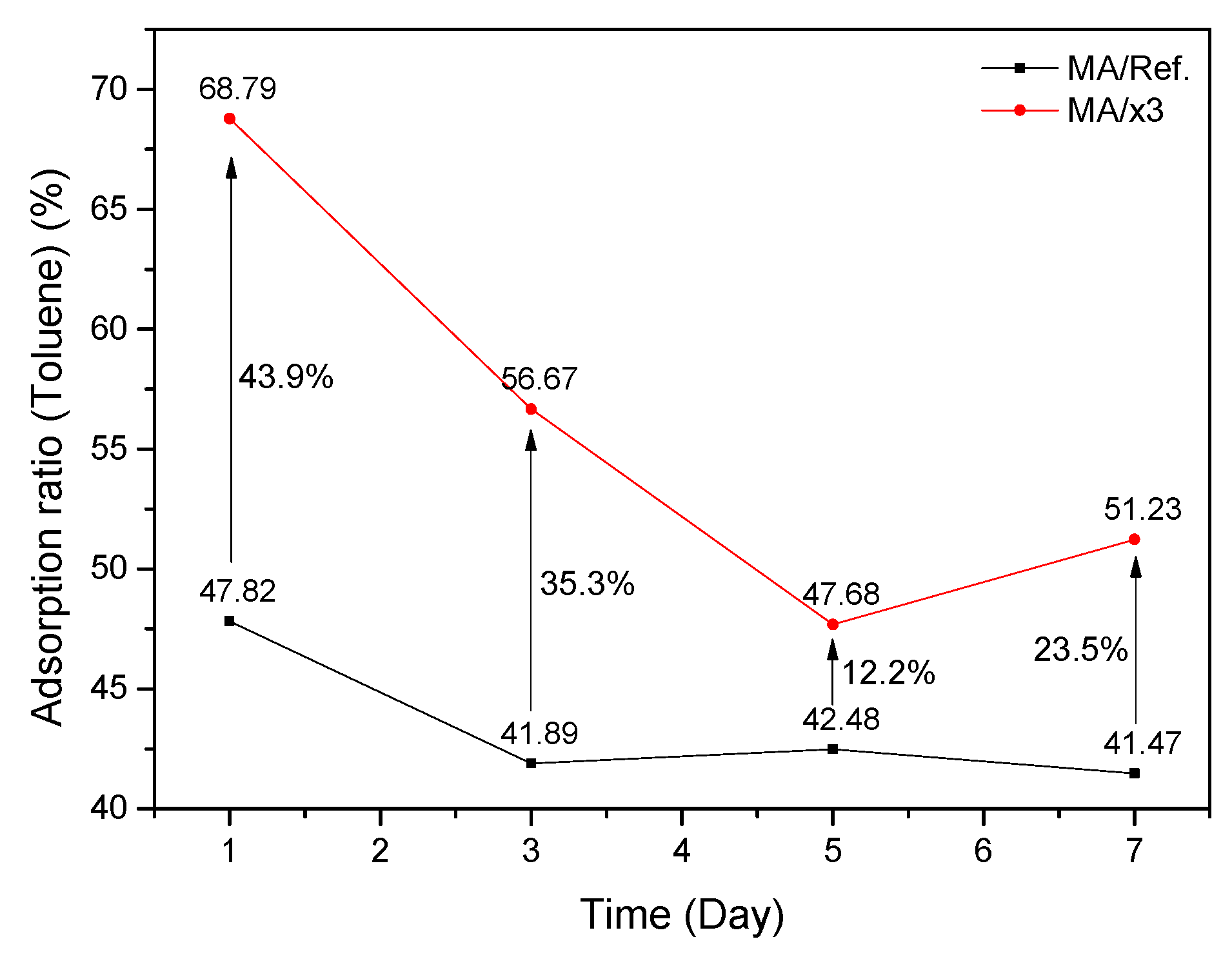
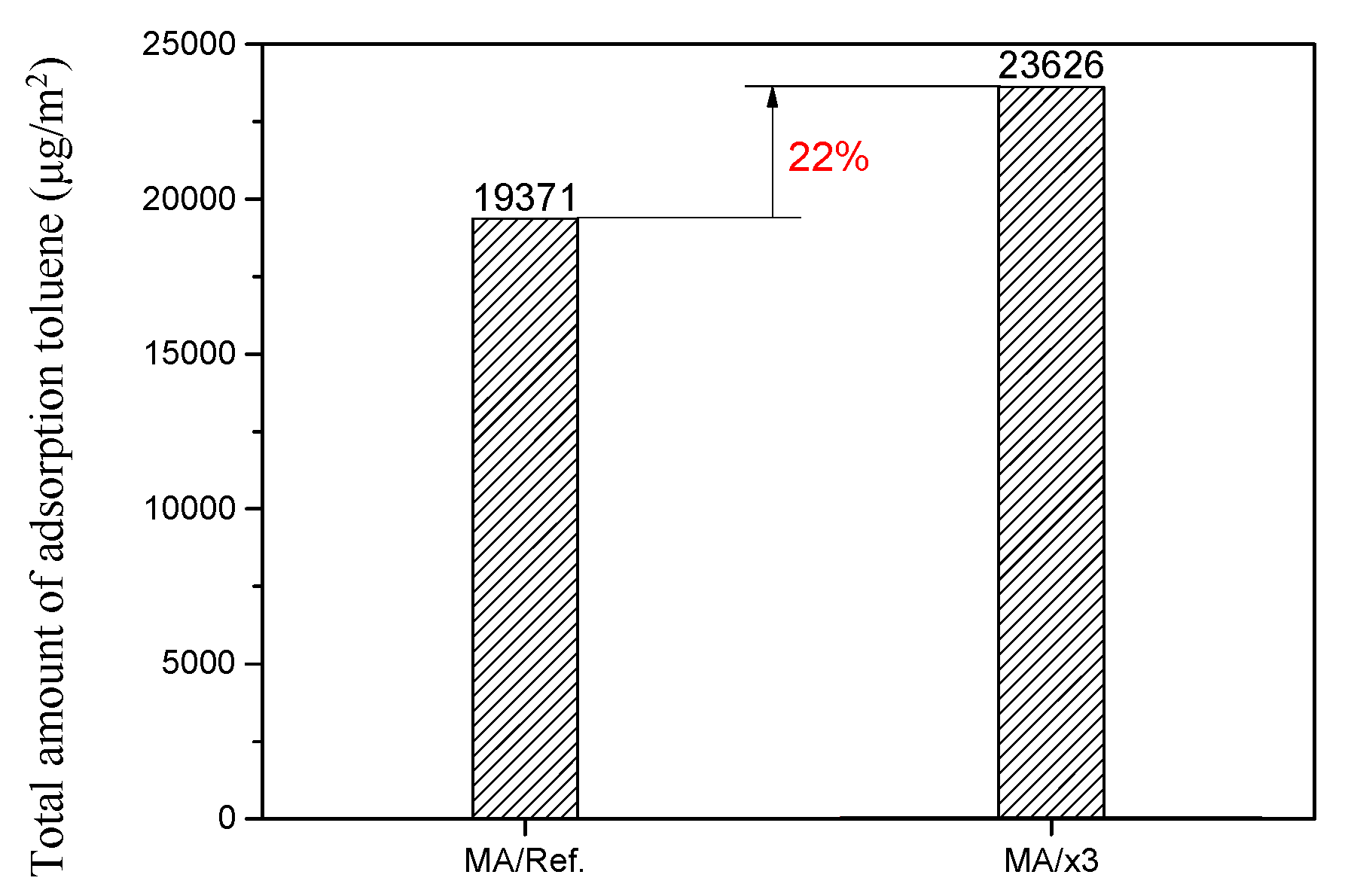
3.2. Evaluation of Toluene Adsorption Performance Using 20 L Small Chamber
3.3. Evaluation of Physical Properties of Adsorption for Mortar Adhesive
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones, A.P. Indoor air quality and health. Atmos. Environ. 1999, 33, 4535–4564. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Moon, S. Evaluation of VOC emissions from building finishing materials using a small chamber and VOC analyser. Indoor Built Environ. 2006, 15, 511–523. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.; Kim, H.; Park, J.C. Formaldehyde and TVOC emission behaviors according to finishing treatment with surface materials using 20 L chamber and FLEC. J. Hazard. Mater. 2010, 177, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Page, N.P.; Mehlman, M. Health effects of gasoline refueling vapors and measured exposures at service stations. Toxicol. Ind. Health 1989, 5, 869–890. [Google Scholar] [CrossRef] [PubMed]
- Shiue, A.; Kang, Y.; Hu, S.; Jou, G.; Lin, C.; Hu, M.; Lin, S. Vapor adsorption characteristics of toluene in an activated carbon adsorbent-loaded nonwoven fabric media for chemical filters applied to cleanrooms. Build. Environ. 2010, 45, 2123–2131. [Google Scholar] [CrossRef]
- Anbia, M.; Khataei, N.K. Ordered nanoporous carbon as an effective adsorbent in solid-phase microextraction of toluene and chlorinated toluenes in water samples. J. Saudi Chem. Soc. 2012, 20 (Suppl. 1), S38–S45. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.Y.; Zhao, X.J.; Zhou, Z. Citrus pectin derived porous carbons as a superior adsorbent toward removal of methylene blue. J. Solid State Chem. 2016, 243, 101–105. [Google Scholar] [CrossRef]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Fu, C.; Wang, Z.; Liu, J.; Jiang, H.; Li, G.; Zhi, C. Large scale fabrication of graphene for oil and organic solvent absorption. Prog. Nat. Sci. Mater. Int. 2016, 26, 319–323. [Google Scholar] [CrossRef]
- Seo, J.; Kato, S.; Ataka, Y.; Yang, J. Influence of environmental factors on performance of sorptive building materials. Indoor Built Environ. 2010, 19, 413–421. [Google Scholar] [CrossRef]
- Liu, C.; Tang, Z.; Chen, Y.; Su, S.; Jiang, W. Characterization of mesoporous activated carbons prepared by pyrolysis of sewage sludge with pyrolusite. Bioresour. Technol. 2010, 101, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Vinh-Thang, H.; Malekian, A.; Eić, M.; Trong-On, D.; Kaliaguine, S. Adsorption of n-heptane, toluene and o-xylene on mesoporous UL-ZSM5 materials. Microporous Mesoporous Mater. 2006, 87, 224–234. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, H.; Yoo, H.; Lee, C. Adsorption equilibria of toluene and gasoline vapors on activated carbon. J. Chem. Eng. Data 2002, 47, 1222–1225. [Google Scholar] [CrossRef]
- Lee, W.; Park, J.; Sok, J.; Reucroft, P. Effects of pore structure and surface state on the adsorption properties of nano-porous carbon materials in low and high relative pressures. Appl. Surf. Sci. 2005, 246, 77–81. [Google Scholar] [CrossRef]
- Kim, K.C.; Yoon, T.; Bae, Y. Applicability of using CO2 adsorption isotherms to determine BET surface areas of microporous materials. Microporous Mesoporous Mater. 2016, 224, 294–301. [Google Scholar] [CrossRef]
- Chui, S.S.; Lo, S.M.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Comelli, B.; Perrin, R.; Wirquin, E. Effect of formulation parameters on adhesive properties of ANSI 118–15 and 118–11 compliant tile adhesive mortars. Int. J. Adhes. Adhes. 2016, 66, 73–80. [Google Scholar] [CrossRef]
- Petit, J.; Wirquin, E. Evaluation of various cellulose ethers performance in ceramic tile adhesive mortars. Int. J. Adhes. Adhes. 2013, 40, 202–209. [Google Scholar] [CrossRef]
- Indoor Air—Part 23: Performance Test for Evaluating the Reduction of Formaldehyde Concentrations by Sorptive Building Materials; ISO 16000-23; International Organization for Standardization: Vernier, Geneva, Switzerland, 2009.
- Indoor Air—Part 24: Performance Test for Evaluating the Reduction of Volatile Organic Compound (Except Formaldehyde) Concentrations by Sorptive Building Materials; ISO 16000-24; International Organization for Standardization: Vernier, Geneva, Switzerland, 2009.
- Cement for Ceramic Tiles; KS L 1592; Korean Agency for Technology and Standards: Eumseong-gun, Chungcheongbuk-do, Korea, 2011.
- Kim, S.; Drzal, L.T. High latent heat storage and high thermal conductive phase change materials using exfoliated graphite nanoplatelets. Sol. Energy Mater. Sol. Cells 2009, 93, 136–142. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, S.; Kim, J.T. Thermal extractor analysis of VOCs emitted from building materials and evaluation of the reduction performance of exfoliated graphite nanoplatelets. Indoor Built Environ. 2013, 22, 68–76. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S. The determination of the adsorption performance of graphite for VOCs and formaldehyde. Energy Build. 2012, 46, 56–61. [Google Scholar] [CrossRef]
- Standard Guide for Small-Scale Environmental Chamber Determinations of Organic Emissions from Indoor Materials/Products; ASTM D5116-97; ASTM International: West Conshohocken, PA, USA, 1997.
- Standard Test Method for Determining Formaldehyde Factors in Air from Wood Products Using a Small Scale Chamber; ASTM-D6007-96; ASTM International: West Conshohocken, PA, USA, 1996.
- Guideline for the Determination of Steady State Concentrations in Test Chambers; ECA-IAQ Report No. 2; Commission of the European Communities: Luxembourg, 1989.
- Guideline for the Characterization of Volatile Organic Compounds Emitted from Indoor Materials and Products Using Small Test Chambers; ECA-IAQ Report No. 8; Commission of the European Communities: Brussels, Belgium, 1991.
- Determination of VOCs Emitted from Indoor Materials and Products—Inter Laboratory Comparison of Small Chamber Measurements; ECA-IAQ Report No. 13; Commission of the European Communities: Brussels, Belgium, 1993.
- Determination of VOCs Emitted from Indoor Materials and Products—Second Inter Laboratory Comparison of Small Chamber Measurements; ECA-IAQ Report No. 16; Commission of the European Communities: Brussels, Belgium, 1995.
| Sample | Mortar Adhesive (g) | Water (g) | xGnP C-500 (g) |
|---|---|---|---|
| Mortar adhesive (MA/Ref.) | 1 | 0.45 | 0 |
| Mortar adhesive/xGnP C-500 3 wt % (MA/x3) | 1 | 0.45 | 0.03 |
| Ingredients | CAS No. | % by Weight |
|---|---|---|
| Dolomite | 16389-88-1 | 45–55 |
| Portland cement | 65997-15-1 | 35–45 |
| Redispersible polymer powder | - | 4–8 |
| Methyl cellulose | 9004-67-5 | 0.1–0.5 |
| Test Condition | 20 L Small Chamber |
|---|---|
| Sample area (m2) | 0.0392 |
| Volume (L) | 20 |
| Loading factor (area of sample/volume, m2/m3) | 1.96 |
| Air change rate (h−1) | 0.5 |
| Flow rate (mL/min) | 1.0 |
| Injection volume (μL) | 25 |
| Equilibration time (hour) | 24, 72, 120, 168 |
| Temperature (°C), Relative humidity (%) | 25 ± 1.0, 50 ± 5 |
| Background concentration (μg/m3) | HCHO: <2, TVOC: <20 |
| Analysis method | VOC: GC/MS |
| Test Items | Unit | Reference Mortar Adhesive (MA/Ref.) | Mortar Adhesive with xGnP C-500 3 wt % (MA/x3) | Korean Standards (KS L 1592:2011) |
|---|---|---|---|---|
| Shear bond strength (7 days air dry curing) | N/mm2 | 1.86 | 1.10 | More than 1.03 |
| Shear bond strength (28 days air dry curing) | N/mm2 | 2.25 | 1.23 | More than 1.03 |
| Shear bond strength (28 days air dry curing after freezing and thawing) | N/mm2 | 1.75 | 0.72 | More than 0.69 |
| The length change rate | % | 0.15 | 0.11 | Below 0.2 |
| The water retention rate | % | 89 | 93 | From 80 to 95 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wi, S.; Chang, S.J.; Jeong, S.-G.; Lee, J.; Kim, T.; Park, K.-W.; Lee, D.R.; Kim, S. Evaluation of Toluene Adsorption Performance of Mortar Adhesives Using Porous Carbon Material as Adsorbent. Materials 2017, 10, 853. https://doi.org/10.3390/ma10080853
Wi S, Chang SJ, Jeong S-G, Lee J, Kim T, Park K-W, Lee DR, Kim S. Evaluation of Toluene Adsorption Performance of Mortar Adhesives Using Porous Carbon Material as Adsorbent. Materials. 2017; 10(8):853. https://doi.org/10.3390/ma10080853
Chicago/Turabian StyleWi, Seunghwan, Seong Jin Chang, Su-Gwang Jeong, Jongki Lee, Taeyeon Kim, Kyung-Won Park, Dong Ryeol Lee, and Sumin Kim. 2017. "Evaluation of Toluene Adsorption Performance of Mortar Adhesives Using Porous Carbon Material as Adsorbent" Materials 10, no. 8: 853. https://doi.org/10.3390/ma10080853





