Novel Durable Antimicrobial Ceramic with Embedded Copper Sub-Microparticles for a Steady-State Release of Copper Ions
Abstract
:1. Introduction
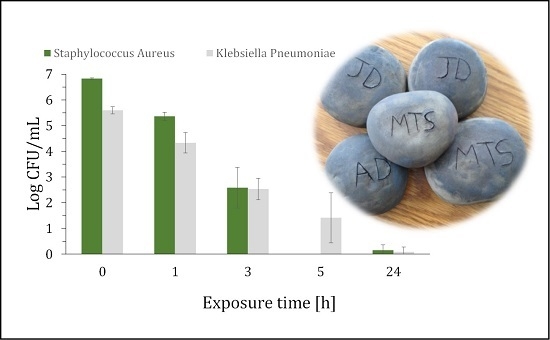
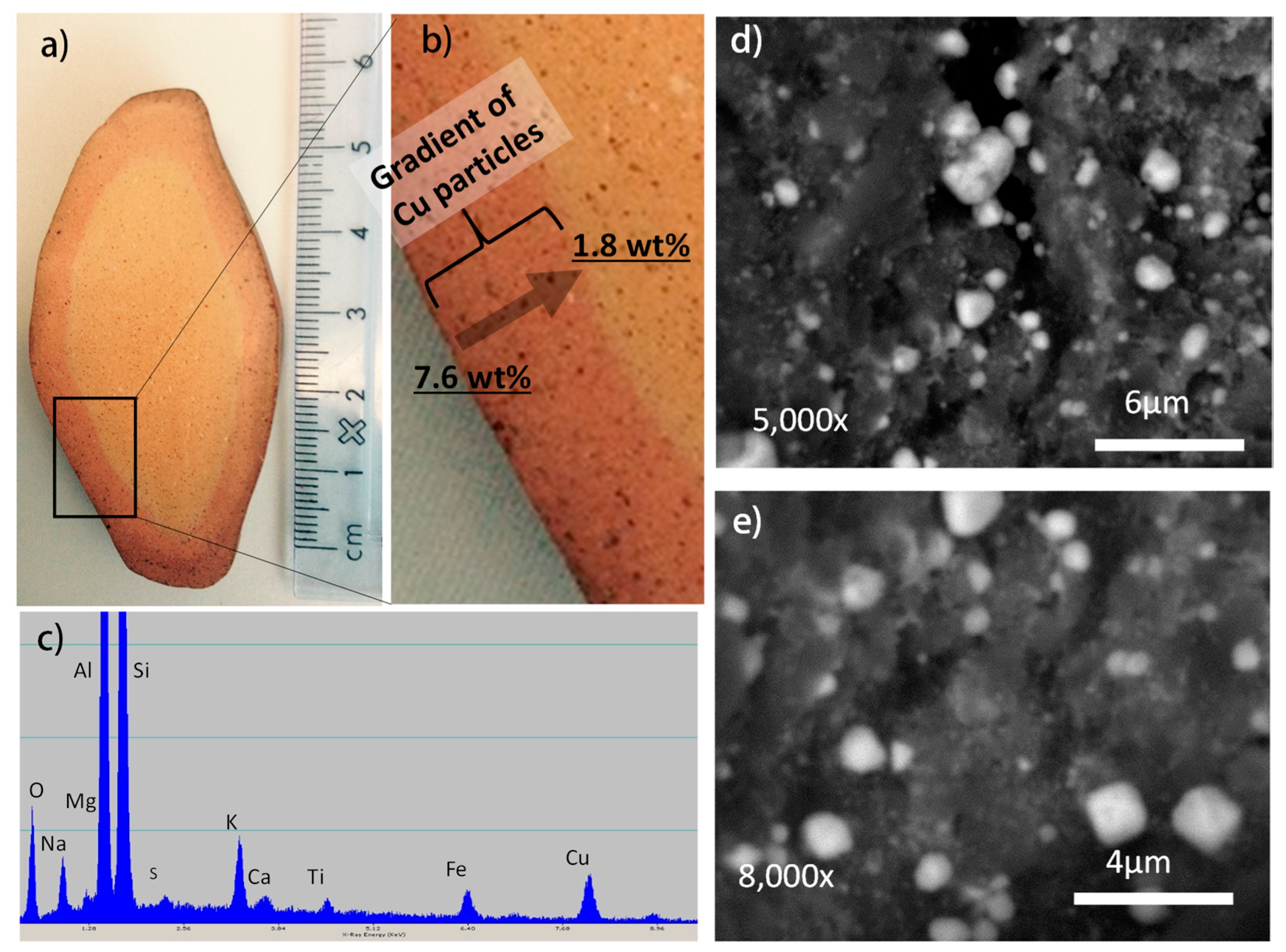
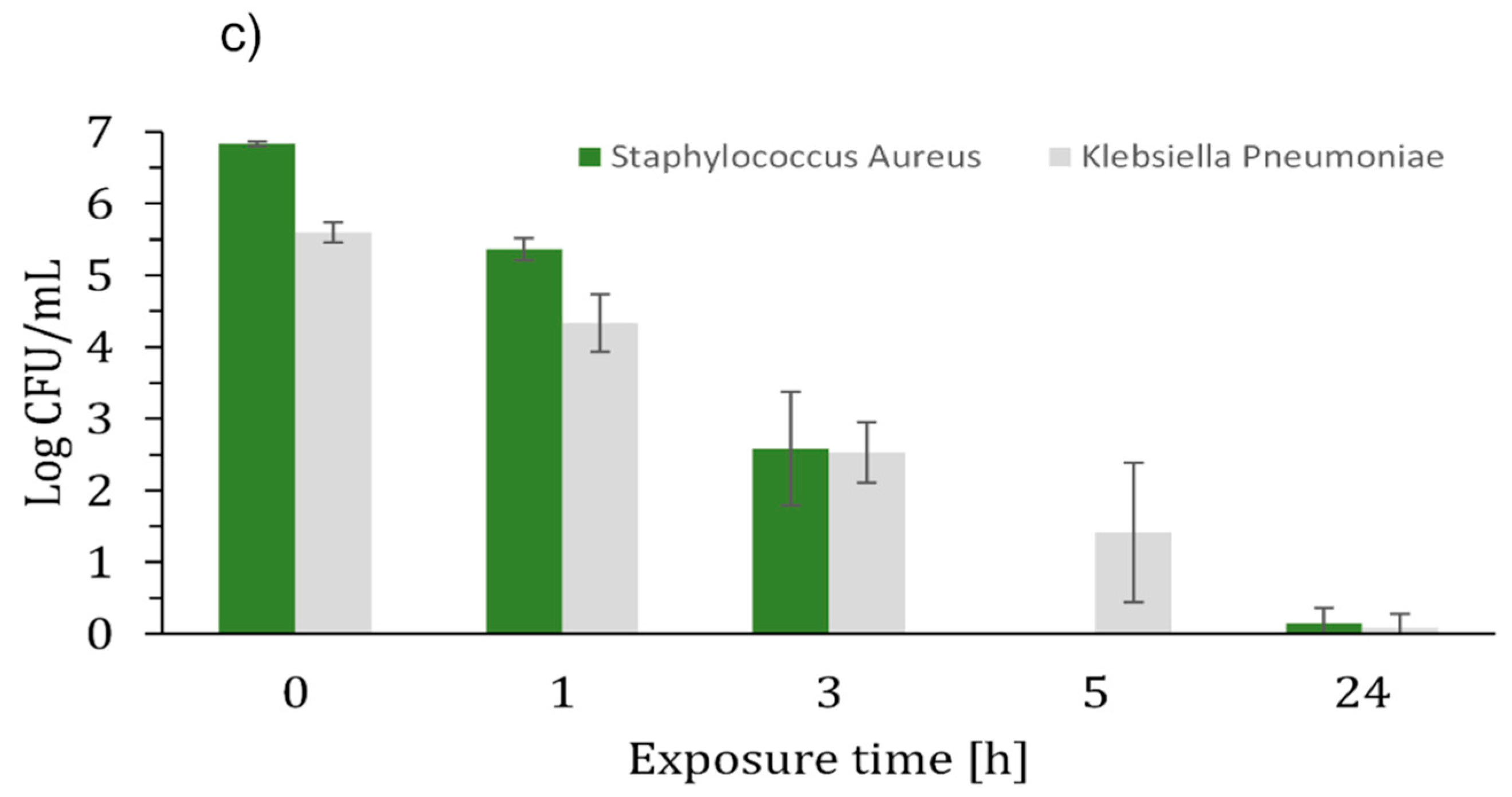
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Conflicts of Interest
References
- World Health Organization. Global Strategy for Containment of Antimicrobial Resistance; Department of Communicable Disease Surveillance and Response, World Health Organization: Geneva, Switzerland, 2001; p. 105. [Google Scholar]
- Kaali, P.; Perez-Madrigal, M.M.; Stromberg, E.; Aune, R.E.; Czel, G.; Karlsson, S. The influence of Ag+, Zn2+ and Cu2+ exchanged zeolite on antimicrobial and long term in vitro stability of medical grade polyether polyurethane. Express Polym. Lett. 2011, 5, 1028–1040. [Google Scholar] [CrossRef]
- Cross, J.B.; Currier, R.P.; Torraco, D.J.; Vanderberg, L.A.; Wagner, G.L.; Gladen, P.D. Killing of bacillus spores by aqueous dissolved oxygen, ascorbic acid, and copper ions. Appl. Environ. Microbiol. 2003, 69, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Gant, V.A.; Wren, M.W.D.; Rollins, M.S.M.; Jeanes, A.; Hickok, S.S.; Haj, T.J. Three novel highly charged copper-based biocides: Safety and efficacy against healthcare-associated organisms. J. Antimicrob. Chemother. 2007, 60, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.; Michels, H.T.; Keevil, C.W. Potential for preventing spread of fungi in air-conditioning systems constructed using copper instead of aluminium. Lett. Appl. Microbiol. 2010, 50, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Wheeldon, L.J.; Worthington, T.; Lambert, P.A.; Hilton, A.C.; Lowden, C.J.; Elliott, T.S.J. Antimicrobial efficacy of copper surfaces against spores and vegetative cells of Clostridium difficile: The germination theory. J. Antimicrob. Chemother. 2008, 62, 522–525. [Google Scholar] [CrossRef] [PubMed]
- CDA Copper Development Association. Available online: http://www.copper.org/homepage.html (accessed on 27 August 2016).
- Borkow, G.; Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G. Using copper to fight microorganisms. Curr. Chem. Biol. 2012, 6, 93–103. [Google Scholar] [CrossRef]
- Ohsumi, Y.; Kitamoto, K.; Anraku, Y. Changes induced in the permeability barrier of the yeast plasma-membrane by cupric ion. J. Bacteriol. 1988, 170, 2676–2682. [Google Scholar] [CrossRef] [PubMed]
- Avery, S.V.; Howlett, N.G.; Radice, S. Copper toxicity towards Saccharomyces cerevisiae: Dependence on plasma membrane fatty acid composition. Appl. Environ. Microbiol. 1996, 62, 3960–3966. [Google Scholar] [PubMed]
- Karlstrom, A.R.; Levine, R.L. Copper inhibits the protease from human immunodeficiency virus-1 by both cysteine-dependent and cysteine-independent mechanisms. Proc. Natl. Acad. Sci. USA 1991, 88, 5552–5556. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cho, H.; Ryu, S.E.; Choi, M.U. Effects of metal ions on the activity of protein tyrosine phosphatase VHR: Highly potent and reversible oxidative inactivation by Cu2+ ion. Arch. Biochem. Biophys. 2000, 382, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Gilbert, B.C.; Haywood, R.M. Radical-induced damage to proteins - ESR spin-trapping studies. Free Radic. Res. Commun. 1991, 15, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Geierstanger, B.H.; Kagawa, T.F.; Chen, S.L.; Quigley, G.J.; Ho, P.S. Base-specific binding of copper (II) to z-DNA-the 1,3-a single-crystal structure of d(m5CGUAm5CG) in the presence of CuCl2. J. Biol. Chem. 1991, 266, 20185–20191. [Google Scholar] [PubMed]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M.; Hasman, H. Susceptibility of different bacterial species isolated from food animals to copper sulphate, zinc chloride and antimicrobial substances used for disinfection. Vet. Microbiol. 2004, 100, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Elguindi, J.; Moffitt, S.; Hasman, H.; Andrade, C.; Raghavan, S.; Rensing, C. Metallic copper corrosion rates, moisture content, and growth medium influence survival of copper ion-resistant bacteria. Appl. Microbiol. Biotechnol. 2011, 89, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Fait, G.; Broos, K.; Zrna, S.; Lombi, E.; Hamon, R. Tolerance of nitrifying bacteria to copper and nickel. Environ. Toxicol. Chem. 2006, 25, 2000–2005. [Google Scholar] [CrossRef] [PubMed]
- Santo, C.E.; Morais, P.V.; Grass, G. Isolation and Characterization of bacteria resistant to metallic copper surfaces. Appl. Environ. Microbiol. 2010, 76, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Uauy, R. Copper as an essential nutrient. Am. J. Clin. Nutr. 1996, 63, 791–796. [Google Scholar]
- Borkow, G.; Gabbay, J.; Zatcoff, R.C. Could chronic wounds not heal due to too low local copper levels? Med. Hypotheses 2008, 70, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.E.D.; Felcman, J. Correlation between five minerals and the healing effect of Brazilian medicinal plants. Biol. Trace Elem. Res. 1998, 65, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.E.; Yu, V.L. Experiences of the first 16 hospitals using copper-silver ionization for Legionella control: Implications for the evaluation of other disinfection modalities. Infect. Control Hosp. Epidemiol. 2003, 24, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Lin, Y.E.; Liu, Y.C.; Huang, W.K.; Shih, H.Y.; Wann, S.R.; Lee, S.S.; Tsai, H.C.; Li, C.H.; Chao, H.L.; et al. Efficacy of point-of-entry copper-silver ionisation system in eradicating Legionella pneumophila in a tropical tertiary care hospital: Implications for hospitals contaminated with Legionella in both hot and cold water. J. Hosp. Infect. 2008, 68, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.I.; Shih, H.Y.; Lee, C.M.; Yang, T.C.; Lay, J.J.; Lin, Y.E. In vitro efficacy of copper and silver ions in eradicating pseudomonas aeruginosa, stenotrophomonas maltophilia and acinetobacter baumannii: Implications for on-site disinfection for hospital infection control. Water Res. 2008, 42, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.; Chavez, G.; Baez, M.; Martinez, C.; Chaidez, C. Internalization of salmonella typhimurium into mango pulp and prevention of fruit pulp contamination by chlorine and copper ions. Int. J. Environ. Health Res. 2007, 17, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Wilks, S.A.; MichelS, H.T.; Keevil, C.W. Survival of listeria monocytogenes scott a on metal surfaces: Implications for cross-contamination. Int. J. Food Microbiol. 2006, 111, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing. Appl. Environ. Microbiol. 2006, 72, 4239–4244. [Google Scholar] [CrossRef] [PubMed]
- Drelich, J.; Li, B.; Bowen, P.; Hwang, J.Y.; Mills, O.; Hoffman, D. Vermiculite decorated with copper nanoparticles: Novel antibacterial hybrid material. Appl. Surf. Sci. 2011, 257, 9435–9443. [Google Scholar] [CrossRef]
- Drelich, J.; Li, B.W.; Villeneuve, B.; Bowen, P. Inexpensive mineral copper materials with antibacterial surfaces. Surf. Innov. 2013, 1, 15–26. [Google Scholar] [CrossRef]
- Drelich, J.; Li, B. In inexpensive mineral-based antibacterial materials infused with copper. In Proceedings of the Annual International Conference on Materials Science, Metal & Manufacturing, Singapore, 19–20 November 2012. [Google Scholar]
- Rao, N.N.; Chaturvedi, V. Photoactivity of TiO2-coated pebbles. Ind. Eng. Chem. Res. 2007, 46, 4406–4414. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Sand supported mixed-phase TiO2 photocatalysts for water decontamination applications. Adv. Eng. Mater. 2014, 16, 248–254. [Google Scholar] [CrossRef]
- Bansal, P.; Verma, A. Synergistic effect of dual process (photocatalysis and photo-Fenton) for the degradation of Cephalexin using TiO2 immobilized novel clay beads with waste fly ash/foundry sand. J. Photochem. Photobiol. A-Chem. 2017, 342, 131–142. [Google Scholar] [CrossRef]
- Stevenson, C.M.; Gurnick, M. Structural collapse in kaolinite, montmorillonite and illite clay and its role in the ceramic rehydroxylation dating of low-fired earthenware. J. Archaeol. Sci. 2016, 69, 54–63. [Google Scholar] [CrossRef]
- Li, B. Characteristics and Antimicrobial Activity of Copper-Based Materials. Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2008. [Google Scholar]
- Inglezakis, V.J.; Stylianou, M.; Loizidou, M. Ion exchange and adsorption equilibrium studies on clinoptilolite, bentonite and vermiculite. J. Phys. Chem. Solids 2010, 71, 279–284. [Google Scholar] [CrossRef]
- Malamis, S.; Katsou, E.; Stylianou, M.; Haralambous, K.J.; Loizidou, M. Copper removal from sludge permeate with ultrafiltration membranes using zeolite, bentonite and vermiculite as adsorbents. Water Sci. Technol. 2010, 61, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Zhao, F.; Chen, M.L.; Zhang, Y.L.; Zhao, C.; Zhou, H.L. Factors affecting the adsorption of Zn2+ and Cd2+ ions from aqueous solution onto vermiculite. Adsorpt. Sci. Technol. 2008, 26, 145–155. [Google Scholar]
- Sari, A.; Tuzen, M. Removal of Cr(VI) from aqueous solution by turkish vermiculite: Equilibrium, thermodynamic and kinetic studies. Sep. Sci. Technol. 2008, 43, 3563–3581. [Google Scholar] [CrossRef]
- Stylianou, M.A.; Inglezakis, V.J.; Moustakas, K.G.; Malamis, S.P.; Loizidou, M.D. Removal of Cu(II) in fixed bed and batch reactors using natural zeolite and exfoliated vermiculite as adsorbents. Desalination 2007, 215, 133–142. [Google Scholar] [CrossRef]
- Abate, G.; Masini, J.C. Influence of pH, ionic strength and humic acid on adsorption of Cd(II) and Pb(II) onto vermiculite. Colloid Surf. A-Physicochem. Eng. Asp. 2005, 262, 33–39. [Google Scholar] [CrossRef]
- Magana, S.M.; Quintana, P.; Aguilar, D.H.; Toledo, J.A.; Angeles-Chavez, C.; Cortes, M.A.; Leon, L.; Freile-Pelegrin, Y.; Lopez, T.; Sanchez, R.M.T. Antibacterial activity of montmorillonites modified with silver. J. Mol. Catal. A-Chem. 2008, 281, 192–199. [Google Scholar] [CrossRef]
- Esteban-Tejeda, L.; Malpartida, F.; Pecharroman, C.; Moya, J.S. High antibacterial and antifungal activity of silver monodispersed nanoparticles embedded in a glassy matrix. Adv. Eng. Mater. 2010, 12, B292–B297. [Google Scholar] [CrossRef]
- Esteban-Cubillo, A.; Pecharroman, C.; Aguilar, E.; Santaren, J.; Moya, J.S. Antibacterial activity of copper monodispersed nanoparticles into sepiolite. J. Mater. Sci. 2006, 41, 5208–5212. [Google Scholar] [CrossRef]
- Kuznicki, S.M.; Kelly, D.J.A.; Bian, J.J.; Lin, C.C.H.; Liu, Y.; Chen, J.; Mitlin, D.; Xu, Z.H. Metal nanodots formed and supported on chabazite and chabazite-like surfaces. Microporous Mesoporous Mater. 2007, 103, 309–315. [Google Scholar] [CrossRef]
- Liu, Y.; Kelly, D.J.A.; Yang, H.Q.; Lin, C.C.H.; Kuznicki, S.M.; Xu, Z.G. Novel regenerable sorbent for mercury capture from flue gases of coal-fired power plant. Environ. Sci. Technol. 2008, 42, 6205–6210. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, F.; Kuznicki, S.M.; Wasylishen, R.E.; Xu, Z.H. A Novel Method to Control the Size of Silver Nanoparticles Formed on Chabazite. J. Nanosci. Nanotechnol. 2009, 9, 2768–2771. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Z.H.; Kuznicki, S.M. Development of a novel mercury cartridge for mercury analysis. Energy Fuels 2010, 24, 10–17. [Google Scholar] [CrossRef]
- Naccache, C.M.; Taarit, Y.B. Oxidizing and acidic properties of copper-exchange Y zeolite. J. Catal. 1971, 22, 171–181. [Google Scholar] [CrossRef]
- Kubo, T.; Arai, H.; Tominaga, H.; Kunugi, T. Metal catalysts supported on zeolite. I. A study on platinum particles distribution. Bull. Chem. Soc. Jpn. 1972, 45, 607–612. [Google Scholar] [CrossRef]
- Herman, R.G.; Lunsford, J.H.; Beyer, H.; Jacobs, P.A.; Uytterhoeven, J.B. Redox Behavior of transition-metal ions in zeolites. 1. Study of reversibility of hydrogen reduction of copper y zeolites. J. Phys. Chem. 1975, 79, 2388–2394. [Google Scholar] [CrossRef]
- Texter, J.; Strome, D.H.; Herman, R.G.; Klier, K. Chemical and spectroscopic properties of copper containing zeolites. J. Phys. Chem. 1977, 81, 333–338. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Wasylishen, R.E.; Xu, Z.H.; Kuznicki, S.M. Solid-State NMR and TGA studies of silver reduction in chabazite. J. Nanosci. Nanotechnol. 2012, 12, 1988–1993. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Zeolite-Supported Silver Nanoparticles for Coal-Fired Power Plant Mercury Emission Control. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2009. [Google Scholar]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drelich, A.J.; Miller, J.; Donofrio, R.; Drelich, J.W. Novel Durable Antimicrobial Ceramic with Embedded Copper Sub-Microparticles for a Steady-State Release of Copper Ions. Materials 2017, 10, 775. https://doi.org/10.3390/ma10070775
Drelich AJ, Miller J, Donofrio R, Drelich JW. Novel Durable Antimicrobial Ceramic with Embedded Copper Sub-Microparticles for a Steady-State Release of Copper Ions. Materials. 2017; 10(7):775. https://doi.org/10.3390/ma10070775
Chicago/Turabian StyleDrelich, Adam J., Jessie Miller, Robert Donofrio, and Jaroslaw W. Drelich. 2017. "Novel Durable Antimicrobial Ceramic with Embedded Copper Sub-Microparticles for a Steady-State Release of Copper Ions" Materials 10, no. 7: 775. https://doi.org/10.3390/ma10070775