A New Biphasic Dicalcium Silicate Bone Cement Implant
Abstract
:1. Introduction
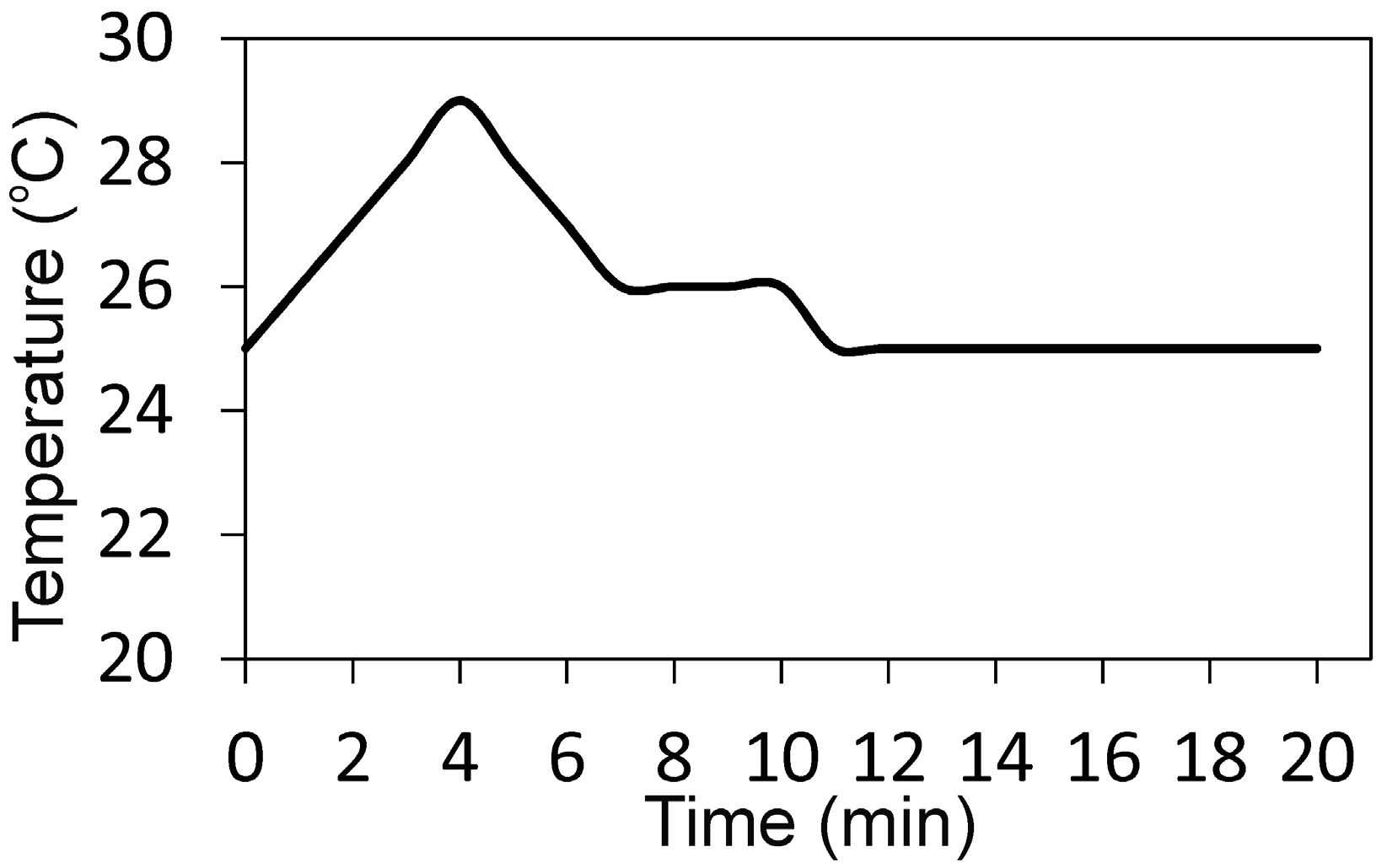
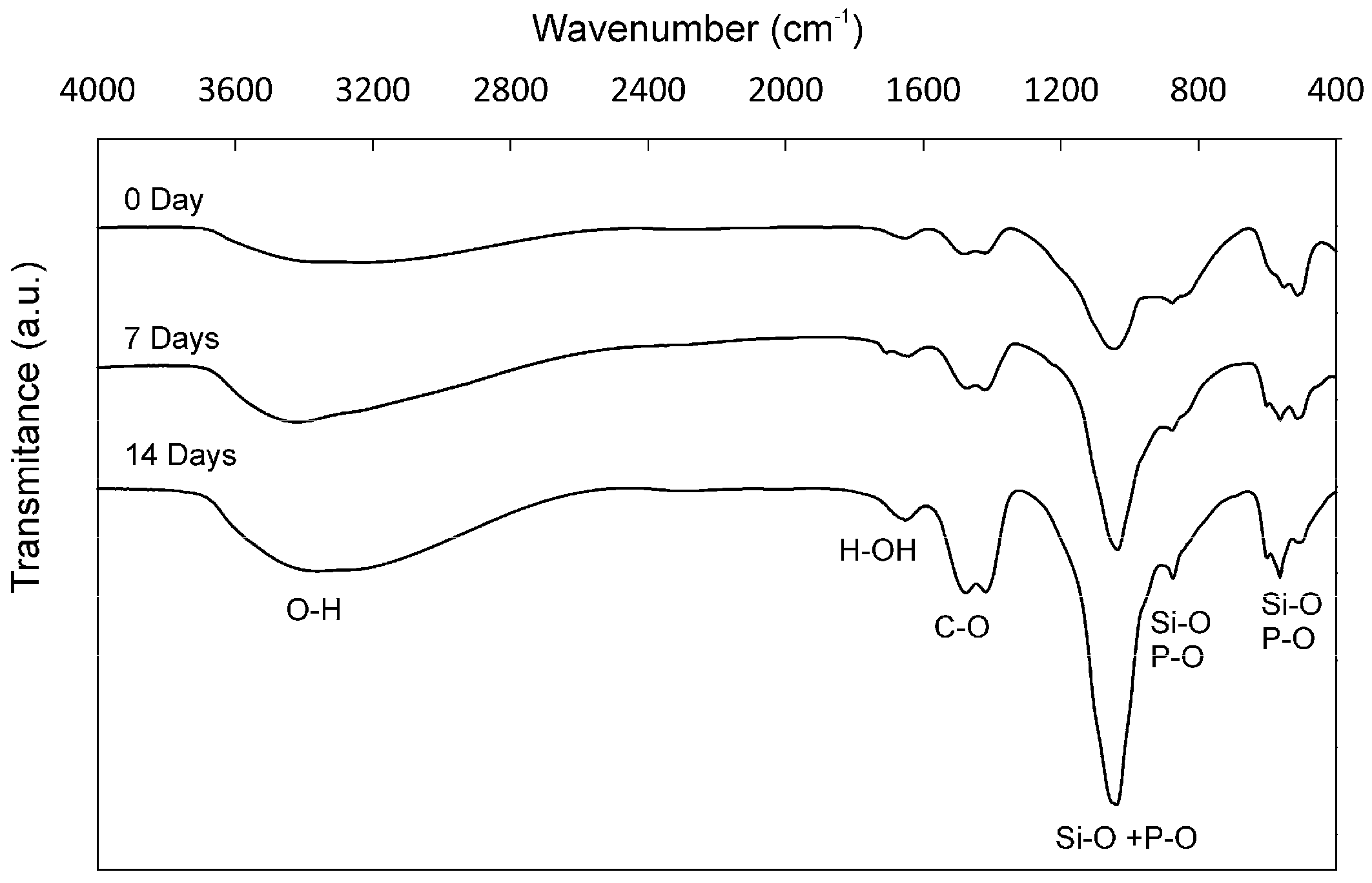
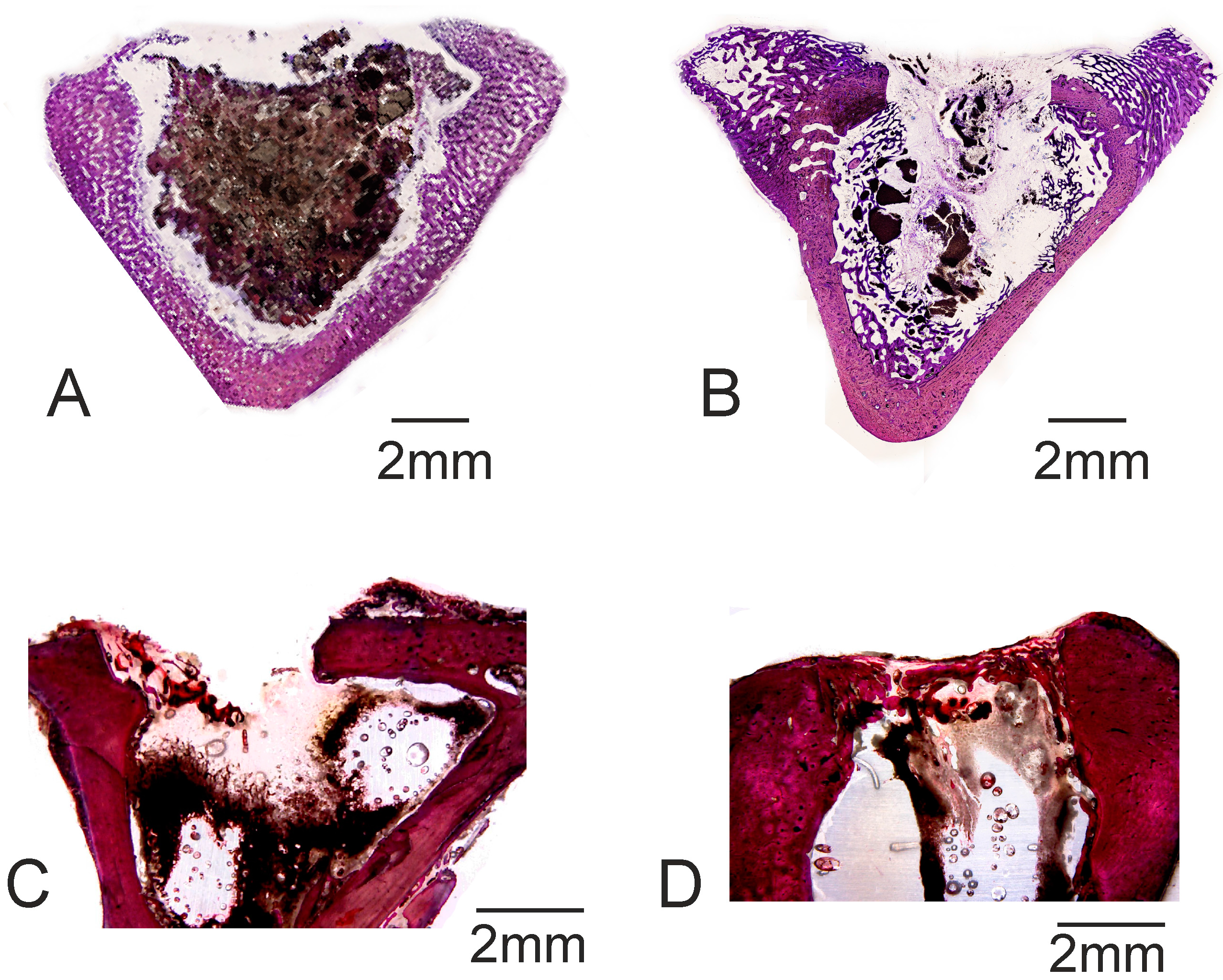
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis of the Powder and Cement Paste Preparation and Characterization
4.2. In Vitro Bioactivity
4.3. Cell Test
4.4. Animal Test
4.5. Cement Implant Characterization
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taylor, H.F.W. Cement Chemistry; Academic Press: London, UK, 1990. [Google Scholar]
- Sarkar, N.K.; Caidedo, R.; Tirwik, P.; Moiseyeva, R.; Kawashima, I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J. Endod. 2005, 31, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Montesin, F.E.; Brady, K.; Sweeney, R.; Curtis, R.V.; Pitt Ford, T.R. The constitution of mineral trioxide aggregate. Dent. Mater. 2005, 21, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Dammaschke, T.; Gerth, H.U.V.; Züchner, H.; Schäfer, E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent. Mater. 2005, 21, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Farascioni, S.; Pashley, D.H.; Gasparotto, G.; Prati, C. Calcium silicate coating derived from portland cement as treatment for hypersensitive dentine. J. Dent. 2008, 36, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Holden, D.T.; Schwartz, S.A.; Kirkpatrick, T.C.; Schindler, W.G. Clinical outcomes of artificial root-end barriers with mineral trioxide aggregate in teeth with immature apices. J. Endod. 2008, 34, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Pace, R.; Giuliani, V.; Pagavino, G. Mineral trioxide aggregate as repair material for furcal perforation: Case series. J. Endod. 2008, 34, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Saunder, W.P. A prospective clinical study of periradicular surgery using mineral trioxide aggregate as a root-end filling. J. Endod. 2008, 34, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Carrodeguas, R.G.; de Aza, A.H.; de Aza, P.N.; Baudin, C.; de Aza, S.; Arribas, J.J.; Lopez-Bravo, A. Assessment of natural and synthetic wollastonite as source for bioceramics preparation. J. Biomed. Mater. Res. A 2007, 83, 484–495. [Google Scholar] [CrossRef] [PubMed]
- De Aza, P.N.; Guitian, F.; de Aza, S.; Valle, F.J. Analytical control of wollastonite for biomedical applications by use of atomic absorption spectrometry and inductively coupled plasma atomic emission spectroscopy. Analyst 1998, 123, 681–685. [Google Scholar] [CrossRef] [PubMed]
- De Aza, P.N.; Luklinska, Z.B.; Guitian, F.; De Aza, S. Electron microscopy of interfaces in a wollastonite-tricalcium phosphate bioeutectic. J. Microsc.-Oxf. 1998, 189, 145–153. [Google Scholar] [CrossRef]
- De Aza, A.H.; Velasquez, P.; Alemany, M.I.; Pena, P.; De Aza, P.N. In situ bone-like apatite formation from a Bioeutectic® ceramic in SBF dynamic flow. J. Am. Ceram. Soc. 2007, 90, 1200–1207. [Google Scholar] [CrossRef]
- Velasquez, P.; Luklinska, Z.B.; Meseguer-Olmo, L.; Mate-Sanchez de Val, J.E.; Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Ramirez-Fernandez, M.P.; De Aza, P.N. αTCP ceramic doped with Dicalcium Silicate for bone regeneration applications prepared by powder metallurgy method. In vitro and in vivo studies. J. Biomed. Mater. Res. A 2013, 101, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Meseguer-Olmo, L.; Aznar-Cervantes, S.; Mazón, P.; De Aza, P.N. In vitro behaviour of adult mesenchymal stem cells of human bone marrow origin seeded on a novel bioactive ceramics in the Ca2SiO4-Ca3(PO4)2 system. J. Mater. Sci. Mater. Med. 2012, 23, 3003–3014. [Google Scholar] [CrossRef] [PubMed]
- Minarelli-Gaspar, A.M.; Saska, S.; Carrodeguas, R.G.; de Aza, A.H.; Pena, P.; de Aza, P.N.; de Aza, S. Biological response to wollastonite doped α-tricalcium phosphate implants in hard and soft tissues in rats. Key Eng. Mater. 2009, 396–398, 7–10. [Google Scholar] [CrossRef]
- Correa, D.; Almirall, A.; Garcia-Carrodeguas, R.; Alberto dos Santos, L.; de Aza, A.H.; Parra, J.; Delgado, J.A. β-Dicalcium silicate-based cement: Synthesis, characterization and in vitro bioactivity and biocompatibility studies. J. Biomed. Mater. Res. Part A 2014, 102, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- De Aza, P.N.; Zuleta, F.; Velasquez, P.; Vicente-Salar, N.; Reig, J.A. α´H-dicalcium silicate bone cement doped with tricalcium phosphate: Characterization, bioactivity and biocompatibility. J. Mater. Sci. Mater. Med. 2014, 5, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Maté-Sánchez de Val, J.E.; Calvo-Guirado, J.L.; Granero Marín, J.M.; Gomez-Moreno, G.; Mazón, P.; de Aza, P.N. Material characterization and in vivo behavior of dicalcium silicate cement modified with phosphorus. Ceram. Int. 2016, 42, 952–960. [Google Scholar] [CrossRef]
- Martinez, I.M.; Meseguer-Olmo, L.; Bernabeu-Esclapez, A.; Velasquez, P.A.; de Aza, P.N. In vitro behavior of α-Tricalcium Phosphate doped with Dicalcium Silicate in the system Ca2SiO4–Ca3(PO4)2. Mater. Charact. 2012, 63, 47–55. [Google Scholar] [CrossRef]
- Fix, W.; Heymann, H.; Heinke, R. Subsolidus relations in the system 2CaO•SiO2–3CaO•P2O5. J. Am. Ceram. Soc. 1969, 52, 346–347. [Google Scholar] [CrossRef]
- Rubio, V.; de la Casa-Lillo, M.A.; de Aza, S.; de Aza, P.N. The system Ca3(PO4)2–Ca2SiO4: The sub-system Ca2SiO4–7CaOP2O52SiO2. J. Am. Ceram. Soc. 2011, 94, 4459–4462. [Google Scholar] [CrossRef]
- Abdullah, D.; Pitt, T.R.; Papaioannou, S.; Nicholson, J.; McDonald, F. An evaluation of accelerated Portland cement as a restorative material. Biomaterials 2002, 23, 4001–4010. [Google Scholar] [CrossRef]
- Chen, C.C.; Ho, C.C.; Chen David, C.H.; Ding, S.J. Physicochemical properties of dicalcium silicate cements for endodontic treatment. J. Endod. 2009, 35, 1288–1291. [Google Scholar] [CrossRef] [PubMed]
- Huan, Z.; Chang, J. Calcium–phosphate–silicate composite bone cement: Self-setting properties and in vitro bioactivity. J. Mater. Sci. Mater. Med. 2009, 20, 833–841. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, R.M.; Orr, J.F.; Buchanan, F.J.; Wilcox, R.K.; David, C.; Barton, D.C.; Dunne, N.J. Development of a bovine collagen–apatitic calcium phosphate cement for potential fracture treatment through verte- broplasty. Acta Biomater. 2012, 8, 4043–4052. [Google Scholar] [CrossRef] [PubMed]
- Morejón-Alonso, L.; Bareiro, O.J.; Carrodeguas, R.G.; dos Santos, L.A. Bioactive composite bone cement based on a-tricalcium phosphate/tricalcium silicate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Kim, H.W.; Ginebra, M.P. Polymeric additives to enhance the functional properties of calcium phosphate cements. J. Tissue Eng. 2012, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Serena, S.; Caballero, A.; de Aza, P.N.; Sainz, M.A. New evaluation of the in vitro response of silicocarnotite monophasic material. Ceram. Int. 2015, 41, 9411–9419. [Google Scholar] [CrossRef]
- Martinez, I.M.; Velasquez, P.A.; Meseguer-Olmo, L.; de Aza, P.N. Production and study of in vitro behaviour of monolithic α-Tricalcium Phosphate based ceramics in the system Ca3(PO4)2–Ca2SiO4. Ceram. Int. 2011, 37, 2527–2535. [Google Scholar] [CrossRef]
- Serena, S.; Sainz, M.A.; Caballero, A. Single-phase silicocarnotite synthesis in the subsystem Ca3(PO4)2–Ca2SiO4. Ceram. Int. 2014, 40, 8245–8252. [Google Scholar] [CrossRef]
- Lugo, G.J.; Mazón, P.; de Aza, P.N. Phase transitions in single phase Si-Ca-P-based ceramic under thermal treatment. J. Eur. Ceram. Soc. 2015, 35, 3693–3700. [Google Scholar] [CrossRef]
- Ros-Tárraga, P.; Murciano, A.; Mazón, P.; Gehrke, S.A.; de Aza, P.N. New 3D stratified Si-Ca-P porous scaffolds obtained by sol-gel and polymer replica method: Microstructural, mineralogical and chemical characterization. Ceram. Int. 2017, 43, 702–707. [Google Scholar] [CrossRef]
- Del Valle, S.; Miño, N.; Muñoz, F.; Gonzalez, A.; Planell, J.A.; Ginebra, M.P. In vivo evaluation of an injectable macroporous calcium phosphate cement. J. Mater. Sci. Mater. Med. 2007, 18, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.S.; Paroli, R.M.; Beaudoin, J.J.; Delgado, A.H. Hand-Book of Thermal Analysis of Construction Materials; Noyes Publications: New York, NY, USA, 2002; pp. 35–89. [Google Scholar]
- Renaudin, G.; Russias, J.; Leroux, F.; Frizon, F.; Cau-dit-Coumes, C. Structural characterization of C-S-H and C-A-S-H samples. I. Long-range order investigated by Rietveld analyses. J. Solid State Chem. 2009, 182, 3312–3319. [Google Scholar] [CrossRef]
- Renaudin, G.; Russias, J.; Leroux, F.; Cau-dit-Coumes, C.; Frizon, F. Structural characterization of C-S-H and C-A-S-H samples. II. Local environment investigated by spectroscopic analyses. J. Solid State Chem. 2009, 182, 3320–3329. [Google Scholar] [CrossRef]
- Viana, N.; Guerreiro-Tanomaru, J.M.; Ferreira, G.; Sasso-Cerri, E.; Tanomaru, M.; Cerri, S. Biocompatibility of an experimental MTA Sealer implanted in the rat subcutaneous: Quantitative and immunohistochemical evaluation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Fadel, M.A.; Moharram, M.A.; Higazy, H.; Khalil, S. Interaction of acrylic bone cement with bone structure. Polym.-Plast. Tech. Eng. 1993, 32, 457–465. [Google Scholar] [CrossRef]
- Chen, W.S.; Monroe, E.A.; Condrate, R.A., Sr.; Guo, Y.M. An investigation of the hardening process for several phosphate glass-containing cements. J. Mater. Sci. Mater. Med. 1993, 4, 111–116. [Google Scholar] [CrossRef]
- Pina, S.; Ferreira, J.M.F. Brushite-Forming Mg-, Zn- and Sr-Substituted Bone Cements for Clinical Applications. Materials 2010, 3, 519–535. [Google Scholar] [CrossRef]
- De Mulder, E.L.W.; Buma, P.; Hannink, G. Anisotropic Porous Biodegradable Scaffolds for Musculoskeletal Tissue Engineering. Materials 2009, 2, 1674–1696. [Google Scholar] [CrossRef]
- Lu, J.X.; Flautre, B.; Anselme, K.; Hardouin, P.; Gallur, A.; Descamps, M.; Thierry, B. Role of interconnections in porous bioceramics on bone recolonization in vitro and in vivo. J. Mater. Sci. Mater. Med. 1999, 10, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ros-Tarraga, P.; Mazon, P.; Rodriguez, M.A.; Meseguer-Olmo, L.; de Aza, P.N. Novel resorbable and osteoconductive calcium silicophosphate scaffold induced bone formation. Materials 2016, 9, 785. [Google Scholar] [CrossRef]
- Rabadan-Ros, R.; Velásquez, P.; Meseguer-Olmo, L.; de Aza, P.N. Morphological and structural study of a novel porous Nurse´s A ceramic with osteoconductive properties for tissue engineering. Materials 2016, 9, 474. [Google Scholar] [CrossRef]
- Legeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; Legeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Paredes, B.; Santana, A.; Arribas, M.I.; Vicente-Salar, N.; de Aza, P.N.; Roche, E.; Such, J.; Reig, J.A. Phenotypic differences during the osteogenic differentiation of single cell-derived clones isolated from human lipoaspirates. J. Tissue Eng. Regen. Med. 2011, 5, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Ros-Tarraga, P.; Rabadan-Ros, R.; Murciano, A.; Meseguer-Olmo, L.; de Aza, P.N. Assessment of Effects of Si-Ca-P Biphasic Ceramic on the Osteogenic Differentiation of a Population of Multipotent Adult Human Stem Cells. Materials 2016, 9, 969. [Google Scholar] [CrossRef]
- Maté-Sánchez, J.E.; Mazon, P.; Guirado, J.L.C.; Ruíz, R.A.D.; Ramírez-Fernandez, M.P.; Negri, B.; Abboud, M.; de Aza, P.N. Comparison of three novel β-tricalcium phosphate/collagen ceramic scaffolds. An in vivo study. J. Biomed. Mater. Res. A 2014, 102A, 1037–1046. [Google Scholar] [CrossRef] [PubMed]



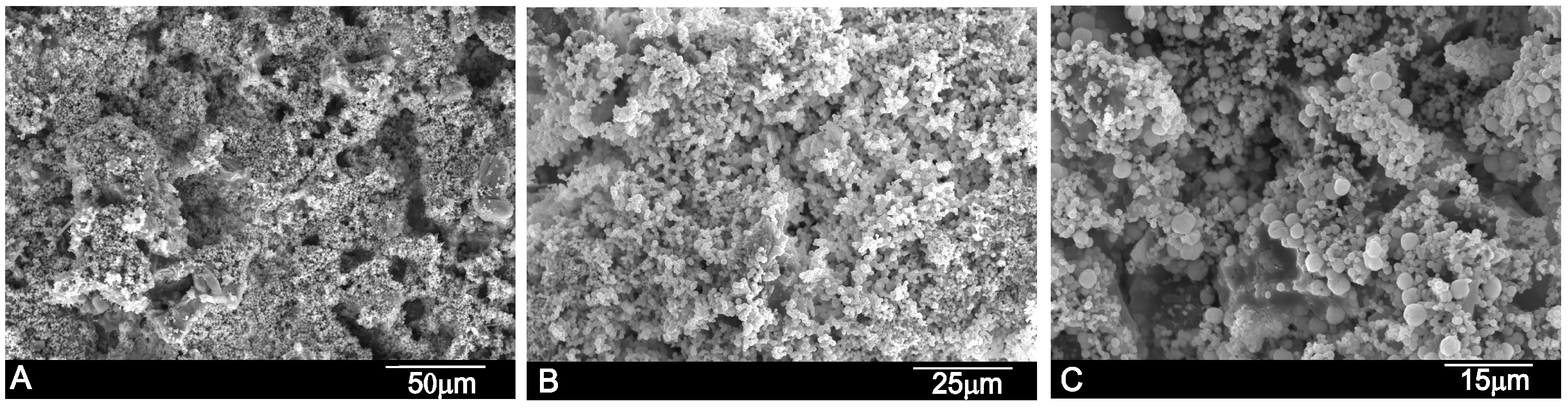




| wt % | Ca | P | Si | O |
|---|---|---|---|---|
| γ-C2S | 46.54 | - | 16.29 | 37.16 |
| Cement paste | 45.92 | 1.59 | 15.01 | 37.48 |
| Days/(%) | Cement Paste Implant | Control | ||
|---|---|---|---|---|
| 30 Days | 60 Days | 30 Days | 60 Days | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| BIC | 48.67 ± 0.83 * | 55.86 ± 0.23 * | 0.00 ± 0.00 | 0.00 ± 0.00 |
| New bone ingrowth | 54.96 ± 2.46 * | 65.53 ± 2.86 * | 17.26 ± 2.73 | 26.17 ± 1.75 |
| Defect closure | 42.46 ± 2.48 * | 58.94 ± 2.48 * | 28.46 ± 2.07 | 30.86 ± 1.86 |
| Residual biomaterial | 45.04 ± 1.67 | 35.42 ± 2.08 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Resorption rate | 45.21 ± 2.64 | 47.31 ± 2.62 | 0.00 ± 0.00 | 0.00 ± 0.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuleta, F.; Murciano, A.; Gehrke, S.A.; Maté-Sánchez de Val, J.E.; Calvo-Guirado, J.L.; De Aza, P.N. A New Biphasic Dicalcium Silicate Bone Cement Implant. Materials 2017, 10, 758. https://doi.org/10.3390/ma10070758
Zuleta F, Murciano A, Gehrke SA, Maté-Sánchez de Val JE, Calvo-Guirado JL, De Aza PN. A New Biphasic Dicalcium Silicate Bone Cement Implant. Materials. 2017; 10(7):758. https://doi.org/10.3390/ma10070758
Chicago/Turabian StyleZuleta, Fausto, Angel Murciano, Sergio A. Gehrke, José E. Maté-Sánchez de Val, José L. Calvo-Guirado, and Piedad N. De Aza. 2017. "A New Biphasic Dicalcium Silicate Bone Cement Implant" Materials 10, no. 7: 758. https://doi.org/10.3390/ma10070758






