Zinc Sorption on Modified Waste Poly(methyl methacrylate)
Abstract
:1. Introduction
2. Results and Discussion
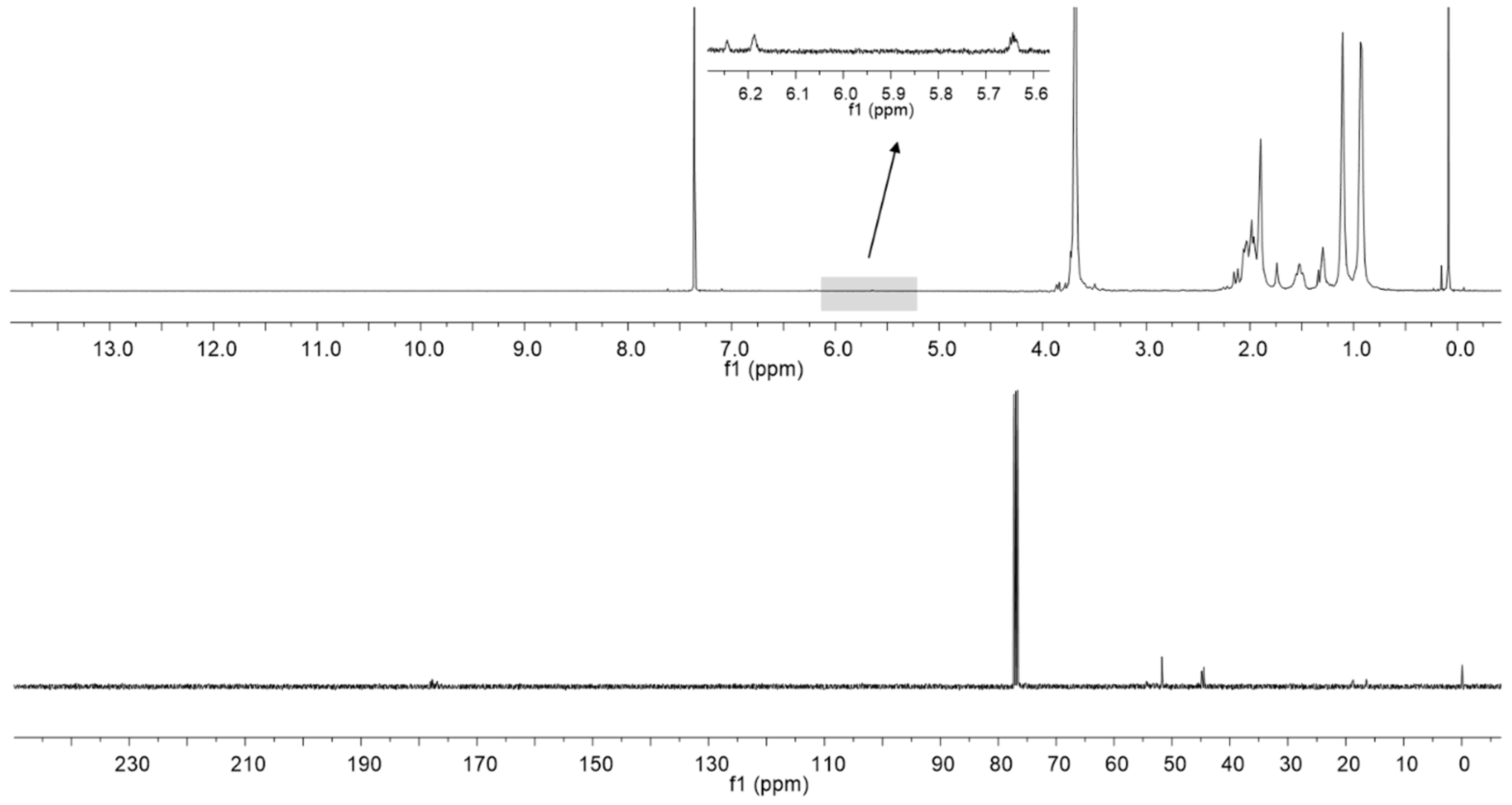
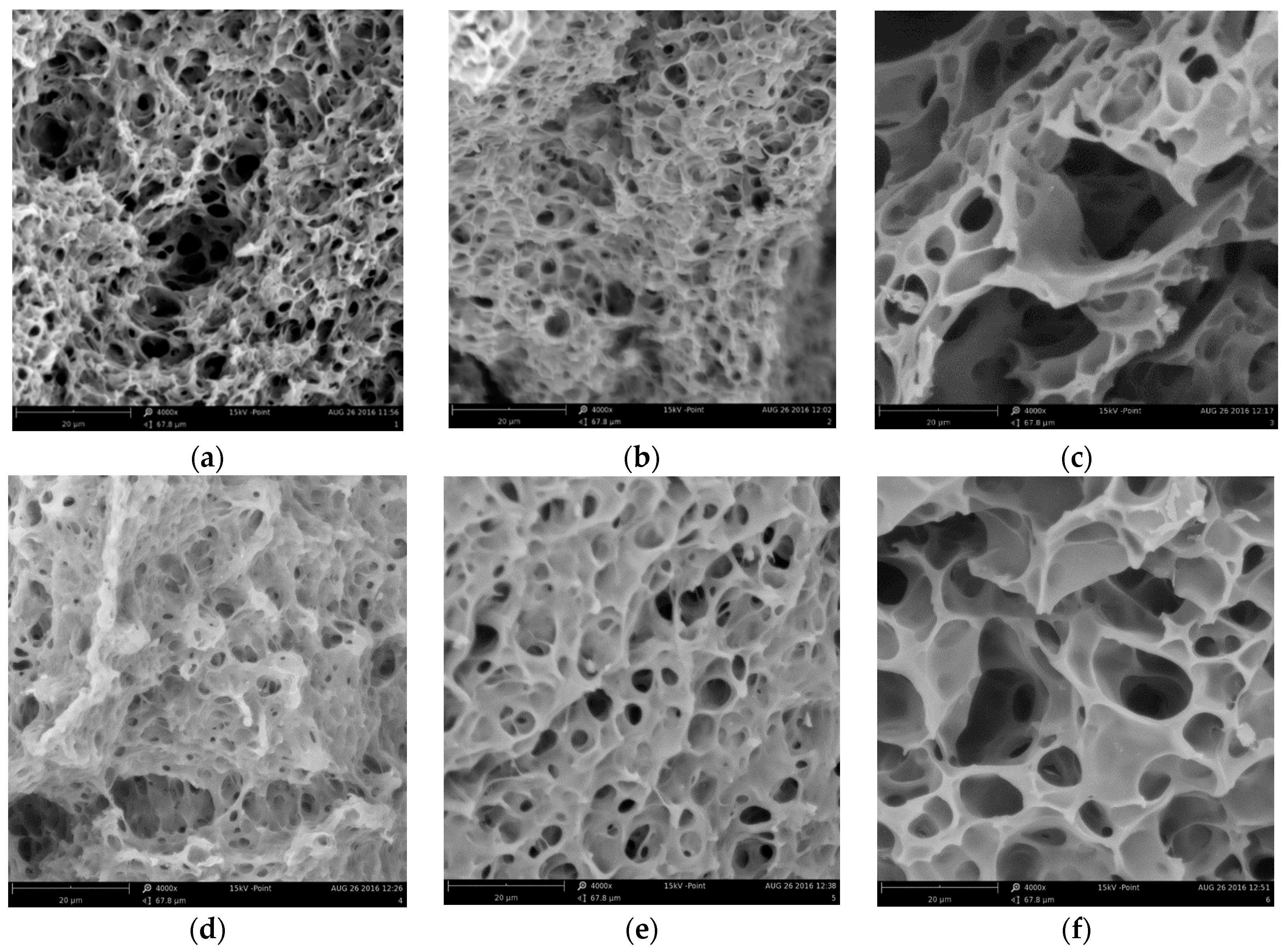
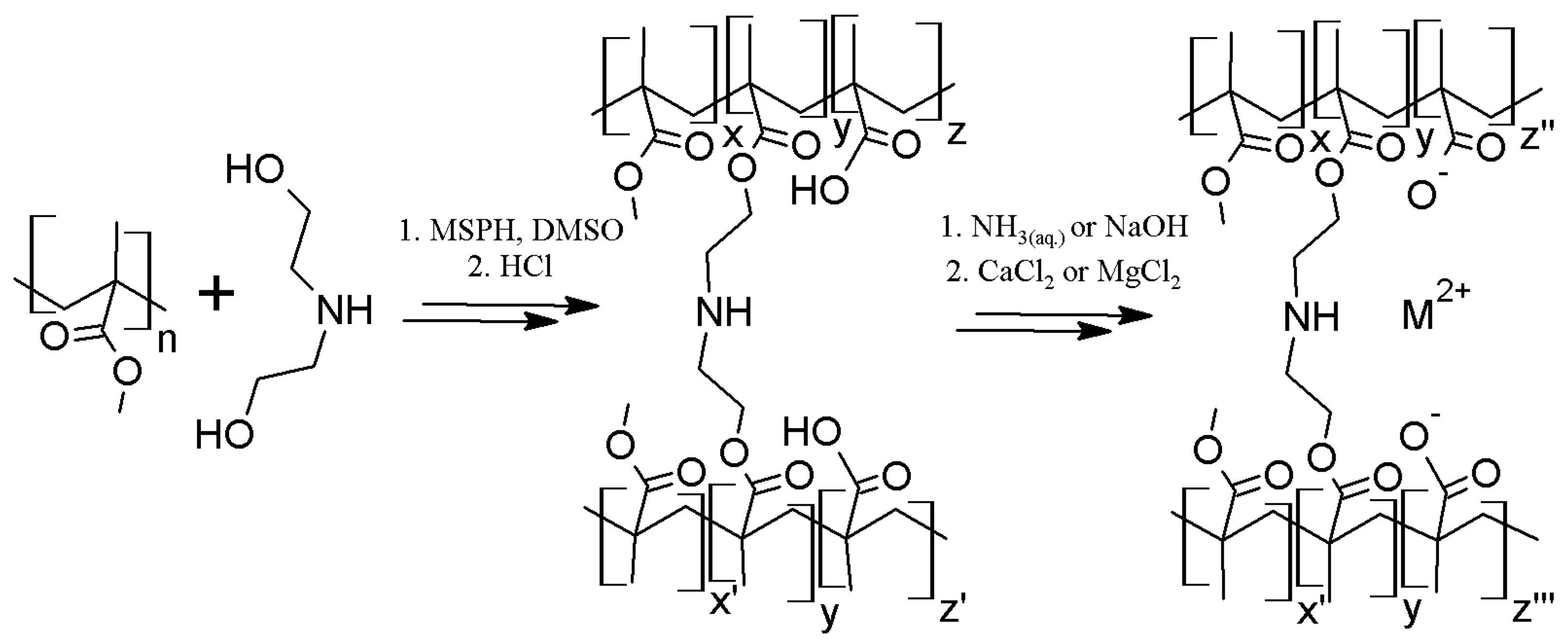
2.1. Polymer Matrix Preparation from PMMA by the One Flask Hydrolysis-Crosslinking Reaction
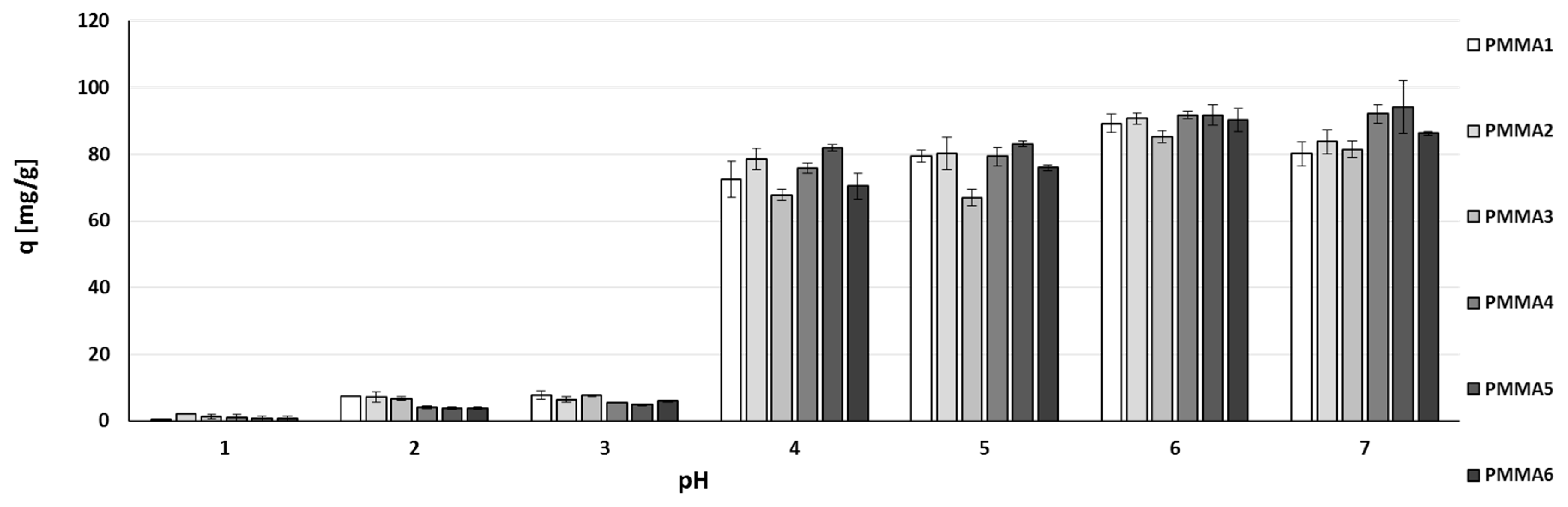
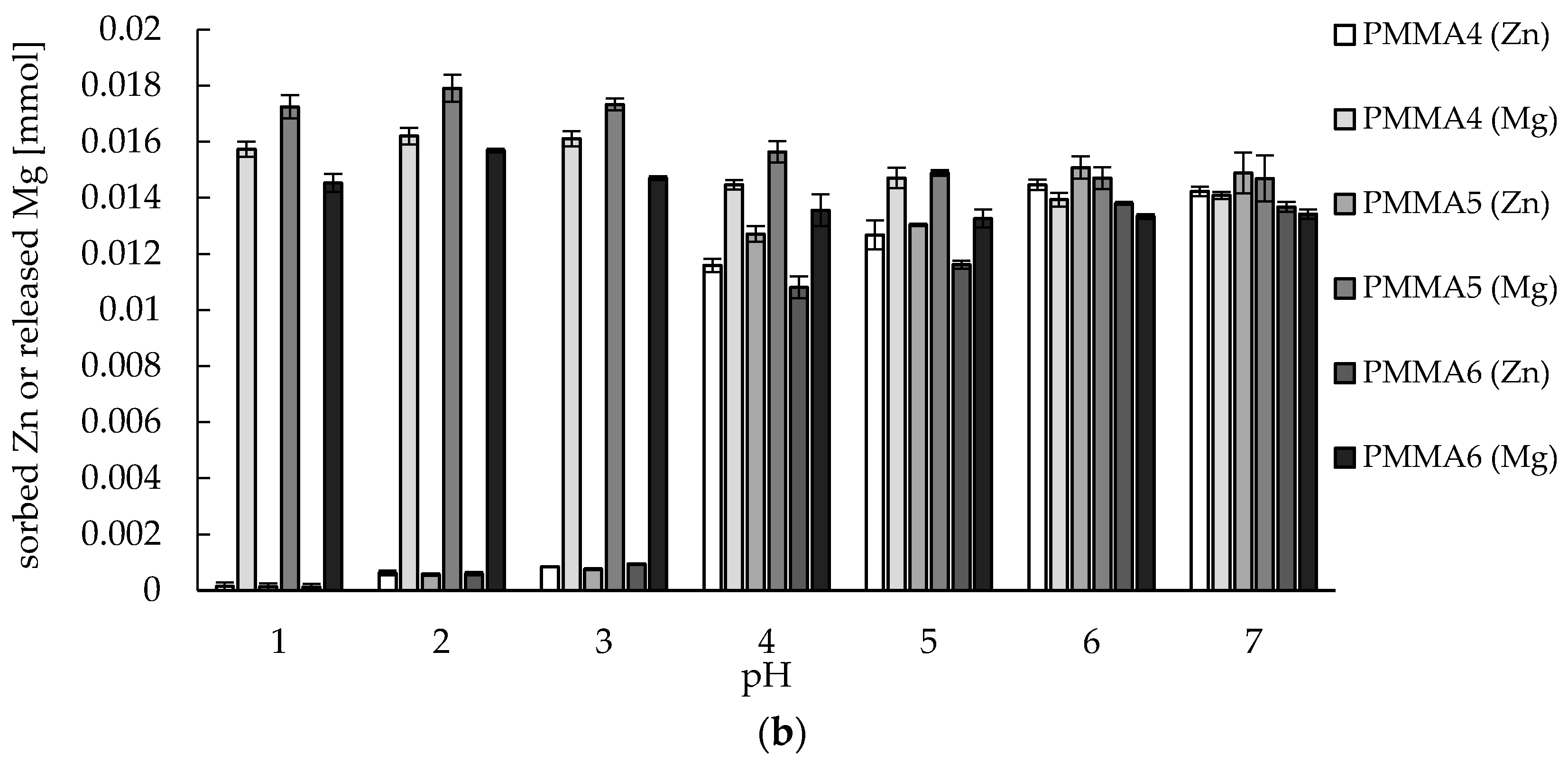
2.2. Zinc(II) Ions Sorption Studies—pH Dependence
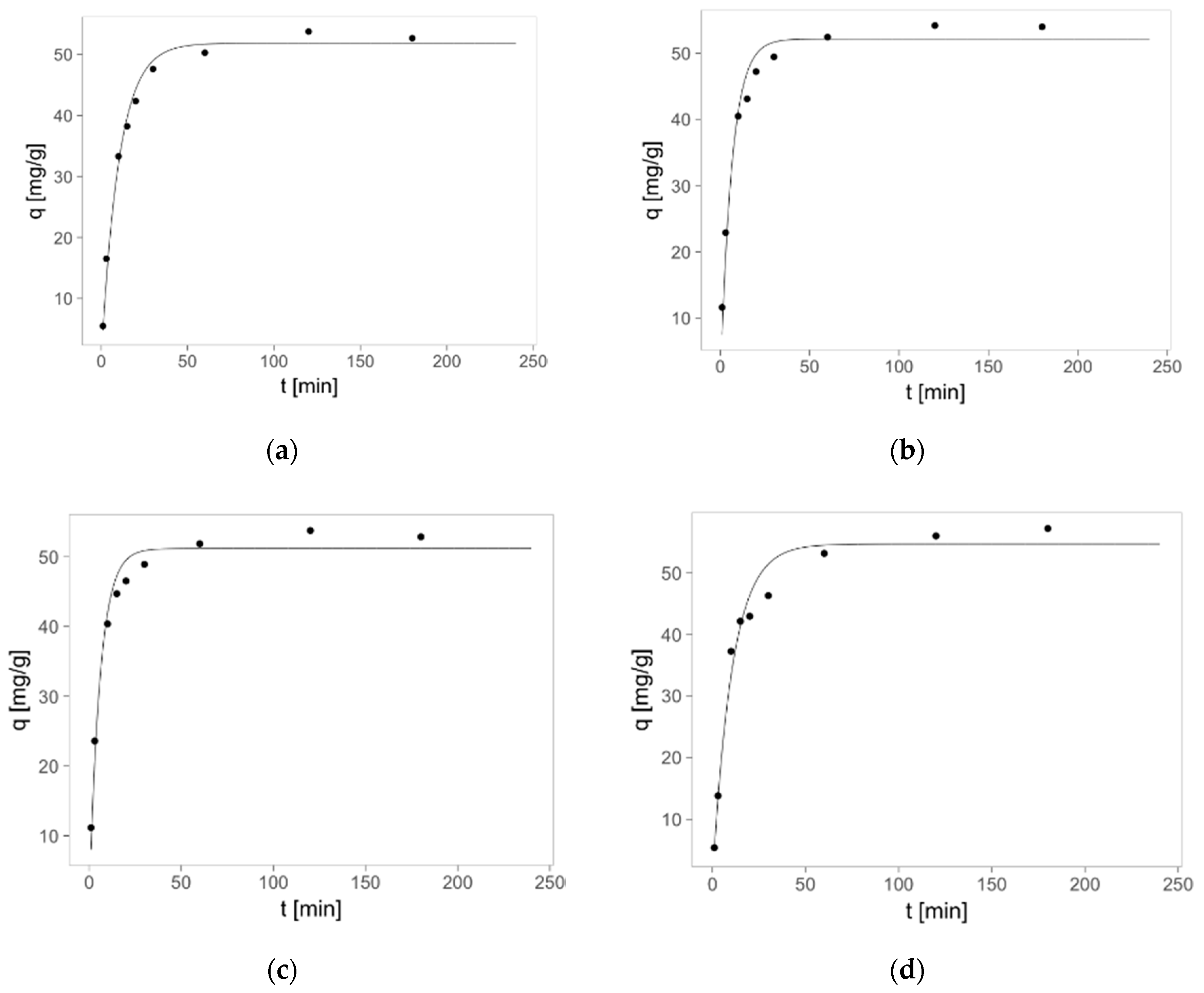
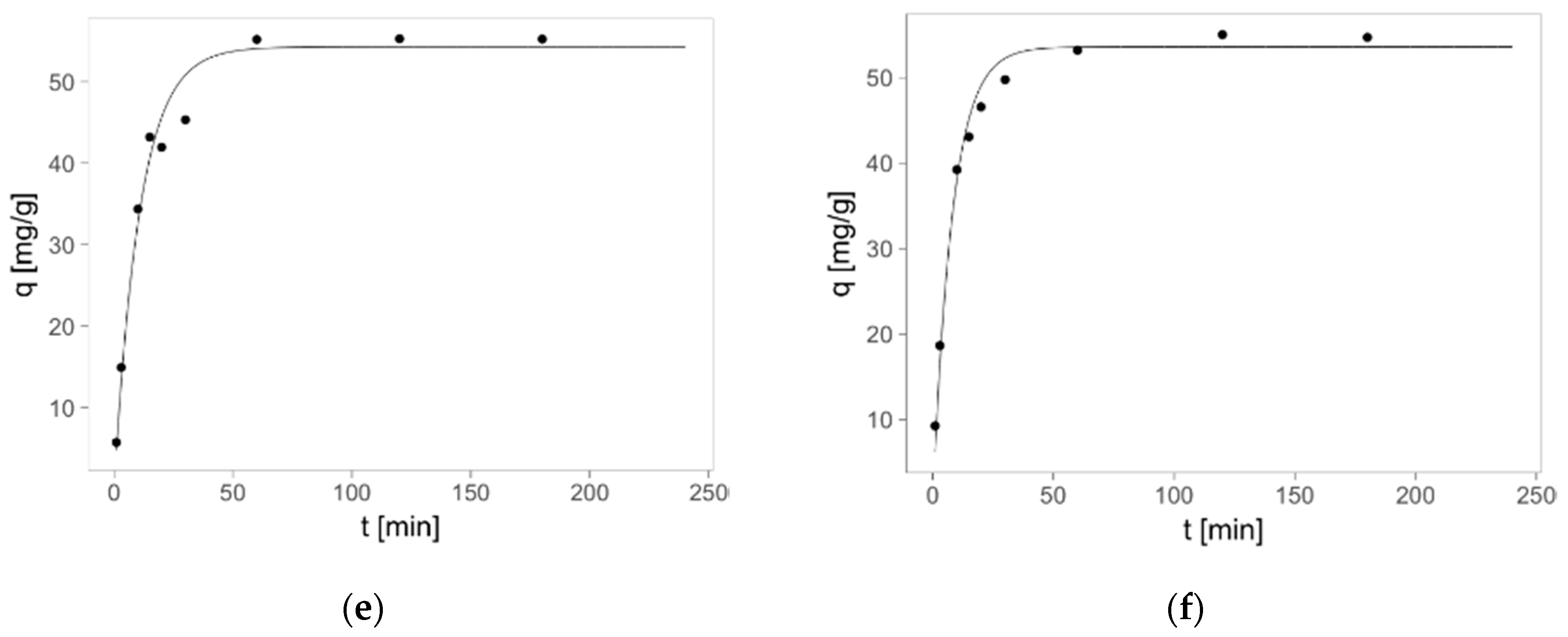
2.3. Zinc(II) Ions Sorption Studies—Kinetics
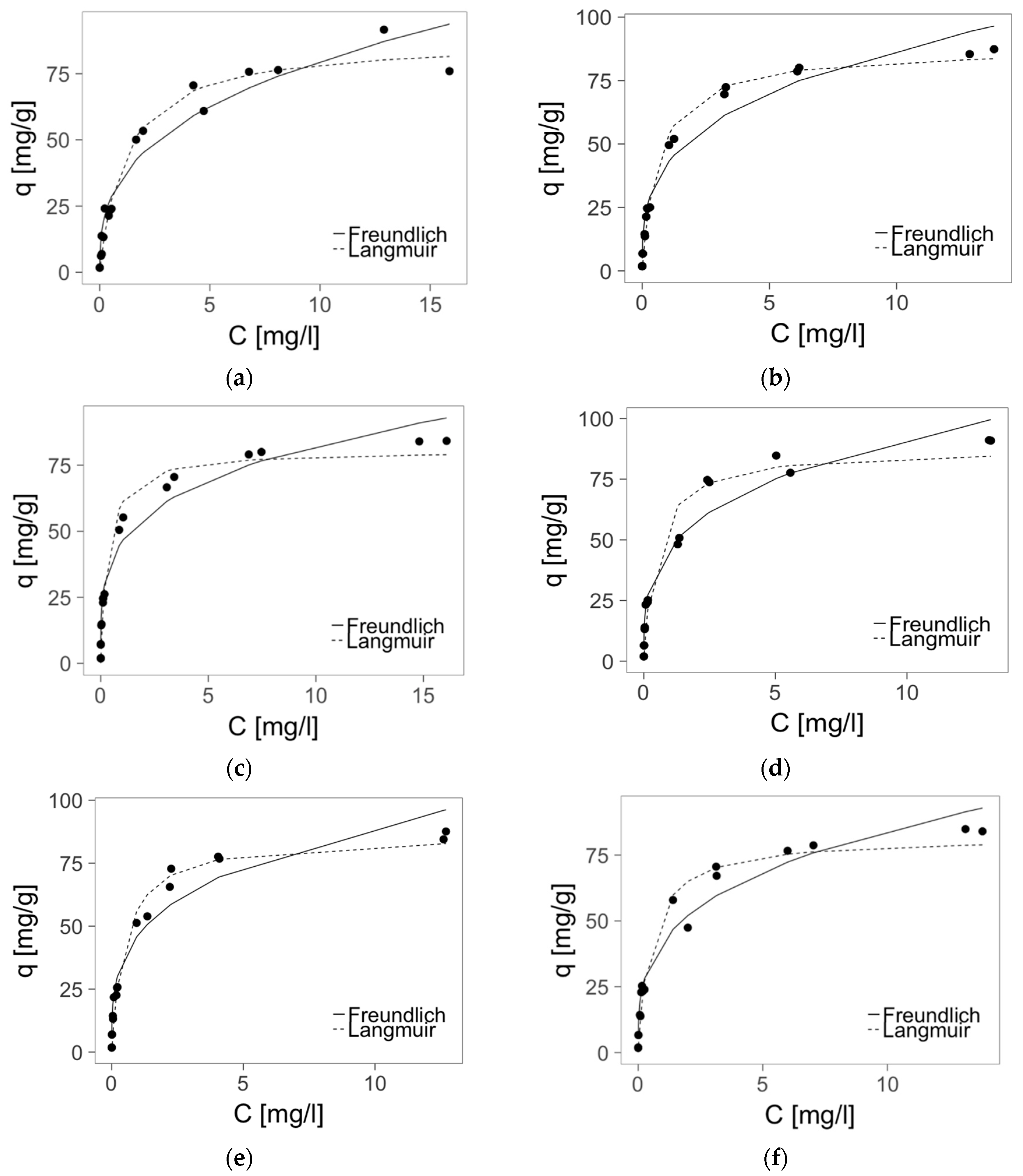
2.4. Zinc(II) Ions Sorption Studies—Isotherms
3. Materials and Methods
3.1. Materials
3.2. The Microcrystalline Suspension of Potassium Hydroxide (MSPH)
3.3. Characterization of Waste Poly(methyl methacrylate)
3.4. Analytical Methods
3.5. Colorimetric Titration of Obtained Polymeric Matrix Samples (Hydrogel Acid Form)
3.6. General Procedure of Polymer Matrix Preparation from PMMA by the One-Pot Hydrolysis—Crosslinking Reaction
3.7. General Procedure of Sorbents Preparation
3.7.1. Crosslinked Materials based on Poly(calcium methacrylate)-co-poly(methyl methacrylate)
3.7.2. Cross-Linked Poly(magnesium methacrylate)-co-poly(methyl methacrylate)
3.8. Zinc(II) ions Sorption Studies—pH Dependence
- c0—the initial concentration of Zn(II) ions in the solution (mg/L),
- c—the final concentration of Zn(II) ions in the solution (mg/L),
- V—the volume of the solution (L),
- m—the mass of the sorbent (g).
3.9. Zinc(II) ions Sorption Studies—Kinetics
- qm—the zinc(II) ions adsorbed on one gram of sorbent at equilibrium (adsorption capacity) (mg/g),
- qt —the zinc(II) ions adsorbed on one gram of sorbent at time “t” (mg/g),
- k1—the rate constant of pseudo-first order adsorption model (1/min),
- k2—the rate constant of pseudo-second order adsorption model (g/mg·min),
- t—the time (min).
3.10. Zinc(II) ions Sorption Studies—Isotherms
- q—the zinc(II) ions adsorbed on one gram of sorbent at equilibrium (adsorption capacity) (mg/g),
- qm—the maximum adsorption capacity (mg/g),
- B—the equilibrium constant that corresponds to the adsorption energy (L/mg),
- c—the equilibrium concentration of zinc(II) ions in the solution (mg/L),
- K—[(mg/g)(L/mg)1/n] corresponds to the relative adsorption capacity,
- n—corresponds to the adsorption intensity of the sorbent.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kikuchi, Y.; Hirao, M.; Ookubo, T.; Sasaki, A. Design of recycling system for poly(methyl methacrylate) (PMMA). Part 1: Recycling scenario analysis. Int. J. Life Cycle Assess. 2014, 19, 120–129. [Google Scholar] [CrossRef]
- Hwang, C.W.; Jeong, M.H.; Kim, Y.J.; Son, W.K.; Kang, K.S.; Lee, C.S.; Hwang, T.S. Process design for lithium recovery using bipolar membrane electrodialysis system. Sep. Purif. Technol. 2016, 166, 34–40. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A review of the properties and applications of poly(methyl methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Achilias, D.S. Chemical recycling of polymers. The case of poly (methyl methacrylate). In Proceedings of the International Conference on Energy & Environmental Systems, Chalkida, Greece, 8–10 May 2006. [Google Scholar]
- Semen, J.; Lando, J.B. The acid hydrolysis of isotactic and syndiotactic poly(methyl methacrylate). Macromolecules 1969, 2, 570–575. [Google Scholar] [CrossRef]
- Thanoo, B.C.; Jayakrishnan, A. Preparation of hydrogel beads from crosslinked poly(methyl methacrylate) microspheres by alkaline hydrolysis. J. Appl. Polym. Sci. 1990, 39, 1153–1161. [Google Scholar] [CrossRef]
- Barth, V.; Klesper, E. Relative rate constants during the hydrolysis of syndiotactic poly(methyl methacrylate) with base. Polymer (Guildf) 1976, 17, 777–786. [Google Scholar] [CrossRef]
- Glavis, F.J. Hydrolysis of crystallizable poly(methyl methacrylate). J. Polym. Sci. 1959, 36, 547–549. [Google Scholar] [CrossRef]
- Doskočilová, D.; Mikeš, F.; Pecka, J.; Kříž, J. Compositional microstructure of polymer prepared by partial hydrolysis of isotactic poly(methyl methacrylate). Die Makromol. Chem. 1992, 193, 2529–2538. [Google Scholar] [CrossRef]
- Guo, L.; Wang, H.; Du, X.; Xu, D.; Lei, J. Preparation of polymethylacrylic acid standard samples for GPC applications via a precipitation fractionation-separated step hydrolysis method. J. Macromol. Sci. Part B Phys. 2014, 53, 1846–1855. [Google Scholar] [CrossRef]
- Góźdź, A.S. Phase transfer catalyzed reactions on polymers, 2. The alkaline hydrolysis of poly(methyl methacrylate). Die Makromol. Chem. Rapid Commun. 1981, 2, 443–448. [Google Scholar] [CrossRef]
- Glavis, F.J. Hydrolysed Polymers of Alkyl Methacrylates and Methods of Producing Them. U.S. Patent US3029228, 1962. [Google Scholar]
- Přádný, M.; Kmínek, I.; Ševčík, S. The potentiometric behaviour of copolymers of methacrylic acid in water-ethanol solutions. Polym. Bull. 1986, 16, 195–200. [Google Scholar] [CrossRef]
- Erdmenger, T.; Guerrero-Sanchez, C.; Vitz, J.; Hoogenboom, R.; Schubert, U.S. Recent developments in the utilization of green solvents in polymer chemistry. Chem. Soc. Rev. 2010, 39, 3317. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhao, H. Glymes as versatile solvents for chemical reactions and processes: From the laboratory to industry. RSC Adv. 2014, 4, 11251. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, O.; Amin, N.K.; El-Ashtoukhy, E.-S.Z. Removal of zinc ions from aqueous solution using a cation exchange resin. Chem. Eng. Res. Des. 2013, 91, 165–173. [Google Scholar] [CrossRef]
- Demirbas, A.; Pehlivan, E.; Gode, F.; Altun, T.; Arslan, G. Adsorption of Cu(II), Zn(II), Ni(II), Pb(II), and Cd(II) from aqueous solution on Amberlite IR-120 synthetic resin. J. Colloid Interface Sci. 2005, 282, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, E.; Altun, T. The study of various parameters affecting the ion exchange of Cu2+, Zn2+, Ni2+, Cd2+, and Pb2+ from aqueous solution on Dowex 50W synthetic resin. J. Hazard. Mater. 2006, 134, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Juang, R.-S. Ion-exchange kinetics of Cu(II) and Zn(II) from aqueous solutions with two chelating resins. Chem. Eng. J. 2007, 132, 205–213. [Google Scholar] [CrossRef]
- Yi, W.; Wu, H.; Wang, H.; Du, Q. Interconnectivity of macroporous hydrogels prepared via graphene oxide-stabilized pickering high internal phase emulsions. Langmuir 2016, 32, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Kluczka, J.; Trojanowska, J.; Zołotajkin, M. Utilization of fly ash zeolite for boron removal from aqueous solution. Desalin. Water Treat. 2015, 54, 1839–1849. [Google Scholar] [CrossRef]
- Morey, G.H.; Smith, E.F. Production of Potassium Hydroxide Composition. U.S. Patent US2479692, 1949. [Google Scholar]
- Lagergren, S. Zur Theorie der sogenannten Adsorption gelöster Stoffe. K. Sven. Vetenskapsakademiens. Handl. 1898, 1–39. [Google Scholar]
- Ho, Y.S. Citation review of lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar]
- Duong, D.D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press: London, UK, 1998; ISBN 1860941303. [Google Scholar]

| Exp. no. | Polymer Matrix | PMMA Mw (by GPC) [Da] | Yield [%] | Degree of Hydrolysis [%] | Sorbent Sample no. Counter ion | |
|---|---|---|---|---|---|---|
| Ca2+ | Mg2+ | |||||
| 1 | PM 1 | 966,000 | 95 | 34 | PMMA1 | PMMA4 |
| 2 | PM 2 | 350,000 | 96 | 32 | PMMA2 | PMMA5 |
| 3 | PM 3 | 117,000 | 87 | 35 | PMMA3 | PMMA6 |
| Sorbent | PMMA1 | PMMA2 | PMMA3 | PMMA4 | PMMA5 | PMMA6 |
|---|---|---|---|---|---|---|
| Pseudo-first-order kinetics | ||||||
| R2 | 0.991 | 0.975 | 0.978 | 0.977 | 0.979 | 0.988 |
| qm [mg/g] | 51.8 ± 0.9 | 52.2 ± 1.3 | 51.2 ± 1.1 | 54.6 ± 1.4 | 54.3 ± 1.4 | 53.6 ± 1.0 |
| k1·102 [1/min] | 9.64 ± 0.68 | 15.51 ± 1.97 | 17.11 ± 1.96 | 9.40 ± 0.96 | 9.18 ± 0.90 | 12.30 ± 1.07 |
| Pseudo-second-order kinetics | ||||||
| R2 | 0.996 | 0.998 | 0.998 | 0.991 | 0.988 | 0.996 |
| qm [mg/g] | 35.9 ± 2.2 | 42.5 ± 1.9 | 46.2 ± 1.6 | 46.8 ± 2.1 | 46.2 ± 2.1 | 42.3 ± 1.9 |
| k2·104 [g/mg∙min] | 26.6 ± 2.0 | 44.3 ± 1.8 | 48.9 ± 2.2 | 23.1 ± 2.6 | 23.4 ± 2.9 | 33.5 ± 2.3 |
| Sorbent | PMMA1 | PMMA2 | PMMA3 | PMMA4 | PMMA5 | PMMA6 |
|---|---|---|---|---|---|---|
| Calculated Parameters of Langmuir Isotherm | ||||||
| R2 | 0.975 | 0.991 | 0.980 | 0.962 | 0.983 | 0.969 |
| qm [mg/g] | 87.6 ± 3.5 | 87.6 ± 2.0 | 80.59 ± 2.6 | 87.50 ± 4.3 | 86.1 ± 3.0 | 81.8 ± 3.2 |
| B [L/mg] | 0.84 ± 0.13 | 1.51 ± 0.15 | 3.06 ± 0.51 | 2.10 ± 0.52 | 1.96 ± 0.30 | 1.94 ± 0.37 |
| Calculated Parameters of Freundlich Isotherm | ||||||
| R2 | 0.947 | 0.963 | 0.971 | 0.963 | 0.953 | 0.964 |
| K [(mg/g)(L/mg)1/n] | 35.6 ± 2.3 | 42.5 ± 2.0 | 46.3 ± 1.7 | 47.1 ± 2.1 | 46.5 ± 2.1 | 42.3 ± 1.9 |
| n | 2.86 ± 0.25 | 3.21 ± 0.23 | 3.99 ± 0.26 | 3.45 ± 0.26 | 3.50 ± 0.29 | 3.34 ± 0.25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakóbik-Kolon, A.; Milewski, A.; Zdybał, D.; Mitko, K.; Laskowska, E.; Mielańczyk, A.; Bok-Badura, J. Zinc Sorption on Modified Waste Poly(methyl methacrylate). Materials 2017, 10, 755. https://doi.org/10.3390/ma10070755
Jakóbik-Kolon A, Milewski A, Zdybał D, Mitko K, Laskowska E, Mielańczyk A, Bok-Badura J. Zinc Sorption on Modified Waste Poly(methyl methacrylate). Materials. 2017; 10(7):755. https://doi.org/10.3390/ma10070755
Chicago/Turabian StyleJakóbik-Kolon, Agata, Andrzej Milewski, Dominik Zdybał, Krzysztof Mitko, Ewa Laskowska, Anna Mielańczyk, and Joanna Bok-Badura. 2017. "Zinc Sorption on Modified Waste Poly(methyl methacrylate)" Materials 10, no. 7: 755. https://doi.org/10.3390/ma10070755





