Lithium Hydrazinidoborane Ammoniate LiN2H3BH3·0.25NH3, a Derivative of Hydrazine Borane
Abstract
:1. Introduction
2. Results
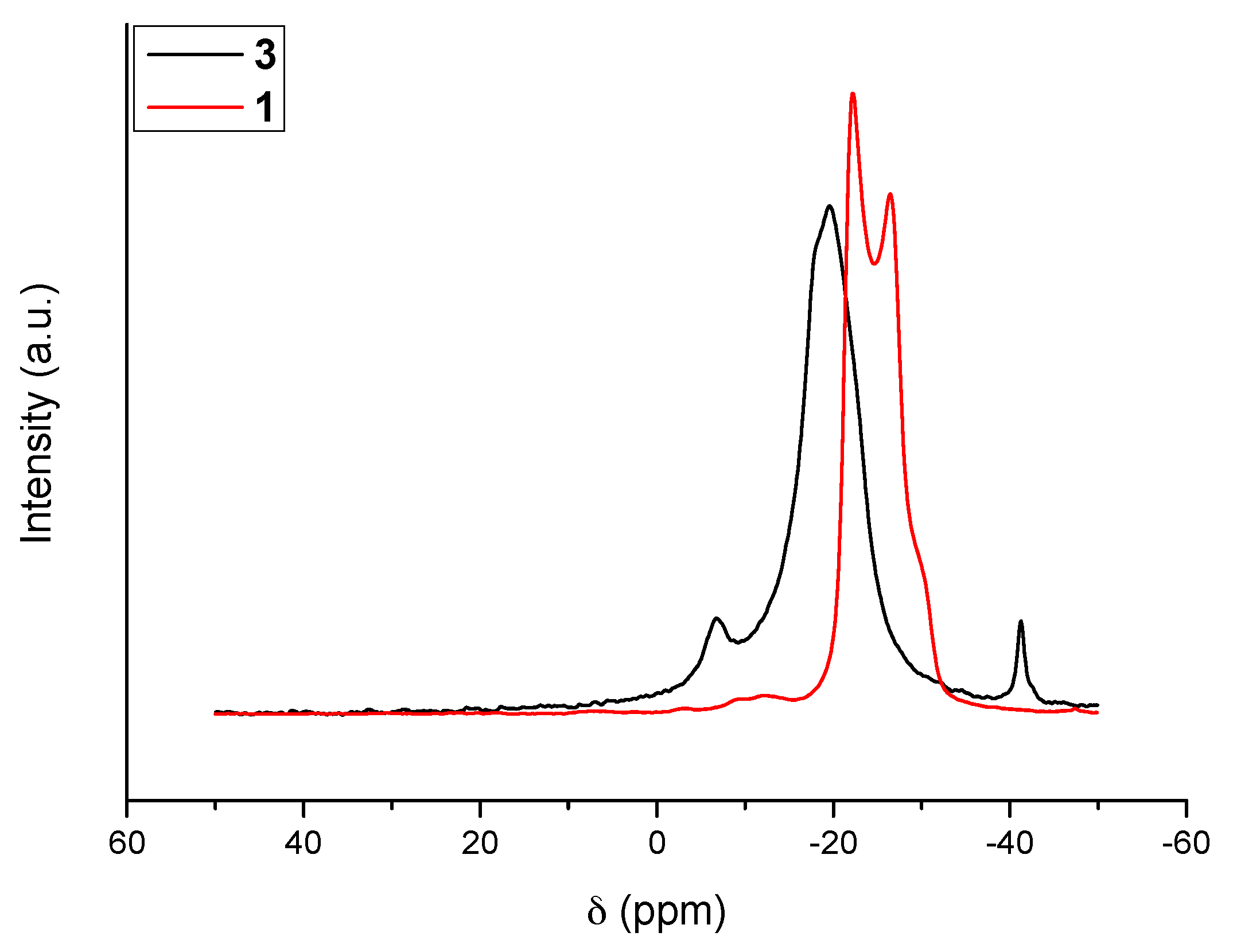
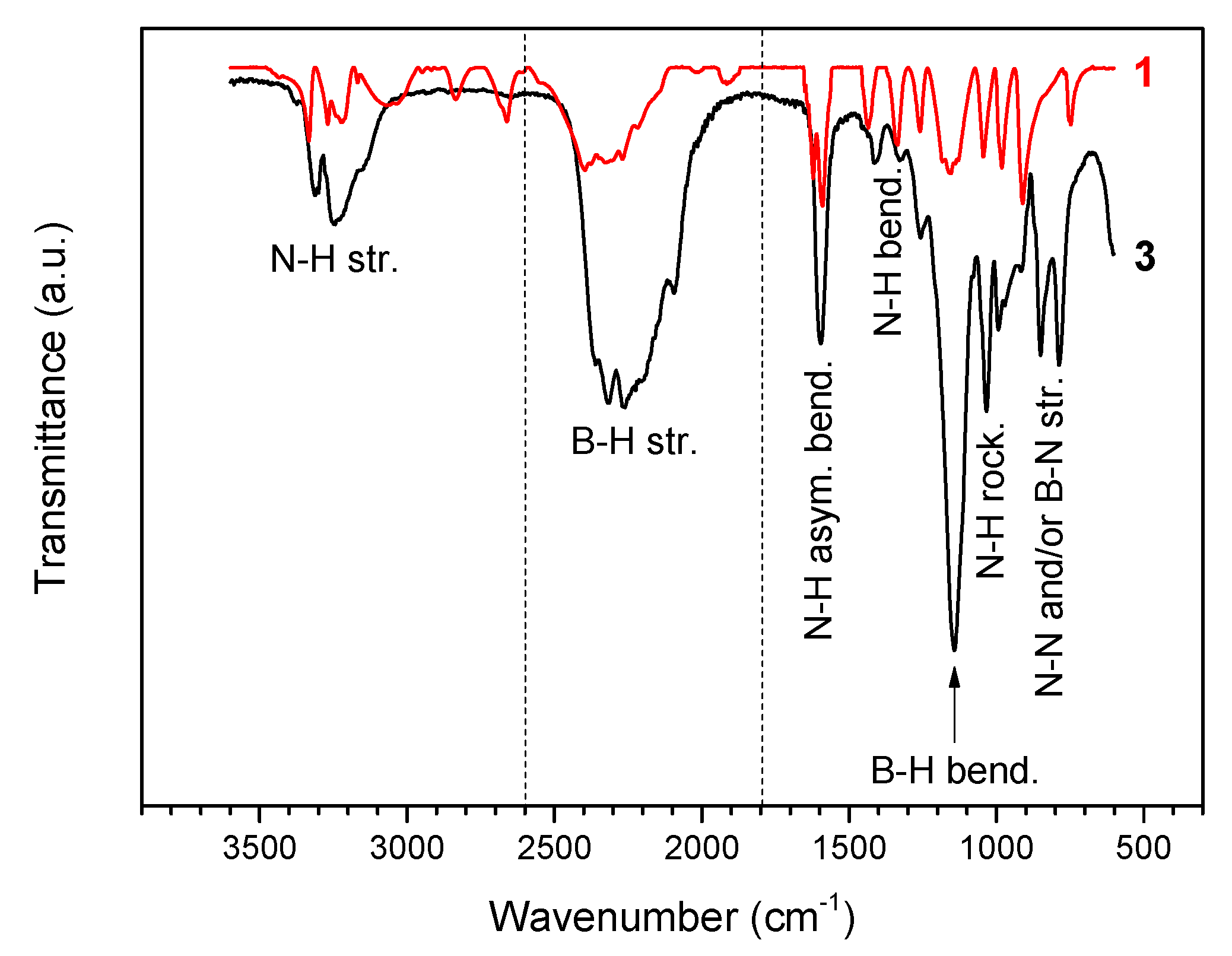
2.1. Molecular Structure
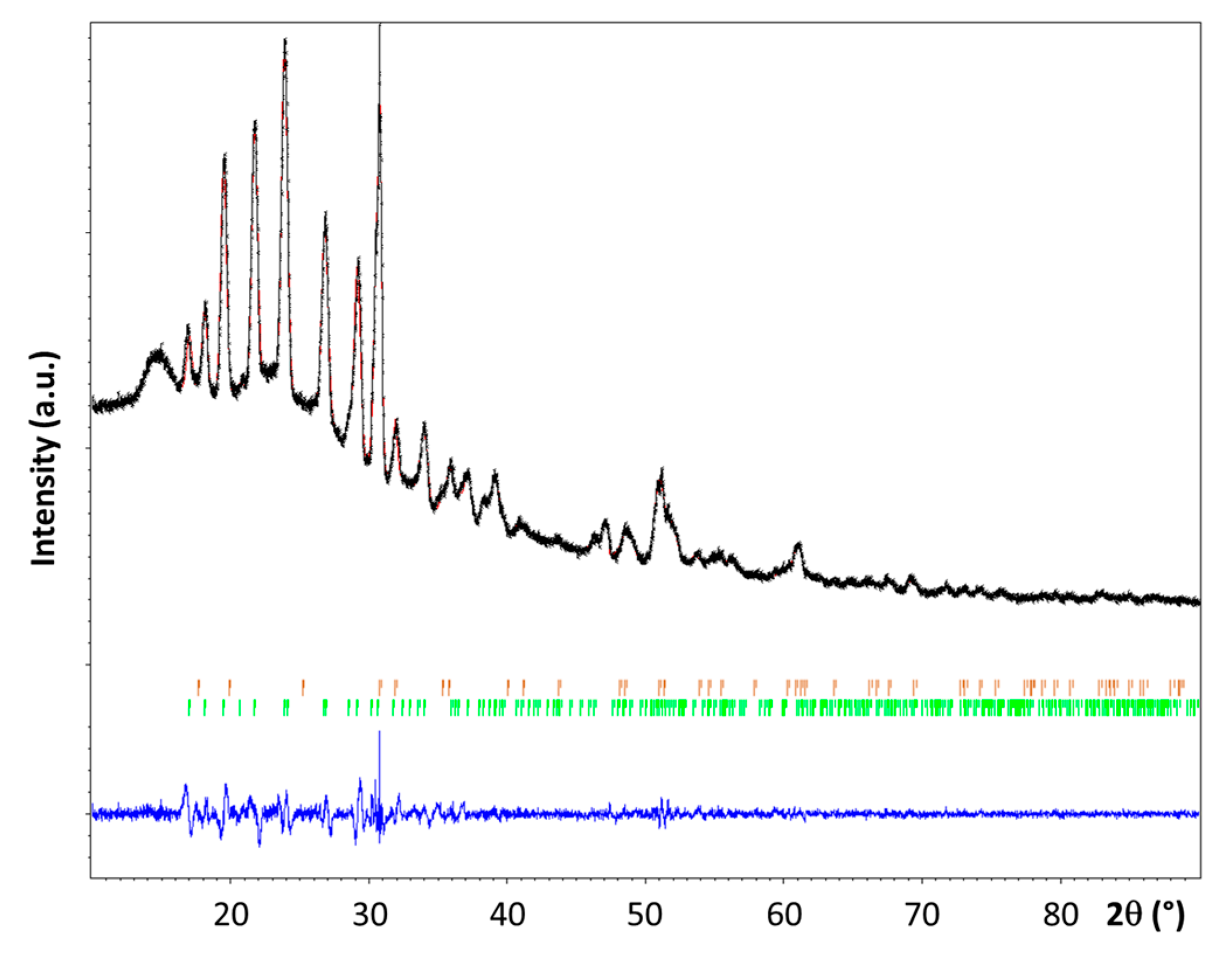
2.2. Crystallography
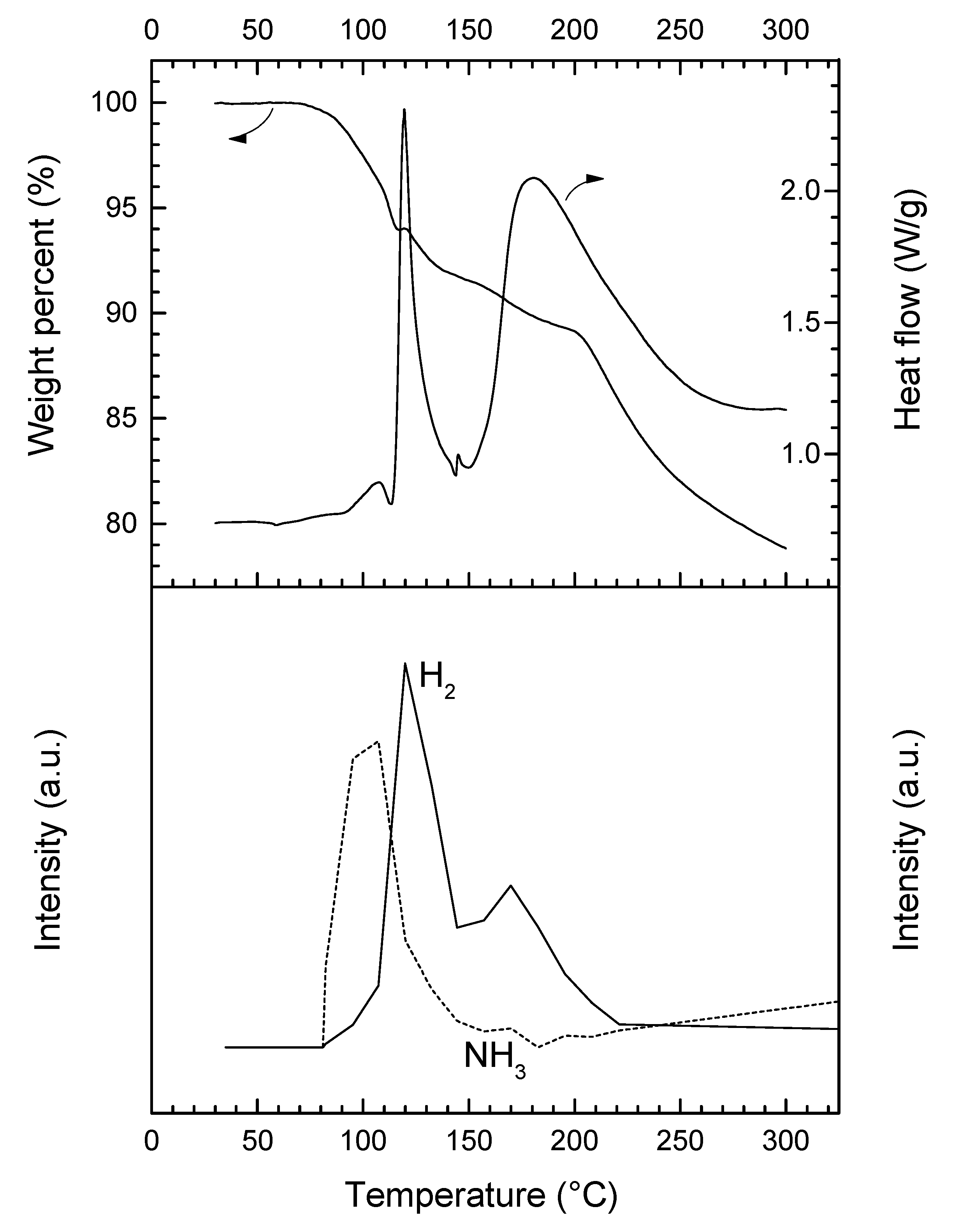
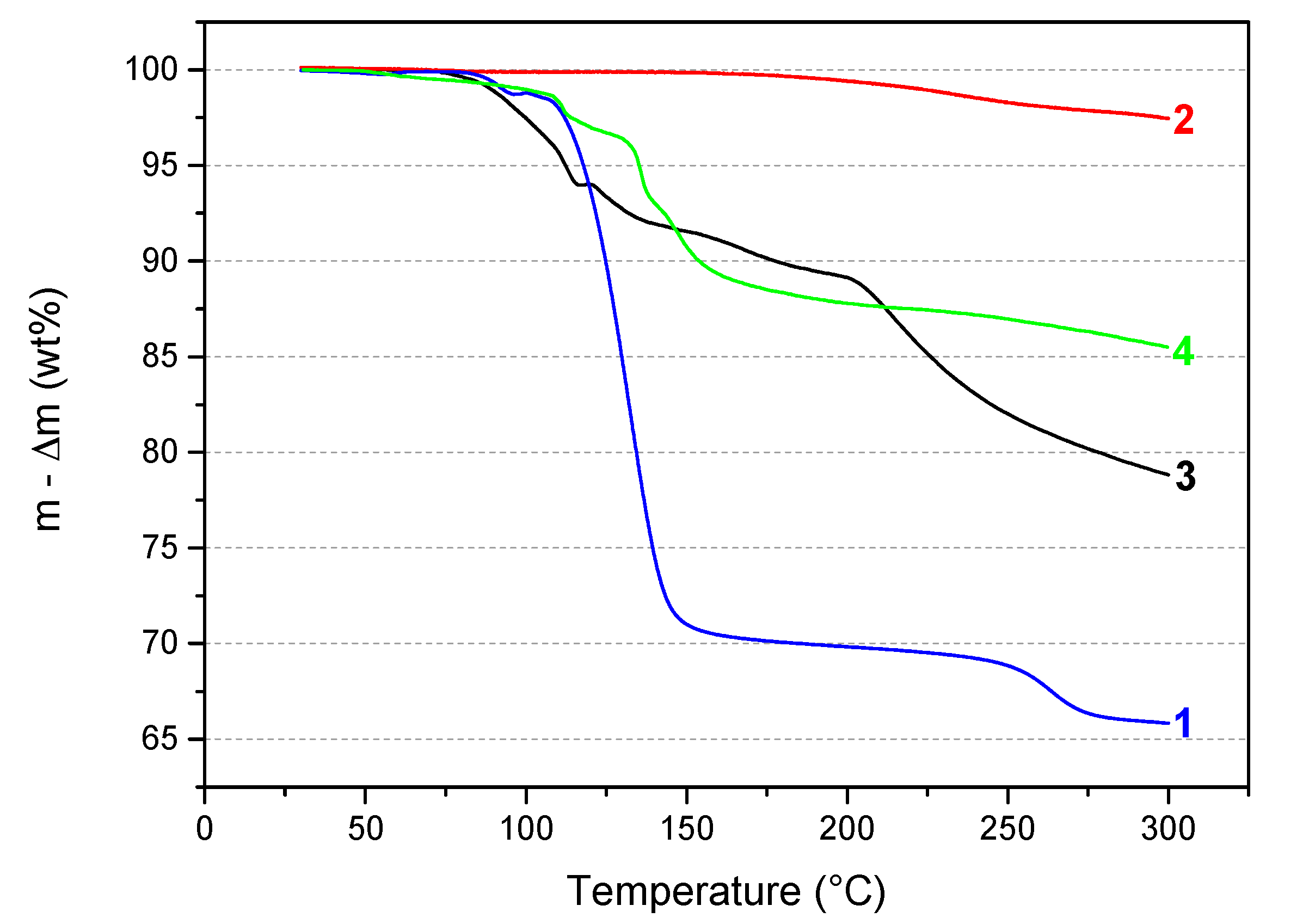
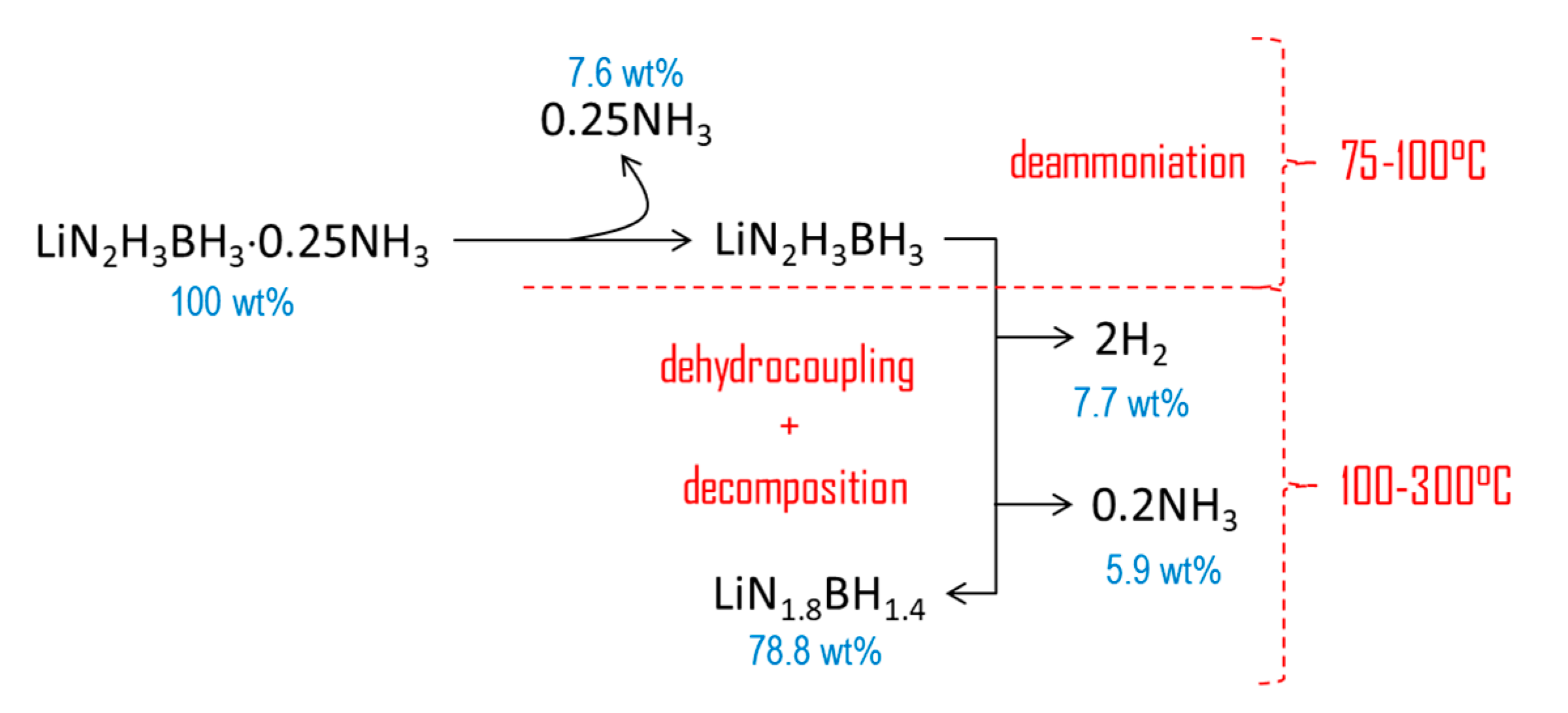
2.3. Thermal Analyses and Evolving Gas Analyses
3. Discussion and Concluding Remarks
4. Materials and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sherif, S.A.; Goswami, D.Y.; Stefanakos, E.K.; Steinfeld, A. Handbook of Hydrogen Energy; CRC Press: New York, NY, USA, 2014. [Google Scholar]
- Staubitz, A.; Robertson, A.P.M.; Sloan, M.E.; Manners, I. Amine- and phosphine-borane adducts: New interest in old molecules. Chem. Rev. 2010, 110, 4023–4078. [Google Scholar] [CrossRef] [PubMed]
- Goubeau, V.J.; Ricker, E. Borinhydrazin und seine pyrolyseprodukte. Z. Anorg. Allg. Chem. 1961, 310, 123–142. [Google Scholar] [CrossRef]
- Hügle, T.; Kühnel, M.F.; Lentz, D. Hydrazine borane: A promising hydrogen storage material. J. Am. Chem. Soc. 2009, 131, 7444–7446. [Google Scholar] [CrossRef] [PubMed]
- Moury, R.; Moussa, G.; Demirci, U.B.; Hannauer, J.; Bernard, S.; Petit, E.; van der Lee, A.; Miele, P. Hydrazine borane: Synthesis, characterization, and application prospects in chemical hydrogen storage. Phys. Chem. Chem. Phys. 2012, 14, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Moury, R.; Demirci, U.B. Hydrazine borane and hydrazinidoboranes as chemical hydrogen storage materials. Energies 2015, 8, 3118–3141. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Pinkerton, F.E.; Udovic, T.J.; Yildirim, T.; Rush, J.J. Metal hydrazinoborane LiN2H3BH3 and LiN2H3BH3·2N2H4BH3: Crystal structures and high-extent dehydrogenation. Energy Environ. Sci. 2012, 5, 7531–7535. [Google Scholar] [CrossRef]
- Moury, R.; Demirci, U.B.; Ban, V.; Filinchuk, Y.; Ichikawa, T.; Zeng, L.; Goshome, K.; Miele, P. Lithium hydrazinidoborane, a polymorphic material with potential for chemical hydrogen storage. ChemSusChem 2014, 26, 3249–3255. [Google Scholar] [CrossRef]
- Moury, R.; Demirci, U.B.; Ichikawa, T.; Filinchuk, Y.; Chiriac, R.; van der Lee, A.; Miele, P. Sodium hydrazinidoborane, a chemical hydrogen storage material. ChemSusChem 2013, 6, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Pylypko, S.; Petit, J.F.; Ould-Amara, S.; Ndhili, N.; Taihei, A.; Chiriac, R.; Ichikawa, T.; Cretin, M.; Miele, P.; Demirci, U.B. Metal hydride-hydrazine borane: Towards hydrazinidoboranes or composites as hydrogen carriers. Int. J. Hydrogen Energy 2015, 40, 14875–14884. [Google Scholar] [CrossRef]
- Chua, Y.S.; Pei, Q.; Ju, X.; Zhou, W.; Udovic, T.J.; Wu, G.; Xiong, Z.; Chen, P.; Wu, H. Alkali metal hydride modification on hydrazine borane for improved dehydrogenation. J. Phys. Chem. C 2014, 118, 11244–11251. [Google Scholar] [CrossRef]
- Chua, Y.S.; Wu, G.; Xiong, Z.; Karkamkar, A.; Guo, J.; Jian, M.; Wong, M.W.; Autrey, T.; Chen, P. Synthesis, structure and dehydrogenation of magnesium amidoborane monoammoniate. Chem. Commun. 2010, 46, 5752–5754. [Google Scholar] [CrossRef] [PubMed]
- Chua, Y.S.; Wu, G.; Zhou, W.; Udovic, T.J.; Wu, G.; Xiong, Z.; Wong, M.W.; Chen, P. Monoammoniate of calcium amidoborane: Synthesis, structure, and hydrogen-storage properties. Inorg. Chem. 2012, 51, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Remhof, A.; Rentsch, D.; Züttel, A.; Giri, S.; Jena, P. A novel strategy for reversible hydrogen storage in Ca(BH4)2. Chem. Commun. 2015, 51, 11008–11011. [Google Scholar] [CrossRef] [PubMed]
- Esteruelas, M.A.; Nolis, P.; Oliván, M.; Oñate, E.; Vallribera, A.; Vélez, A. Ammonia borane dehydrogenation promoted by a pincer-square-planar rhodium(I) monohydride: A stepwise hydrogen transfer from the substrate to the catalyst. Inorg. Chem. 2016, 55, 7176–7181. [Google Scholar] [CrossRef] [PubMed]
- Stowe, A.C.; Shaw, W.J.; Linehan, J.C.; Schmid, B.; Autrey, T. In situ solid state 11B MAS-NMR studies of the thermal decomposition of ammonia borane: Mechanistic studies of the hydrogen release pathways from a solid state hydrogen storage material. Phys. Chem. Chem. Phys. 2007, 9, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, W.; Pinkerton, F.E.; Meyer, M.S.; Srinivas, G.; Yildirim, T.; Udovic, T.J.; Rush, J.J. A new family of metal borohydride ammonia borane complexes: Synthesis, structures, and hydrogen storage properties. J. Mater. Chem. 2010, 20, 6550–6556. [Google Scholar] [CrossRef]
- Yot, P.G.; Miele, P.; Demirci, U.B. In situ Synchrotron X-ray thermodiffraction of boranes. Crystals 2016, 6, 16. [Google Scholar] [CrossRef]
- Yang, J.B.; Zhou, X.D. Crystal and electronic structures of LiNH2. Appl. Phys. Lett. 2006, 88, 041914. [Google Scholar] [CrossRef]
- Boultif, A.; Louer, D. Indexing of powder diffraction patterns for low-symmetry lattices by the successive dichotomy method. J. Appl. Crystallogr. 1991, 24, 987–993. [Google Scholar] [CrossRef]
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A kit of tools for phasing crystal structures from powder data. J. Appl. Crystallogr. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Kristallogr. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Xia, G.; Yu, X.; Guo, Y.; Wu, Z.; Yang, C.; Liu, H.; Dou, S. Amminelithium amidoborane Li(NH3)NH2BH3: A new coordination compound with favorable dehydrogenation characteristics. Chem. Eur. J. 2010, 16, 3763–3769. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wu, H.; Zhou, W.; Wang, P. A simple and efficient approach to synthesize amidoborane ammoniates: Case study for Mg(NH2BH3)2(NH3)3 with unusual coordination structure. J. Mater. Chem. 2012, 22, 13174–13179. [Google Scholar] [CrossRef]
- Gao, L.; Fang, H.; Li, Z.; Yu, X.; Fan, K. Liquefaction of solid-state BH3NH3 by gaseous NH3. Inorg. Chem. 2011, 50, 4301–4306. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.G.; Parry, R.W. The crystalline compound ammonia-borane, H3NBH3. J. Am. Chem. Soc. 1955, 77, 6084–6085. [Google Scholar] [CrossRef]
- Summerscales, O.T.; Gordon, J.C. Regeneration of ammonia borane from spent fuel materials. Dalton Trans. 2013, 42, 10075–10084. [Google Scholar] [CrossRef] [PubMed]
- Halseid, R.; Vie, P.J.S.; Tunold, R. Effect of ammonia on the performance of polymer electrolyte membrane fuel cells. J. Power Sources 2006, 154, 343–350. [Google Scholar] [CrossRef]
| LiN2H3BH3·xNH3 | LiNH2 | |
|---|---|---|
| s.g. | P21/n (N° 14) | (N° 82) |
| Z | 4 | 8 |
| a (Å) | 7.6498(18) | 5.1158(11) |
| b (Å) | 7.482(3) | 5.1158(11) |
| c (Å) | 5.968(17) | 10.103(3) |
| β (°) | 97.803(12) | - |
| V (Å3) | 338.91(17) | 264.41(12) |
| R.P.A. (wt%) 1 | 95.4(5)% | 4.6(6)% |
| GoF | 2.94 | 2.94 |
| Rp | 3.66 | 3.66 |
| wRp | 486 | 4.86 |
| R(obs)/R(all) | 13.85/15.47 | 11.17/12.49 |
| wR(obs)/wR(all) | 11.66/11.77 | 11.92/12.00 |
| Sample | Atom | Site | Occupancy | x | y | z | Uiso (Å2) |
|---|---|---|---|---|---|---|---|
| 3 | Li1_1 | 4e | 1 | 0.4025(11) | 0.4401(9) | 0.767(2) | 0.0229(1) |
| B2_1 | 4e | 1 | 0.7210(11) | 0.3147(17) | 0.5489(13) | 0.0213(1) | |
| N3_1 | 4e | 1 | 0.6451(11) | 0.2980(11) | 0.7827(11) | 0.0202(1) | |
| N4_1 | 4e | 1 | 0.6373(18) | 0.1096(14) | 0.867(3) | 0.0202(1) | |
| 2 | Li1_2 | 2a | 1 | 0.00000 | 0.500000 | 0.25000 | 0.0177(1) |
| Li2_2 | 2d | 1 | 0.00000 | 0.00000 | 0.00000 | 0.0177(1) | |
| Li3_2 | 4e | 1 | 0,00000 | 0.00000 | 0.25300 | 0.0177(1) | |
| N4_2 | 8g | 1 | 0.23400 | 0.25400 | 0.13700 | 0.0065(1) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ould-Amara, S.; Granier, D.; Chiriac, R.; Toche, F.; Yot, P.G.; Demirci, U.B. Lithium Hydrazinidoborane Ammoniate LiN2H3BH3·0.25NH3, a Derivative of Hydrazine Borane. Materials 2017, 10, 750. https://doi.org/10.3390/ma10070750
Ould-Amara S, Granier D, Chiriac R, Toche F, Yot PG, Demirci UB. Lithium Hydrazinidoborane Ammoniate LiN2H3BH3·0.25NH3, a Derivative of Hydrazine Borane. Materials. 2017; 10(7):750. https://doi.org/10.3390/ma10070750
Chicago/Turabian StyleOuld-Amara, Salem, Dominique Granier, Rodica Chiriac, François Toche, Pascal G. Yot, and Umit B. Demirci. 2017. "Lithium Hydrazinidoborane Ammoniate LiN2H3BH3·0.25NH3, a Derivative of Hydrazine Borane" Materials 10, no. 7: 750. https://doi.org/10.3390/ma10070750







