Symmetric Supercapacitor Electrodes from KOH Activation of Pristine, Carbonized, and Hydrothermally Treated Melia azedarach Stones
Abstract
:1. Introduction
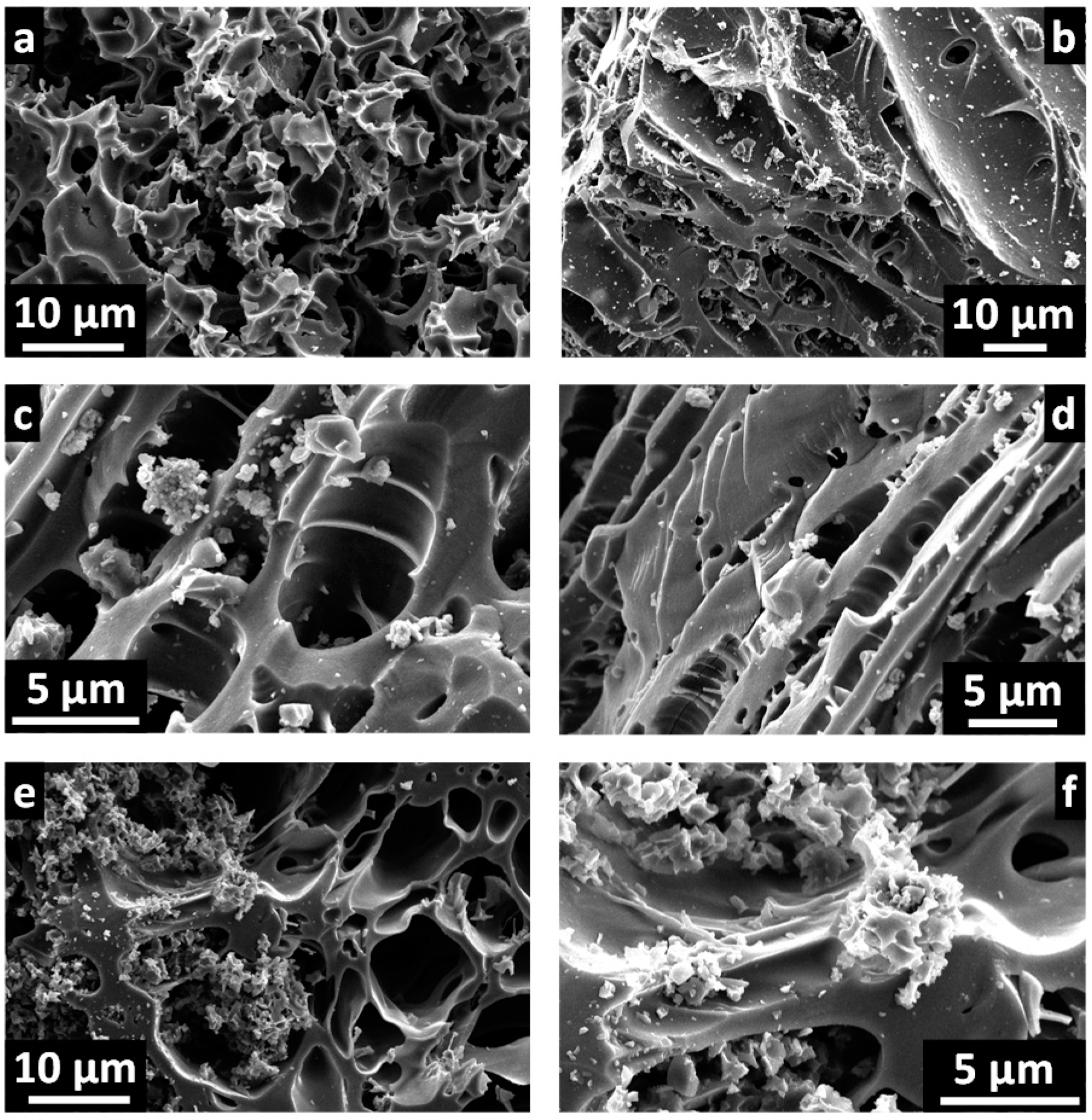
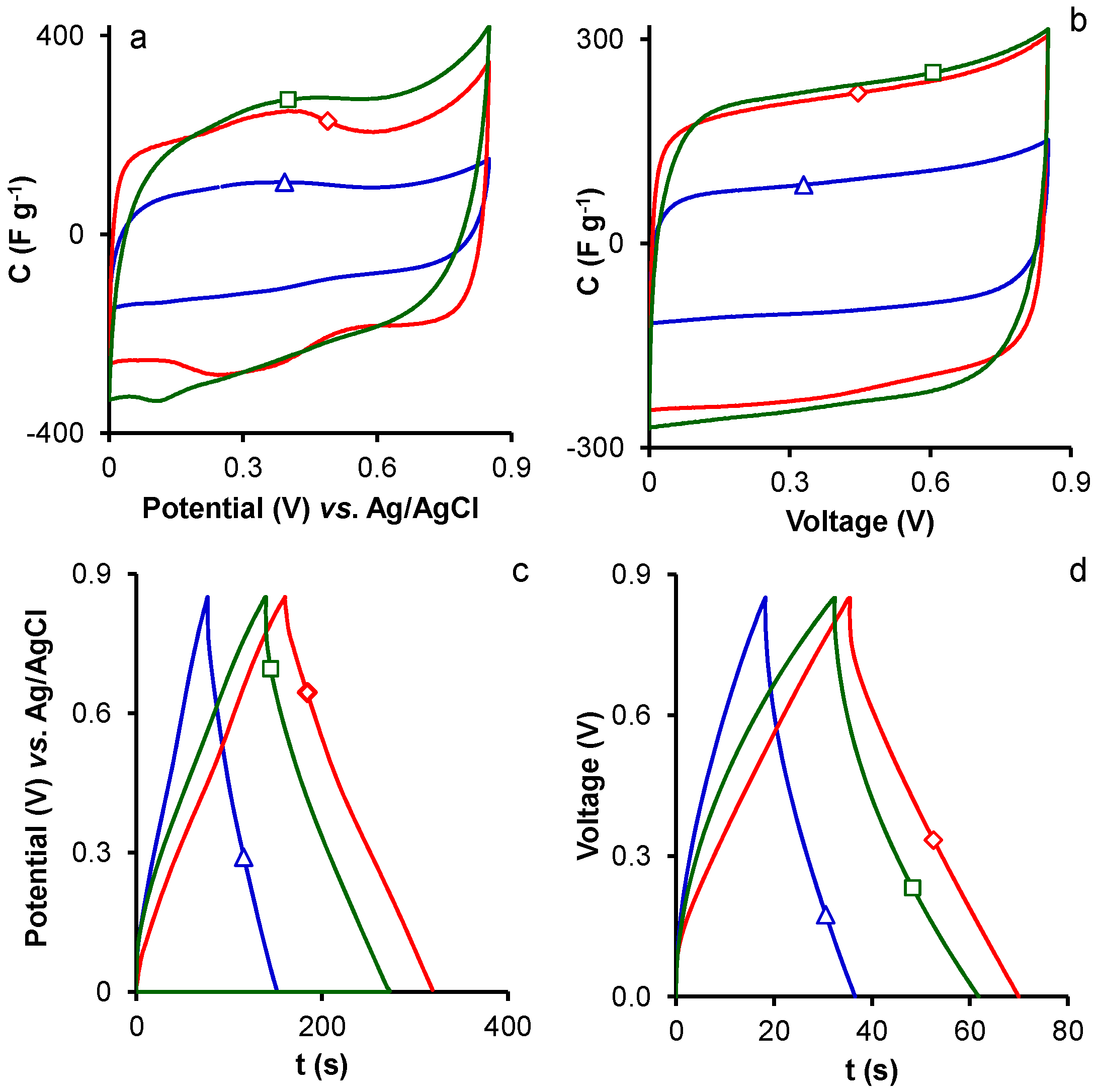
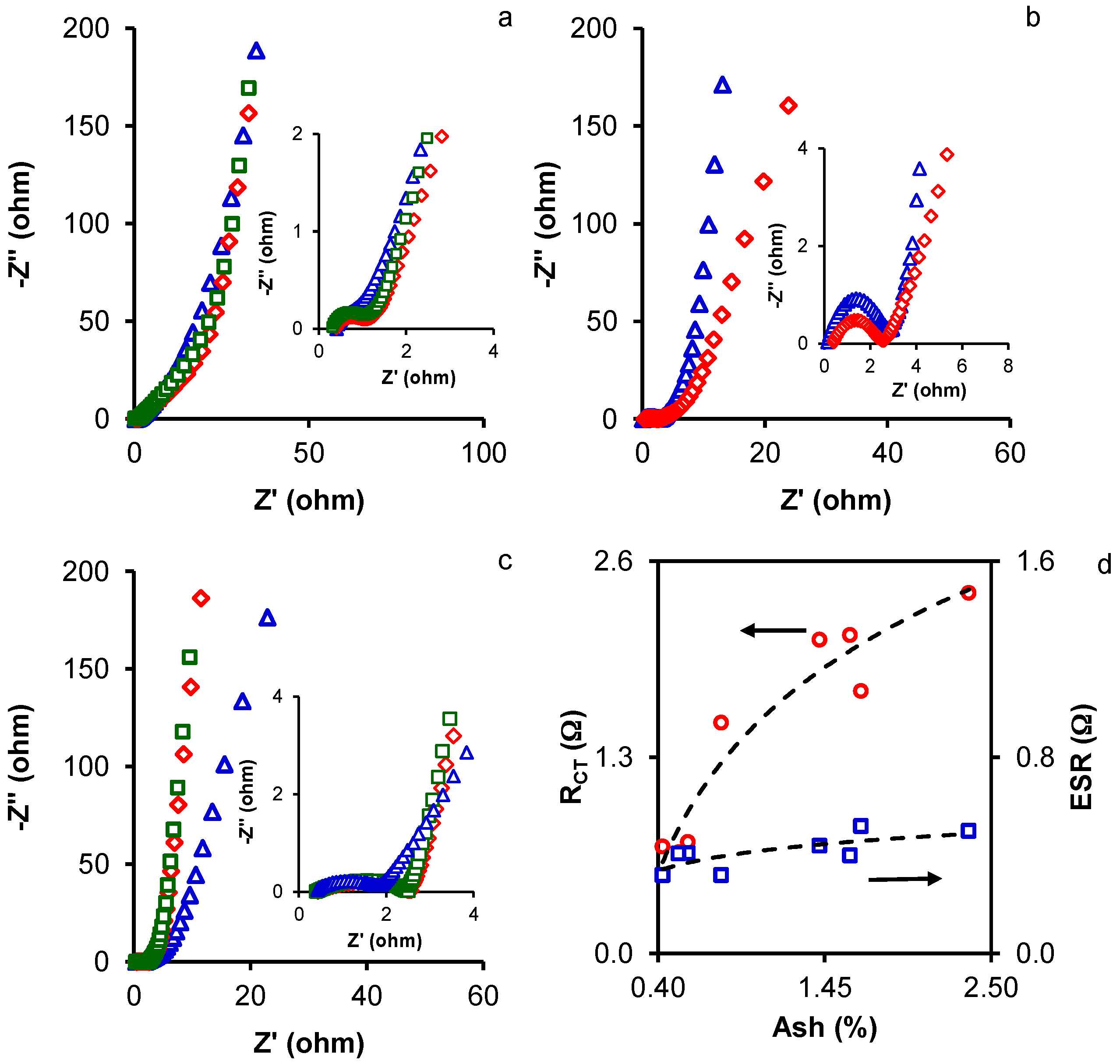
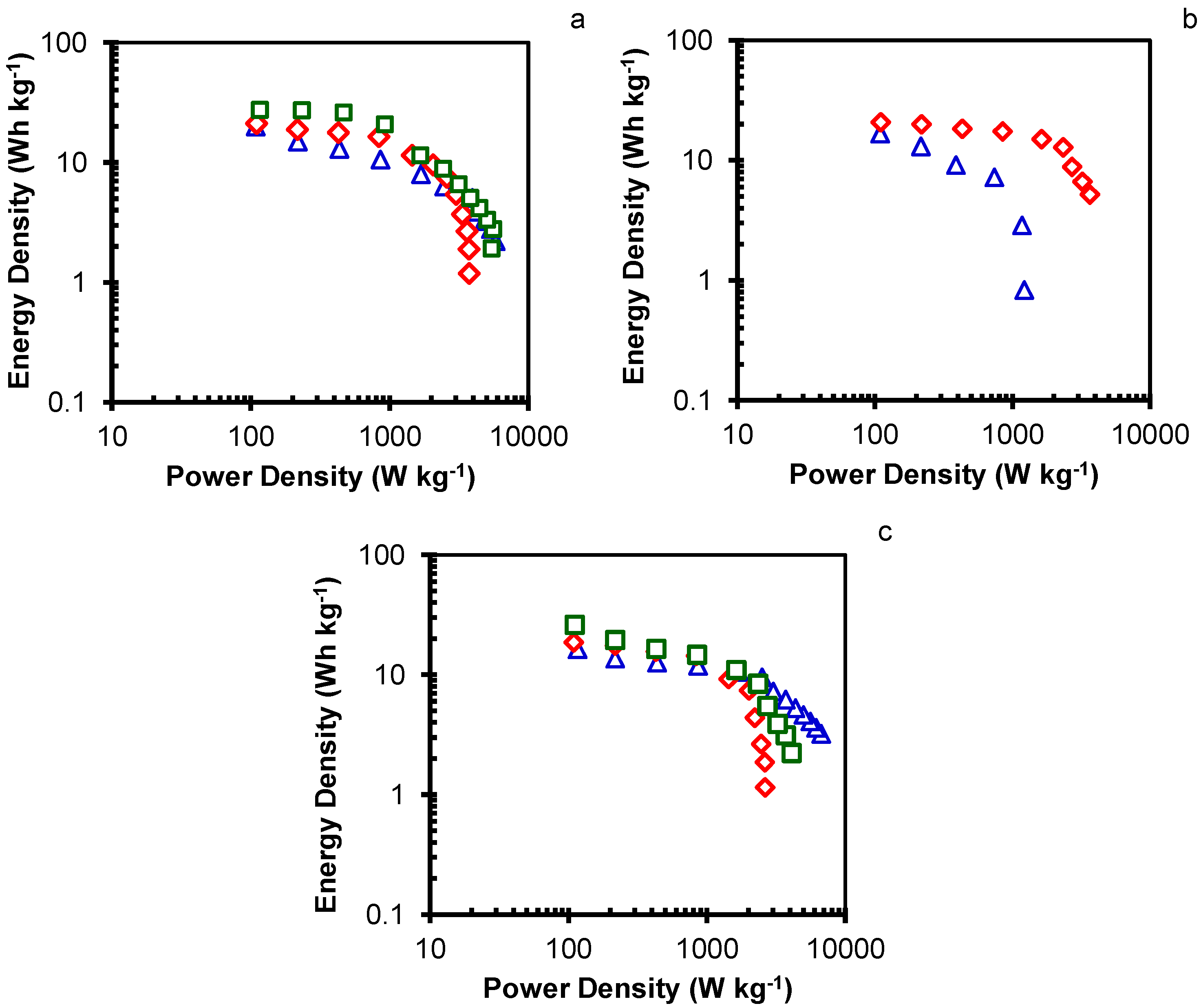
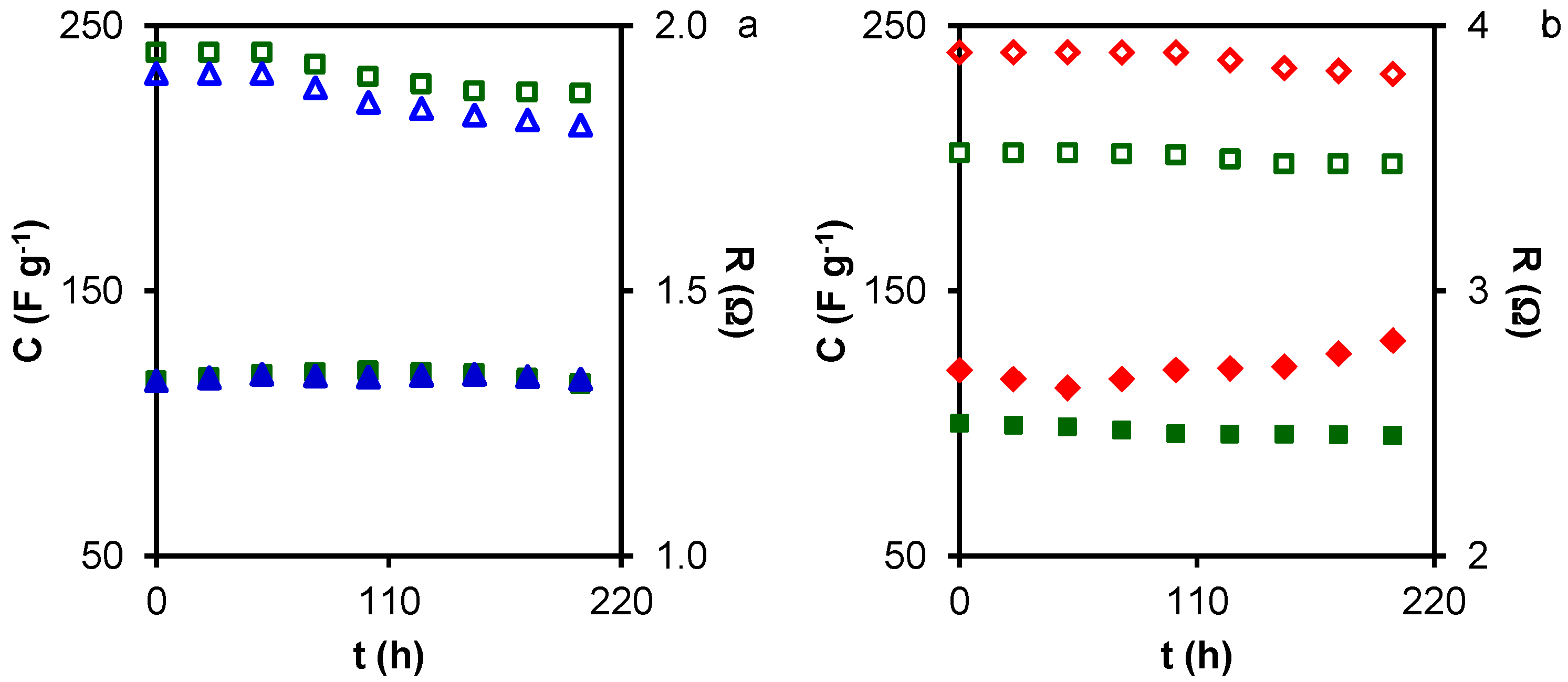
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Activated Carbons
3.2. Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Conway, B.E. Electrochemical supercapacitors. In Scientific Fundamentals and Technological Applications; Kluwer Academic/Plenum: New York, NY, USA, 1999. [Google Scholar]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Castilla, C.; Dawidziuk, M.B.; Carrasco-Marín, F.; Morallón, E. Electrochemical performance of carbon gels with variable surface chemistry and physics. Carbon 2012, 50, 3324–3332. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Fuertes, A.B.; Sevilla, M. From soybean residue to advanced supercapacitors. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, Q.; Tan, Y.; Tian, W.; Xu, J.; Yang, K.; Yang, C. Nitrogen and oxygen co-doped microporous carbons derived from the leaves of Euonymus japonicas as high performance supercapacitor electrode material. Microporous Mesoporous Mater. 2015, 210, 1–9. [Google Scholar] [CrossRef]
- Wu, M.-B.; Li, R.-C.; He, X.-J.; Zhang, H.-B.; Sui, W.-B.; Tan, M.-H. Microwave-assisted preparation of peanut shell-based activated carbons and their use in electrochemical capacitors. New Carbon Mater. 2015, 30, 86–91. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, X.; Chen, H.; Liu, Y.; Li, H. Promising porous carbons derived from lotus seedpods with outstanding supercapacitance performance. Electrochim. Acta 2016, 208, 55–63. [Google Scholar] [CrossRef]
- Liu, B.; Chen, H.; Gao, Y.; Li, H. Preparation and capacitive performance of porous carbon materials derived from eulaliopsis binata. Electrochim. Acta 2016, 189, 93–100. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Q.; Zhang, G.; Jiao, H.; Deng, X.; Liu, L. Promising activated carbons derived from cabbage leaves and their application in high-performance supercapacitors electrodes. J. Solid State Electrochem. 2016, 20, 319–325. [Google Scholar] [CrossRef]
- Wang, K.; Yan, R.; Zhao, N.; Tian, X.; Li, X.; Lei, S.; Song, Y.; Guo, Q.; Liu, L. Bio-inspired hollow activated carbon microtubes derived from willow catkins for supercapacitors with high volumetric performance. Mater. Lett. 2016, 174, 249–252. [Google Scholar] [CrossRef]
- Feng, W.; He, P.; Ding, S.; Zhang, G.; He, M.; Dong, F.; Wen, J.; Du, L.; Liu, M. Oxygen-doped activated carbons derived from three kinds of biomass: Preparation, characterization and performance as electrode materials for supercapacitors. RSC Adv. 2016, 6, 5949–5956. [Google Scholar] [CrossRef]
- Sun, K.; Guo, D. Nitrogen-doped porous carbon derived from rapeseed residues. Int. J. Electrochem. Sci. 2016, 11, 4743–4754. [Google Scholar] [CrossRef]
- Tian, Z.; Xiang, M.; Zhou, J.; Hu, L.; Cai, J. Nitrogen and oxygen-doped hierarchical porous carbons from algae biomass: Direct carbonization and excellent electrochemical properties. Electrochim. Acta 2016, 211, 225–233. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, L.; Liu, Y.; Zhao, G.; Chen, J.Y.; Yu, G. Biobased nano porous active carbon fibers for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 15205–15215. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, S.; Fang, G.; Geng, G.; Ma, J. From trash to treasure: Direct transformation of onion husks into three-dimensional interconnected porous carbon frameworks for high-performance supercapacitors in organic electrolyte. Electrochim. Acta 2016, 216, 405–411. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhang, G.; Liu, W.; Wang, D.; Dong, Y. Preparation of activated carbon from willow leaves and evaluation in electric double-layer capacitors. Mater. Lett. 2016, 176, 60–63. [Google Scholar] [CrossRef]
- Sun, W.; Lipka, S.M.; Swartz, C.; Williams, D.; Yang, F. Hemp-derived activated carbons for supercapacitors. Carbon 2016, 103, 181–192. [Google Scholar] [CrossRef]
- Lu, B.; Hu, L.; Yin, H.; Mao, X.; Xiao, W.; Wang, D. Preparation and application of capacitive carbon from bamboo shells by one step molten carbonates carbonization. Int. J. Hydrogen Energy 2016, 41, 18713–18720. [Google Scholar] [CrossRef]
- Teo, E.Y.L.; Muniandy, L.; Ng, E.P.; Adam, F.; Mohamed, A.R.; Jose, R.; Chong, K.F. High surface area activated carbon from rice husk as a high performance supercapacitor electrode. Electrochim. Acta 2016, 192, 110–119. [Google Scholar] [CrossRef]
- Manyala, N.; Bello, A.; Barzegar, F.; Khaleed, A.A.; Momodu, D.Y.; Dangbegnon, J.K. Coniferous pine biomass: A novel insight into sustainable carbon materials for supercapacitors electrode. Mater. Chem. Phys. 2016, 182, 139–147. [Google Scholar] [CrossRef]
- Wang, K.; Song, Y.; Yan, R.; Zhao, N.; Tian, X.; Li, X.; Guo, Q.; Liu, Z. High capacitive performance of hollow activated carbon fibers derived from willow catkins. Appl. Surf. Sci. 2017, 394, 569–577. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Zhao, G.; Chen, J.Y. Sustainable activated carbon fiber from sawdust by reactivation for high-performance supercapacitors. J. Mater. Sci. 2017, 52, 478–488. [Google Scholar] [CrossRef]
- Linares-Solano, A.; Lozano-Castelló, D.; Lillo-Ródenas, M.A.; Cazorla-Amorós, D. Carbon activation by alkaline hydroxides: Preparation and reactions, porosity and performance. In Chemistry and Physics of Carbon; Radovic, L.R., Ed.; CRC Press: Boca Raton, FL, USA, 2008; Volume 30, pp. 1–62. [Google Scholar]
- Wang, Y.C.; Lin, J.C. Air quality enhancement zones in Taiwan: A carbon reduction benefit assessment. For. Policy Econ. 2012, 23, 40–45. [Google Scholar] [CrossRef]
- Titirici, M.M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly-to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204–1212. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. Activated carbon: Structure, characterization, preparation and applications. In Introduction to Carbon Technologies; Marsh, H., Heintz, E.A., Rodríguez-Reinoso, F., Eds.; University of Alicante: Alicante, Spain, 1997; pp. 35–101. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special refereence to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Zapata-Benabithe, Z.; Carrasco-Marín, F.; de Vicente, J.; Moreno-Castilla, C. Carbon xerogel microspheres and monoliths from resorcinol-formaldehyde mixtures with varying dilution ratios: Preparation, surface characteristics, and electrochemical double-layer capacitances. Langmuir 2013, 29, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Reinoso, F.; Linares-Solano, A. Microporous structure of activated carbons as revealed by adsorption methods. In Chemistry and Physics of Carbon; Thrower, P.A., Ed.; Marcel Dekker: New York, NY, USA, 1989; Volume 21, pp. 1–146. [Google Scholar]
- Cazorla-Amorós, D.; Alcañiz-Monge, J.; De la Casa-Lillo, M.A.; Linares-Solano, A. CO2 as an adsorptive to characterize carbon molecular sieves and activated carbons. Langmuir 1998, 14, 4589–4596. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material's performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Béguin, F. Application of nanotextured carbons for supercapacitors and hydrogen storage. In Activated Carbon Surfaces in Environmental Remediation; Bandosz, T.J., Ed.; Elsevier: Oxford, UK, 2006; pp. 293–343. [Google Scholar]
- Kotz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, M.; Gan, L.; Ma, X.; Zhu, D.; Xu, Z.; Chen, L. Ultramicroporous carbon nanoparticles for the high-performance electrical double-layer capacitor electrode. Energy Fuel 2013, 28, 1561–1568. [Google Scholar] [CrossRef]
- Hulicova, D.; Kodama, M.; Hatori, H. Electrochemical performance of nitrogen-enriched carbons in aqueous and non-aqueous supercapacitors. Chem. Mater. 2006, 18, 2318–2326. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Ruch, P.W.; Cericola, D.; Foelske-Schmitz, A.; Kötz, R.; Wokaun, A. Aging of electrochemical double layer capacitors with acetonitrile-based electrolyte at elevated voltages. Electrochim. Acta 2010, 55, 4412–4420. [Google Scholar] [CrossRef]
- Weingarth, D.; Foelske-Schmitz, A.; Kötz, R. Cycle versus voltage hold—Which is the better stability test for electrochemical double layer capacitors? J. Power Sources 2013, 225, 84–88. [Google Scholar] [CrossRef]
- Bello, A.; Barzegar, F.; Madito, M.J.; Momodu, D.Y.; Khaleed, A.A.; Masikhwa, T.M.; Dangbegnon, J.K.; Manyala, N. Stability studies of polypyrole-derived carbon based symmetric supercapacitor via potentiostatic floating test. Electrochim. Acta 2016, 213, 107–114. [Google Scholar] [CrossRef]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Fiset, E.; Zhu, Z.; Lu, G.Q. Double-layer capacitance of waste coffee ground activated carbons in an organic electrolyte. Electrochem. Commun. 2009, 11, 974–977. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Fuertes, A.B.; Sevilla, M. N-doped microporous carbon microspheres for high volumetric performance supercapacitors. Electrochim. Acta 2015, 168, 320–329. [Google Scholar] [CrossRef]
- Liang, T.; Chen, C.; Li, X.; Zhang, J. Popcorn-derived porous carbon for energy storage and CO2 capture. Langmuir 2016, 32, 8042–8049. [Google Scholar] [CrossRef] [PubMed]
| Biomass Precursor | Activation | SBET (m2·g−1) a | Capacitances at 1 A·g−1 (F·g−1 or μF·cm−2) | Energy Density (Wh·Kg−1) at Power Density (kW·kg−1) | Electrolyte | Reference |
|---|---|---|---|---|---|---|
| Lotus seed pods | KOH | 1813 | 380 or 20.9 | 12.5 at 0.26 | 6 M KOH | [9] |
| Eulaliopsis binata | KOH | 2273 | 360 or 15.8 | nd b | 6 M KOH | [10] |
| Cabbage leaves | KOH | 3012 | 336 or 11.2 | nd | 2 M KOH | [11] |
| Euonimus japonica leaves | KOH | 1268 | 275 or 21.7 | 5.0 at 8.6 | 6 M KOH | [7] |
| Willow catkins | KOH | 1115 | 275 or 24.7 | 8.8 at 0.05 | 6 M KOH | [12,23] |
| Dragonfruit skin | KOH | 911 | 240 or 26.3 | nd | 2 M KOH | [13] |
| Rapeseed | ZnCl2 | 682 | 229 or 33.5 | 13.5 at 0.4 c | 6 M KOH | [14] |
| Peanut shells | KOH | 1277 | 228 or 17.9 | 6 at 0.8 | 6 M KOH | [8] |
| Fir sawdust | KOH | 2395 | 225 or 9.4 | 8.4 at 0.25 | 6 M KOH | [24] |
| Soybean residue | KOH | 1340 | 220 or 16.4 | 12 at 0.025 | 1 M H2SO4 | [5] |
| Enteromorpha prolifera | 700 °C (N2 atm.) | 422 | 216 or 51.2 | nd | 6 M KOH | [15] |
| Chinese fir sawdust | KOH | 2294 | 213 or 9.3 | 7.8 at 0.25 | 6 M KOH | [16] |
| Momordica grosvenori skin | KOH | 597 | 208 or 34.8 | nd | 2 M KOH | [13] |
| Firmiana catkins | KOH | 287 | 195 or 67.9 | nd | 2 M KOH | [13] |
| Onion husks | K2CO3 | 2571 | 188 or 7.3 | 47.6 at 0.67 | 1 M TEABF4/AN | [17] |
| Willow leaves | ZnCl2 | 1031 | 172 or 16.7 | nd | 6 M KOH | [18] |
| Hemp stem | KOH | 2801 | 167 or 6.0 | 19.8 at 21 | 1.8 M TEMABF4/PC | [19] |
| Bamboo shells | Na2CO3-K2CO3 | 843 | 164 or 19.5 | nd | 1 M H2SO4 | [20] |
| Rice husk | KOH | 2696 | 120 or 4.5 | 5.1 at 0.05 | 6 M KOH | [21] |
| Pinecones | KOH | 1515 | 78 or 5.1 | 2.7 at 0.5 | 1 M Na2SO4 | [22] |
| Sample | Ash | C | H | N | O a | NXPS | OXPS |
|---|---|---|---|---|---|---|---|
| MA | 0.70 | 50.60 | 6.90 | 1.28 | 40.52 | nd | nd |
| MA1 | 0.59 | 88.17 | 0.26 | 0.73 | 10.25 | 0.38 | 7.60 |
| MA2 | 0.53 | 86.62 | 0.27 | 0.76 | 11.82 | 1.03 | 10.80 |
| MA3 | 0.43 | 85.62 | 0.34 | 0.86 | 12.75 | 1.07 | 11.72 |
| CMA | 5.28 | 85.81 | 0.79 | 1.32 | 6.80 | 1.06 | 6.51 |
| CMA2 | 2.36 | 85.37 | 0.28 | 0.44 | 11.55 | 0.32 | 8.43 |
| CMA4 | 1.61 | 90.07 | 0.24 | 0.53 | 7.55 | 0.65 | 8.90 |
| HMA100 | 0.62 | 53.58 | 7.46 | 1.38 | 36.96 | nd | nd |
| HMA150 | 0.63 | 61.33 | 7.68 | 1.42 | 28.94 | nd | nd |
| HMA200 | 0.63 | 68.17 | 7.94 | 1.73 | 21.53 | nd | Nd |
| HMA100-2 | 1.68 | 85.75 | 0.32 | 0.65 | 11.60 | 0.71 | 7.81 |
| HMA150-2 | 1.42 | 88.26 | 0.20 | 0.70 | 9.42 | 0.85 | 10.44 |
| HMA200-2 | 0.80 | 89.28 | 0.20 | 0.79 | 8.93 | 0.89 | 10.95 |
| Sample | SBET (m2·g−1) | W0(N2) (cm3·g−1) | W0(CO2) (cm3·g−1) | L0(N2) (nm) | L0(CO2) (nm) | V0.95 (cm3·g−1) | Micro a (%) |
|---|---|---|---|---|---|---|---|
| MA1 | 829 | 0.32 | 0.41 | 0.80 | 0.63 | 0.40 | 80 |
| MA2 | 1032 | 0.41 | 0.44 | 0.89 | 0.73 | 0.50 | 82 |
| MA3 | 1234 | 0.50 | 0.41 | 0.94 | 0.72 | 0.60 | 83 |
| CMA2 | 969 | 0.37 | 0.46 | 0.45 | 0.71 | 0.44 | 84 |
| CMA4 | 1975 | 0.76 | 0.53 | 0.79 | 0.74 | 0.87 | 87 |
| HMA100-2 | 1069 | 0.40 | 0.32 | 0.91 | 0.56 | 0.55 | 73 |
| HMA150-2 | 1154 | 0.45 | 0.44 | 0.85 | 0.69 | 0.62 | 73 |
| HMA200-2 | 1317 | 0.51 | 0.43 | 0.83 | 0.71 | 0.70 | 73 |
| Sample | 3EC | 2EC | |||||
|---|---|---|---|---|---|---|---|
| C (F·g−1) | C (F·g−1) | CA (μF·cm−2) | Cmax (F·g−1) | ESR (Ω) | RCT (Ω) | τ (s) | |
| MA1 | 149 | 140 | 16.9 | 117 | 0.41 | 0.74 | 1.98 |
| MA2 | 244 | 232 | 22.5 | 236 | 0.41 | 0.71 | 1.48 |
| MA3 | 246 | 240 | 19.4 | 235 | 0.32 | 0.67 | 1.11 |
| CMA2 | 178 | 131 | 13.5 | 159 | 0.50 | 2.39 | 1.48 |
| CMA4 | 257 | 240 | 12.2 | 261 | 0.40 | 2.11 | 1.11 |
| HMA100-2 | 185 | 157 | 14.7 | 169 | 0.52 | 1.74 | 1.48 |
| HMA150-2 | 260 | 207 | 17.9 | 227 | 0.44 | 2.08 | 1.48 |
| HMA200-2 | 256 | 202 | 15.3 | 205 | 0.32 | 1.53 | 0.83 |
| Sample | Energy Density |
|---|---|
| MA1 | 20.0 |
| MA2 | 21.2 |
| MA3 | 27.4 |
| CMA2 | 16.7 |
| CMA4 | 20.8 |
| HMA100-2 | 16.3 |
| HMA150-2 | 18.6 |
| HMA200-2 | 26.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Castilla, C.; García-Rosero, H.; Carrasco-Marín, F. Symmetric Supercapacitor Electrodes from KOH Activation of Pristine, Carbonized, and Hydrothermally Treated Melia azedarach Stones. Materials 2017, 10, 747. https://doi.org/10.3390/ma10070747
Moreno-Castilla C, García-Rosero H, Carrasco-Marín F. Symmetric Supercapacitor Electrodes from KOH Activation of Pristine, Carbonized, and Hydrothermally Treated Melia azedarach Stones. Materials. 2017; 10(7):747. https://doi.org/10.3390/ma10070747
Chicago/Turabian StyleMoreno-Castilla, Carlos, Helena García-Rosero, and Francisco Carrasco-Marín. 2017. "Symmetric Supercapacitor Electrodes from KOH Activation of Pristine, Carbonized, and Hydrothermally Treated Melia azedarach Stones" Materials 10, no. 7: 747. https://doi.org/10.3390/ma10070747





