Performance of a New Al2O3/Ce–TZP Ceramic Nanocomposite Dental Implant: A Pilot Study in Dogs.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powder Processing and Microstructural Characterization
2.2. Characterization of the Alumina/Ce-TZP Nanocomposite
2.2.1. Mechanical Properties of Alumina/Ce-TZP Material
2.2.2. Determination of Surface Topography (Discs and Implants)
2.3. Determination of the In Vitro Response
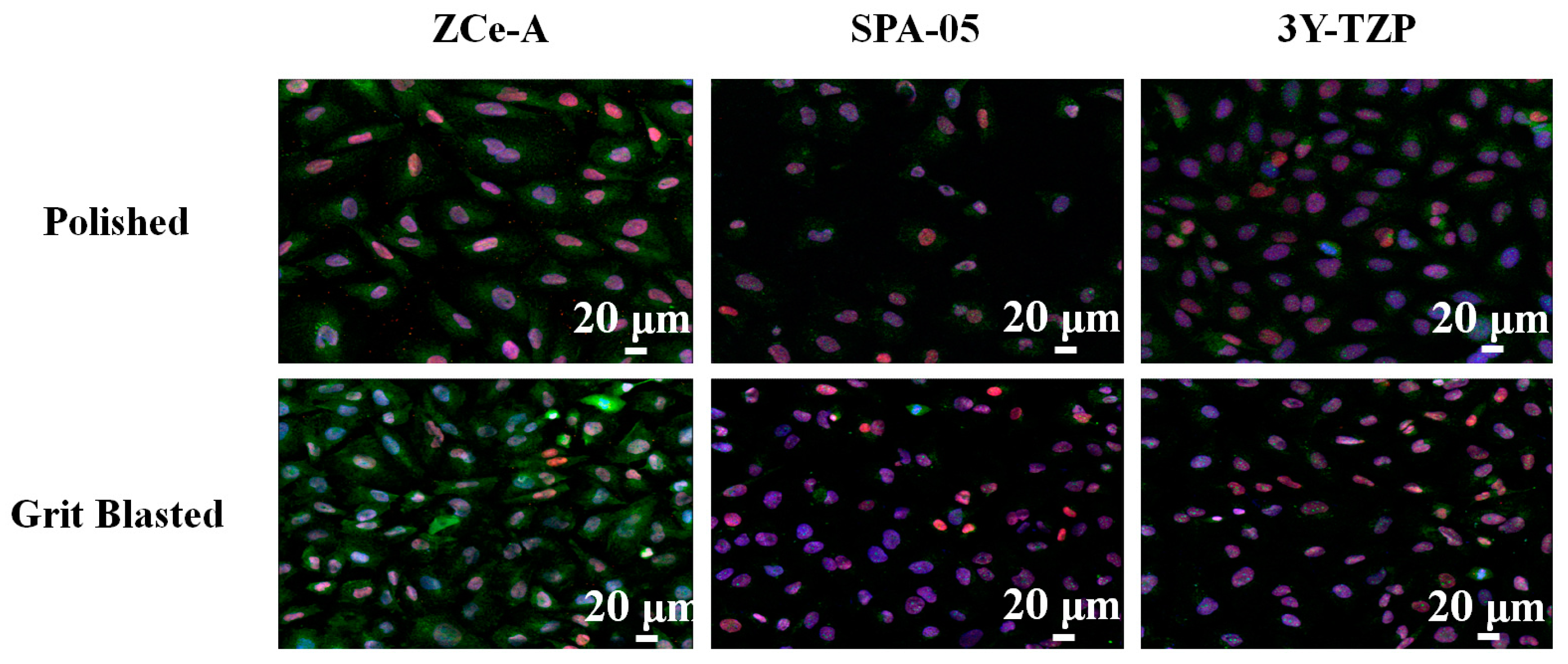
2.3.1. Cell Immunostaining and Imaging
2.3.2. In Vitro Cytotoxicity
2.3.3. In Vitro Osseous Differentiation Determination
2.4. In Vivo Test
2.4.1. Animals
2.4.2. Surgery
2.4.3. Histological Preparation and Analysis
3. Results and Discussion
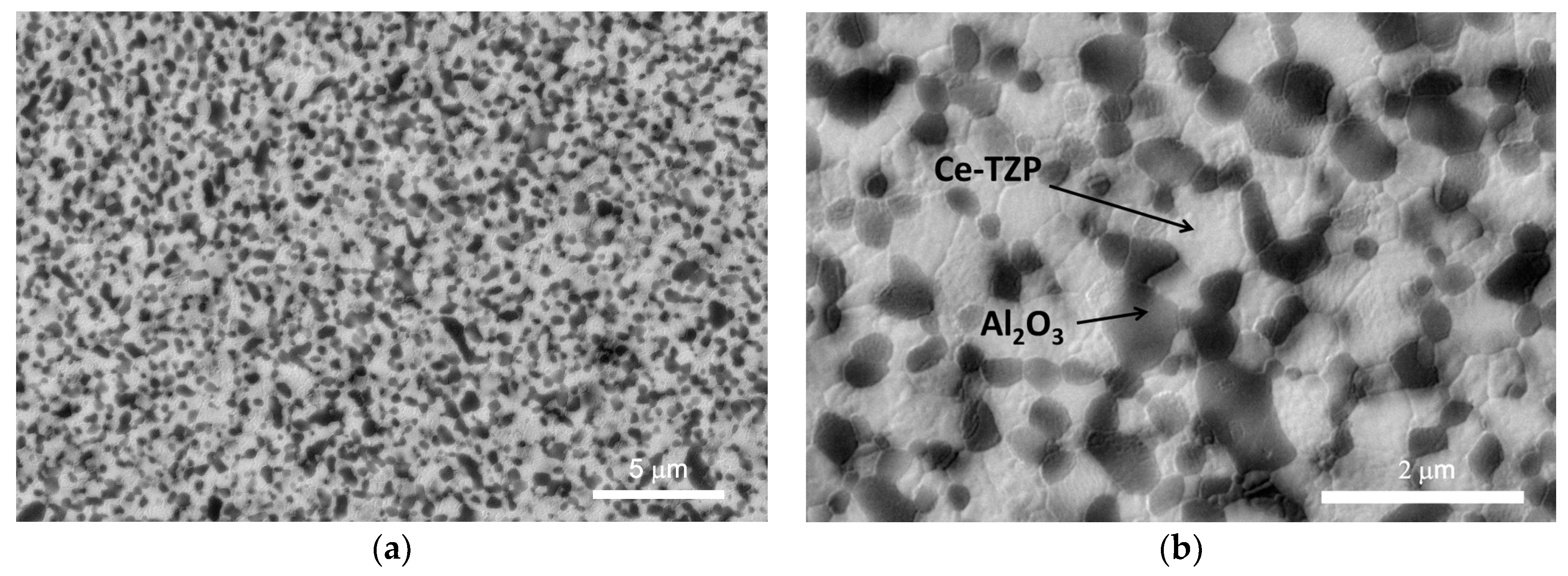
3.1. Nanocomposite Characterization
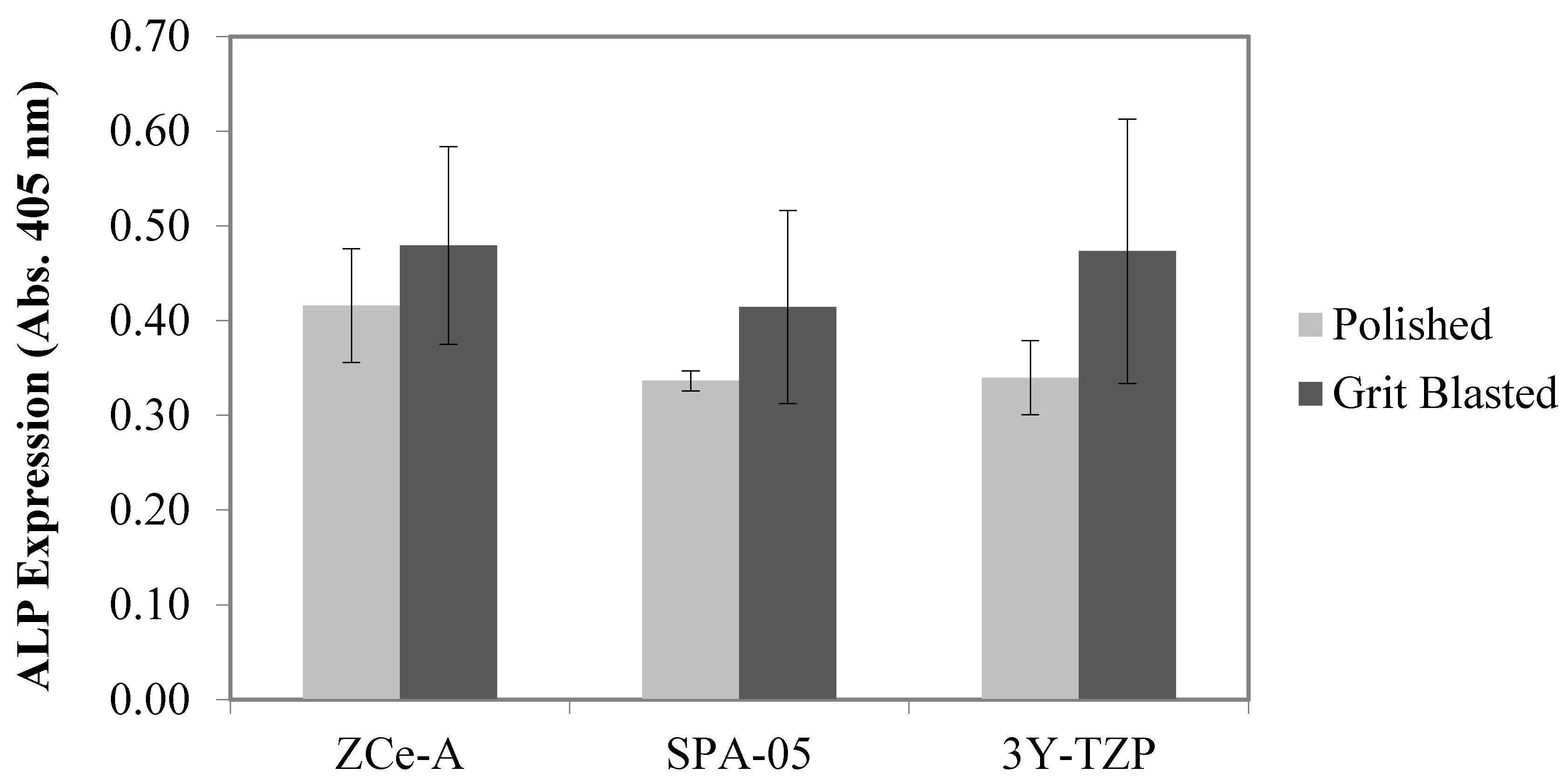
3.2. In Vitro Biological Assays
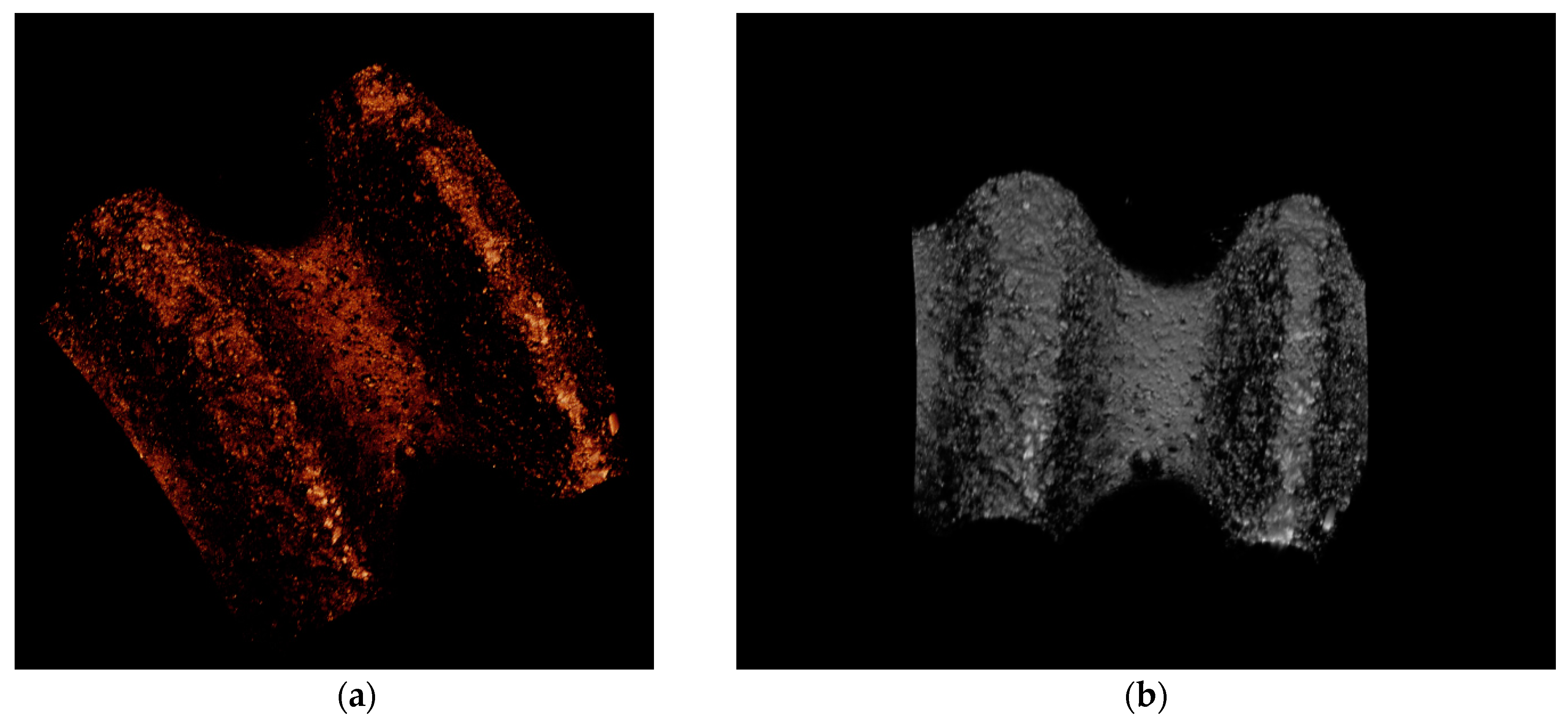
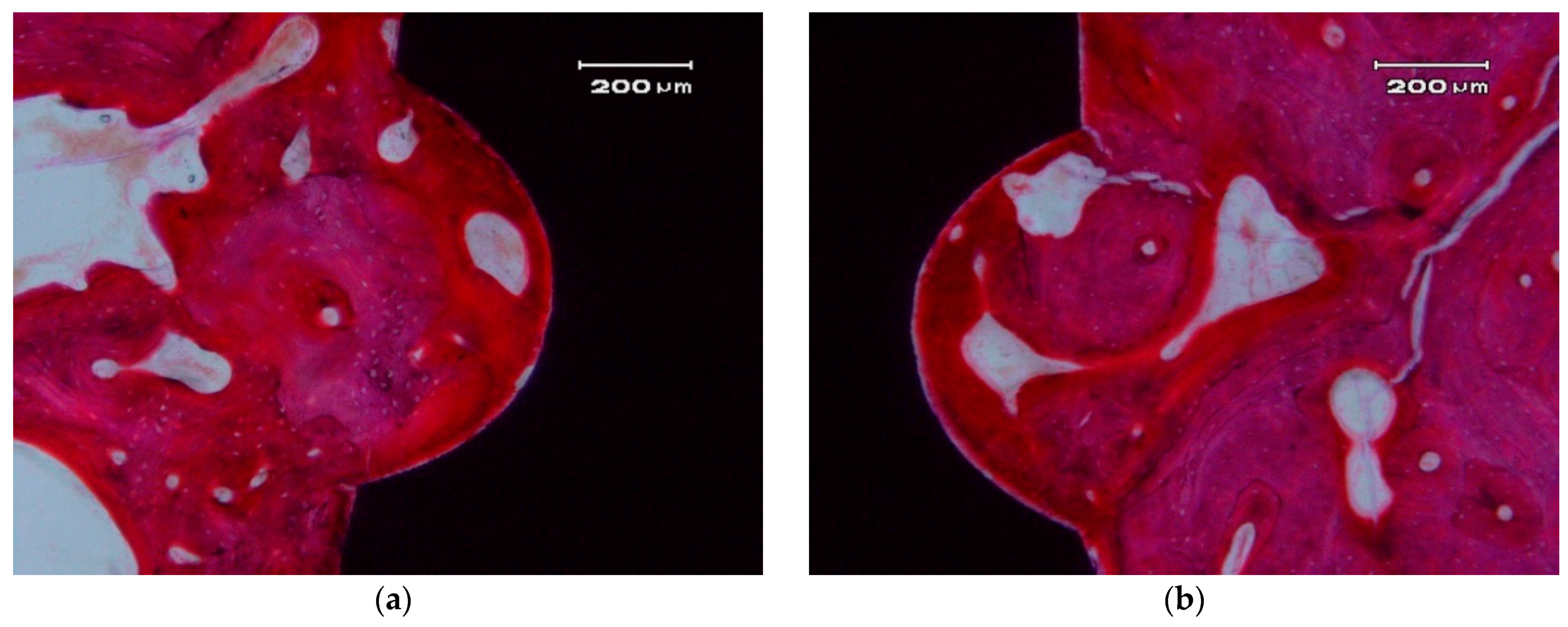
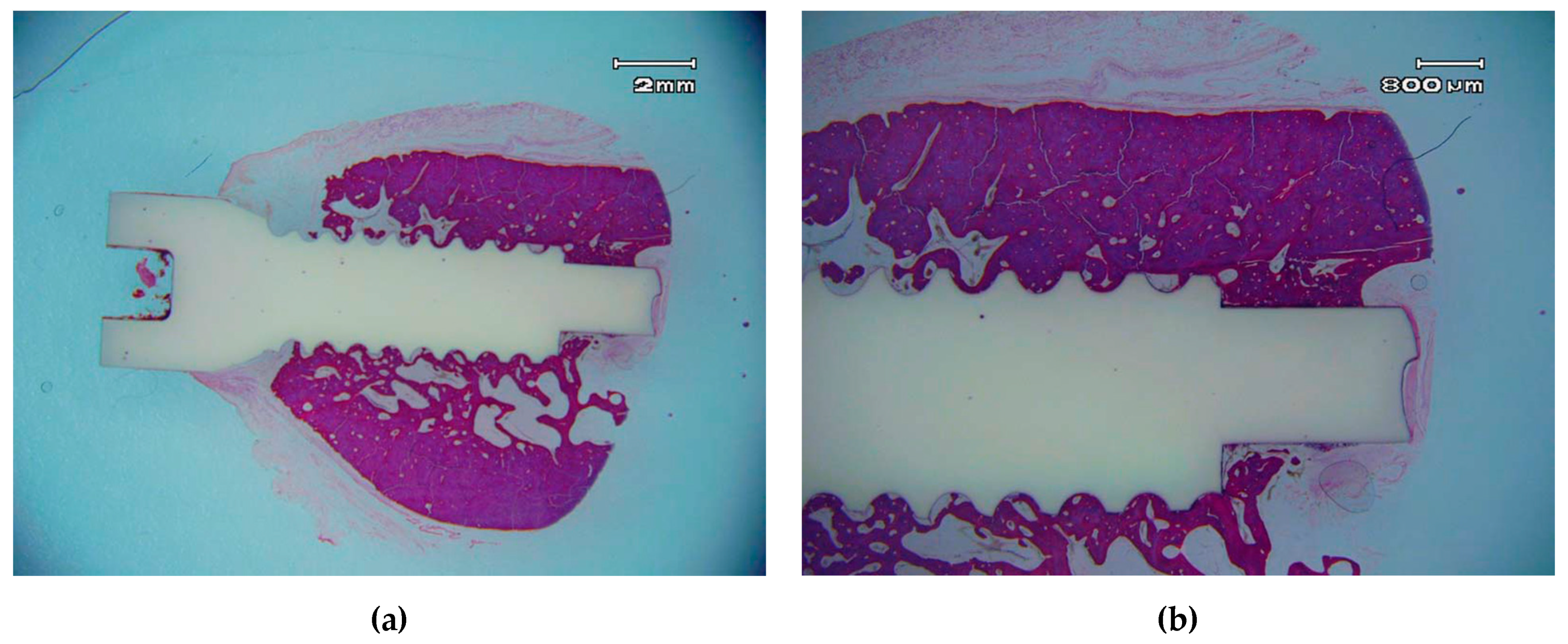
3.3. Histological Descriptive Interpretation of Thin Ground Preparations.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Den Hartog, L.; Slater, J.J.; Vissink, A.; Meijer, H.J.; Raghoebar, G.M. Treatment outcome of immediate, early and conventional single-tooth implants in the aesthetic zone: A systematic review to survival, bone level, soft-tissue, aesthetics and patient satisfaction. J. Clin. Periodontol. 2008, 35, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Fuentealba, R.; Jofré, J. Esthetic failure in implant dentistry. Dent. Clin. North Am. 2015, 59, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Evrard, L.W.D.; Parent, D. Allergies to dental metals. Titanium: A new allergen. Rev. Med. Brux. 2010, 31, 44–49. [Google Scholar] [PubMed]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral. Implant. Res. 2008, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Al-Hezaimi, K.; Almas, K.; Romanos, G.E. Is titanium sensitivity associated with allergic reactions in patients with dental implants? A systematic review. Clin. Implant. Dent. Relat. Res. 2013, 15, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Bagedahl-Strindlund, M.; Hie, M.; Furhotf, A.K.; Tomson, Y.; Larsson, K.S.; Sandborgh-Englund, G.; Torstenson, B.; Wretlind, K. A multidisciplinary clinical study of patients suffering from illness associated with mercury release from dental restorations: psychiatric aspects. Acta Psychiatr. Scand. 1997, 96, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.; Manaka, M.; Green, D.; Williams, P.; Pezzotti, G.; Kim, Y.; Ries, M.; Sugano, N.; Sedel, L.; Delauney, C.; et al. Current status of Zirconia used in total hip implants. J. Bone Joint Surg. Am. 2003, 85, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Moussi, J.; Drury, J.L.; Wataha, J.C. Zirconia in biomedical applications. Expert Rev. Med. Devices 2016, 13, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Gremillard, L.; Deville, S. Low-temperature degradation of Zirconia and implications for biomedical implants. Annu. Rev. Mater. Res. 2007, 37, 1–32. [Google Scholar] [CrossRef]
- Hashim, D.; Cionca, N.; Courvoisier, D.S.; Mombelli, A. A systematic review of the clinical survival of zirconia implants. Clin. Oral. Investig. 2016, 20, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42, S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, R.; Moya, J.S.; Díaz, L.A.; Bartolomé, J.F.; Fernández, A.; Lopez-Esteban, S. Nanotechnology in joint replacement. Wires Nanomed. Nanobiotechnol. 2009, 1, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Taddei, P.; Gremillard, L.; Deville, S.; Fantozzi, G.; Bartolomé, J.F.; Pecharroman, C.; Moya, J.S.; Díaz, L.A.; Torrecillas, R.; et al. Reliability assessment in advanced nanocomposite materials for orthopaedic applications. J. Mech. Behav. Biomed. 2011, 4, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Hannink, R.H.J.; Swain, M.W. Transformation Toughening of Ceramics; CRC Press: Boca Raton, FL, USA, 1989; p. 232. [Google Scholar]
- Claussen, N.; Steeb, J. Toughening of Ceramic Composites by Oriented Nucleation of Microcracks. J. Am. Ceram. Soc. 1976, 59, 49–51. [Google Scholar] [CrossRef]
- Claussen, N.; Steeb, J.; Pabst, R.F. Effect of induced microcracking on the fracture toughness of ceramics. Am. Ceram. Soc. Bull. 1977, 56, 559–562. [Google Scholar]
- Lange, F.F. Transformation toughening—Part 4 Fabrication, fracture toughness and strength of Al2O3-ZrO2 composites. J. Mater. Sci. 1982, 17, 247–254. [Google Scholar] [CrossRef]
- Hori, S.; Yoshimura, M.; Somiya, S. Strength-Toughness Relations in Sintered and Isostatically Hot-Pressed ZrO2-Toughened Al2O3. J. Am. Ceram. Soc. 1986, 69, 169–172. [Google Scholar] [CrossRef]
- Becher, P.F.; Alexander, K.B.; Bleier, A.; Waters, S.B.; Warwick, W.H. Influence of ZrO2 Grain Size and Content on the Transformation Response in the Al2O3-ZrO2 (12 mol % CeO2) System. J. Am. Ceram. Soc. 1993, 76, 657–663. [Google Scholar] [CrossRef]
- De Aza, A.H.; Chevalier, J.; Fantozzi, G.; Schehl, M.; Torrecillas, R. Crack growth resistance of alumina, zirconia and zirconia toughened alumina ceramic for joint prostheses. Biomaterials 2002, 23, 937–945. [Google Scholar] [CrossRef]
- Pecharromán, C.; Bartolomé, J.F.; Requena, J.; Moya, J.S.; Deville, S.; Chevalier, J.; Fantozzi, G.; Torrecillas, R. Percolative Mechanism of Ageing in Zirconia Containing Ceramics for Medical Applications. Adv. Mater. 2003, 15, 507–511. [Google Scholar] [CrossRef]
- Fegley, B., Jr.; White, P.; Bowen, H.K. Preparation of Zirconia-Alumina Powders by Zirconium Alkoxide Hydrolysis. J. Am. Ceram. Soc. 1985, 68, C60–C62. [Google Scholar] [CrossRef]
- Ceramic Powder Science III; Messing, G.L.; Hirano, S.I.; Hausner, H. (Eds.) American Ceramic Society: Westerville, OH, USA, 1990; p. 995. [Google Scholar]
- Nawa, M.; Yamaguchi, K.; Toki, M. ZrO2-Al2O3 Composite Ceramic Material and Production Method Thereof. U.S. Patent 7,012,036, 2006. [Google Scholar]
- Nawa, M.; Nakamoto, S.; Sekino, T.; Niihara, K. Tough and Strong Ce-TZP/Alumina Nanocomposites Doped with Titania. Ceram. Int. 1998, 24, 497–506. [Google Scholar] [CrossRef]
- Goyos-Ball, L.; García-Tuñón, E.; Fernández-García, E.; Díaz, R.; Fernández, A.; Prado, C.; Saiz, E.; Torrecillas, R. Mechanical and biological evaluation of 3D printed 10CeTZP-Al2O3 structures. J. Eur. Ceram. Soc. 2017, 37, 3151–3158. [Google Scholar] [CrossRef]
- Goyos, L.; Díaz, L.A.; Torrecillas, R. Alumina-Ceria-TZP nanocomposites obtained in an alcohol médium by two different processing routes. In Proceedings of the ECCM15—15th European Conference on Composite Materials, Venice, Italy, 24–28 June 2012. [Google Scholar]
- Hempel, U.; Hefti, T.; Kalbacova, M.; Wolf-Brandstetter, C.; Dieter, P.; Schlottig, F. Response of osteoblast-like SAOS-2 cells to zirconia ceramics with different surface topographies. Clin. Oral. Implan. Res. 2010, 21, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Buser, D. Titanium for dental applications (II): Implants with roughened surfaces. In Titanium in Medicine; Brunette, D.M., Tengvall, P., Textor, M., Thomsen, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 876–888. [Google Scholar]
- Soskolne, W.A.; Cohen, S.; Sennerby, L.; Wennerberg, A.; Shapira, L. The effect of titanium surface roughness on the adhesion of monocytes and their secretion of TNF-alpha and PGE2. Clin. Oral. Implant. Res. 2002, 13, 86–93. [Google Scholar] [CrossRef]
- Saulacic, N.; Bosshardt, D.D.; Bornstein, M.M.; Berner, S.; Buser, D. Bone apposition to a titanium-zirconium alloy implant, as compared to two other titanium-containing implants. Eur. Cell Mater. 2012, 23, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Saulacic, N.; Erdosi, R.; Bosshardt, D.D.; Gruber, R.; Buser, D. Acid and alkaline etching of sandblasted zirconia implants: A histomorphometric study in miniature pigs. Clin. Implant. Dent. Relat. Res. 2014, 16, 313–322. [Google Scholar] [CrossRef] [PubMed]


| Material | Supplier | Designation | Purity | d50 (nm) | Specific Surface |
|---|---|---|---|---|---|
| Al2O3 | Sasol | SPA 0.5 | >99.9% | 380 | 7.7 m2/g |
| ZrO2 10% CeO2 | Daiichi Kigenso | 10 Ce-TZP | >99.9% | 35 | 14.3 m2/g |
| Parameter | Ra 1 (µm) | Ra 2 (µm) | Ra 3 (µm) | Avg. Ra (µm) | Ra S.D. (µm) | Overall Avg. Ra (µm) | Overall Ra S.D. (µm) |
|---|---|---|---|---|---|---|---|
| Peak 1 | 1.58 | 1.09 | 1.32 | 1.33 | 0.25 | 1.38 | 0.24 |
| Peak 2 | 1.72 | 1.20 | 1.36 | 1.43 | 0.27 | ||
| Peak 3 | 1.47 | 1.16 | 1.54 | 1.39 | 0.20 | ||
| Valley 1 | 0.94 | 0.92 | 0.83 | 0.90 | 0.06 | 0.93 | 0.10 |
| Valley 2 | 0.88 | 0.83 | 0.83 | 0.85 | 0.03 | ||
| Valley 3 | 0.92 | 0.94 | 1.32 | 1.06 | 0.23 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Píriz, R.; Fernández, A.; Goyos-Ball, L.; Rivera, S.; Díaz, L.A.; Fernández-Domínguez, M.; Prado, C.; Moya, J.S.; Torrecillas, R. Performance of a New Al2O3/Ce–TZP Ceramic Nanocomposite Dental Implant: A Pilot Study in Dogs. Materials 2017, 10, 614. https://doi.org/10.3390/ma10060614
Lopez-Píriz R, Fernández A, Goyos-Ball L, Rivera S, Díaz LA, Fernández-Domínguez M, Prado C, Moya JS, Torrecillas R. Performance of a New Al2O3/Ce–TZP Ceramic Nanocomposite Dental Implant: A Pilot Study in Dogs. Materials. 2017; 10(6):614. https://doi.org/10.3390/ma10060614
Chicago/Turabian StyleLopez-Píriz, Roberto, Adolfo Fernández, Lidia Goyos-Ball, Sergio Rivera, Luis A. Díaz, Manuel Fernández-Domínguez, Catuxa Prado, José S. Moya, and Ramón Torrecillas. 2017. "Performance of a New Al2O3/Ce–TZP Ceramic Nanocomposite Dental Implant: A Pilot Study in Dogs." Materials 10, no. 6: 614. https://doi.org/10.3390/ma10060614





