Improvement in Char Strength with an Open Cage Silsesquioxane Flame Retardant
Abstract
:1. Introduction
2. Results and Discussion
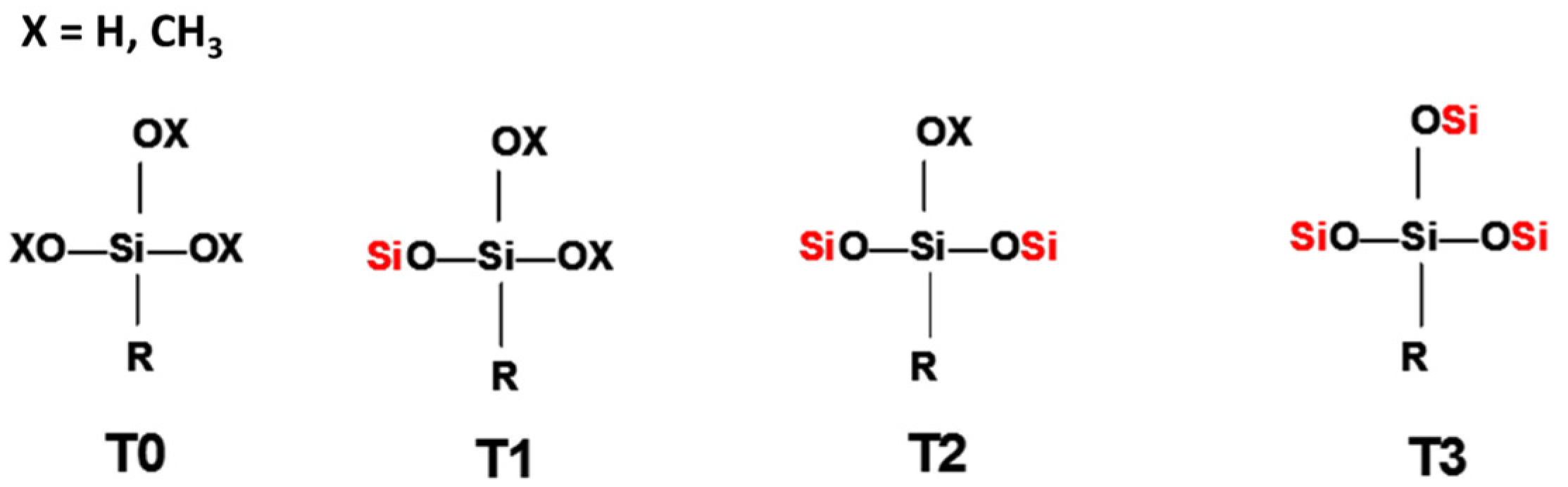
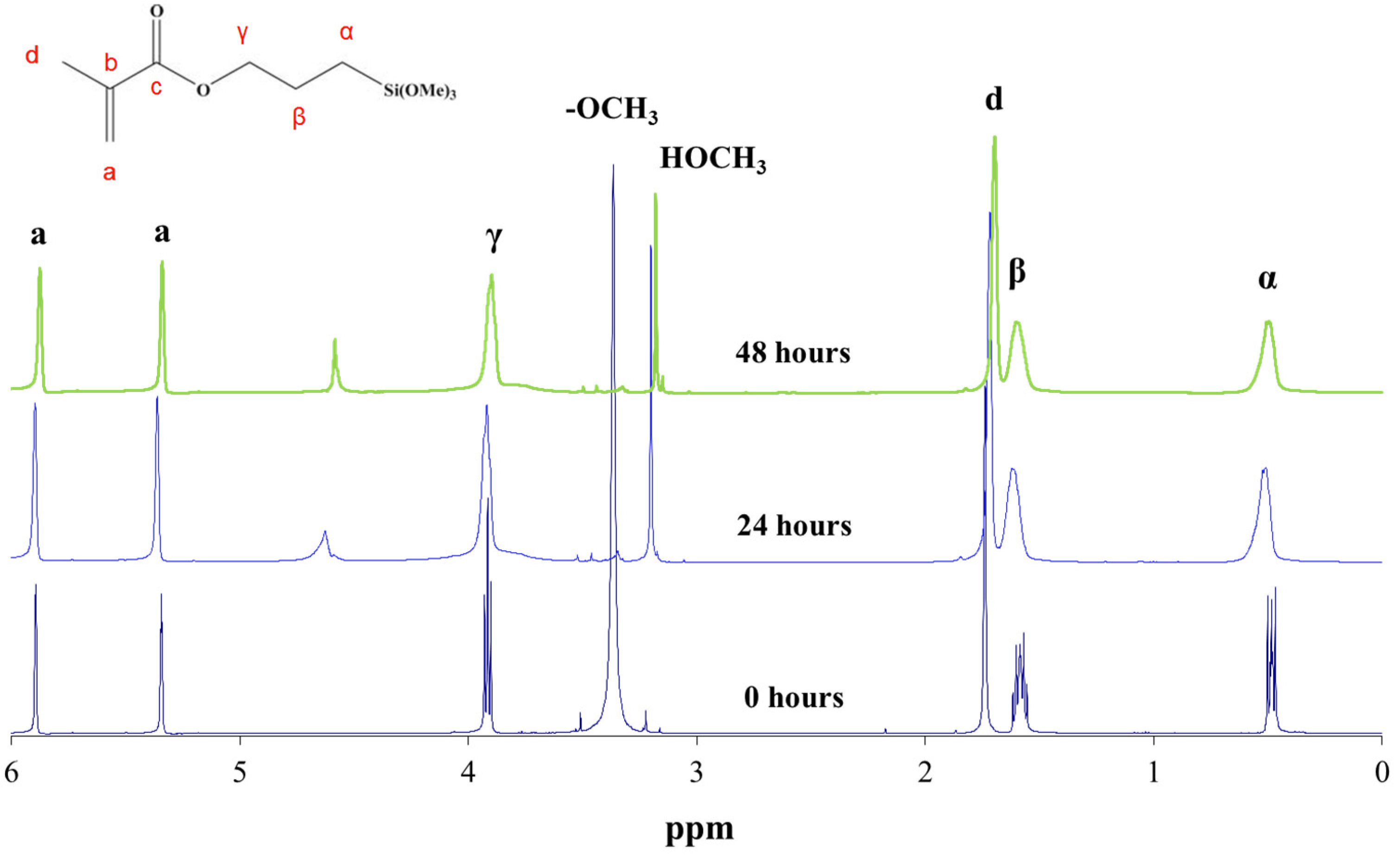
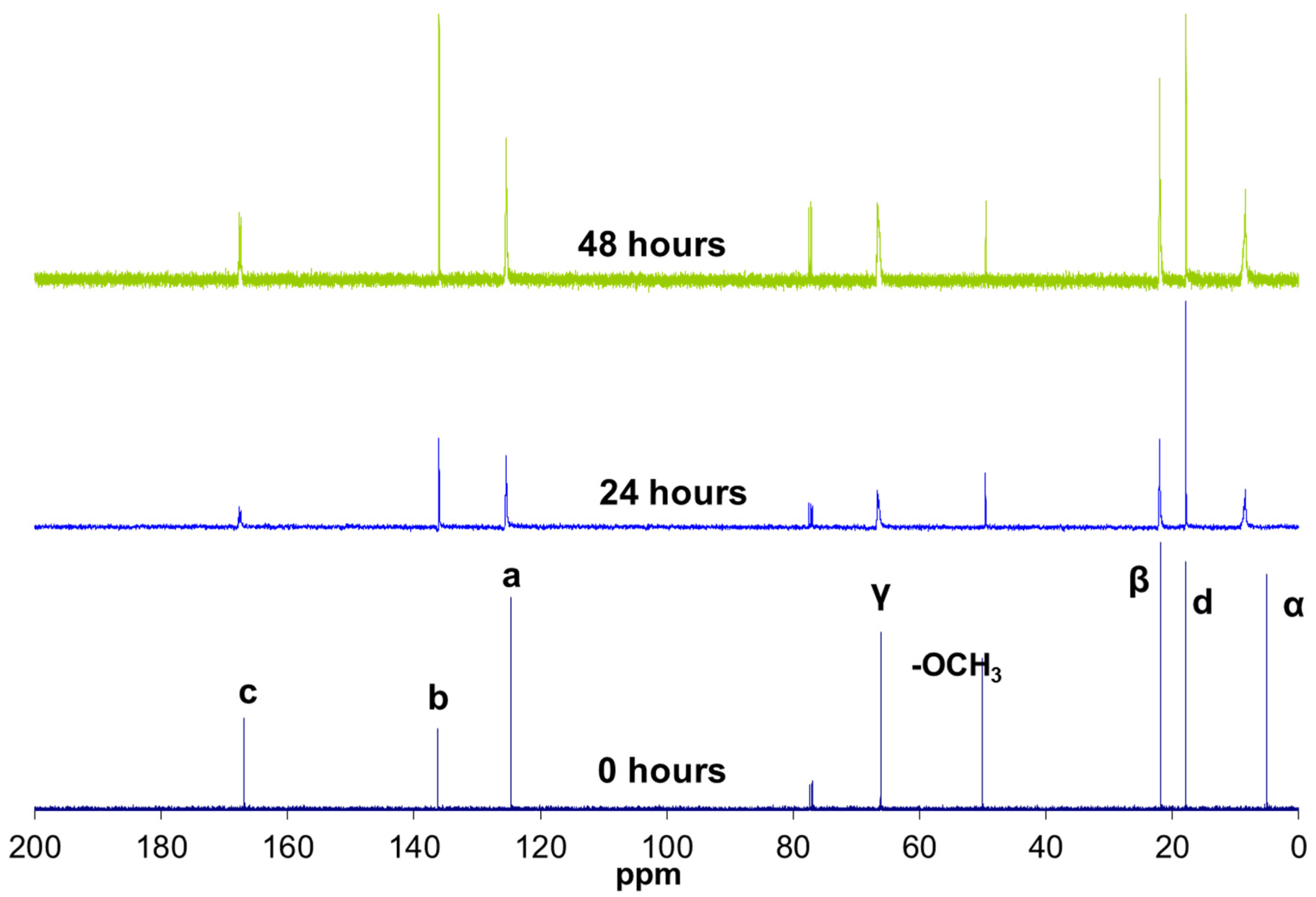
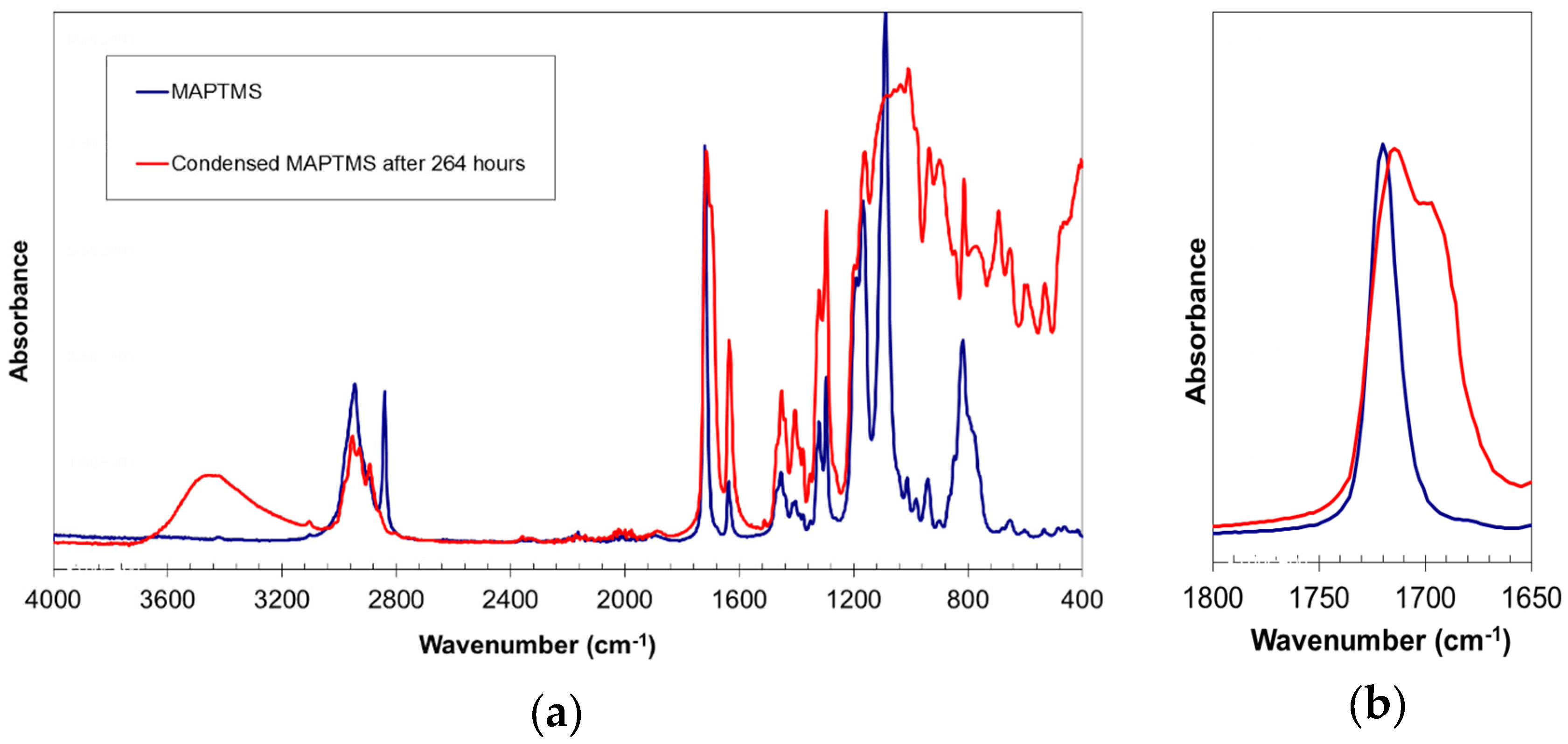
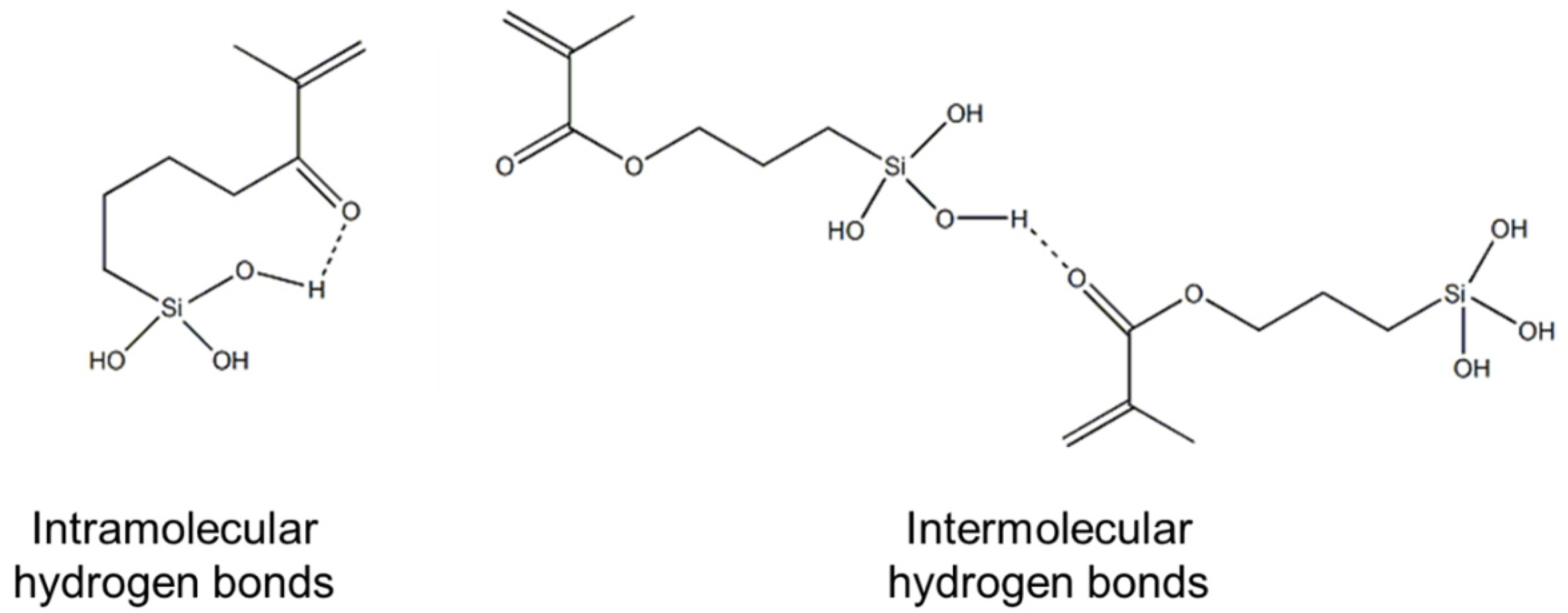
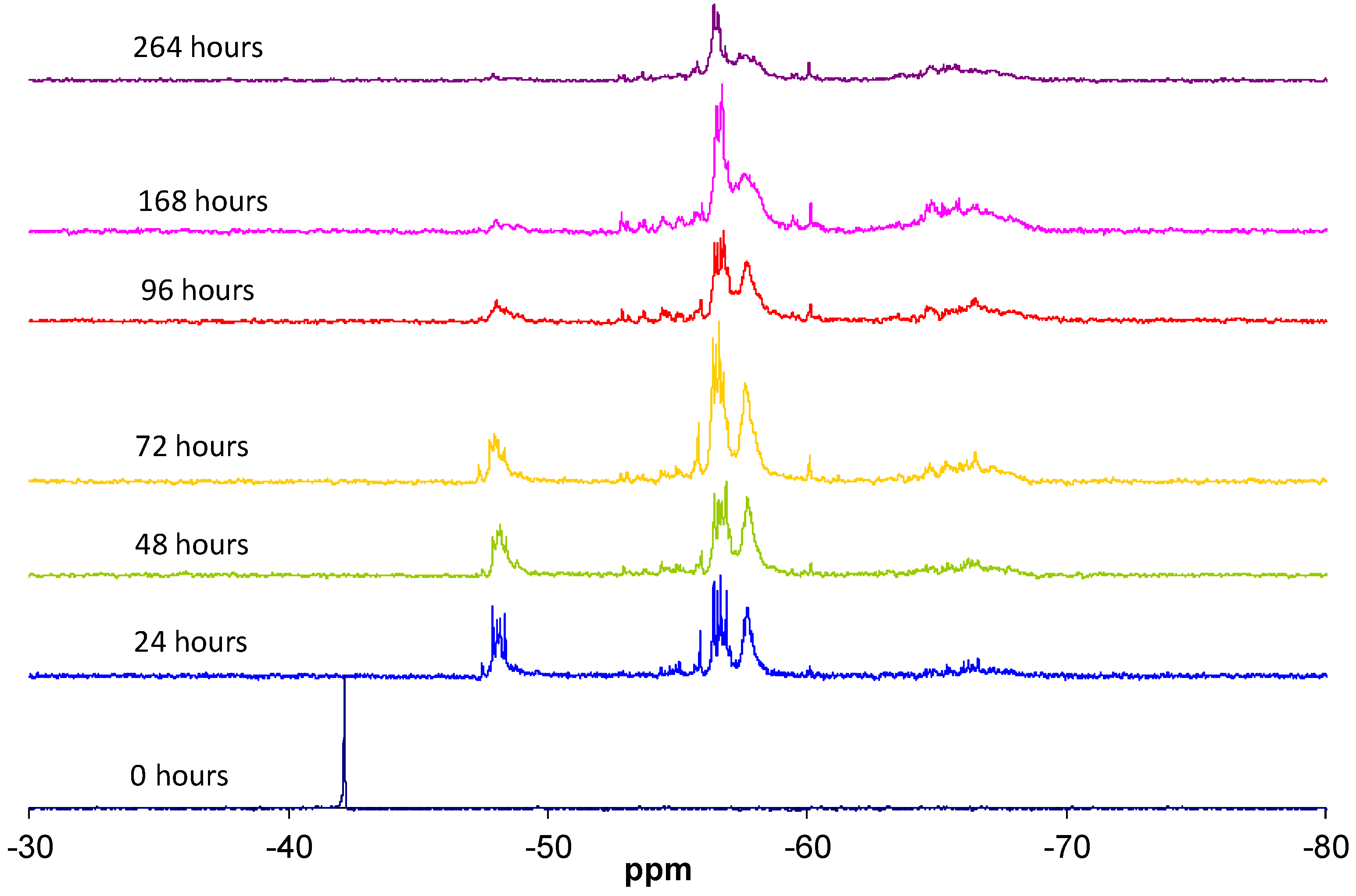
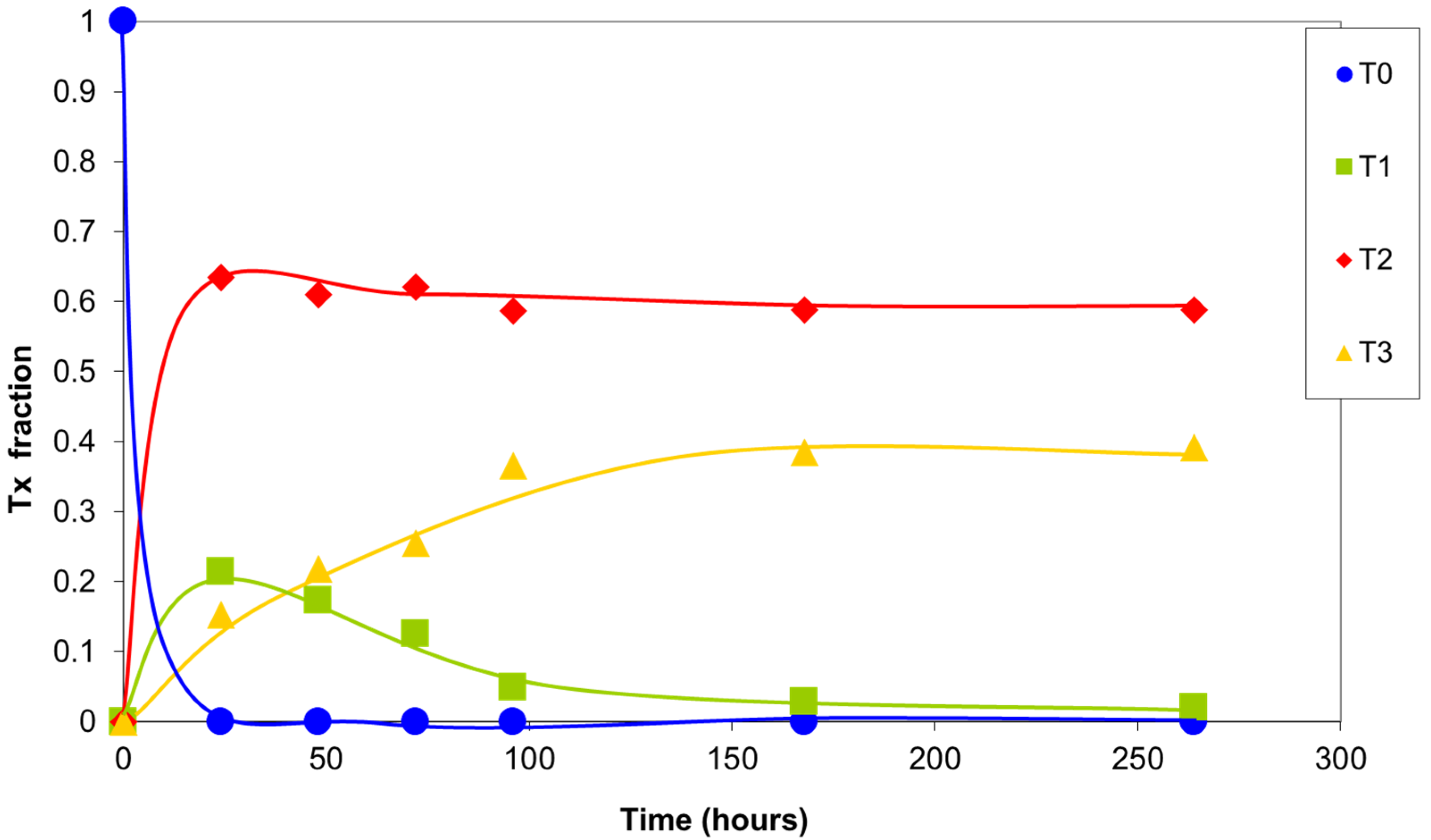
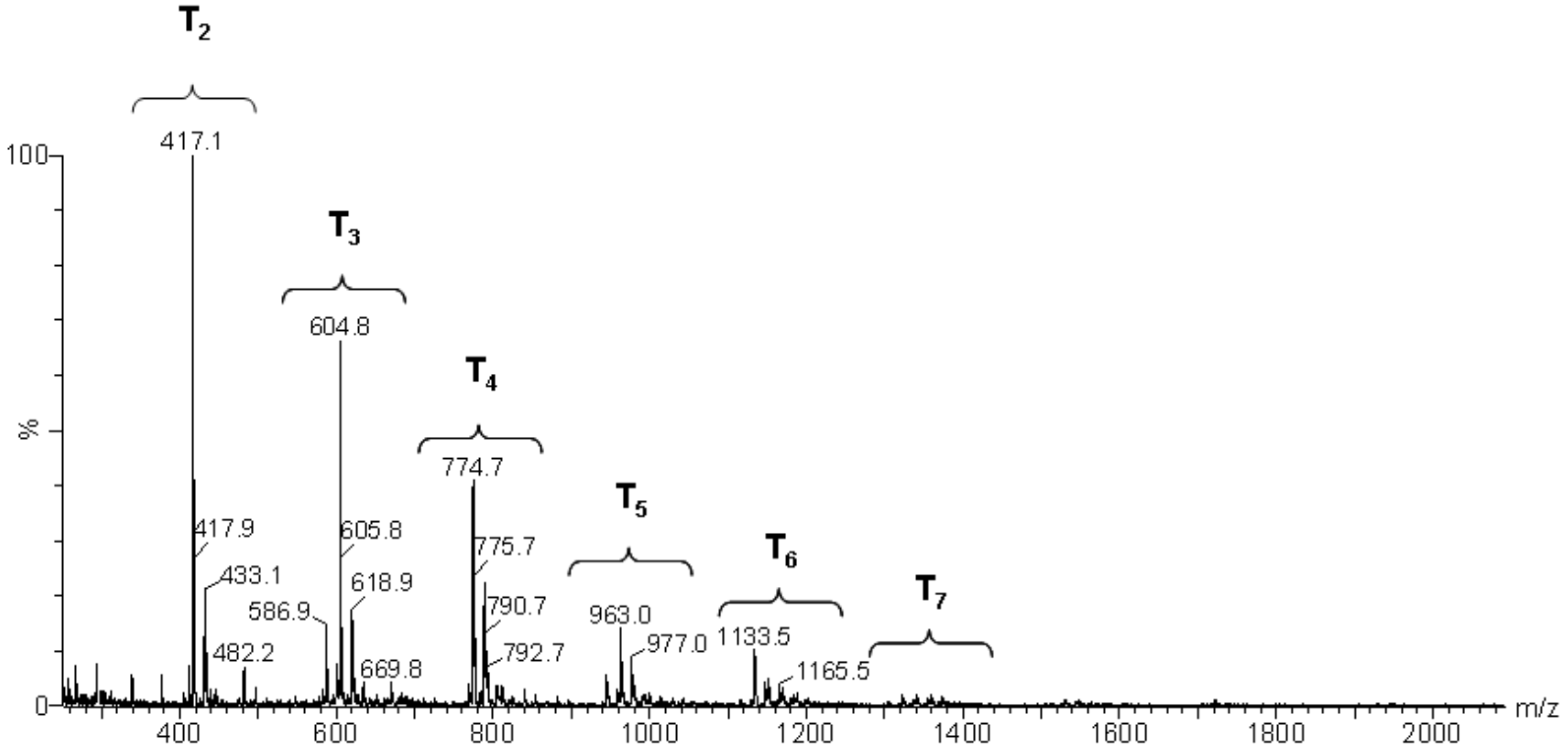
2.1. Silsesquioxane Oligomers
2.2. Unsaturated Polyester Composite Materials
3. Materials and Methods
3.1. Materials
3.2. Preparation of Silsesquioxane Oligomers
3.3. Preparation of Polymer Composites
3.4. Silsesquioxane Oligomer Structural Characterisation
3.5. Polymer Composite Characterisation
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; López-Cuesta, J.-M.; Dubois, P.H. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Wilkie, C.A.; Morgan, A.B. (Eds.) Fire Retardancy of Polymeric Materials, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Zhou, W.; Yang, H.; Guo, X.; Lu, J. Thermal degradation behaviors of some branched and linear polysiloxanes. Polym. Degrad. Stab. 2006, 91, 1471–1475. [Google Scholar] [CrossRef]
- Zhanga, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Shea, K.J.; Moreau, J.; Loy, D.A.; Corriu, R.J.; Boury, P.B. Bridged Polysilsesquioxanes. Molecular-Engineering Nanostructured Hybrid Organic-Inorganic Materials. In Functional Hybrid Materials; Gomez Romero, P., Sanchez, C., Eds.; Wiley-VCH: Weinheim, Germany, 2004; ISBN 978-3-527-30484-4. [Google Scholar]
- Brinker, C.J.; Scherer, G.W. Sol–Gel Science, the Physics and Chemistry of Sol–gel Processing; Academia Press: Boston, MA, USA, 1990. [Google Scholar]
- Li, G.; Wang, L.; Ni, H.; Pittman, C.U., Jr. Polyhedral Oligomeric Silsesquioxane (POSS). Polymers and Copolymers: A Review. J. Inorg. Organomet. Polym. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Engelhardt, G.; Michel, D. High Resolution Solid-State NMR of Silicates and Zeolites; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Pantoja, M.; Diaz-Benito, B.; Velasco, F.; Abenojar, J.; Real, J.C.D. Analysis of hydrolysis process of γ-methacryloxypropyltrimethoxysilane and its influence on the formation of silane coatings on 6063 aluminum alloy. Appl. Surf. Sci. 2009, 255, 6386–6390. [Google Scholar] [CrossRef]
- Soppera, O.; Croutxé-Barghorn, C. Real-time Fourier transform infrared study of free-radical UV-induced polymerization of hybrid sol–gel. I. Effect of silicate backbone on photopolymerization kinetics. J. Polym. Sci. A Polym. Chem. 2003, 41, 716–724. [Google Scholar] [CrossRef]
- Chan, C.-K.; Chu, I.M. Effect of hydrogen bonding on the glass transition behaviour of poly(acrylic acid)/silica hybrid materials prepared by sol–gel process. Polymer 2001, 42, 6089–6093. [Google Scholar] [CrossRef]
- Landry, C.J.T.; Coltrain, B.K.; Wesson, J.A.; Lippert, J.L.; Zumbulyadis, N. In situ polymerization of tetraethoxysilane in polymers: Chemical nature of the interactions. Polymer 1992, 33, 1496–1506. [Google Scholar] [CrossRef]
- Sugahara, Y.; Okada, S.; Sato, S.; Kuroda, K.; Kato, C. 29Si-NMR study of hydrolysis and initial polycondensation processes of organoalkoxysilanes. II. Methyltriethoxysilane. J. Non Cryst. Solids 1994, 167, 21–28. [Google Scholar] [CrossRef]
- Babonneau, F.; Maquet, J. Nuclear magnetic resonance techniques for the structural characterization of siloxane–oxide hybrid materials. Polyhedron 2000, 19, 315–322. [Google Scholar] [CrossRef]
- Brunet, F. Polymerization reactions in methyltriethoxysilane studied through 29Si NMR with polarization transfer. J. Non-Cryst. Solids 1998, 231, 58–77. [Google Scholar] [CrossRef]
- Hanjiang, D.; Manho, L.; Ruthanne, D.T.; Zhengping, Z.; Reidy, R.F.; Mueller, D.W. Methyltrimethoxysilane sol–gel polymerization in acidic ethanol solutions studied by 29Si NMR spectroscopy. J. Sol Gel Sci. Technol. 2003, 28, 5–14. [Google Scholar] [CrossRef]
- Brunet, F.; Cabane, J. Populations of oligomers in sol–gel condensation. J. Non Cryst. Solids 1993, 163, 211–225. [Google Scholar] [CrossRef]
- Devereux, F.; Boilot, J.P.; Chaput, F. Sol–gel condensation of rapidly hydrolyzed silicon alkoxides: A joint 29Si NMR and small-angle x-ray scattering study. Phys. Rev. A 1990, 41, 6901–6909. [Google Scholar] [CrossRef]
- Eisenberg, P.; Erra-Balsells, R.; Ishikawa, Y.; Lucas, J.C.; Nonami, H.; Williams, R.J.J. Silsesquioxanes derived from the bulk polycondensation of [3-(Methacryloxy)propyl]trimethoxysilane with concentrated formic acid: Evolution of molar mass distributions and fraction of intramolecular cycles. Macromolecules 2002, 35, 1160–1174. [Google Scholar] [CrossRef]
- UNE-EN ISO 4589-2. Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test; AENOR: Madrid, Spain, 2001. [Google Scholar]



| Reference | Reaction Time | ||||
|---|---|---|---|---|---|
| t = 0 h | t = 24 h | t = 48 h | |||
| Chemical Shifts (ppm) | Chemical Shifts (ppm) | Intensity Ratio | Chemical Shifts (ppm) | Intensity Ratio | |
| α | 5.07 | 8.50 | --- | 8.50 | --- |
| γ | 66.16 | 66.38 66.68 | 0.40 0.60 | 66.36 66.65 | 0.35 0.65 |
| a | 124.64 | 125.31 125.44 | 0.32 0.68 | 125.29 125.42 | 0.34 0.66 |
| c | 166.85 | 167.43 167.67 | 0.32 0.68 | 167.34 167.61 | 0.34 0.66 |
| Species | Chemical Band Shift (ppm) |
|---|---|
| T1 | −47.4 to −49.3 |
| T21(3c) | −52.7 to −54.2 |
| T20(3c) | −54.2 to −55.4 |
| T21(4c) | −55.4 to −56.1 |
| T20(4c) | −56.1 to −57.3 |
| T2(5c+6c+l) | −57.3 to −58.1 |
| T3(3c) | − 58.1 to −59.2 |
| T3(4c) | −59.2 to −60.7 |
| T3(r) | −62 to −69 |
| Experimental m/z Value | Integration | Species | Structures |
|---|---|---|---|
| 417.1 | 20% | T2(OH)4Na+ (l) |  |
| 433.1 | 6% | T2(OH)3(OCH3)Na+ (l) | |
| 604.8 | 15% | T3(OH)5Na+ (l) |  |
| 618.9 | 8% | T3(OH)4(OCH3)Na+ (l) | |
| 774.7 | 11% | T4(OH)4Na+ (c) |  |
| 788.7 | 8% | T4(OH)3(OCH3)Na+ (c) |
| Experimental m/z Value | Integration | Species | Structures |
|---|---|---|---|
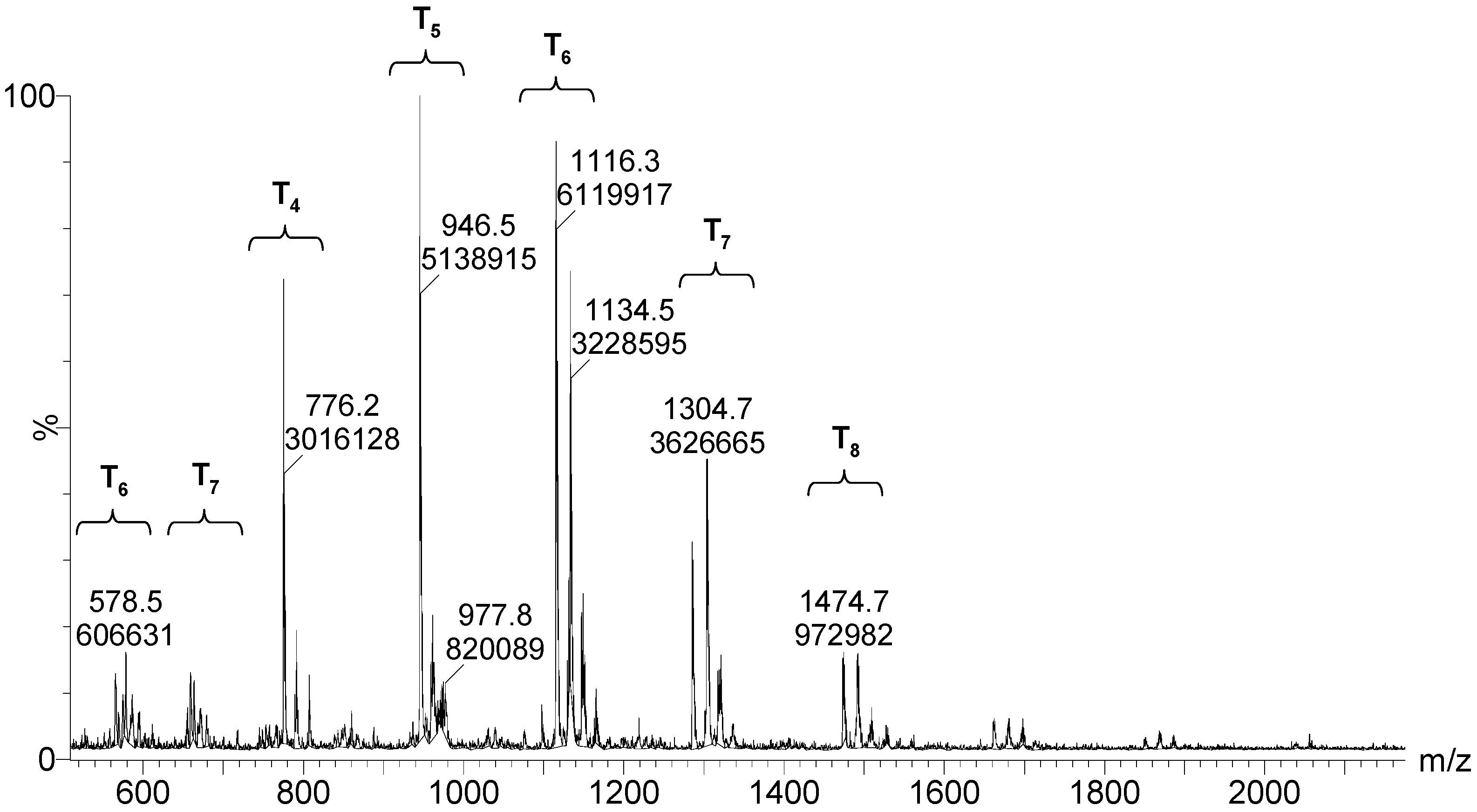
| 1115.3 | 15% | T6(OH)2Na+ (3c) |  |
| 945.5 | 13% | T5(OH)3Na+ (2c) |  |
| 664.3 1303.7 | 9% | T7(OH)3Na2+2 (3c) T7(OH)3Na+ (3c) |  |
| 578.5 1133.5 | 9% | T6(OH)4Na2+2 (2c) T6(OH)4Na2+2 (2c) |  |
| 775.2 | 7% | T4(OH)4Na+ (c) |  |
| 55.6 1285.0 | 6% | T7(OH)Na2+2 (4c) T7(OH)Na (4c) |  |
| 587.0 1149.4 | 5% | T6(OH)6Na2+2 (1c) T6(OH)6Na+ (1c) |  |
| 1491.2 | 4% | T8(OH)4Na+ (3c) |  |
| Composite | Resin | ATH | MAPTMS (Synthesis Time) | POSS | Limiting Oxygen Index (LOI) | Mechanical Resistance of Combustion Char (kg/cm2) |
|---|---|---|---|---|---|---|
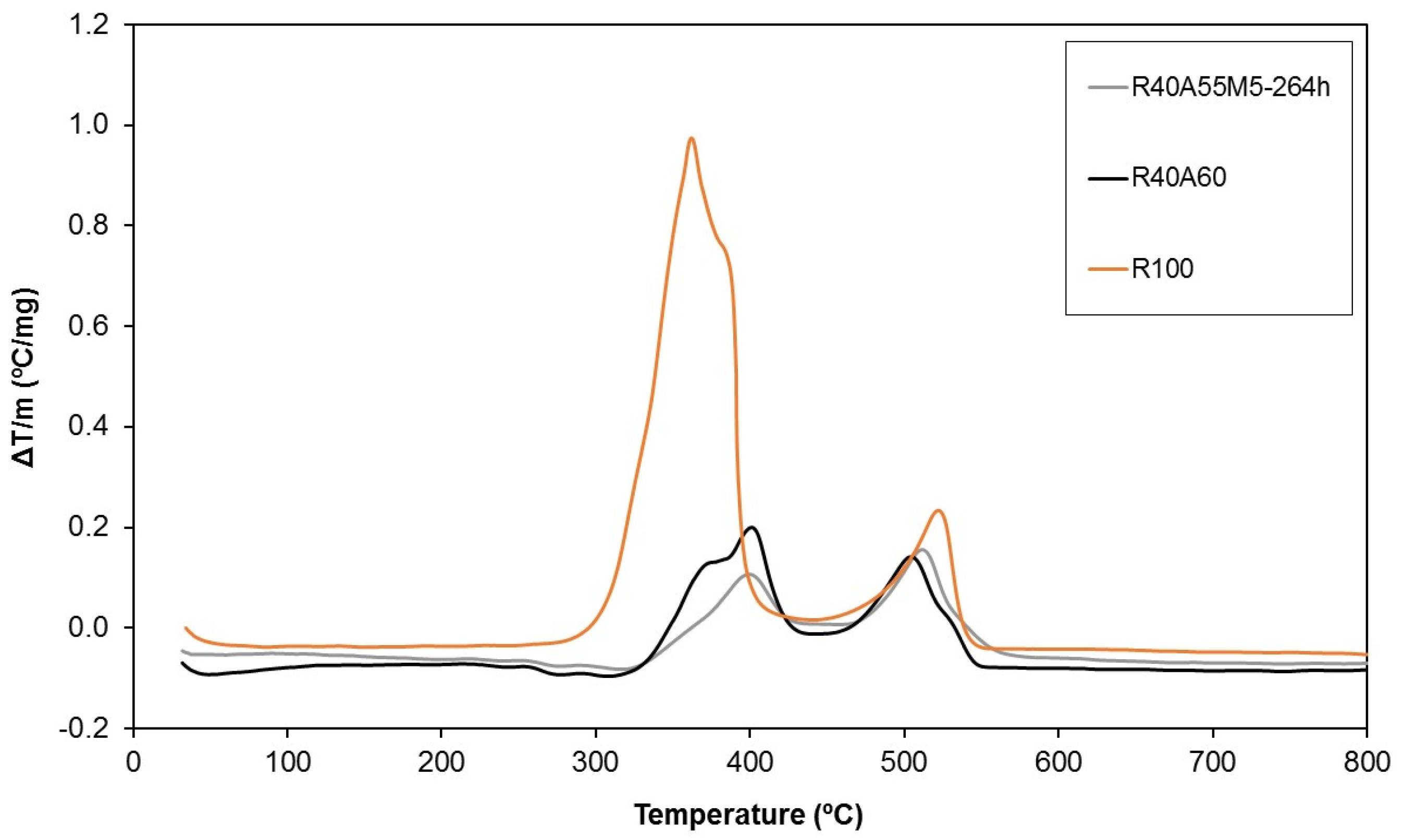
| R100 | 100% | 0% | 0% | 0% | 21% | --- |
| R40A60 | 40% | 60% | 0% | 0% | 44% | --- |
| R40A55M5-0 h | 40% | 55% | 5% (0 h) | 0% | 46% | 0.7 ± 0.3 |
| R40A55M5-24 h | 40% | 55% | 5% (24 h) | 0% | 48% | 0.7 ± 0.2 |
| R40A55M5-48 h | 40% | 55% | 5% (48 h) | 0% | 50% | 1.2 ± 0.1 |
| R40A55M5-96 h | 40% | 55% | 5% (96 h) | 0% | 50% | 2.3 ± 0.2 |
| R40A55M5-264 h | 40% | 55% | 5% (264 h) | 0% | 50% | 2.8 ± 0.2 |
| R40A55P5 | 40% | 55% | 0% | 5% | 51% | 0.8 ± 0.1 |
| Temperature (°C) | R100 | R + M |
|---|---|---|
| 25 |  |  |
| 350 |  |  |
| 380 |  |  |
| 400 |  |  |
| 800 | --- |  |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bautista, Y.; Gozalbo, A.; Mestre, S.; Sanz, V. Improvement in Char Strength with an Open Cage Silsesquioxane Flame Retardant. Materials 2017, 10, 567. https://doi.org/10.3390/ma10060567
Bautista Y, Gozalbo A, Mestre S, Sanz V. Improvement in Char Strength with an Open Cage Silsesquioxane Flame Retardant. Materials. 2017; 10(6):567. https://doi.org/10.3390/ma10060567
Chicago/Turabian StyleBautista, Yolanda, Ana Gozalbo, Sergio Mestre, and Vicente Sanz. 2017. "Improvement in Char Strength with an Open Cage Silsesquioxane Flame Retardant" Materials 10, no. 6: 567. https://doi.org/10.3390/ma10060567






