Modification of Cellulose with Succinic Anhydride in TBAA/DMSO Mixed Solvent under Catalyst-Free Conditions
Abstract
:1. Introduction
2. Results and Discussion
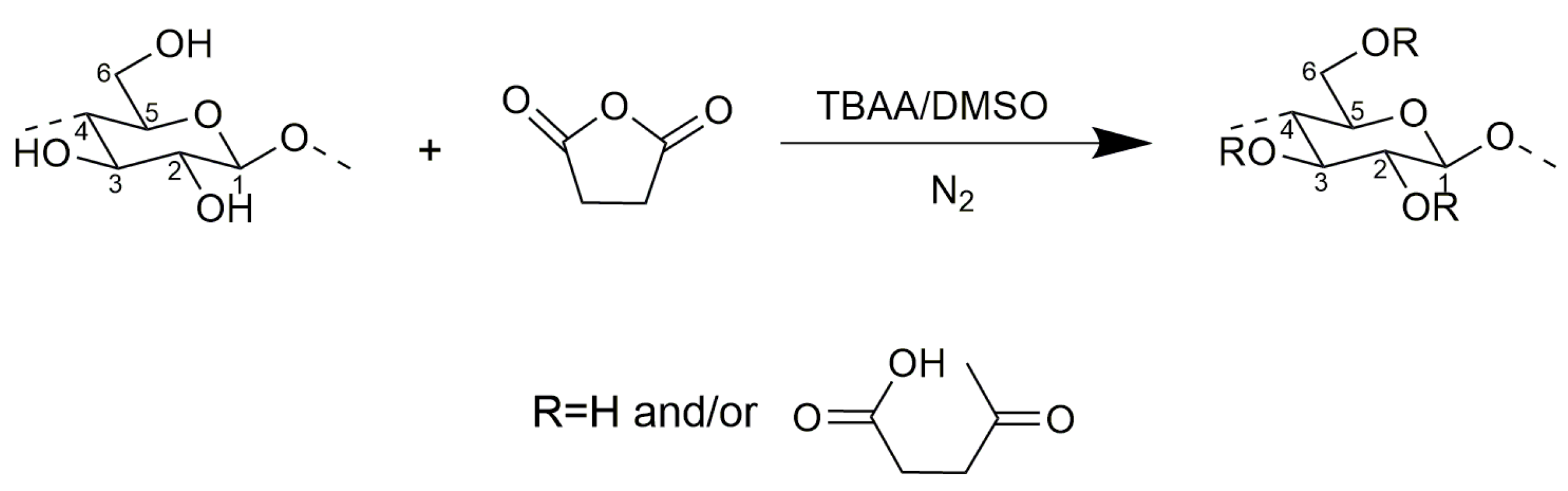
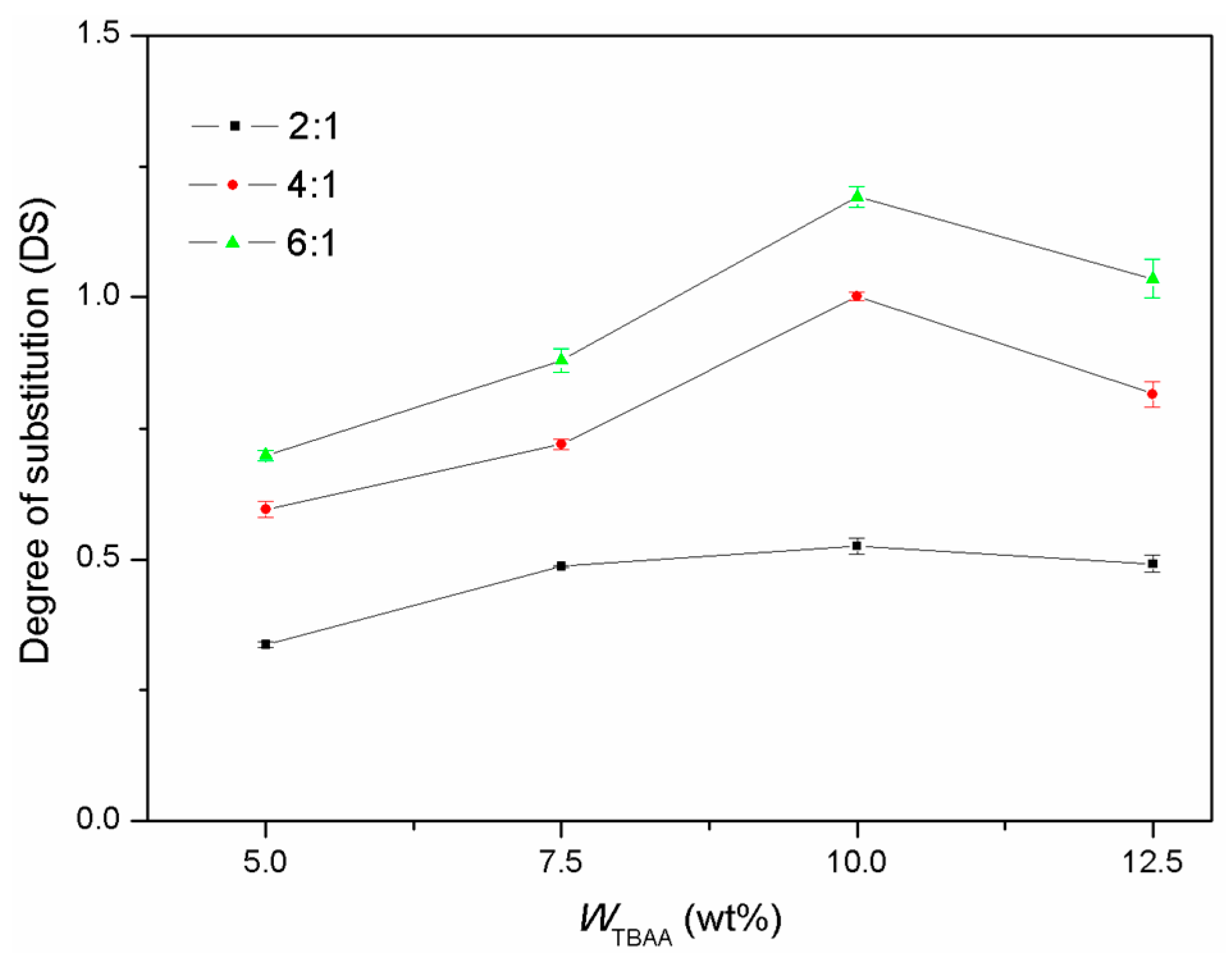
2.1. Succinylation of Cellulose
2.2. ATR-FTIR Analysis
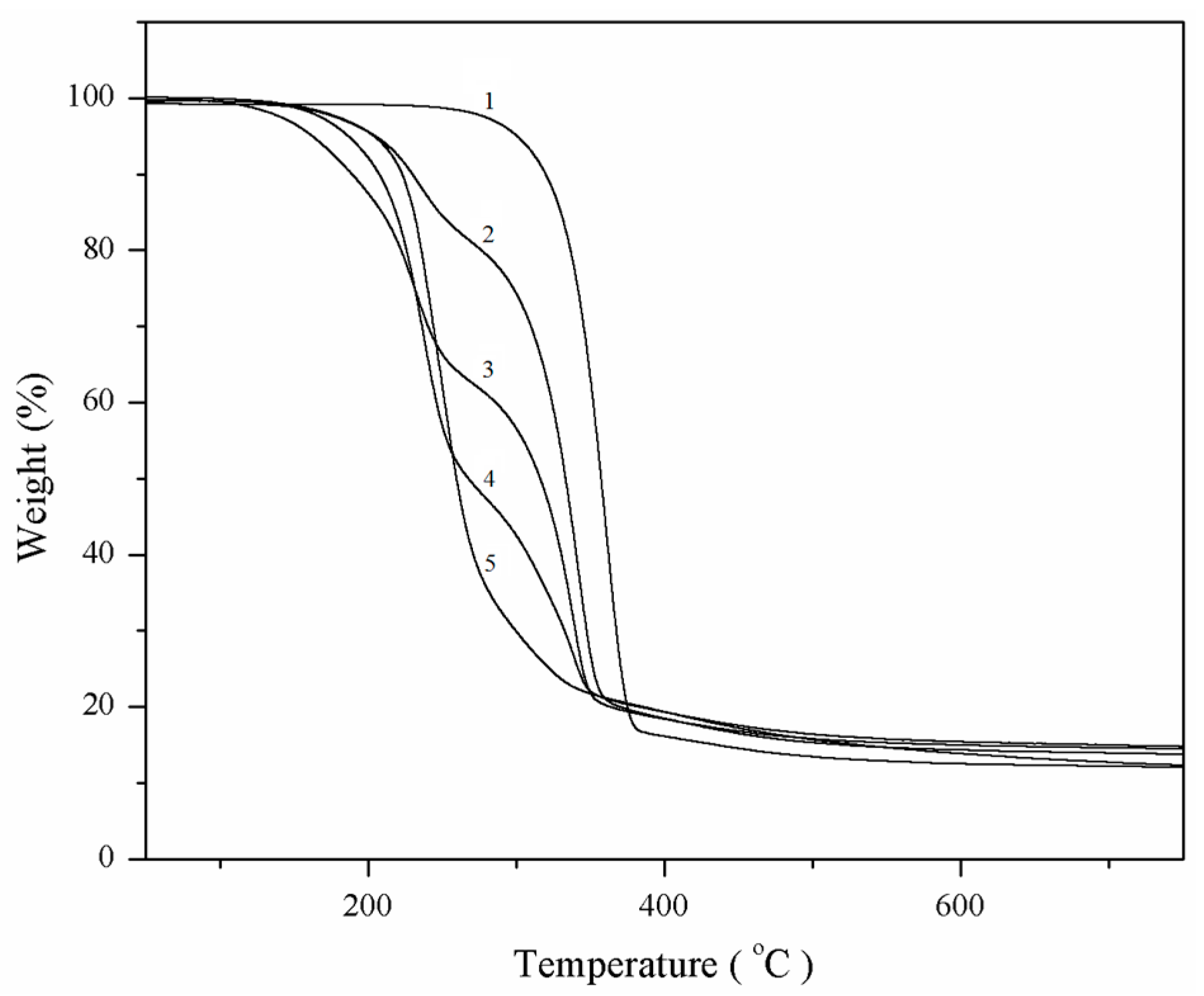
2.3. TGA Analysis
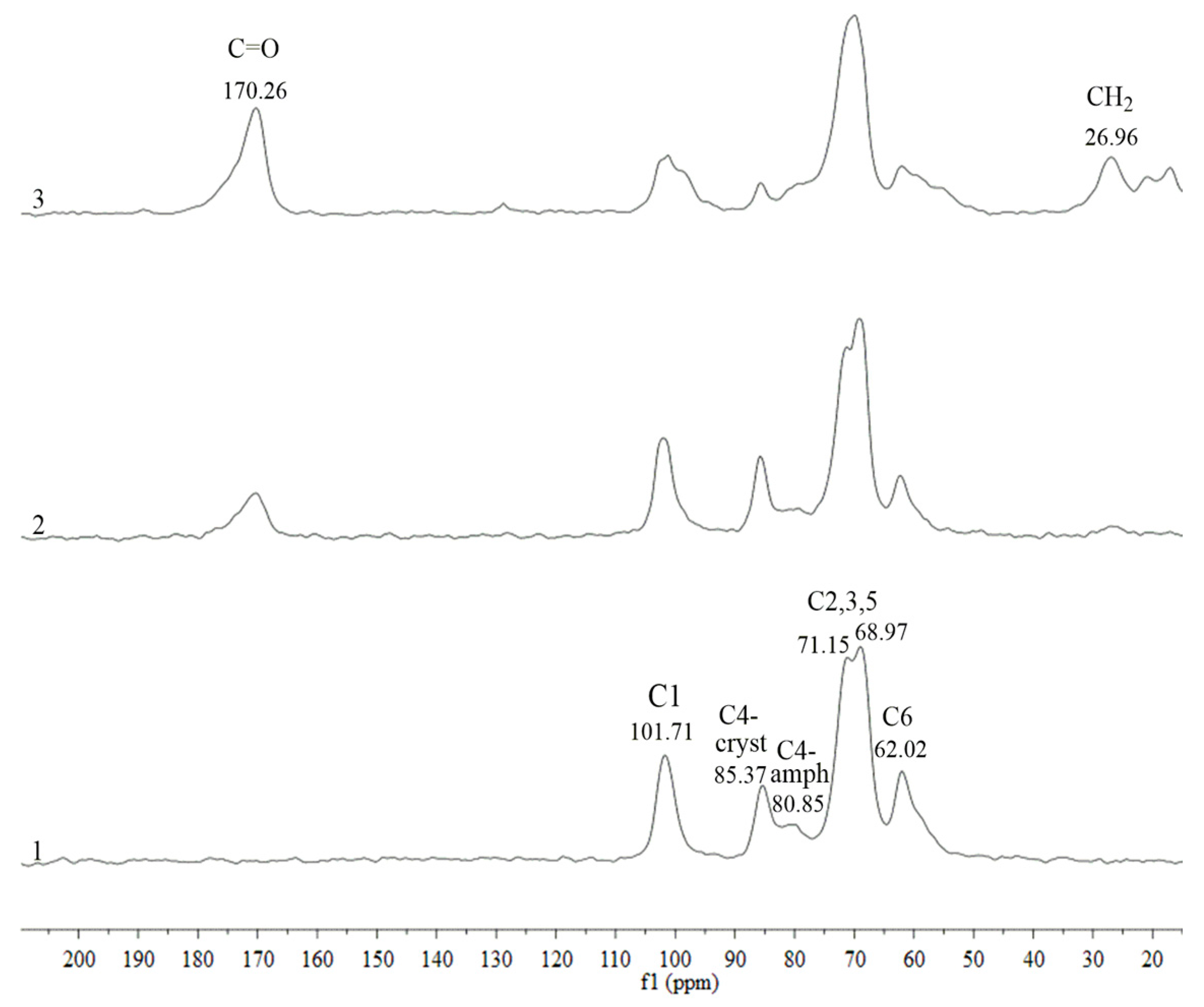
2.4. CP/MAS 13C NMR Analysis
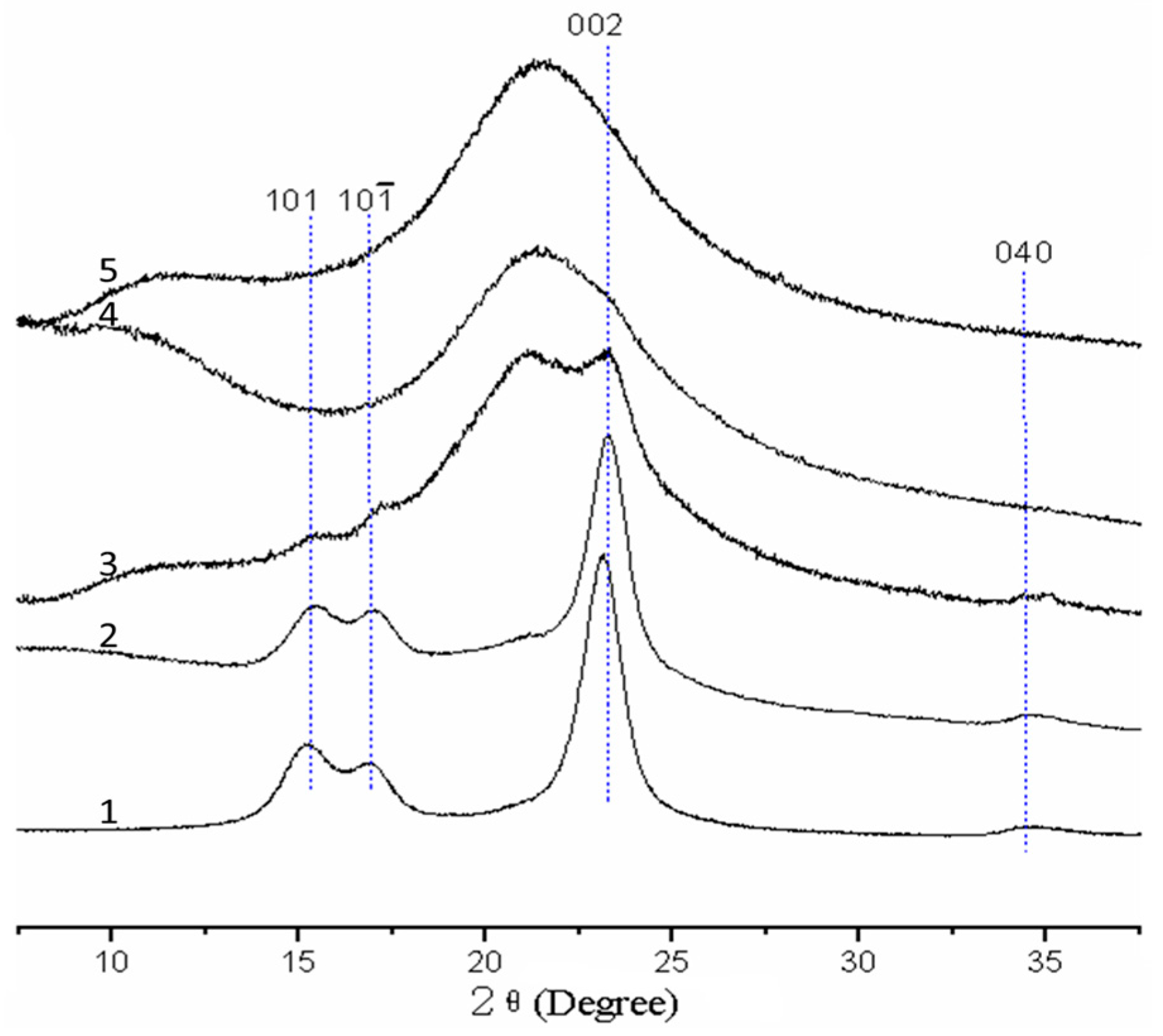
2.5. XRD Analysis
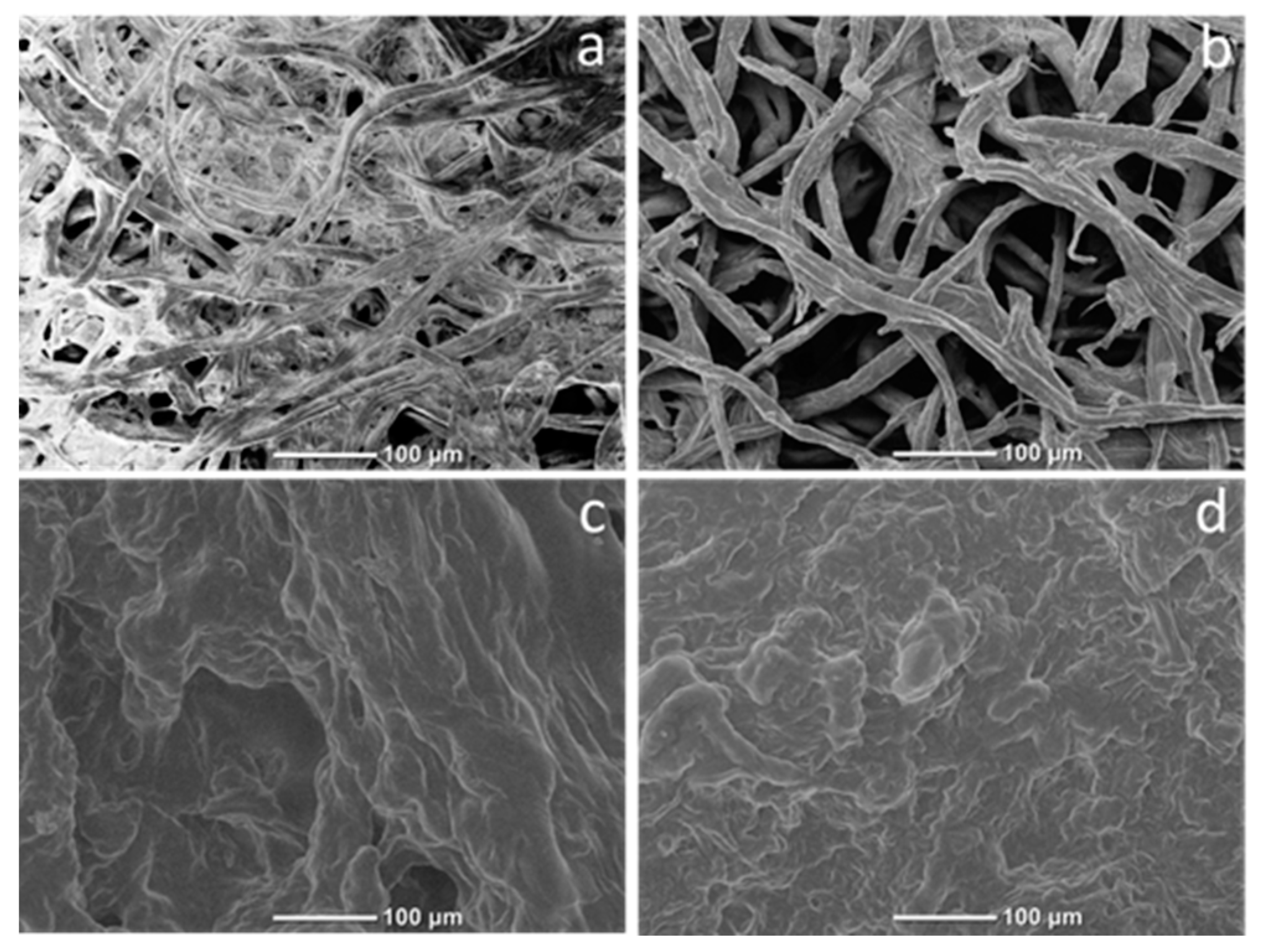
2.6. SEM Analysis
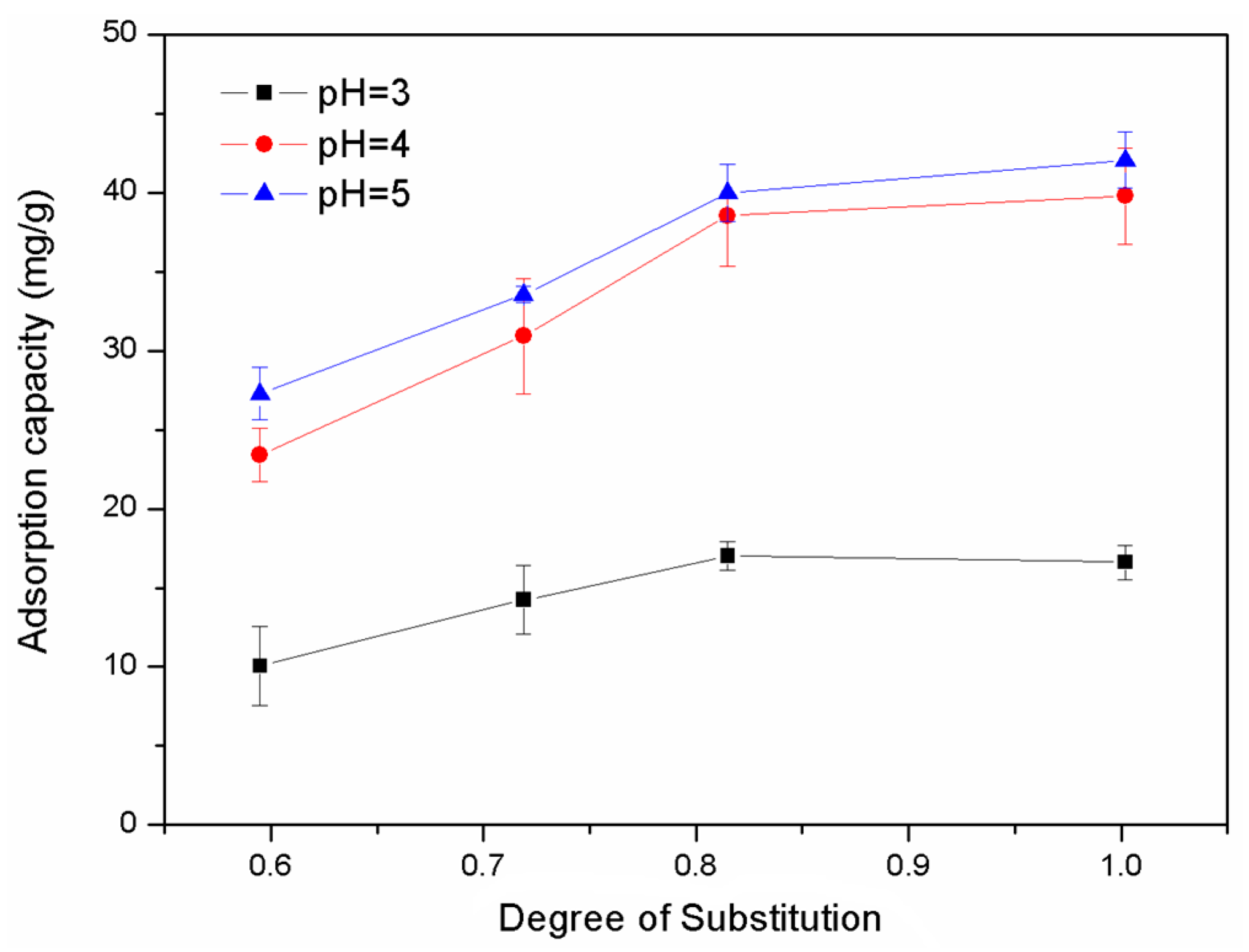
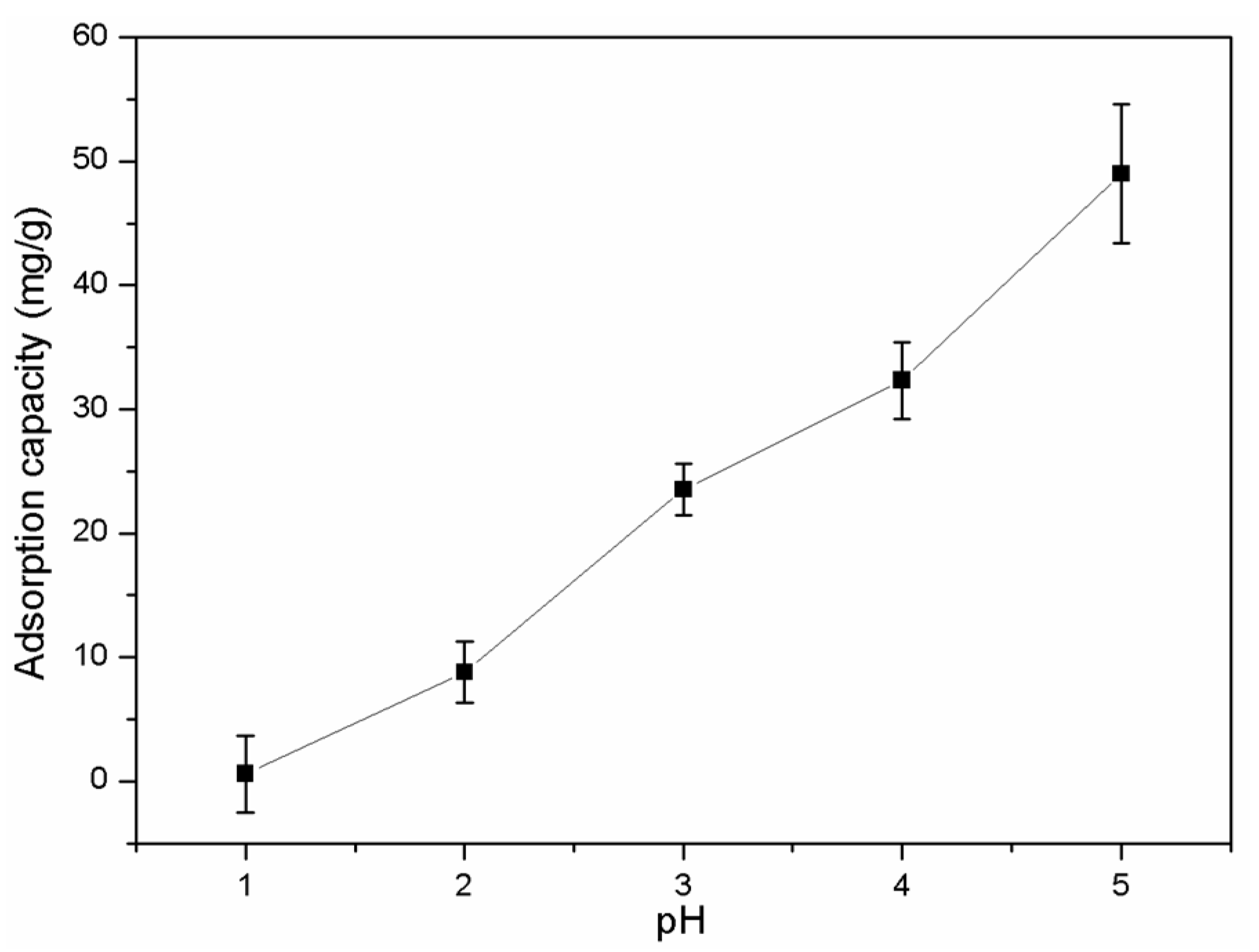
2.7. Application for the Adsorption of Heavy Metal Ions
3. Materials and Methods
3.1. Materials
3.2. Succinoylation of Cellulose with Succinic Anhydride in TBAA/DMSO Mixed Solvent
3.3. Characterization
3.3.1. Determination of the Degree of Substitution (DS)
3.3.2. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR) Analysis
3.3.3. Thermal Gravimetric (TGA) Analysis
3.3.4. Solid State CP/MAS 13C NMR Spectroscopy (CP/MAS 13C NMR) Analysis
3.3.5. X-ray Diffraction (XRD) Analysis
3.3.6. Scanning Electron Microscopy (SEM) Analysis
3.4. Metal Ion Adsorption Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.L.; Wang, X.L.; Li, F.; Li, H.Z.; Wang, Y.Z. Green composite films prepared from cellulose, starch and lignin in room-temperature ionic liquid. Bioresour. Technol. 2009, 100, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, M.; Aida, T.M.; Watanabe, M.; Smith, R.L., Jr. Dissolution and recovery of cellulose from 1-butyl-3-methylimidazolium chloride in presence of water. Carbohydr. Polym. 2013, 92, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.B.; Fu, Y. ChemInform Abstract: Hydrolysis of Cellulose to Glucose by Solid Acid Catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Habibi, Y. ChemInform Abstract: Key Advances in the Chemical Modification of Nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef] [PubMed]
- Oksman, K.; Aitomäki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Compos. Part A: Appl. Sci. Manuf. 2016, 83, 2–18. [Google Scholar] [CrossRef]
- Liimatainen, H.; Visanko, M.; Sirviö, J.; Hormi, O.; Niinimäki, J. Sulfonated cellulose nanofibrils obtained from wood pulp through regioselective oxidative bisulfite pre-treatment. Cellulose 2013, 20, 741–749. [Google Scholar] [CrossRef]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated Cellulose Surface Modification: A Review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef]
- Ramírez, J.A.Á.; Fortunati, E.; Kenny, J.M.; Torre, L.; Foresti, M.L. Simple citric acid-catalyzed surface esterification of cellulose nanocrystals. Carbohydr. Polym. 2017, 157, 1358–1364. [Google Scholar]
- Eyholzer, C.; Couraça, A.B.D.; Duc, F.; Bourban, P.E.; Tingaut, P.; Zimmermann, T.; Månson, J.A.E.; Oksman, K. Biocomposite Hydrogels with Carboxymethylated, Nanofibrillated Cellulose Powder for Replacement of the Nucleus Pulposus. Biomacromolecules 2011, 12, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.C.; Li, B.; Xu, D.; Edgar, K.J. Regioselective Esterification and Etherification of Cellulose: A Review. Biomacromolecules 2011, 12, 1956–1972. [Google Scholar] [CrossRef] [PubMed]
- Goussé, C.; Chanzy, H.; Cerrada, M.L.; Fleury, E. Surface silylation of cellulose microfibrils: Preparation and rheological properties. Polymer 2004, 45, 1569–1575. [Google Scholar]
- Chundawat, S.P.; Bellesia, G.; Uppugundla, N.; da Costa Sousa, L.; Gao, D.; Cheh, A.M.; Agarwal, U.P.; Bianchetti, C.M.; Phillips, G., Jr.; Langan, P. Restructuring the crystalline cellulose hydrogen bond network enhances its depolymerization rate. J. Am. Chem. Soc. 2011, 133, 11163–11174. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, A.; Ballone, P. Room Temperature Ionic Liquids Meet Biomolecules: A Microscopic View of Structure and Dynamics. ACS Sustain. Chem. Eng. 2015, 4, 392–412. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A.; Song, Z.; Olubajo, O.; Crittle, T.; Peters, D. Designing enzyme-compatible ionic liquids that can dissolve carbohydrates. Green Chem. 2008, 10, 696–705. [Google Scholar] [CrossRef]
- Chadlia, A.; Farouk, M.H.M. Rapid homogeneous esterification of cellulose extracted from Posidonia induced by microwave irradiation. J. Appl. Polym. Sci. 2011, 119, 3372–3381. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef] [PubMed]
- Striegel, A.M. Advances in the Understanding of the Dissolution Mechanism of Cellulose in DMAc/LiCl. J. Chil. Chem. Soc. 2003, 48, 73–77. [Google Scholar] [CrossRef]
- Kotelnikova, N.E.; Bykhovtsova, Y.V.; Mikhailidi, A.M.; Saprykina, N.N. Comparative study on the methods for dissolving powder lignocelluloses in DMAc/LiCl and chemical properties of samples regenerated from the solutions. Rus. J. Bioorgan. Chem. 2015, 41, 700–707. [Google Scholar] [CrossRef]
- Ostlund, A.; Lundberg, D.; Nordstierna, L.; Holmberg, K.; Nydén, M. Dissolution and gelation of cellulose in TBAF/DMSO solutions: The roles of fluoride ions and water. Biomacromolecules 2009, 10, 2401–2407. [Google Scholar] [CrossRef] [PubMed]
- Yusup, E.M.; Mahzan, S.; Jafferi, N.; Been, C.W. The Effectiveness of TBAF/DMSO in Dissolving Oil Palm Empty Fruit Bunch-Cellulose Phosphate. J. Med. Bioeng. 2015, 4, 165–169. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Zhang, H.; He, J.; Ren, Q.; Guo, M. Homogeneous Acetylation of Cellulose in a New Ionic Liquid. Biomacromolecules 2004, 5, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Köhler, S.; Liebert, T.; Schöbitz, M.; Schaller, J.; Meister, F.; Günther, W.; Heinze, T. Interactions of Ionic Liquids with Polysaccharides 1. Unexpected Acetylation of Cellulose with 1-Ethyl-3-methylimidazolium Acetate. Macromol. Rapid Commun. 2007, 28, 2311–2317. [Google Scholar]
- Amarasekara, A.S.; Owereh, O.S. Homogeneous phase synthesis of cellulose carbamate silica hybrid materials using 1-n-butyl-3-methylimidazolium chloride ionic liquid medium. Carbohydr. Polym. 2009, 78, 635–638. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.; Feng, Y.; Wu, J.; Yu, J.; He, J.; Zhang, J. Homogeneous esterification of cellulose in room temperature ionic liquids. Polym. Int. 2015, 64, 963–970. [Google Scholar] [CrossRef]
- Shang, W.; Sheng, Z.; Shen, Y.; Ai, B.; Zheng, L.; Yang, J.; Xu, Z. Study on oil absorbency of succinic anhydride modified banana cellulose in ionic liquid. Carbohydr. Polym. 2016, 141, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.A.; Gaugler, M.; Smith, D.A. Green route to modification of wood waste, cellulose and hemicellulose using reactive extrusion. Carbohydr. Polym. 2016, 136, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Belhalfaoui, B.; Aziz, A.; Elandaloussi, E.H.; Ouali, M.S. Succinate-bonded cellulose: A regenerable and powerful sorbent for cadmium-removal from spiked high-hardness groundwater. J. Hazard. Mater. 2009, 169, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.A.M.A.; Hassan, M.L. Ion exchange properties of carboxylated bagasse. J. Appl. Polym. Sci. 2006, 102, 1399–1404. [Google Scholar] [CrossRef]
- Yoshimura, T.; Matsuo, K.; Fujioka, R. Novel biodegradable superabsorbent hydrogels derived from cotton cellulose and succinic anhydride: Synthesis and characterization. J. Appl. Polym. Sci. 2010, 99, 3251–3256. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, M.; Song, G.; Huang, H. Modification of pineapple peel fibre with succinic anhydride for Cu2+, Cd2+ and Pb2+ removal from aqueous solutions. Environ. Technol. 2011, 32, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Yu, C.; Zhang, X.; Yang, J.; Lin, Q.; Wang, J.; Zhu, Q. Comparison of succinylation methods for bacterial cellulose and adsorption capacities of bacterial cellulose derivatives for Cu 2+ ion. Polym. Bull. 2011, 67, 401–412. [Google Scholar] [CrossRef]
- Li, W.Y.; Jin, A.X.; Liu, C.F.; Sun, R.C.; Zhang, A.P.; Kennedy, J.F. Homogeneous modification of cellulose with succinic anhydride in ionic liquid using 4-dimethylaminopyridine as a catalyst. Carbohydr. Polym. 2009, 78, 389–395. [Google Scholar] [CrossRef]
- Chen, J.; Su, M.; Zhang, X.; Chen, R.; Hong, J.; Yang, L.; Yang, Z. The role of cations in homogeneous succinoylation of mulberry wood cellulose in salt-containing solvents under mild conditions. Cellulose 2014, 21, 4081–4091. [Google Scholar] [CrossRef]
- Chen, J.; Su, M.; Chen, R.; Hong, J.; Cheng, R. Effects of salt on homogeneous succinoylation of lignocellulosic fibers in dimethyl sulfoxide/tetraethylammonium chloride under mild condition. J. Appl. Polym. Sci. 2015, 132, 41912–41919. [Google Scholar] [CrossRef]
- Liu, C.F.; Zhang, A.P.; Li, W.Y.; Yue, F.X.; Sun, R.C. Homogeneous Modification of Cellulose in Ionic Liquid with Succinic Anhydride Using N-Bromosuccinimide as a Catalyst. J. Agric. Food Chem. 2009, 57, 1814. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Sun, R.C.; Zhang, A.P.; Ren, J.L.; Geng, Z.C. Structural and thermal characterization of sugarcane bagasse cellulose succinates prepared in ionic liquid. Polym. Degrad. Stab. 2006, 91, 3040–3047. [Google Scholar] [CrossRef]
- Liu, C.F.; Sun, R.C.; Zhang, A.P.; Ren, J.L.; Wang, X.A.; Qin, M.H.; Chao, Z.N.; Luo, W. Homogeneous modification of sugarcane bagasse cellulose with succinic anhydride using a ionic liquid as reaction medium. Carbohydr. Res. 2007, 342, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Chen, C.Y.; Liu, C.F.; Sun, R.C. Homogeneous modification of sugarcane bagasse with maleic anhydride in 1-butyl-3-methylimidazolium chloride without any catalysts. Ind. Crop. Prod. 2013, 46, 380–385. [Google Scholar] [CrossRef]
- Huang, Y.B.; Xin, P.P.; Li, J.X.; Shao, Y.Y.; Huang, C.B.; Pan, H. Room-Temperature Dissolution and Mechanistic Investigation of Cellulose in a Tetra-Butylammonium Acetate/Dimethyl Sulfoxide System. ACS Sustain. Chem. Eng. 2016, 4, 2286–2294. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of Cellulose with Ionic Liquids and Its Application: A Mini-Review. Green Chem. 2006, 37, 325–327. [Google Scholar] [CrossRef]
- Lin, L.; Yamaguchi, H.; Suzuki, A. Dissolution of cellulose in the mixed solvent of [bmim]Cl-DMAc and its application. RSC Adv. 2013, 3, 14379–14384. [Google Scholar] [CrossRef]
- Tomkinson, J. Separation and Characterization of Cellulose from Wheat Straw. Sep. Sci. Technol. 2005, 39, 391–411. [Google Scholar]
- Liu, C.F. Succinoylation of cellulose catalyzed with iodine in ionic liquid. Ind. Crop.Prod. 2010, 31, 363–369. [Google Scholar] [CrossRef]
- Miao, J.; Sun, H.; Yu, Y.; Song, X.; Zhang, L. Quaternary ammonium acetate: An efficient ionic liquid for the dissolution and regeneration of cellulose. RSC Adv. 2014, 4, 36721–36724. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Licciardello, A. Surface chemical modification of natural cellulose fibers. J. Appl. Polym. Sci. 2002, 83, 38–45. [Google Scholar] [CrossRef]
- Frisoni, G.; Baiardo, M.; Scandola, M.; Lednická, D.; Cnockaert, M.C.; Mergaert, J.; Swings, J. Natural Cellulose Fibers: Heterogeneous Acetylation Kinetics and Biodegradation Behavior. Biomacromolecules 2001, 2, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Yu, Y.; Jiang, Z.; Zhang, L. One-pot preparation of hydrophobic cellulose nanocrystals in an ionic liquid. Cellulose 2016, 23, 1209–1219. [Google Scholar] [CrossRef]
- Pan, H.; Shupe, T.F.; Hse, C.Y. Characterization of liquefied wood residues from different liquefaction conditions. J. Appl. Polym. Sci. 2007, 105, 3740–3746. [Google Scholar] [CrossRef]
- Chadlia, A.; Mohamed, K.; Najah, L.; Farouk, M.M. Preparation and characterization of new succinic anhydride grafted Posidonia for the removal of organic and inorganic pollutants. J. Hazard. Mater. 2009, 172, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Cossich, S.; Tavares, E.G.; Ravagnani, C.R.K.; Massako, T. Biosorption of chromium(III) by Sargassum sp. biomass. Electron. J. Biotechnol. 2002, 5, 133–140. [Google Scholar] [CrossRef]
- Thirumavalavan, M.; Lai, Y.L.; Lin, L.C.; Lee, J.F. Cellulose-Based Native and Surface Modified Fruit Peels for the Adsorption of Heavy Metal Ions from Aqueous Solution: Langmuir Adsorption Isotherms. J. Chem. Eng. Data 2013, 55, 1186–1192. [Google Scholar] [CrossRef]
- Jeon, Y.S.; Lowell, A.V.; Gross, R.A. Studies of Starch Esterification: Reactions with Alkenylsuccinates in Aqueous Slurry Systems. Starch-Stärke 1999, 51, 90–93. [Google Scholar] [CrossRef]
- Stojanović, Ž.; Jeremić, K.; Jovanović, S.; Lechner, M.D. Comparison of Some Methods for the Determination of the Degree of Substitution of Carboxymethyl Starch. Starch-Starke 2005, 57, 79–83. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, P.-P.; Huang, Y.-B.; Hse, C.-Y.; Cheng, H.N.; Huang, C.; Pan, H. Modification of Cellulose with Succinic Anhydride in TBAA/DMSO Mixed Solvent under Catalyst-Free Conditions. Materials 2017, 10, 526. https://doi.org/10.3390/ma10050526
Xin P-P, Huang Y-B, Hse C-Y, Cheng HN, Huang C, Pan H. Modification of Cellulose with Succinic Anhydride in TBAA/DMSO Mixed Solvent under Catalyst-Free Conditions. Materials. 2017; 10(5):526. https://doi.org/10.3390/ma10050526
Chicago/Turabian StyleXin, Ping-Ping, Yao-Bing Huang, Chung-Yun Hse, Huai N. Cheng, Chaobo Huang, and Hui Pan. 2017. "Modification of Cellulose with Succinic Anhydride in TBAA/DMSO Mixed Solvent under Catalyst-Free Conditions" Materials 10, no. 5: 526. https://doi.org/10.3390/ma10050526





