Fabrication Processes to Generate Concentration Gradients in Polymer Solar Cell Active Layers
Abstract
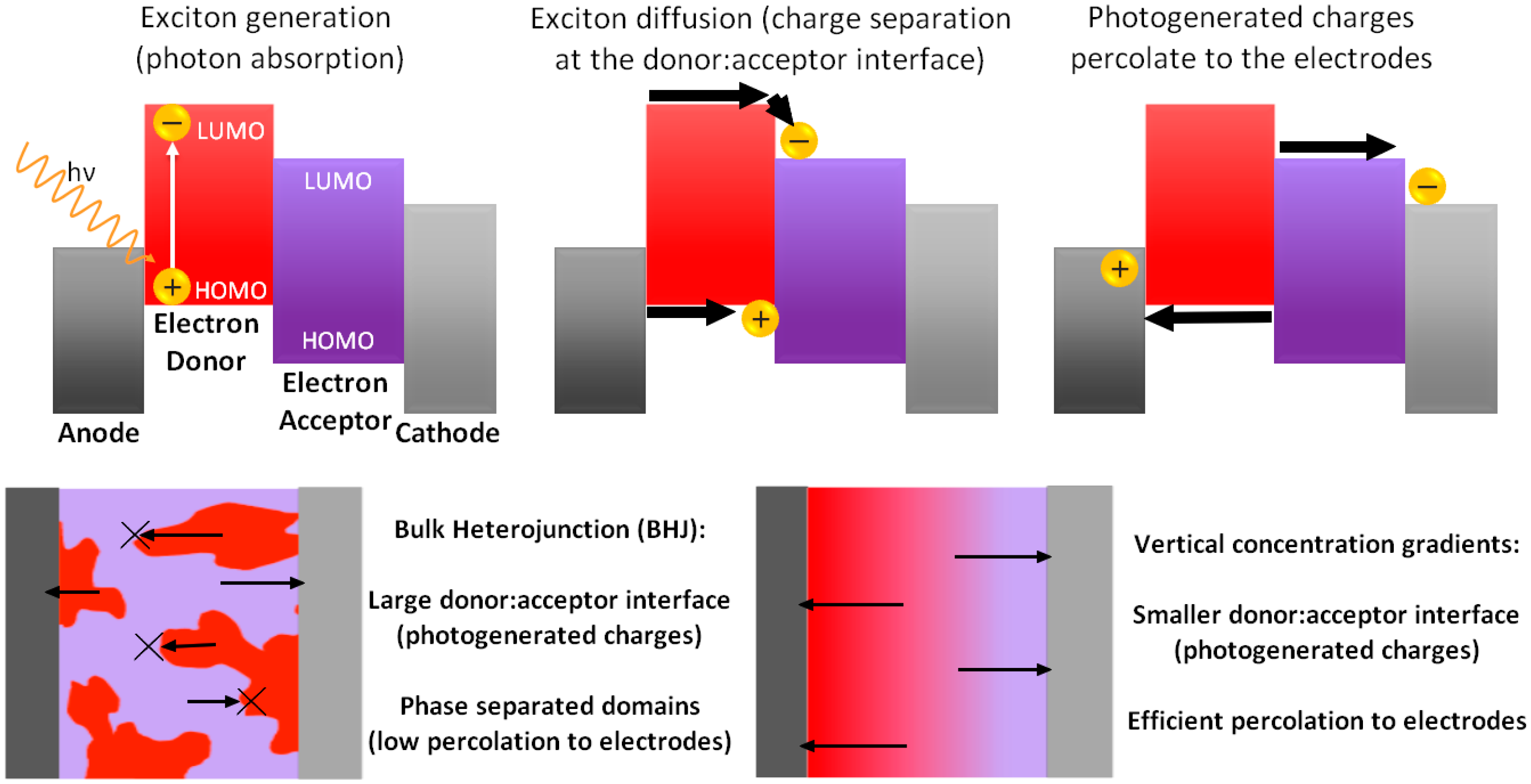
:1. Introduction
2. Generation of Vertical ED–EA Distribution in Single Active Layer PSCs
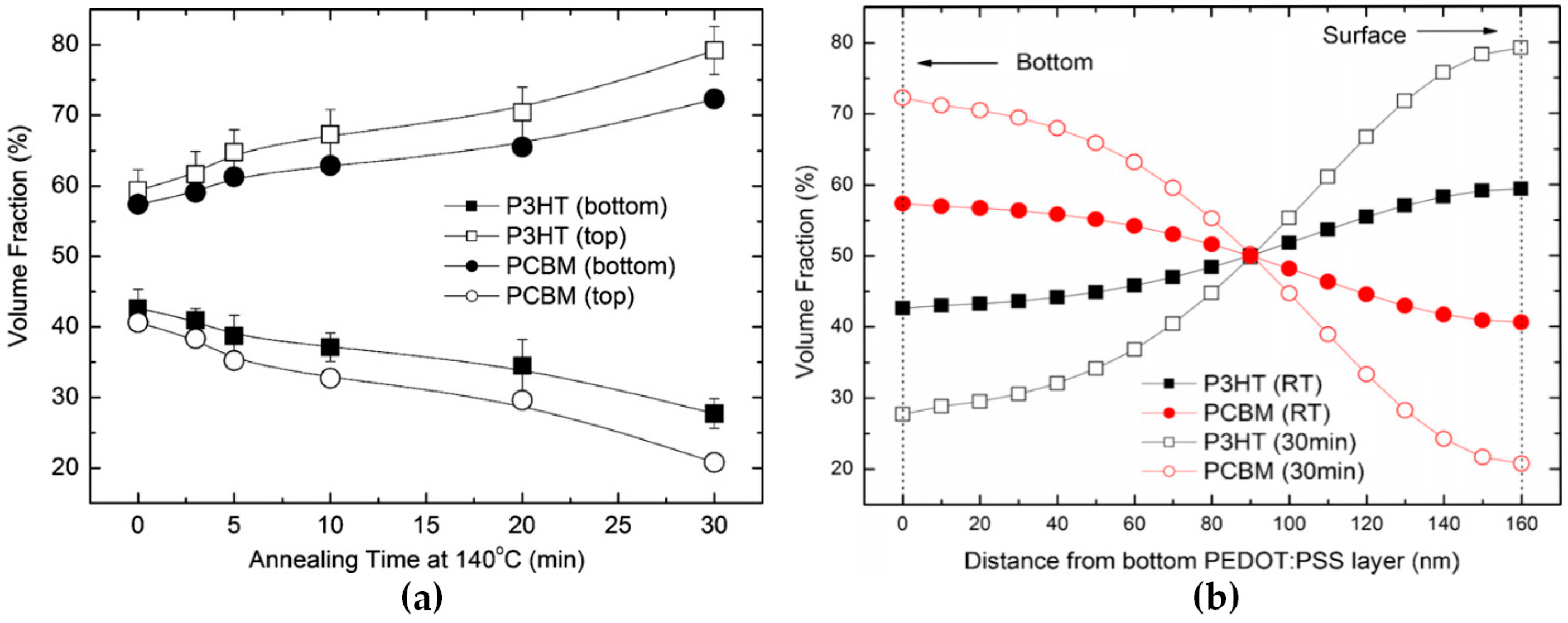
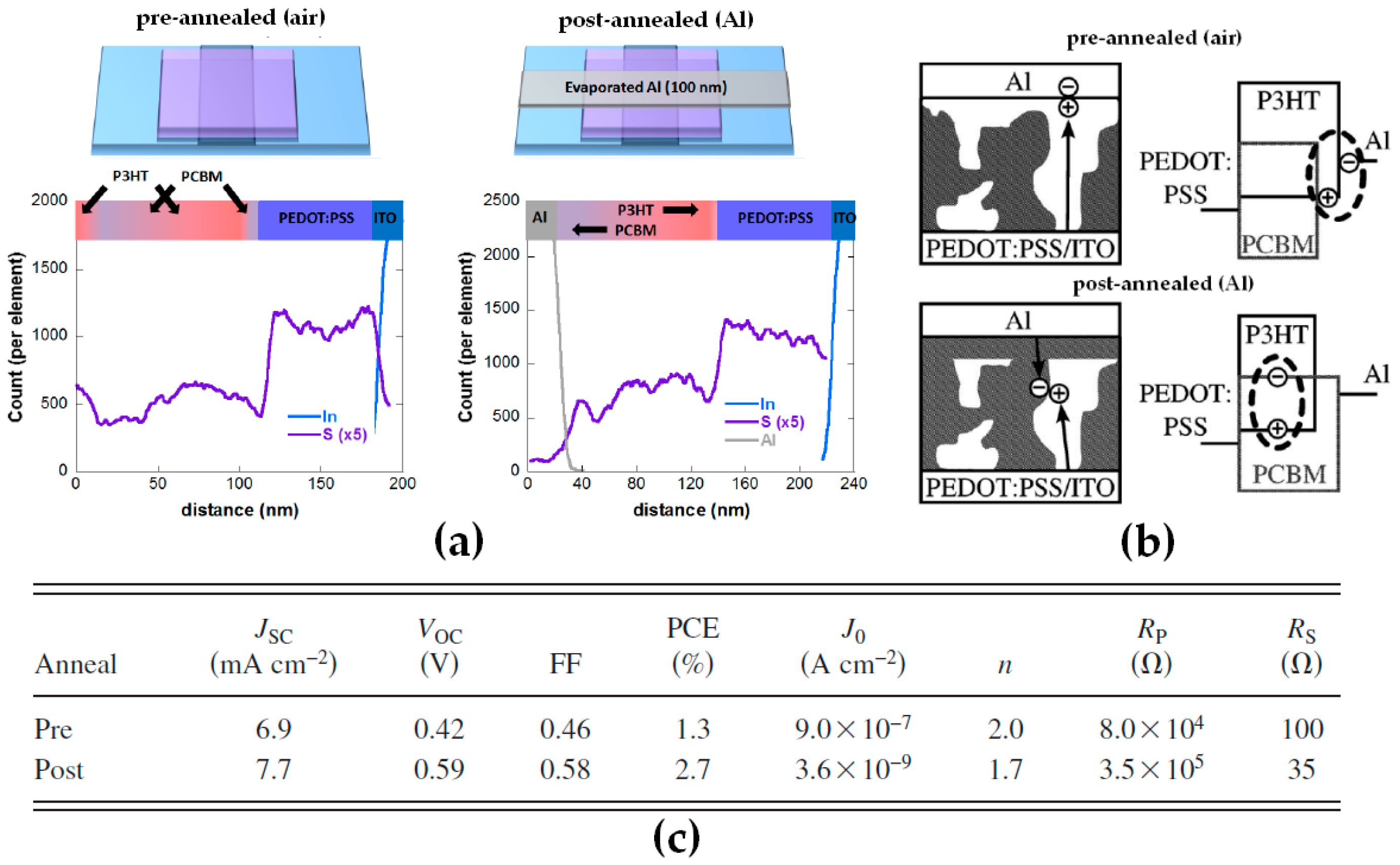
2.1. Effect of Thermal Annealing and Interfacial Interactions
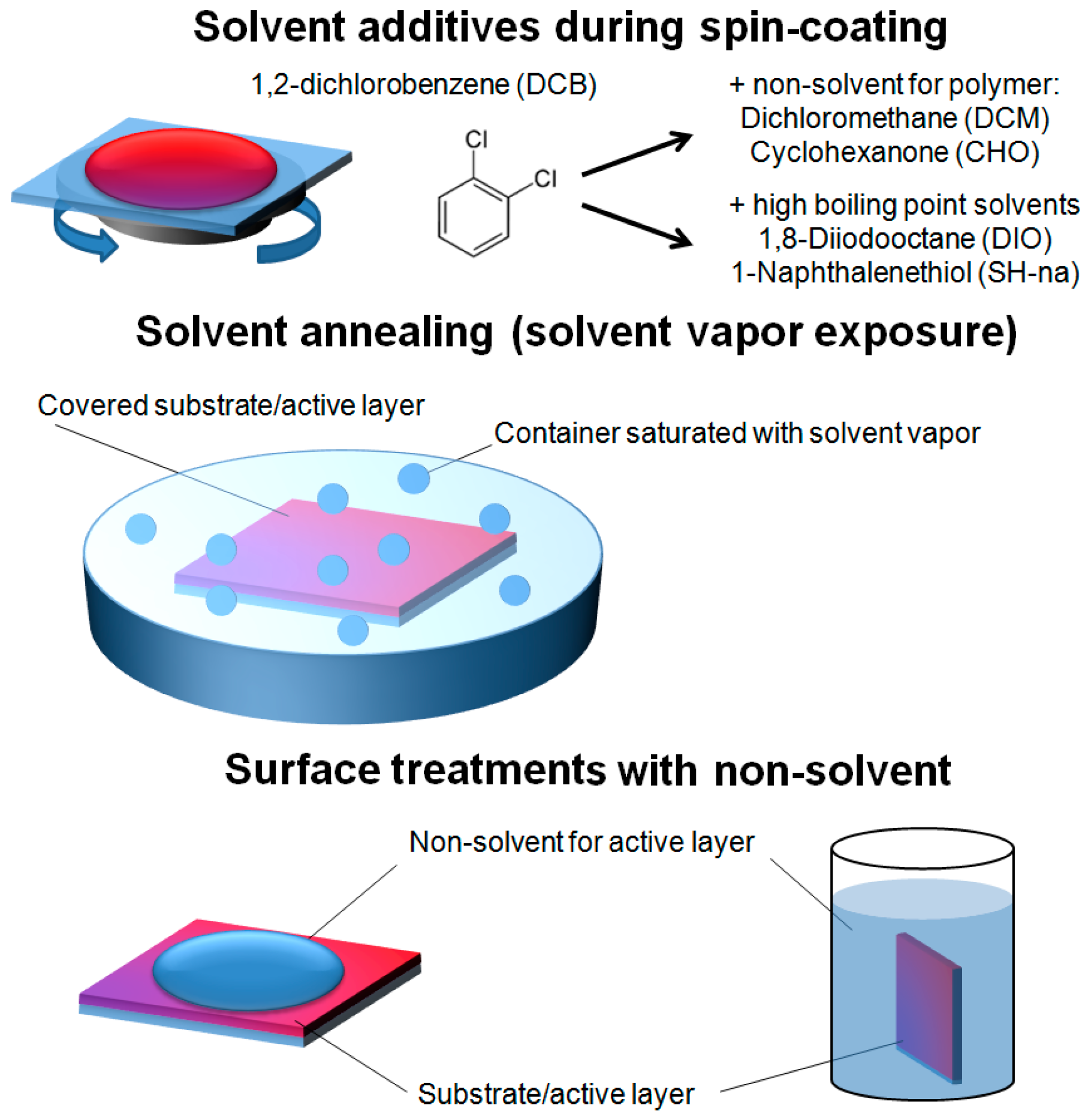
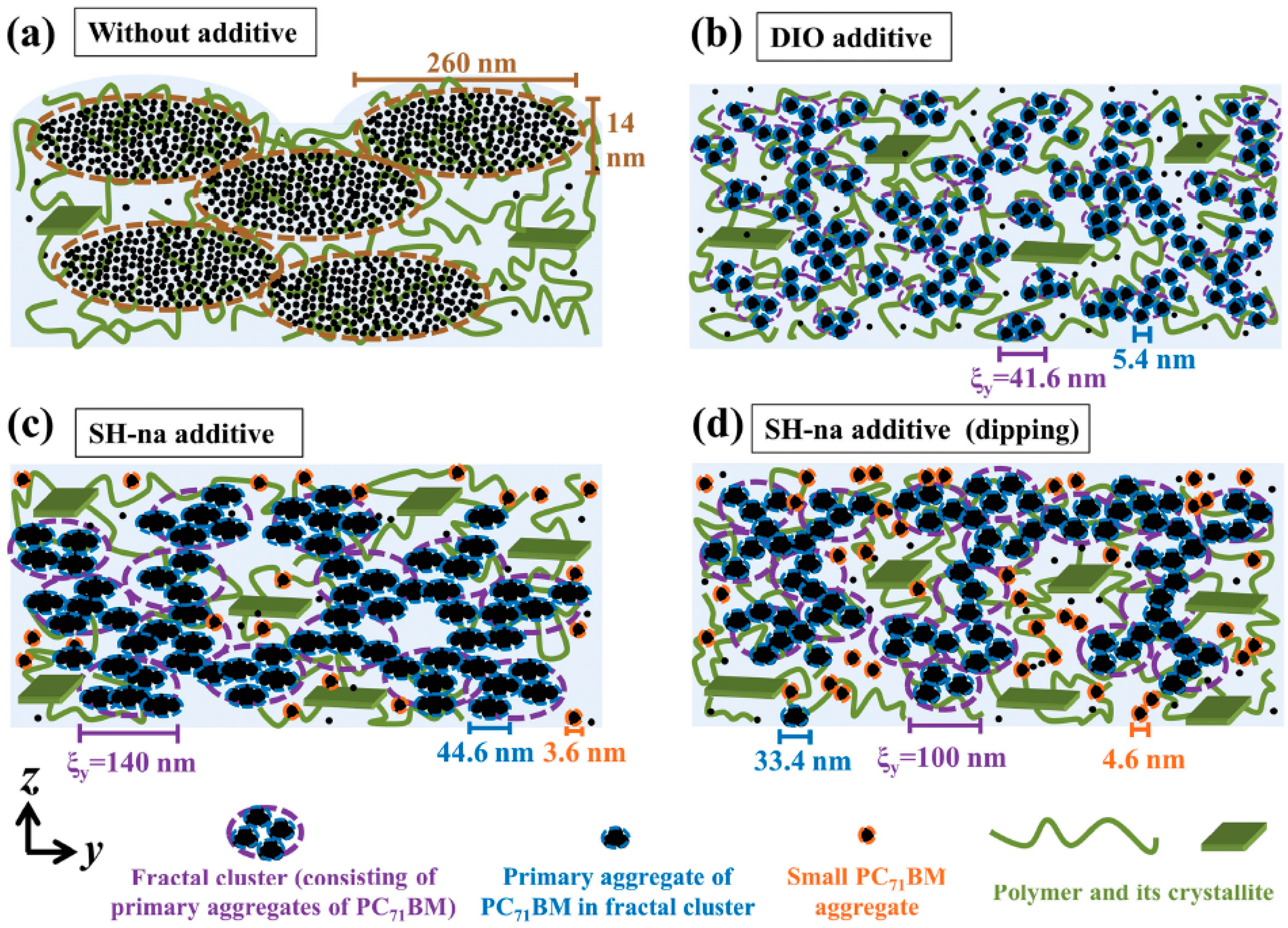
2.2. Solvent Assisted Vertical Molecular Distribution
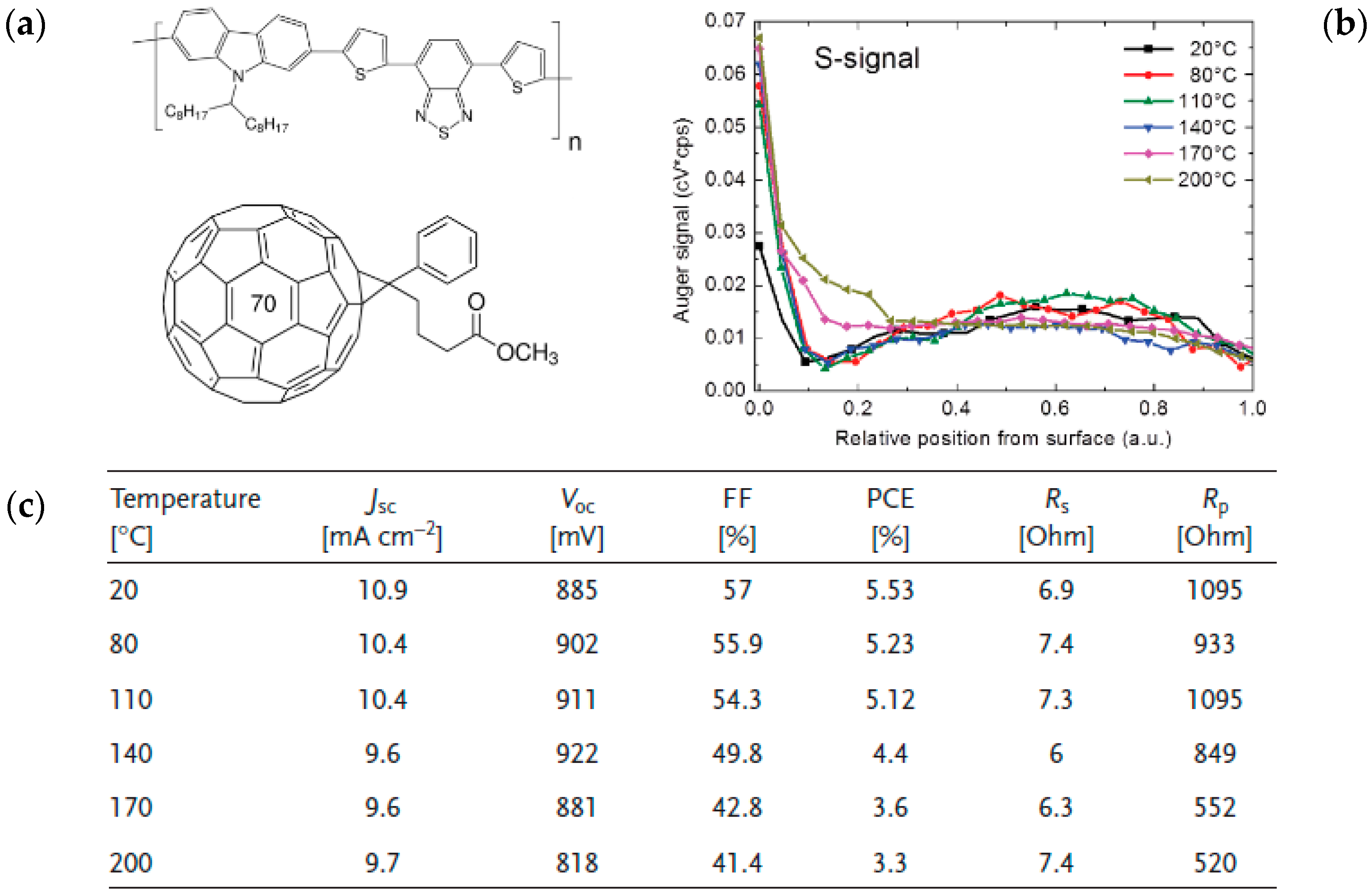
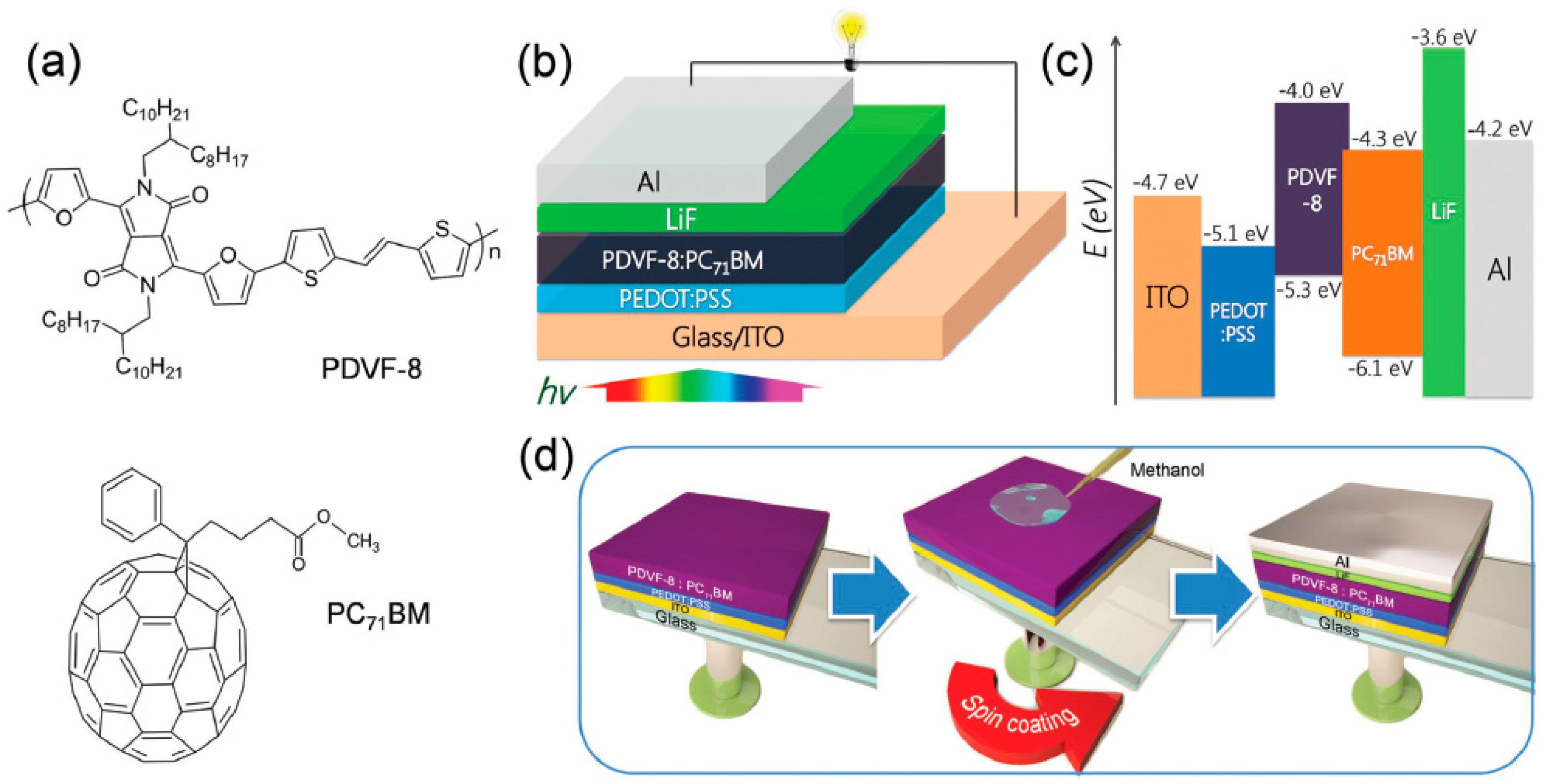
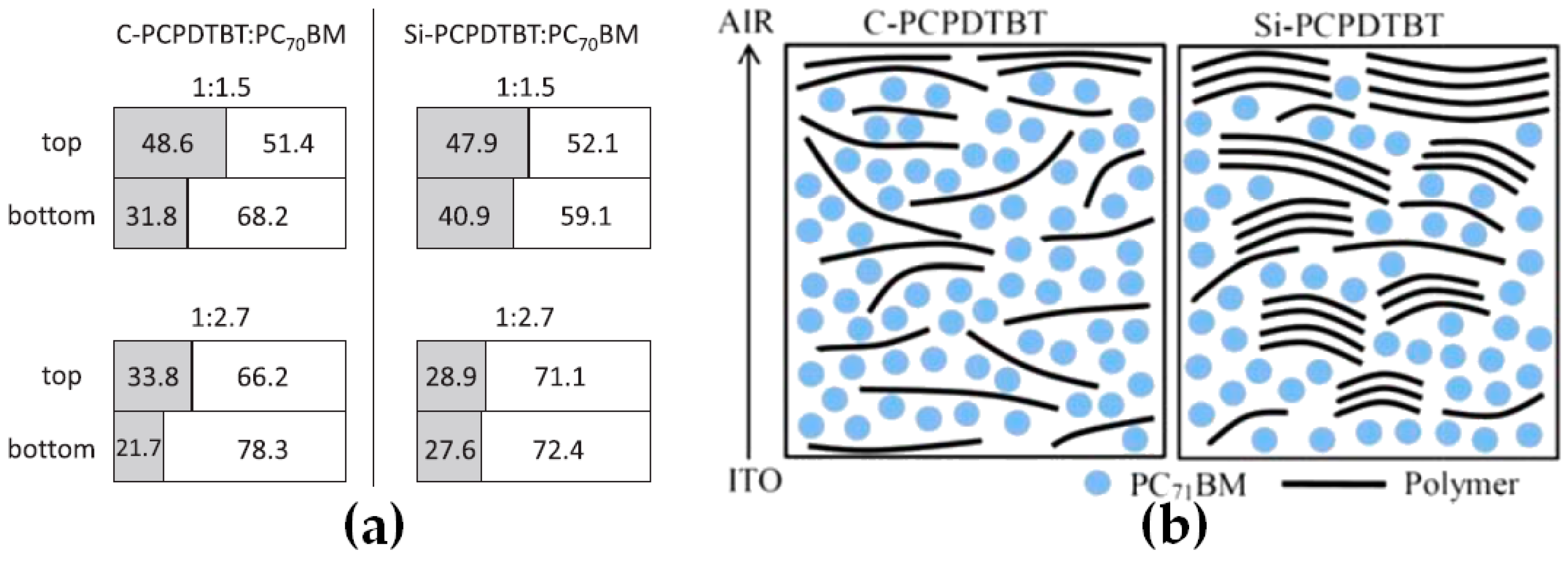
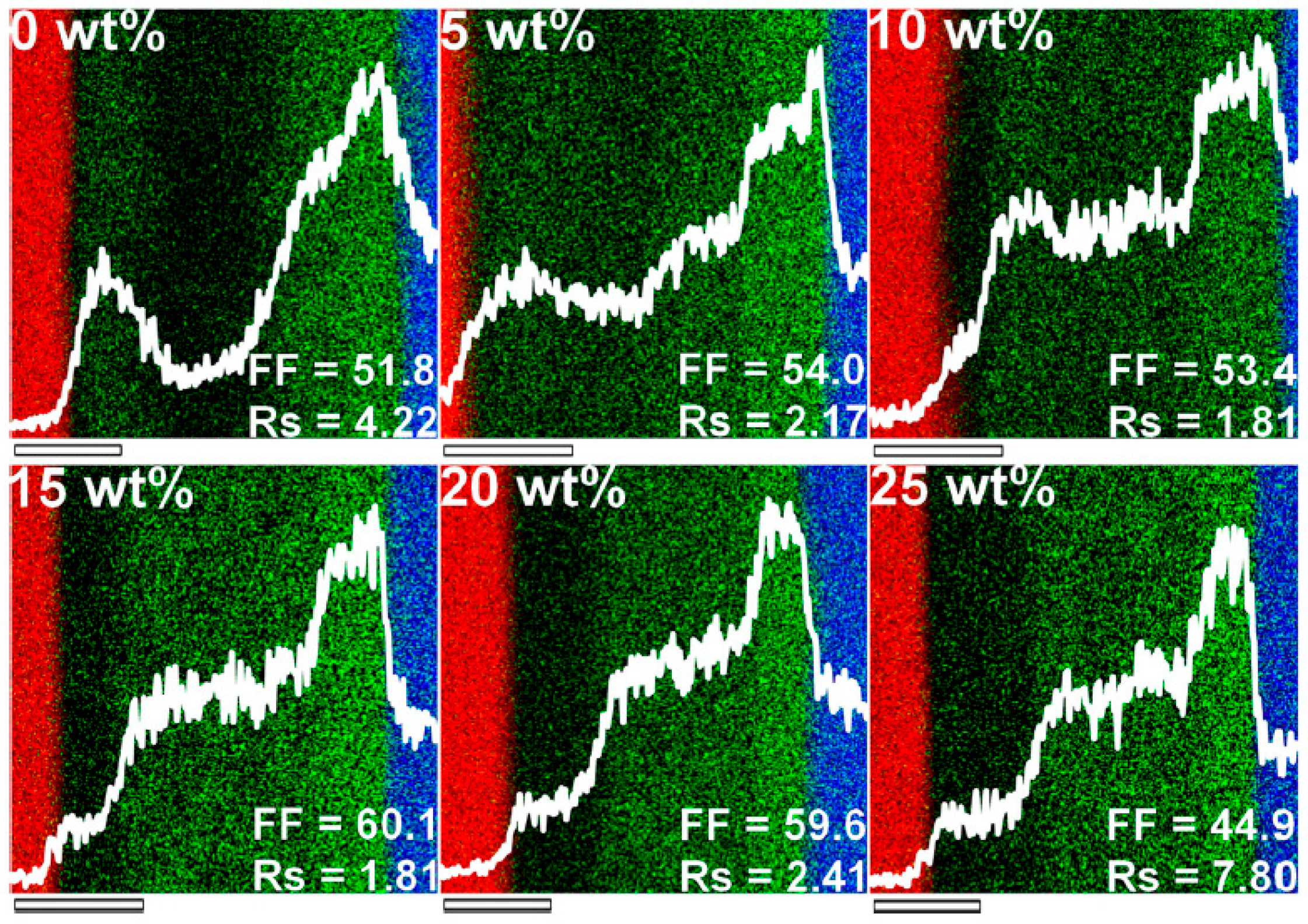
2.3. Chemical Modification of Active Materials to Induce Adequte Vertical Distribution
3. Sequential Deposition Processes to Fabricate Multilayer Active Layer PSCs
3.1. Fabrication Processes to Generate Inverted Multilayer Active Layers
3.2. Fabrication Processes to Generate Regular Multilayer Active Layers
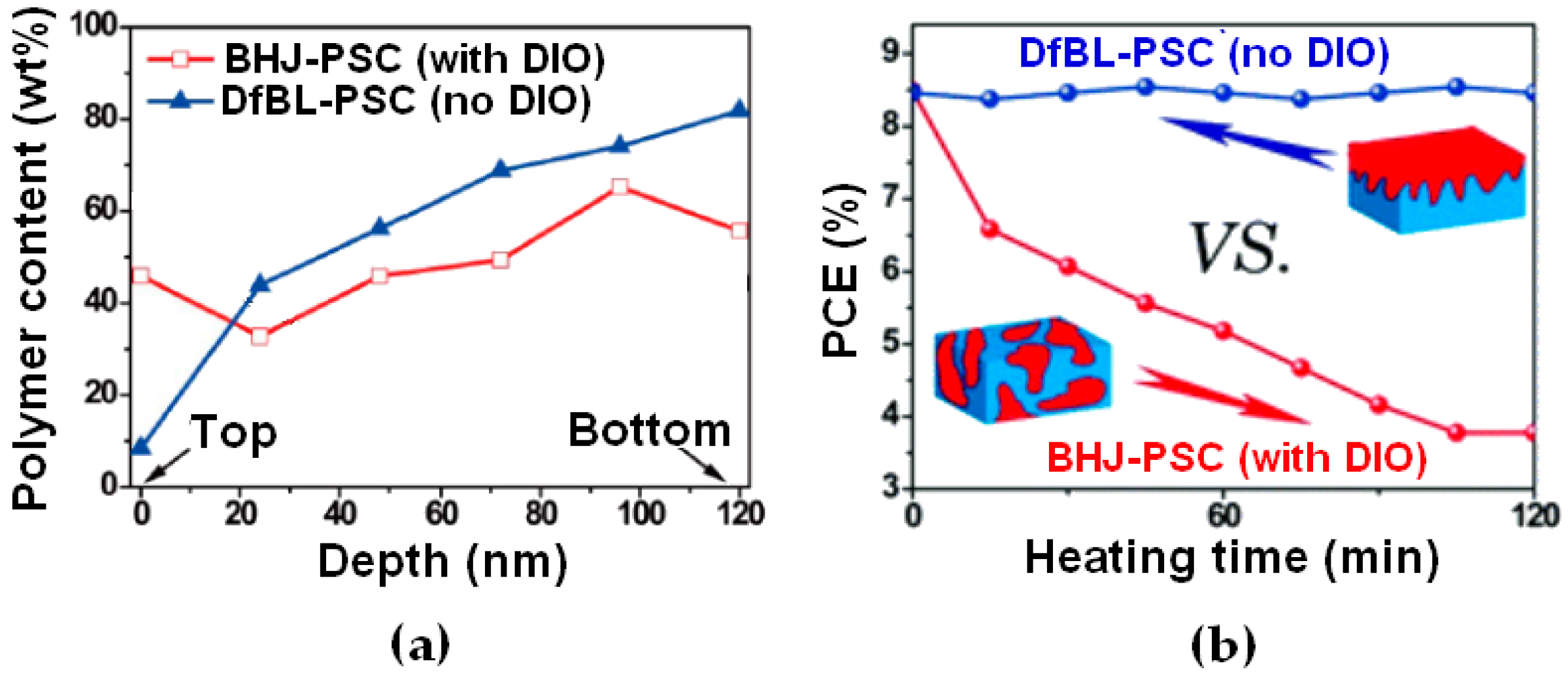
3.3. DfBL Active Layers for Regular Device Architectures: Alternative Deposition Process with the Potential to Overcome BHJ PSC Performances
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, C.W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Vohra, V.; Kawashima, K.; Kakara, T.; Koganezawa, T.; Osaka, I.; Takimiya, K.; Murata, H. Efficient inverted polymer solar cells employing favourable molecular orientation. Nat. Photonics 2015, 9, 403–408. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and morphology control enables multiple cases of high efficiency polymer solar cells. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Carpenter, J.H.; Li, C.Z.; Yu, J.S.; Ade, H.; Jen, A.K.Y. Highly efficient organic solar cells with improved vertical donor-acceptor compositional gradient via an inverted off-center spinning method. Adv. Mater. 2015, 28, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Cui, C.; Li, Y.Q.; Zhou, L.; Ou, Q.D.; Li, C.; Li, Y. Single-junction polymer solar cells exceeding 10% power conversion efficiency. Adv. Mater. 2014, 27, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Qian, D.; Zhang, S.; Li, S.; Ingäns, O.; Geo, F.; Hou, J. Fullerene-free polymer solar cells with over 11% efficiency and excellent thermal stability. Adv. Mater. 2016, 28, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Huang, L.; Yang, D.; Fu, P.; Zhou, L.; Zhang, J.; Li, C. Efficiency exceeding 10% for inverted polymer solar cells with a ZnO/ionic liquid combined cathode interfacial layer. J. Mater. Chem. A 2015, 3, 10660–10665. [Google Scholar] [CrossRef]
- Liu, C.; Yi, C.; Wang, K.; Yung, Y.; Bhatta, R.S.; Tsige, M.; Xiao, S.; Gomg, X. Single-junction polymer solar cells with over 10% efficiency by a novel Two-Dimensional donor-acceptor conjugated copolymer. Appl. Mater. Interfaces 2015, 7, 4928–4935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ye, L.; Zhao, W.; Yang, B.; Wang, Q.; Hou, J. Realizing over 10% efficiency in polymer solar cell by device optimization. Sci. China Chem. 2015, 58, 248–256. [Google Scholar] [CrossRef]
- Chen, C.C.; Dou, L.; Zhu, R.; Chung, C.H.; Song, T.B.; Zheng, Y.B.; Hawks, S.; Li, G.; Weiss, P.S.; Yang, Y. Visibly transparent polymer solar cells produced by solution processing. ACS Nano 2012, 6, 7185–7190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, Z.; Wu, Z.; Guan, G.; Pan, S.; Zhang, Y.; Li, H.; Deng, J.; Sun, B.; Peng, H. Weaving efficient polymer solar cell wires into flexible power textiles. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Guan, G.; Pan, S.; Zhu, Z.; Ren, D.; Peng, H. A lightweight polymer solar cell textile that functions when illuminated from either side. Angew. Chem. 2014, 126, 11755–11758. [Google Scholar] [CrossRef]
- Chen, L.M.; Hong, Z.; Li, G.; Yang, Y. Recent progress in polymer solar cells: Manipulation of polymer: Fullerene morphology and the formation of efficient inverted polymer solar cells. Adv. Mater. 2009, 21, 1434–1449. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.; Wang, T. Conjugated-polymer blends for organic photovoltaics: Rational control of vertical stratification for high performance. Adv. Mater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Padinger, F.; Rittberger, R.S.; Sariciftci, N.S. Effects of postproduction treatment on plastic solar cells. Adv. Funct. Mater. 2003, 13, 85–88. [Google Scholar] [CrossRef]
- Zhao, G.; He, Y.; Li, Y. 6.5% Efficiency of polymer solar cells based on poly(3-hexylthiophene) and indene-C60 bisadduct by device optimization. Adv. Mater. 2010, 22, 4355–4358. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.T.; Hirsch, L.; Wantz, G. P3HT:PCBM, best seller in polymer photovoltaic research. Adv. Mater. 2011, 23, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Etxebarria, I.; Ajuria, J.; Pacios, R. Solution-processable polymeric solar cells: A review on materials, strategies and cell architectures to overcome 10%. Org. Electron. 2015, 19, 34–60. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Vohra, V.; Razali, N.T.; Murata, H. Systematic study of organic solar cells cross-section by EDS to correlate donor-acceptor vertical concentration gradients and device performances. In Science and Applications of Tailored Nanostructures, 1st ed.; Di Sia, P., Ed.; One Central Press: Manchester, UK, 2017; pp. 128–147. [Google Scholar]
- Orimo, A.; Masuda, K.; Honda, S.; Benten, H.; Ito, S. Surface segregation at the aluminum interface of poly(3-hexylthiophene)/fullerene solar cells. Appl. Phys. Lett. 2010, 96, 043305. [Google Scholar] [CrossRef]
- Kuwabara, T.; Nakayama, T.; Uozumi, K.; Yamaguchi, T.; Takahashi, K. Highly durable inverted-type organic solar cell using amorphous titanium oxide as electron collection electrode inserted between ITO and organic layer. Sol. Energy Mater. Sol. C 2008, 92, 1476–1482. [Google Scholar] [CrossRef]
- Gao, B. Theoretical analysis and the morphology control of vertical phase segregation in high-efficiency polymer/fullerene solar cells. High Perfom. Polym. 2013, 26, 197–204. [Google Scholar] [CrossRef]
- Vohra, V.; Higsshimine, K.; Tsuzaki, S.; Ohdaina, K.; Murata, H. Formation of vertical concentration gradients in poly(3-hexylthiophene-2,5-diyl):Phenyl-C61-butyric acid methyl ester-graded bilayer solar cells. Thin Solid Films 2014, 554, 41–45. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, N.; Zheng, Y.; Wang, D.; Li, L.; Bo, Z.; Zhou, J.; Huo, H. Relation between morphology and performance parameters of poly(3-hexylthiophene):Phenyl-C61-butyric acid methyl ester photovoltaic devices. Org. Electron. 2016, 28, 189–196. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.M.; Yang, G.; Huang, C.H.; Hou, J.; Wu, Y.; Li, G.; Hsu, C.S.; Yang, Y. Vertical Phase Separation in Poly(3-hexylthiophene):Fullerene Derivative Blends and its Advantage for Inverted Structure Solar Cells. Adv. Funct. Mater. 2009, 19, 1227–1234. [Google Scholar] [CrossRef]
- Karagiannidis, P.G.; Georgiu, D.; Pitsalidis, C.; Laskarakis, A.; Logothetidis, S. Evolution of vertical phase separation in P3HT:PCBM thin films induced by thermal annealing. Mater. Chem. Phys. 2011, 129, 1207–1213. [Google Scholar] [CrossRef]
- Parnell, A.J.; Dunbar, A.D.F.; Pearson, A.J.; Staniec, P.A.; Dennison, A.J.C.; Hamamatsu, H.; Skoda, M.W.A.; Lidzey, D.G.; Jones, R.A.L. Depletion of PCBM at the cathode interface in P3HT/PCBM thin films as quantified via neutron reflectivity measurements. Adv. Mater. 2010, 22, 2444–2447. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choulis, S.A.; Nelson, J.; Bradley, D.D.C. Device annealing effect in organic solar cells with blends of regioregular poly(3-hexylthiophene) and soluble fullerene. Appl. Phys. Lett. 2005, 86. [Google Scholar] [CrossRef]
- Bavel, S.V.; Sourty, E.; With, G.D.; Frolic, K.; Loos, J. Relation between photoactive layer thickness, 3D morphology, and device performance in P3HT/PCBM bulk-heterojunction solar cells. Macromolecules 2009, 42, 7396–7403. [Google Scholar] [CrossRef]
- Nagai, M.; Wei, H.; Yoshida, Y. Thermally induced vertical phase separation and photovoltaic characteristics of polymer solar cells for P3HT/PCBM composites. Jpn. J. Appl. Phys. 2016, 55, 061601. [Google Scholar] [CrossRef]
- Berriman, G.A.; Holdsworth, J.L.; Zhou, X.; Belcher, W.J.; Dastoor, P.C. Molecular versus crystallite PCBM diffusion in P3HT:PCBM blends. AIP Adv. 2015, 5, 097220. [Google Scholar] [CrossRef]
- Kline, R.J.; Mcgehee, M.D. Morphology and charge transport in conjugated polymers. J. Macromol. Sci. Polym. Rev. 2006, 46, 27–45. [Google Scholar] [CrossRef]
- Xue, B.; Vaughan, B.; Poh, C.H.; Burke, K.B.; Thomsen, L.; Stapleton, A.; Zhou, X.; Bryant, G.W.; Belcher, W.; Dastoor, P.C. Vertical stratification and interfacial structure in P3HT:PCBM organic solar cells. J. Phys. Chem. C 2010, 114, 15797–15805. [Google Scholar] [CrossRef]
- Germack, D.S.; Chan, C.K.; Hmadani, B.H.; Richter, L.J.; Fischer, D.A. Substrate-dependent interface composition and charge transport in films for organic photovoltaics. Appl. Phys. Lett. 2009, 94, 233303. [Google Scholar] [CrossRef]
- Kovalenko, A.; Stoyanova, D.; Pospisil, J.; Zhivkov, I.; Fekete, L.; Karashanova, D.; Kratochvílová, I.; Vala, M.; Weiter, M. Morphology versus vertical phase segregation in solvent annealed small molecule bulk heterojunction organic solar cells. Int. J. Photoenergy 2015, 2015, 238981. [Google Scholar] [CrossRef]
- Borges, B.G.A.L.; Marchiori, C.F.N.; Glaser, M.; Garcia-Basabe, Y.; Moura, C.E.V.; Rocha, A.B.; Roman, S.L.; Chassé, T.; Casu, M.B. Electronic and structural properties in thermally annealed PSiF-DBT:PC71BM blends for organic photovoltaics. Thin Solid Films 2016, 615, 165–170. [Google Scholar] [CrossRef]
- Synooka, O.; Eberhardt, K.; Singh, C.R.; Hermann, F.; Eche, G.; Echer, B.; Hauff, E.V.; Gobsch, G.; Hoppe, H. Influence of thermal annealing on PCDTBT:PCBM composition profiles. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, C.; Xie, H.; Li, Y.; Duhm, S.; Tang, J. Unraveling the role of substrates on interface energetics and morphology of PCDTBT:PC70BM bulk heterojunction. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Wang, T.; Scarratt, N.W.; Yi, H.; Coleman, I.F.; Zhang, Y.; Grant, R.T.; Yao, J.; Skoda, M.W.A.; Dunber, A.D.F.; Jones, R.A.L.; et al. Vertical stratification and its impact on device performance in a polycarbazole based copolymer solar cells. J. Phys. Chem. C 2015, 3, 4007–4015. [Google Scholar] [CrossRef]
- Blouin, N.; Michaud, A.; Leclerc, M. A low-bandgap poly(2,7-Carbazole) derivative for use in high-performance solar cells. Adv. Mater. 2007, 19, 2295–2300. [Google Scholar] [CrossRef]
- Park, S.H.; Roy, A.; Beaupré, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photonics 2009, 3, 297–302. [Google Scholar] [CrossRef]
- Moon, J.S.; Jo, J.; Heeger, A.J. Nanomorphology of PCDTBT:PC70BM bulk heterojunction solar cells. Adv. Energy Mater. 2012, 2, 304–308. [Google Scholar] [CrossRef]
- Liu, J.; Shao, S.; Fang, G.; Meng, B.; Xie, Z.; Wang, L. High-efficiency inverted polymer solar cells with transparent and work-function tunable MoO3-Al composite film as cathode buffer layer. Adv. Mater. 2012, 24, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, F.; Li, L.; Yu, J.; Wang, J.; An, Q.; Tang, W. Tuning nanoscale morphology using mixed solvents and solvent vapor treatment for high performance polymer solar cells. RSC Adv. 2014, 4, 48724–48733. [Google Scholar] [CrossRef]
- Shao, M.; Keum, J.K.; Kumar, R.; Chen, J.; Browning, J.F.; Das, S.; Chen, W.; Hou, J.; Do, C.; Littrell, K.C.; et al. Understanding how processing additives tune the nanoscale morphology of high efficiency organic photovoltaic blends: From casting solution to spun-cast thin film. Adv. Funct. Mater. 2014, 24, 6647–6657. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, S.; Su, Y.; Wang, H.; Ye, L.; Tsang, S.; Xie, F.; Xu, J. Enhanced efficiency of organic solar cells by mixed orthogonal solvents. Org. Electron. 2014, 15, 2007–2013. [Google Scholar] [CrossRef]
- Nair, S.S.; Kumar, D.; Majumder, A. A study on the effect of cosolvent addition pathway on the optical and morphological properties of P3HT:PCBM composite films. Adv. Polym. Technol. 2014, 33. [Google Scholar] [CrossRef]
- Song, T.; Wu, Z.; Tu, Y.; Jin, Y.; Sun, B. Vertical phase segregation of hybrid poly(3-hexylthiophene) and fullerene derivative composites controlled via velocity of solvent drying. Semicond. Sci. Technol. 2011, 26. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, H.; Lv, J.; Chen, Y.; Zhang, W.; Yu, G.; Li, F. Improving the efficiency of polymer solar cells based on furan-flanked diketopyrrolopyrrole copolymer via solvent additive and methanol treatment. Nanoscale 2015, 7, 15945–15952. [Google Scholar] [CrossRef] [PubMed]
- Jhuo, H.; Liao, S.; Li, Y.; Yeh, P.; Chen, S.; Wu, W.; Su, C.; Lee, J.; Yamada, N.L.; Jeng, U. The novel additive 1-naphthalenethiol opens a new processing route to efficiency-enhanced polymer solar cells. Adv. Funct. Mater. 2016, 26, 3094–3104. [Google Scholar] [CrossRef]
- Guo, B.; Zhou, W.; Wu, M.; Lv, J.; Yu, C.; Li, F.; Hu, Z. Improving the efficiency of polymer solar cells via a treatment of methanol:water on the active layers. J. Mater. Chem. A 2016, 4, 9644–9652. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, M.; Ma, W.; Ye, L.; Zhang, S.; Liu, S.; Ade, H.; Huang, F.; Hou, J. Enhanced photovoltaic performance by modulating surface composition in bulk heterojunction polymer solar cells based on PBDTTT-C-T/PC71BM. Adv. Mater. 2014, 26, 4043–4049. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, Q.; Wang, H.; Li, M.; Han, Y.; Xie, Z.; Wang, L. Improving the morphology of PCDTBT:PC70BM bulk heterojunction by mixed-solvent vapor-assisted imprinting: Inhibiting intercalation, optimizing vertical phase separation, and enhancing photon absorption. J. Phys. Chem. C 2014, 118, 4585–4595. [Google Scholar] [CrossRef]
- Kokubu, R.; Yang, Y. Vertical phase separation of conjugated polymer and fullerene bulk heterojunction films induced by high pressure carbon dioxide treatment at ambient temperature. Phys. Chem. Chem. Phys. 2012, 14, 8313–8318. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, D.; Laskarakis, A.; Morana, M.; Karagiannidis, P.G.; Logothetidis, S. Non-destructive optical characterization of phase separation in bulk heterojunction organic photovoltaic cells. Sol. Energy Mater. Sol. C 2014, 125, 190–197. [Google Scholar] [CrossRef]
- Lin, R.; Wright, M.; Puthen-Veettil, B.; Wen, X.; Tayebjee, M.J.Y.; Uddin, A. Effects of blend composition on the morphology of Si-PCPDTBT:PC71BM bulk heterojunction organic solar cells. Phys. Status Solidi 2015, 212, 1931–1940. [Google Scholar] [CrossRef]
- Busby, Y.; List-Kratochvil, E.J.W.; Pireaux, J. Chemical analysis of the interface in bulk-heterojunction solar cells by X-ray photoelectron spectroscopy depth profiling. ACS Appl. Mater. Interfaces 2017, 9, 3842–3848. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Wright, M.; Gong, B.; Chan, K.H.; Tayebjee, M.J.Y.; Uddin, A. Influence of bridging atom on the vertical phase separation of low band gap bulk heterojunction solar cells. Phys. Status Solidi 2014, 8, 904–907. [Google Scholar] [CrossRef]
- Sun, Y.; Pitliya, P.; Liu, C.; Gong, X.; Raghavan, D.; Karim, A. Block copolymer compatibilized polymer: Fullerene blend morphology and properties. Polymer 2017, 113, 135–146. [Google Scholar] [CrossRef]
- Anselmo, A.S.; Lindgren, L.; Rysz, J.; Bernasik, A.; Budkowski, A.; Andersson, M.R.; Svensson, K.; Stam, J.V.; Moons, E. Tuning the vertical phase separation in polyfluorene:fullerene blend films by polymer functionalization. Chem. Mater. 2011, 23, 2295–2302. [Google Scholar] [CrossRef]
- Vohra, V.; Higashimine, K.; Ohdaira, K.; Tsuzaki, S.; Murata, H. Efficient organic devices based on π-electron systems: Comparative study of fullerene derivatives blended with a high efficiency naphthobisthiadiazole-based polymer for organic photovoltaic applications. In Chemical Science of π-Electron Systems, 1st ed.; Akasaka, T., Osuka, A., Fukuzumi, S., Kandori, H., Aso, Y., Eds.; Springer: Tokyo, Japan, 2015; pp. 575–588. [Google Scholar]
- Susarova, D.K.; Troshin, P.A.; Moskvin, Y.L.; Babenko, S.D.; Razumov, V.F. Vertical concentration gradients in bulk heterojunction solar cells induced by differential material solubility. Thin Solid Films 2011, 519, 4132–4135. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, X.; Zheng, L.; Chen, Z.; Xiao, L.; Qu, B.; Gong, Q. Vertical phase separation in bulk heterojunction solar cells formed by in situ polymerization of fulleride. Sci. Rep. 2014, 4, 5071. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.; Chao, Y.; Fang, J.; Tseng, W.; Lan, Y.; Chen, Y.; Wu, K.; Chen, M. Origins of vertical phase separation in P3HT:PCBM mixed films. Jpn. J. Appl. Phys. 2014, 53. [Google Scholar] [CrossRef]
- Treat, N.D.; Brady, M.A.; Smith, G.; Toney, M.F.; Kramer, E.J.; Hawker, C.J.; Chabinyc, M.L. Interdiffusion of PCBM and P3HT reveals miscibility in a photovoltaically active blend. Adv. Energy Mater. 2011, 1, 82–89. [Google Scholar] [CrossRef]
- Chang, J.; Wang, H.; Lin, W.; Chiang, K.; Chen, K.; Huang, W.; Huang, Z.; Meng, H.; Ho, R.; Lin, H. Efficient inverted quasi-bilayer organic solar cells fabricated by using non-halogenated solvent processes. J. Mater. Chem. A 2014, 2, 13398–13406. [Google Scholar] [CrossRef]
- Hau, S.K.; Yip, H.; Acton, O.; Beak, N.S.; Ma, H.; Jen, A.K.Y. Interfacial modification to improve inverted polymer solar cells. J. Mater. Chem. 2008, 18, 5113–5119. [Google Scholar] [CrossRef]
- Cho, S.; Kim, K.; Heo, J.; Lee, J.Y.; Cha, G.; Seo, B.Y.; Kim, Y.D.; Kim, Y.S.; Choi, S.; Lim, D.C. Role of additional PCBM layer between ZnO and photoactive layers in inverted bulk-heterojunction solar cells. Sci. Rep. 2014, 4, 4306. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lee, C.; Lee, H. Performance improvement mechanisms of P3HT:PCBM inverted polymer solar cells using extra PCBM and extra P3HT interfacial layers. Org. Electron. 2015, 21, 126–131. [Google Scholar] [CrossRef]
- Kim, J.B.; Guan, Z.; Lee, S.; Pavlopoulou, E.; Toney, M.F.; Kahn, A.; Loo, Y. Modular construction of P3HT/PCBM planar-heterojunction solar cells by lamination allows elucidation of processing-structure-function relationships. Org. Electron. 2011, 12, 1963–1972. [Google Scholar] [CrossRef]
- Tada, A.; Geng, Y.; Wei, Q.; Hashimoto, K.; Tajima, K. Tailoring organic heterojunction interfaces in bilayer polymer photovoltaic devices. Nat. Mater. 2011, 10, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, W.; Wang, D.H.; Lee, H.; Cho, S.M.; Choi, D.; Park, J.H. Layer-by-Layer all-transfer-based organic solar cells. Langmuir 2013, 29, 5377–5382. [Google Scholar] [CrossRef] [PubMed]
- Haung, J.; Ho, Z.; Kuo, T.; Kekuda, D.; Chu, C.; Ho, K. Fabrication of multilayer organic solar cells through a stamping technique. J. Mater. Chem. 2009, 19, 4077–4080. [Google Scholar] [CrossRef]
- Vohra, V.; Anzai, T.; Inaba, S.; Porzio, W.; Barba, L. Transfer-printing of active layers to achieve high quality interfaces in sequentially deposited multilayer inverted polymer solar cells fabricated in air. Sci. Technol. Adv. Mater. 2016, 17, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Choi, D.; Lee, K.; Park, O.O.; Park, J.H. Photovoltaic devices with an active layer from a stamping transfer technique: Single layer versus double layer. Langmuir 2010, 26, 9584–9588. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; Chen, F.; Li, J.; Huang, A.T.; Huang, J.; Ho, K.; Chu, C. Efficient organic optoelectronics with multilayer structures. J. Mater. Chem. 2012, 22, 1364–1369. [Google Scholar] [CrossRef]
- Wei, H.; Huang, J.; Ho, K.; Chu, C. A strategic buffer layer of polythiophene enhances the efficiency of bulk heterojunction solar cells. Appl. Mater. Interfaces 2010, 2, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xing, X.; Chen, Z.; Xiao, L.; Qu, B. Highly efficient polymer solar cells by using the homogeneous self-assembly of a sulphydryl-capped photoactive polymer covalently bound to the anode. Eng. Technol. 2013, 1, 613–616. [Google Scholar] [CrossRef]
- Liang, C.; Su, W.; Wang, L. Enhancing the photocurrent in poly(3-hexylthiophene)/[6,6]-phenyl C61butyric acid methyl ester bulk heterojunction solar cells by using poly(3-hexylthiophene) as a buffer layer. Appl. Phys. Lett. 2009, 95, 133303. [Google Scholar] [CrossRef]
- Vaughan, B.; Stapleton, A.; Sesa, E.; Holmes, N.P.; Zhou, X.; Dastoor, P.C.; Belcher, W.J. Engineering vertical morphology with nanoparticulate organic photovoltaic devices. Org. Electron. 2016, 32, 250–257. [Google Scholar] [CrossRef]
- Haung, J.; Li, K.; Kekuda, D.; Padhy, H.H.; Lin, H.; Ho, K.; Chu, C. Efficient bilayer polymer solar cells possessing planar mixed-heterojunction structures. J. Mater. Chem. 2010, 20, 3295–3300. [Google Scholar] [CrossRef]
- Kumar, A.; Li, G.; Hong, Z.; Yang, Y. High efficiency polymer solar cells with vertically modulated nanoscale morphology. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Villers, B.T.D.; Tassone, C.J.; Tolbert, S.H.; Schwartz, B.J. Improving the reproducibility of P3HT:PCBM solar cells by controlling the PCBM/Cathode interface. J. Phys. Chem. C 2009, 113, 18978–18982. [Google Scholar] [CrossRef]
- Lai, Y.; Shih, P.; Li, Y.; Tsai, C.; Wu, J.; Cheng, Y.; Hsu, C. Interface engineering to enhance the efficiency of conventional polymer solar cells by alcohol-/water-soluble C60 materials doped with alkali carbonates. Appl. Mater. Interfaces 2013, 5, 5122–5128. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qi, Z.; Wang, J. Layer-by-layer processed polymer solar cells with self-assembled electron buffer layer. Appl. Phys. Lett. 2013, 102, 213901. [Google Scholar] [CrossRef]
- Ayzner, A.L.; Tassone, C.J.; Tolbert, S.H.; Schwartz, B.J. Reappraising the need for bulk heterojunctions in polymer-fullerene photovoltaics: The role of carrier transport in all-solution-processed P3HT/PCBM bilayer solar cells. J. Phys. Chem. C 2009, 113, 20050–20060. [Google Scholar] [CrossRef]
- Yim, J.H.; Joe, S.; Nguyen, D.C.; Ryu, S.Y.; Ha, N.Y.; Ahn, Y.H.; Park, J.; Lee, S. True nature of active layers in organic solar cells fabricated by sequential casting of donor and acceptor layers. Phys. Status Solibi 2017, 11, 1600415. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Wang, J. Optimizing performance of layer-by-layer processed polymer solar cells. Appl. Phys. Lett. 2012, 101, 033907. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Li, Y.F.; Wang, J. Tunable open-circuit voltage in ternary organic solar cells. Appl. Phys. Lett. 2012, 101, 163302. [Google Scholar] [CrossRef]
- Thummalakunta, L.N.S.A.; Yong, C.H.; Ananthanarayanan, K.; Luther, J. P3HT based solution-processed pseudo bi-layer organic solar cell with enhanced performance. Org. Electron. 2012, 13, 2008–2016. [Google Scholar] [CrossRef]
- Xie, L.; Lee, J.S.; Jang, Y.; Ahn, H.; Kim, Y.; Kim, K. Organic photovoltaics utilizing a polymer nanofiber/fullerene interdigitated bilayer prepared by sequential solution deposition. J. Phys. Chem. C 2016, 120, 12933–12940. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Wang, H.; Nordland, D.; Sun, Z.; Ferdous, S.; Russell, T.P. Sequential deposition: Optimization of solvent swelling for high-performance polymer solar cells. Appl. Mater. Interfaces 2015, 7, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Hou, J.; Li, Y.; Zhen, X. Layer-by-layer solution-processed low-bandgap polymer-PC61BM solar cells with high efficiency. Adv. Energy Mater. 2014, 4, 1301349. [Google Scholar] [CrossRef]
- Seak, J.; Shin, T.J.; Park, S.; Cho, C.; Lee, J.; Ryu, D.Y.; Kim, M.H.; Kim, K. Efficient organic photovoltaics utilizing nanoscale heterojunctions in sequentially deposited polymer/fullerene bilayer. Sci. Rep. 2015, 5, 8373. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Yan, C.; Wu, Y.; Dai, S.; Ma, W.; Zhan, X. Efficient and stable organic solar cells via sequential process. J. Mater. Chem. C 2016, 4, 8086–8093. [Google Scholar] [CrossRef]
- Lee, K.H.; Schwenn, P.E.; Smith, A.R.G.; Cavaye, H.; Shaw, P.E.; James, M.; Krueger, K.B.; Gentle, I.R.; Meredith, P.; Burn, P.L. Morphology of all-solution-processed “bilayer” organic solar cells. Adv. Mater. 2011, 23, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Takacs, C.J.; Sun, Y.; Heeger, A.J. Spontaneous formation of bulk heterojunction nanostructures: Multiple routes to equivalent morphologies. Nano Lett. 2011, 11, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Hawks, S.A.; Aguirre, J.C.; Schelhas, L.T.; Thompson, R.J.; Huber, R.C.; Ferreira, A.S.; Zhang, G.; Herzing, A.A.; Tolbert, S.H.; Schwartz, B.J. Comparing matched polymer: Fullerene solar cells made by solution-sequential processing and traditional blend casting: Nanoscale structure and device performance. J. Phys. Chem. C 2014, 118, 17413–17425. [Google Scholar] [CrossRef]
- Zhang, G.; Huber, R.C.; Ferreira, A.S.; Boyd, S.D.; Luscombe, C.K.; Tolbert, S.H.; Schwartz, B.J. Crystallinity effects in sequentially processed and blend-cast bulk-heterojunction polymer/fullerene photovoltaics. J. Phys. Chem. C 2014, 118, 18424–18435. [Google Scholar] [CrossRef]
- Rochester, C.W.; Mauger, S.A.; Moulé, A.J. Investigating the morphology of polymer/fullerene layers coated using orthogonal solvents. J. Phys. Chem. C 2012, 116, 7283–7292. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Y.; Tang, A.; Deng, Z.; Teng, F. Investigating the reduction in the absorption intensity of P3HT in polymer/fullerene “bilayers” coated using orthogonal solvents. J. Appl. Polym. Sci. 2015, 132, 41757. [Google Scholar] [CrossRef]
- Vohra, V.; Higashimine, K.; Murakami, T.; Murata, H. Addition of regiorandom poly(3-hexylthiophene) to solution processed poly(3-hexylthiophene):[6,6]-phenyl-C61-butyric acid methyl ester graded bilayers to tune the vertical concentration gradient. Appl. Phys. Lett. 2012, 101, 173301. [Google Scholar] [CrossRef]
- Vohra, V.; Arrighetti, G.; Barba, L.; Higashimine, K.; Porzio, W.; Murata, H. Enhanced vertical concentration gradient in rubbed P3HT:PCBM graded bilayer solar cells. J. Phys. Chem. C 2012, 3, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Vohra, V.; Dörling, B.; Higashimine, K.; Murata, H. Investigating the effect of solvent boiling temperature on the active layer morphology of diffusive bilayer solar cells. Appl. Phys. Express 2015, 9. [Google Scholar] [CrossRef]
- Jang, Y.; Seo, J.; Seok, J.; Lee, J.; Kim, K. Roughening conjugated polymer surface for enhancing the charge collection efficiency of sequentially deposited polymer/fullerene photovoltaics. Polymers 2015, 7, 1497–1509. [Google Scholar] [CrossRef]
- Vohra, V.; Campoy-Quiles, M.; Garriga, M.; Murata, H. Organic solar cells based on nanoporous P3HT obtained from self assembled P3HT:PS templates. J. Mater. Chem. C 2012, 22, 20017–20025. [Google Scholar] [CrossRef]
- Lee, J.; Jung, Y.K.; Lee, D.Y.; Jang, J.; Cho, S.; Son, S.; Jeong, J.; Park, S.H. Enhanced efficiency of bilayer polymer solar cells by the solvent treatment method. Synth. Met. 2015, 199, 408–412. [Google Scholar] [CrossRef]
- Loiudice, A.; Rizzo, A.; Biasiucci, M.; Gigli, G. Bulk heterojunction versus diffused bilayer: The role of device geometry in solution p-doped polymer-based solar cells. J. Phys. Lett. 2012, 3, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Syu, Y.; Huang, P.; Li, H.; Hsu, C.; Chiu, K.; Kim, C.; Chen, M.; Chao, Y. Enhanced performance of pseudo-bilayer organic photovoltaic devices via small molecule doping. J. Phys. Chem. C 2014, 118, 9958–9965. [Google Scholar] [CrossRef]
- Kim, W.; Kim, J.K.; Kim, E.; Ahn, K.; Wang, D.H.; Park, J.H. Conflicted effects of a solvent additive on PTB7:PC71BM bulk heterojunction solar cells. J. Phys. Chem. C 2015, 119, 5954–5961. [Google Scholar] [CrossRef]


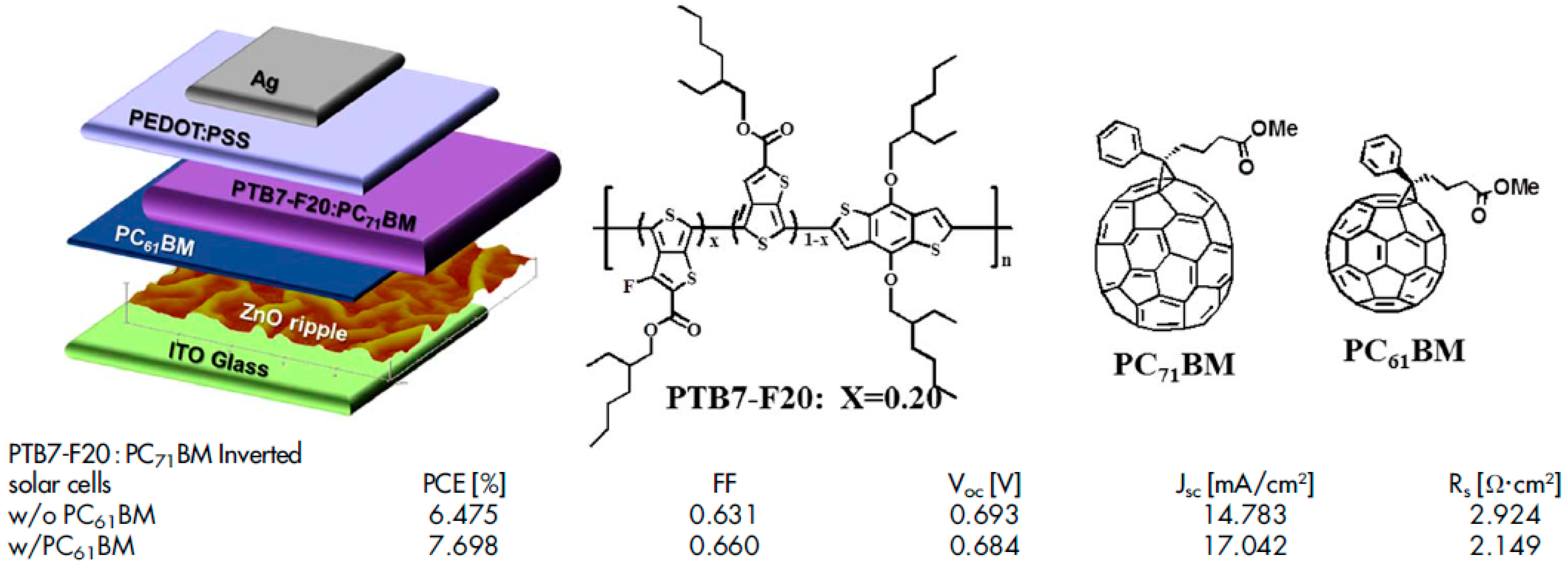
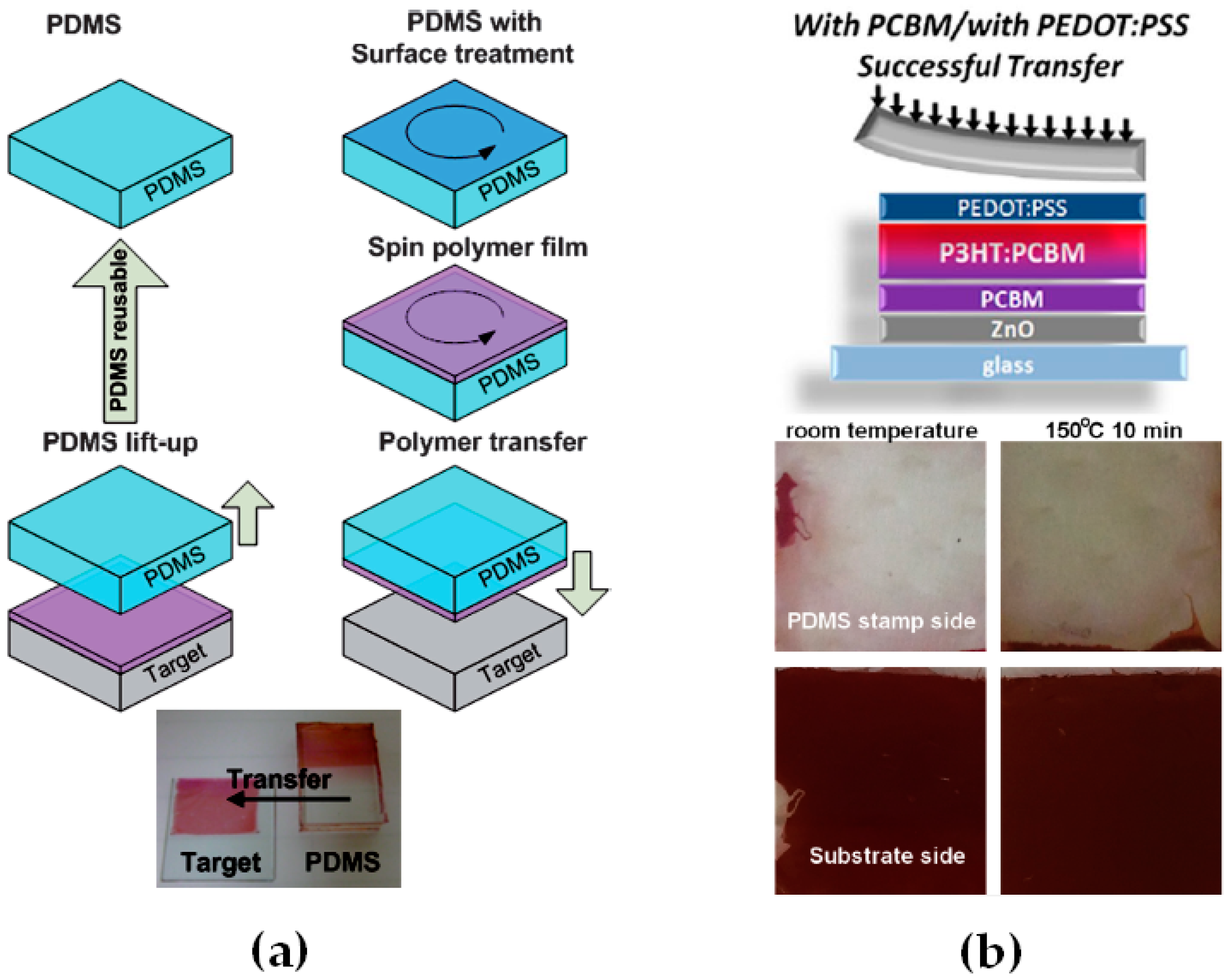
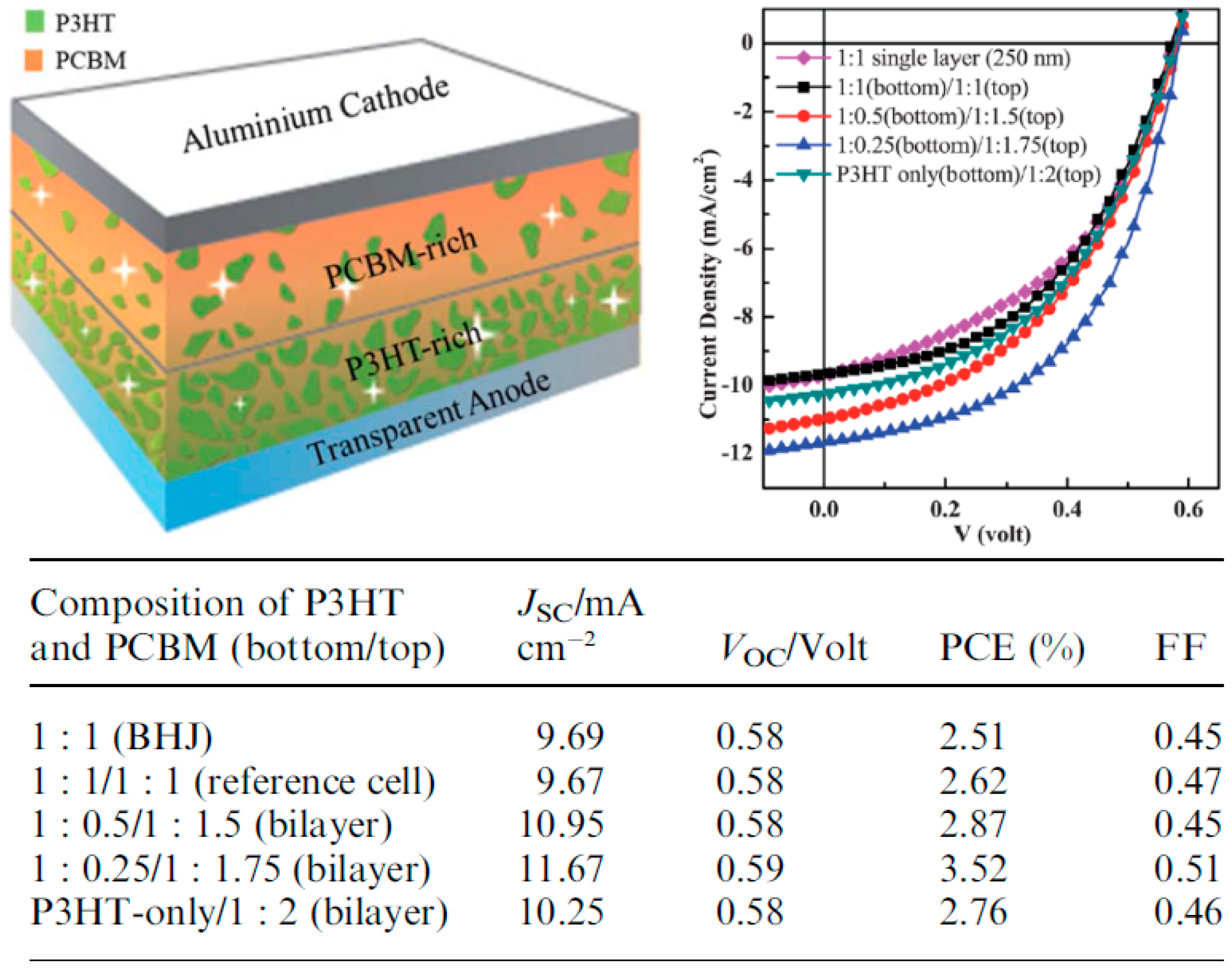

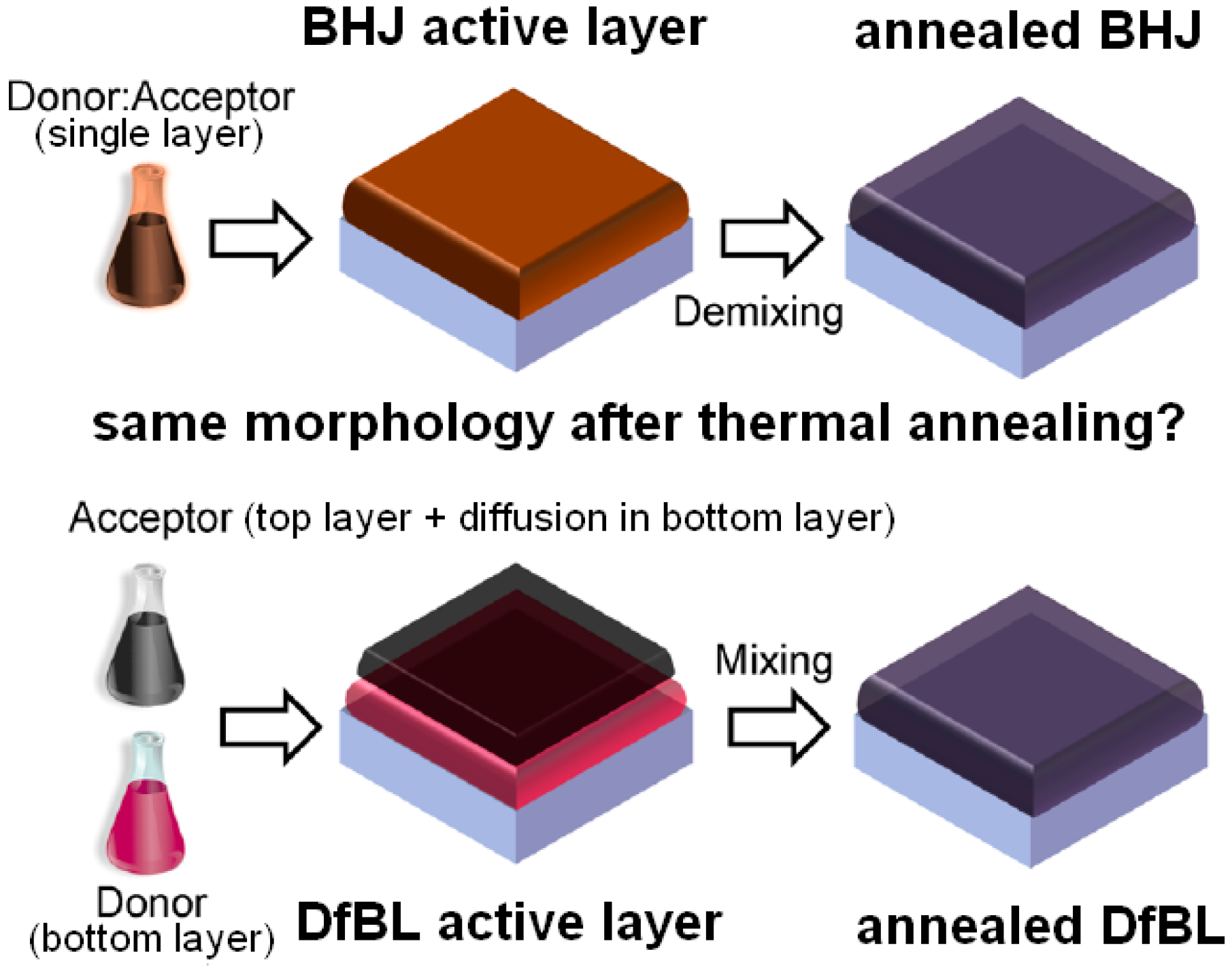
| Active Layer Thickness | P3HT Gradient | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| 50 nm (unannealed) | N/A | 2.6 | 0.55 | 60 | 0.8 |
| 50 nm (annealed) | Weak | 3.4 | 0.58 | 58 | 1.2 |
| 100 nm (unannealed) | N/A | 6.6 | 0.56 | 52 | 1.9 |
| 100 nm (annealed) | Strong | 9.4 | 0.60 | 62 | 3.5 |
| 200 nm (unannealed) | No | 5.5 | 0.53 | 56 | 1.6 |
| 200 nm (annealed) | No | 5.6 | 0.57 | 61 | 1.9 |
| Device Architecture | DIO (vol %) | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| rPSC | 0 | 13.1 | 0.84 | 49.8 | 5.48 |
| rPSC | 3 | 15.2 | 0.76 | 62.3 | 7.20 |
| iPSC | 0 | 14.2 | 0.81 | 45.1 | 5.19 |
| iPSC | 3 | 17.7 | 0.77 | 67.0 | 9.13 |
| Fullerene Derivative | Doping | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| - | - | 9.02 | 0.60 | 67 | 3.61 |
| EGMC-OH | - | 9.43 | 0.60 | 66 | 3.71 |
| EGMC-OH | CS2CO3 (40%) | 9.71 | 0.60 | 65 | 3.74 |
| EGMC-COOH | - | 9.61 | 0.60 | 66 | 3.80 |
| EGMC-COOH | Li2CO3 (40%) | 10.9 | 0.60 | 66 | 4.29 |
| PCDTBT Layer | PC71BM Layer | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| - | - | 2.68 | 0.84 | 48 | 1.09 |
| DIO | - | 4.27 | 0.94 | 45 | 1.82 |
| - | DIM | 5.18 | 0.88 | 63 | 2.88 |
| DIO | DIM | 12.02 | 0.90 | 66 | 7.12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inaba, S.; Vohra, V. Fabrication Processes to Generate Concentration Gradients in Polymer Solar Cell Active Layers. Materials 2017, 10, 518. https://doi.org/10.3390/ma10050518
Inaba S, Vohra V. Fabrication Processes to Generate Concentration Gradients in Polymer Solar Cell Active Layers. Materials. 2017; 10(5):518. https://doi.org/10.3390/ma10050518
Chicago/Turabian StyleInaba, Shusei, and Varun Vohra. 2017. "Fabrication Processes to Generate Concentration Gradients in Polymer Solar Cell Active Layers" Materials 10, no. 5: 518. https://doi.org/10.3390/ma10050518






