A Reagentless Amperometric Formaldehyde-Selective Chemosensor Based on Platinized Gold Electrodes
Abstract
:1. Introduction
2. Results and Discussion
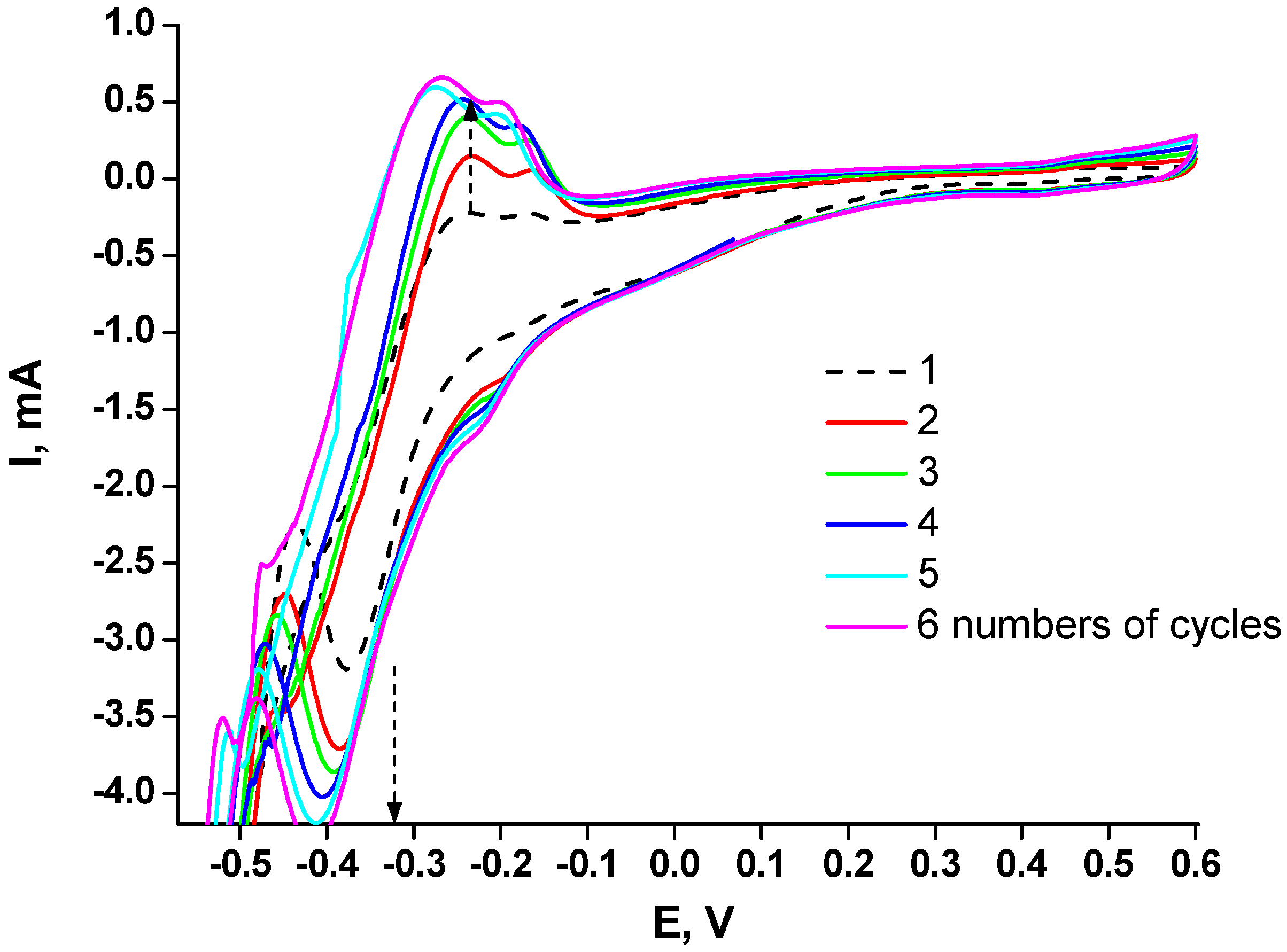
2.1. Development of Procedures for Chemocatalyst Formation on the Electrode Surface
2.2. Determination of Structural and Electrical Properties of the Obtained PtLayers
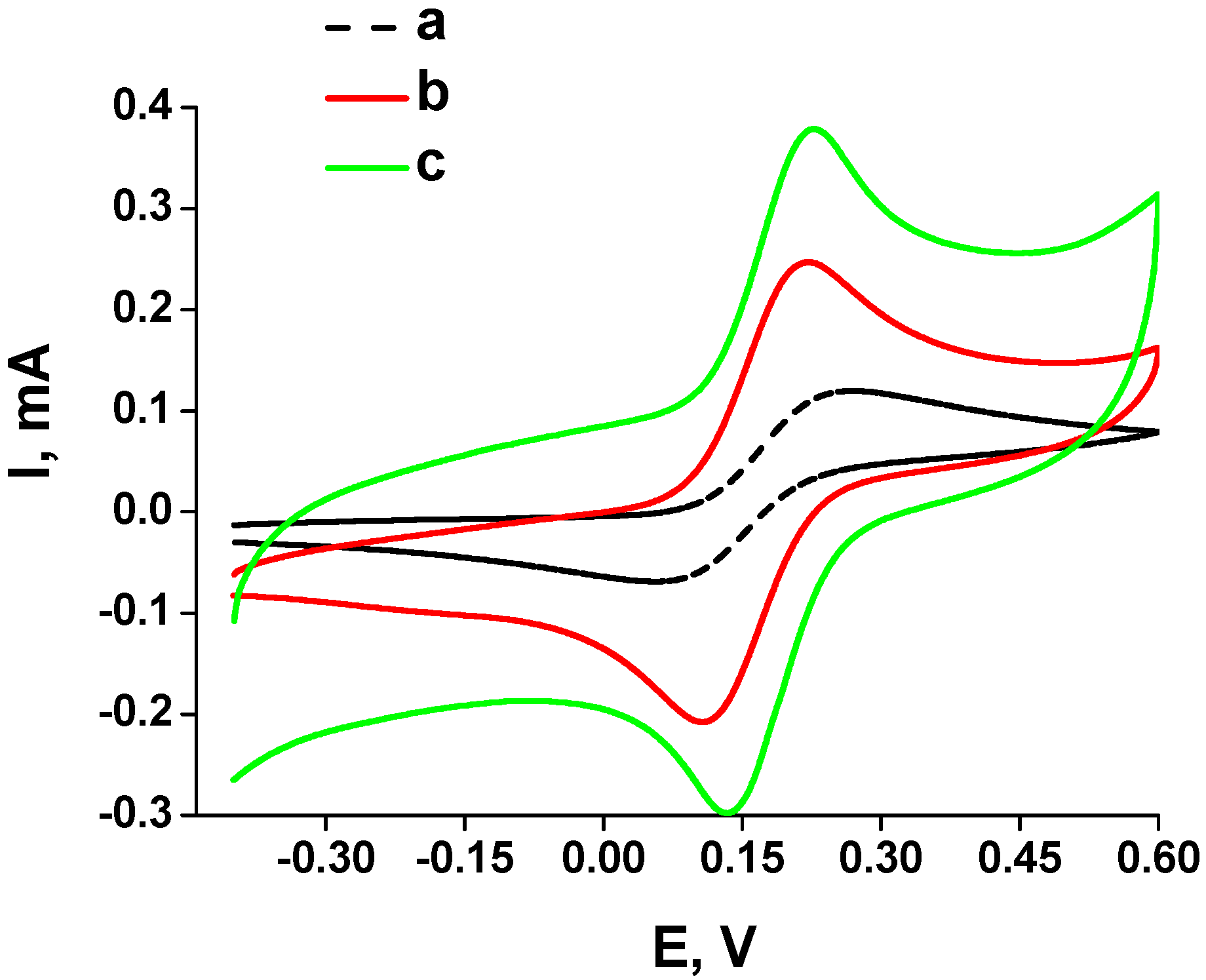
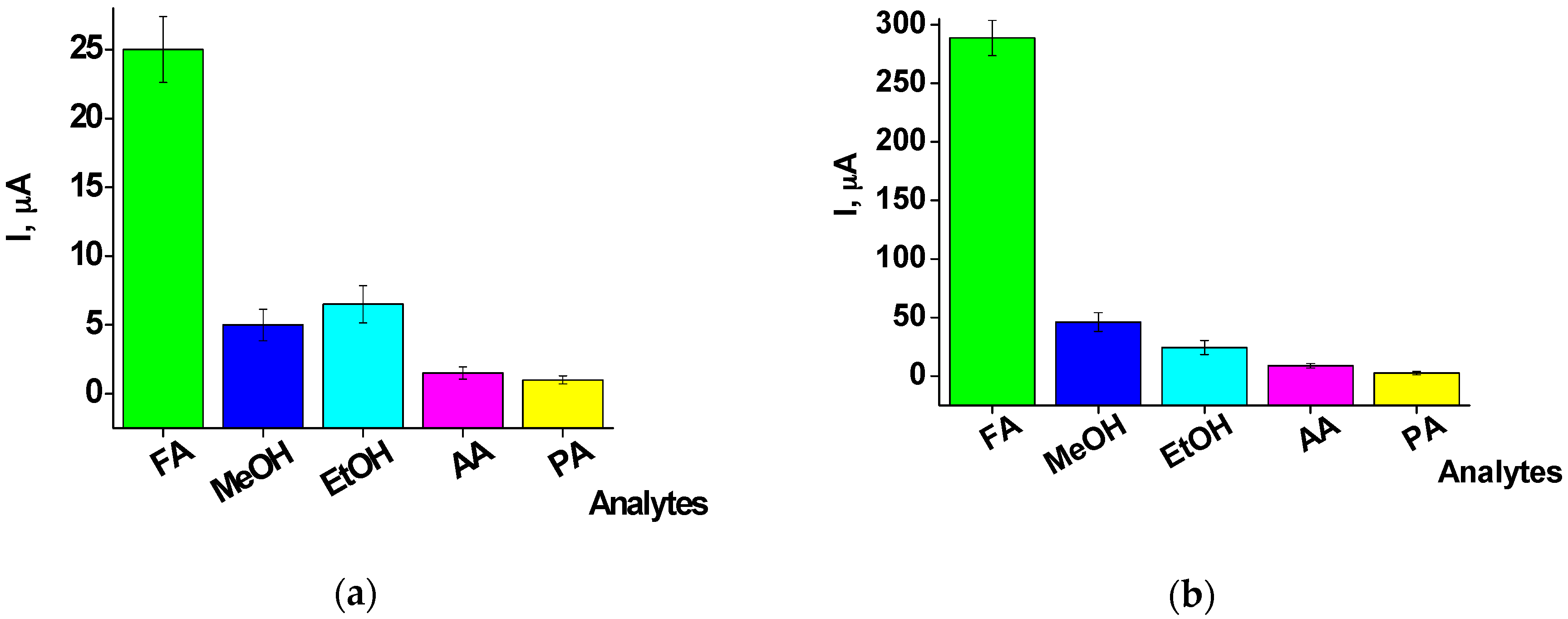
2.3. Characterization of the FA-Sensitive Chemosensors
3. Materials and Methods
3.1. Materials
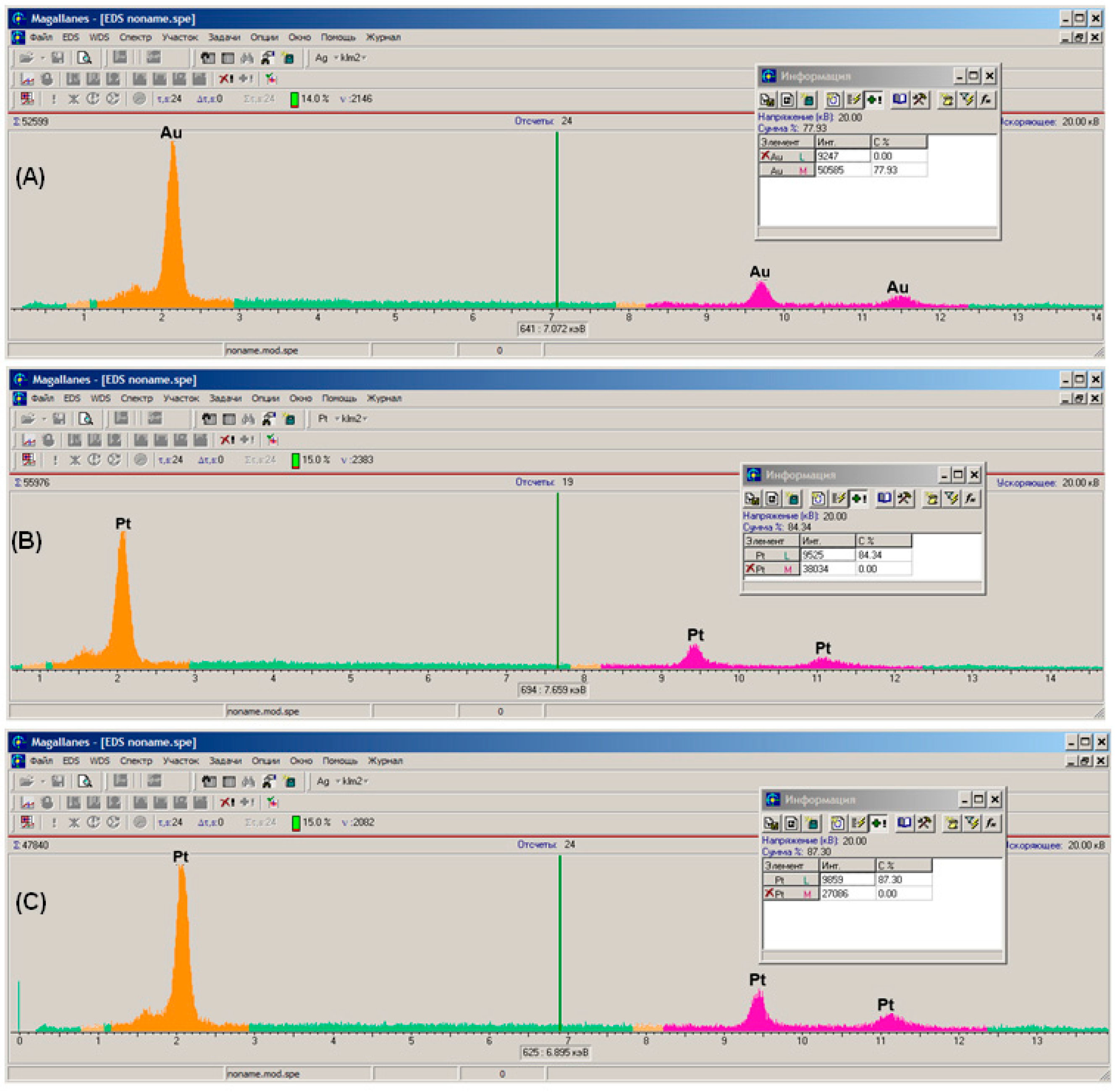
3.2. Scanning Electron Microscopy and X-ray Microanalysis
3.3. Preparation and Evaluation of the Chemosensors
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cogliano, V.J.; Grosse, Y.; Baan, R.A.; Straif, K.; Secretan, M.B.; El Ghissass, F. Formaldehyde. Formaldehyde, 2-Butoxyethanol, and 1-tert-Butoxy-2-Propanol. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; WHO Press: Lyon, France, 2006; Volume 88, pp. 39–93. [Google Scholar]
- Blair, A.; Casanova, M.; Comba, P.; Demers, P.; Goldstein, B.D.; Grafström, R.C.; Järvholm, B.; Kaufman, D.G.; Kauppinen, T.; Leclerc, A. Formaldehyde. In IARC Monographs on Evaluation of Carcinogenic Risks to Humans; WHO Press: Lyon, France, 1995; Volume 62, pp. 217–335. [Google Scholar]
- Barrett, J.C.; Bond, J.A.; Beane-Freeman, L.; Carren-Valencia, T.; Elwell, M.R.; Groopman, J.D.; Friesen, M.D.; Gustavsson, P.; Ghanei, M.; Hayes, R.B.; et al. Formaldehyde. In Chemical Agents and Related Occupations; WHO Press: Lyon, France, 2012; Volume 100F, pp. 401–435. [Google Scholar]
- Feron, V.J.; Til, H.P.; Vrijer, F.; Woutersen, R.A.; Cassee, F.R.; van Bladeren, P.J. Aldehydes: Occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat. Res. 1991, 259, 363–385. [Google Scholar] [CrossRef]
- Hauptmann, M.; Lubin, J.H.; Stewart, P.A.; Hayes, R.; Blair, A. Mortality from solid cancers among workers in formaldehyde industries. Am. J. Epidemiol. 2004, 159, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, A. Formox and formaldehyde market update—A turbo in the future? In Proceedings of the 16th IMPCA Asian Methanol Conference, Singapore, 30 October–1 November 2013; Available online: http://www.methanolmsa.com/wp-content/uploads/2013/11/Andreas-Magnusson.pdf (accessed on 28 March 2017).
- Kim, W.J.; Terada, N.; Nomura, T.; Takahashi, R.; Lee, S.D.; Park, J.H. Effect of formaldehyde on the expression of adhesion molecules in nasal microvascular endothelial cells: The role of formaldehyde in the pathogenesis of sick building syndrome. Clin. Exp. Allergy 2002, 32, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.H. Deamination of methylamine and angiopathy; toxicity of formaldehyde, oxidative stress and relevance to protein glycoxidation in diabetes. J. Neural Transm. 1998, 52, 201–216. [Google Scholar] [CrossRef]
- Zerin, T.; Kim, J.S.; Gil, H.W.; Song, H.Y.; Hong, S.Y. Effects of formaldehyde on mitochondrial dysfunction and apoptosis in SK-N-SH neuroblastoma cells. Cell Biol. Toxicol. 2015, 31, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, E.; Hauser, T.R.; McPherson, S. Spectrophotometric determination of formaldehyde and formaldehyde-releasing compounds with chromotropic acid, 6-amino-1-naphtol-3-sulfonic-acid (J acid), and 6-anilino-1-naphtol-3-sulfonic acid (phenyl J acid). Anal. Chem. 1962, 34, 1460–1474. [Google Scholar] [CrossRef]
- NurIndang, M.; Abdulamir, A.S.; Abu Bakar, A.; Salleh, A.B.; Lee, Y.H.; NorAzah, Y. A review: Methods of determination of health-endangering formaldehyde in diet. Res. J. Pharmacol. 2009, 3, 31–47. [Google Scholar]
- Avigad, G. A simple spectrometric determination of formaldehyde and other aldehydes: Application to periodate oxidized glycerol systems. Anal. Biochem. 1983, 134, 499–504. [Google Scholar] [CrossRef]
- Dumas, T. Determination of formaldehyde in air by gas chromatography. J. Chromatogr. 1982, 247, 289–295. [Google Scholar] [CrossRef]
- Mann, B.; Grajeski, M.L. New chemiluminescentderivatizing agent for the analysis of aldehyde and ketones by high-performance liquid chromatography with peroxioxalatechemiluminescence. J. Chromatogr. 1987, 386, 149–158. [Google Scholar] [CrossRef]
- Lorrain, J.M.; Fortune, C.R.; Dellinger, B. Sampling and ion chromatographic determination of formaldehyde and acetaldehyde. Anal. Chem. 1981, 53, 1302–1305. [Google Scholar] [CrossRef]
- Septon, J.C.; Ku, J.C. Workplace air sampling and polarographic determination of formaldehyde. Am. Ind. Hyg. Assoc. J. 1982, 43, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Gayda, G.; Demkiv, O.; Klepach, H.; Gonchar, M.; Levy-Halaf, R.; Wolf, D.; Nisnevitch, M. Formaldehyde: Detection and Biodegradation. In Formaldehyde: Synthesis, Applications and Potential Health Effects; Patton, A., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2015; pp. 117–142. [Google Scholar]
- Paryzhak, S.; Demkiv, O.; Schuhmann, W.; Gonchar, M. Intact recombinant cells of the yeast Hansenulapolymorpha, over-producing formaldehyde dehydrogenase, as the sensitive bioelements for amperometric assay of formaldehyde. Sens. Electron. Microsyst. Technol. (Ukr.) 2008, 2, 28–38. [Google Scholar]
- Smutok, O.; Ngounou, B.; Pavlishko, H.; Gayda, G.; Gonchar, M.; Schuhmann, W. A reagentlessbienzymeamperometric biosensor based on alcohol oxidase/peroxidase and an Os-complex modified electrodeposition paint. Sens. Actuators B Chem. 2006, 113, 590–598. [Google Scholar] [CrossRef]
- Sigawi, S.; Smutok, O.; Demkiv, O.; Gayda, G.; Vus, B.; Nitzan, Y.; Gonchar, M.; Nisnevitch, M. Detection of waterborne and airborne formaldehyde: From amperometricchemosensing to a visual biosensor based on alcohol oxidase. Materials 2014, 7, 1055–1068. [Google Scholar] [CrossRef]
- Korpan, Y.I.; Gonchar, M.V.; Starodub, N.F.; Shulga, A.A.; Sibirny, A.A.; Elskaya, A.V. A cell biosensor specific for formaldehyde based on pH-sensitive transistors coupled to methylotrophic yeast cells with genetically adjusted metabolism. Anal. Biochem. 1993, 21, 216–222. [Google Scholar] [CrossRef]
- Dzyadevych, S.V.; Arkyhypova, V.N.; Korpan, Y.I.; El’skaya, A.V.; Soldatkin, A.P.; Jaffrezic-Renault, N.; Martelet, C. Conductometric formaldehyde sensitive biosensor with specifically adapted analytical characteristics. Anal. Chim. 2001, 445, 47–55. [Google Scholar] [CrossRef]
- Vianello, F.; Boscolo-Chio, R.; Signorini, S. On-line detection of atmospheric formaldehyde by a conductometricbiosensor. Biosens. Bioelectron. 2007, 22, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, O.; Shleev, S.; Gayda, G.; Demkiv, O.; Gonchar, M.; Gorton, L.; Csöregi, E.; Nistor, M. Bi-enzyme biosensor based on NAD(+)- and glutathione-dependent recombinant formaldehyde dehydrogenase and diaphorase for formaldehyde assay. Sens. Actuators B 2007, 125, 1–9. [Google Scholar] [CrossRef]
- Demkiv, O.; Smutok, O.; Paryzhak, S.; Gayda, G.; Sultanov, Y.; Guschin, D.; Shkil, H.; Schuhmann, W.; Gonchar, M. Reagentlessamperometric formaldehyde-selective biosensors based on the recombinant yeast formaldehyde dehydrogenase. Talanta 2008, 76, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Ferrin, P.; Mavrikakis, M. Structure Sensitivity of Methanol Electrooxidation on Transition Metals. J. Am. Chem. Soc. 2009, 131, 14381–14389. [Google Scholar] [CrossRef] [PubMed]
- Raoof, J.B.; Hosseini, S.R.; Rezaee, S. A simple and effective route for preparation of platinumnanoparticle and its application for electrocatalytic oxidation of methanol and formaldehyde. J. Mol. Liq. 2015, 212, 767–774. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Z.Z.; Shi, J.; Li, H.L. Electrocatalytic oxidation of formaldehyde on platinum well-dispersed into single-wall carbon nanotube/polyaniline composite film. Appl. Surf. Sci. 2007, 253, 8811–8817. [Google Scholar] [CrossRef]
- Kuang, Y.; Wu, B.; Cui, Y.; Yu, Y.; Zhang, X.; Chen, J. Preparation of hollow platinum nanospheres/carbon nanotubes nanohybrids and their improved stability for electro-oxidation of methanol. Electrochim. Acta 2011, 56, 8645–8650. [Google Scholar] [CrossRef]
- Tegou, A.; Armyanov, S.; Valova, E.; Steenhaut, O.; Hubin, A.; Kokkinidis, G.; Sotiropoulos, S. Mixed platinum–gold electrocatalysts for borohydride oxidation prepared by the galvanic replacement of nickel deposits. J. Electroanal. Chem. 2009, 634, 104–110. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Zhang, W.; Zhang, Y.; Li, L.; Cao, Z.; Wang, H.; Jia, G.; Gao, Y.; Liu, J. Electrocatalytic oxidation of formaldehyde on direct electrodeposited graphene–platinum nanoparticles composites electrode. Anal. Methods 2013, 5, 3915–3919. [Google Scholar] [CrossRef]
- Özdokur, K.V.; Tatlı, A.Y.; Yılmaz, B.; Koçak, S.; Ertaş, F.N. Development of pulsed deposited manganese and molybdenum oxide surfaces decorated with platinum nanoparticles and their catalytic application for formaldehyde oxidation. Int. J. Hydrogen Energy 2016, 41, 5927–5933. [Google Scholar] [CrossRef]
- Kovalyshyn, Y.; Koval’chuk, E.; Ostapovych, B.; Chervinka, M. Electrochemical oxidation of formaldehyde on the platinized graphite electrode. Visn. Lviv Univ. Ser. Chem. 2010, 51, 342–347. [Google Scholar]
- Dong, Q.; Li, Y.; Zhu, L.; Ma, T.; Guo, C. Electrocatalytic oxidation of methanol and formaldehyde on platinum-modified poly(o-methoxyaniline)-multiwalled carbon nanotube composites. Int. J. Electrochem. Sci. 2013, 8, 8191–8200. [Google Scholar]
- Mourdikoudis, S.; Chirea, M.; Altantzis, T.; Pastoriza-Santos, I.; Perez-Juste, J.; Silva, F.; Bals, S.; Liz-Marzan, L.M. Dimethylformamide-mediated synthesis ofwater-soluble platinum nanodendrites for ethanoloxidation electrocatalysis. Nanoscale 2013, 5, 4776–4784. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Khalizadeh, B.; Shadjou, N.; Karim-Nezhad, G.; Saghatforoush, L.; Kazeman, I.; Abnosi, M.H. A new kinetic-mechanistic approach to elucidate formaldehyde electrooxidation on copper electrode. Electroanalysis 2009, 22, 168–176. [Google Scholar] [CrossRef]
- Sathe, B.R.; Shinde, D.B.; Pillai, V.K. Preparation and characterization of rhodium nanostructures through the evolution of microgalvanic cells and their enhanced electrocatalytic activity for formaldehyde oxidation. J. Phys. Chem. C 2009, 113, 9616–9622. [Google Scholar] [CrossRef]
- Qiu, C.; Guo, Y.; Zhang, J.; Ma, H.; Cai, Y. Bimetallic Pt–Au thin film electrocatalysts with hierarchical structures for the oxidation of formic acid. Mater. Chem. Phys. 2011, 127, 484–488. [Google Scholar] [CrossRef]
- Mohl, M.; Dobo, D.; Kukovecz, A.; Konya, Z.; Kordas, K.; Wei, J.; Ajayan, P.M. Formation of CuPd and CuPt bimetallic nanotubes by galvanic replacement reaction. Phys. Chem. 2011, 115, 9403–9409. [Google Scholar] [CrossRef]
- Xie, S.; Jin, M.; Tao, J.; Wang, Y.; Xie, Z.; Zhu, Y.; Xia, Y. Synthesis and characterization of Pd@MxCu1−x (M = Au, Pd, and Pt) nanocages with porous walls and a yolk–shell structure through galvanic replacement reactions. Chem. Eur. J. 2012, 18, 14877. [Google Scholar] [CrossRef]
- Mei, H.; Wu, W.; Yu, B.; Wu, H.; Wang, S.; Xia, Q. Nonenzymatic electrochemical sensor based on Fe@Pt core–shell nanoparticles for hydrogen peroxide, glucose and formaldehyde. Sens. Actuators B Chem. 2016, 223, 68–75. [Google Scholar] [CrossRef]
- De Lima, R.B.; Massafera, M.P.; Batista, E.A.; Iwasita, T. Catalysis of formaldehyde oxidation by electrodeposits of PtRu. J. Electroanal. Chem. 2007, 603, 142–148. [Google Scholar] [CrossRef]
- Pötzelberger, I.; Mardareb, C.C.; Burgstallerb, W.; Hassela, A.W. Maximum electrocatalytic oxidation performance for formaldehyde in a combinatorial copper-palladium thin film library. Appl.Catal. A Gen. 2016, 525, 110–118. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, Y.T.; Gao, G.H.; Wang, T.; Zhao, B.; Fu, X.Z.; Suna, R.; Wong, C.-P. Electro-oxidation of formaldehyde and methanol over hollow porous palladium nanoparticles with enhanced catalytic activity. Catal. Commun. 2015, 58, 40–45. [Google Scholar] [CrossRef]
- Hassaninejad-Darzi, S.K.; Rahimnejad, M.; Gholami-Esfidvajani, M. Electrocatalytic oxidation of formaldehyde onto carbon paste electrode modified with nickel decorated nanoporous cobalt-nickel phosphate molecular sieve for fuel cell. Fuel Cells 2016, 16, 89–99. [Google Scholar] [CrossRef]
- Qiao, J.; Guo, Y.; Song, J.; Zhang, Y.; Sun, T.; Shuang, S.; Dong, C. Synthesis of a palladium-graphene material and its application for formaldehyde determination. Anal. Lett. 2013, 46, 1454–1465. [Google Scholar] [CrossRef]



| Product | FA Concentration Determined by Various Methods, M | |||||
|---|---|---|---|---|---|---|
| Biosensor | Chemical (Spectrophotometric) | Declared by Producer | Chemosensor | |||
| FdDH-Based [25] | Chromotropic [10] | MBTH [10,11] | Purpald [12] | - | Current Study | |
| “Formalin” | 13.5 ± 2.1 | 14.0 ± 2.4 | 12.6 ± 2.1 | 12.9 ± 1.9 | 13.0 ± 1.5 | 13.6 ± 1.8 |
| “Sanodez Forte” | 3.2 ± 0.6 | 3.6 ± 0.9 | 3.6 ± 0.8 | 3.3 ± 0.5 | 2.6 ± 0.5 | 2.7 ± 0.48 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demkiv, O.; Smutok, O.; Gonchar, M.; Nisnevitch, M. A Reagentless Amperometric Formaldehyde-Selective Chemosensor Based on Platinized Gold Electrodes. Materials 2017, 10, 503. https://doi.org/10.3390/ma10050503
Demkiv O, Smutok O, Gonchar M, Nisnevitch M. A Reagentless Amperometric Formaldehyde-Selective Chemosensor Based on Platinized Gold Electrodes. Materials. 2017; 10(5):503. https://doi.org/10.3390/ma10050503
Chicago/Turabian StyleDemkiv, Olha, Oleh Smutok, Mykhailo Gonchar, and Marina Nisnevitch. 2017. "A Reagentless Amperometric Formaldehyde-Selective Chemosensor Based on Platinized Gold Electrodes" Materials 10, no. 5: 503. https://doi.org/10.3390/ma10050503







