Synthesis and Thermochromic Properties of Cr-Doped Al2O3 for a Reversible Thermochromic Sensor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Structural Characterization
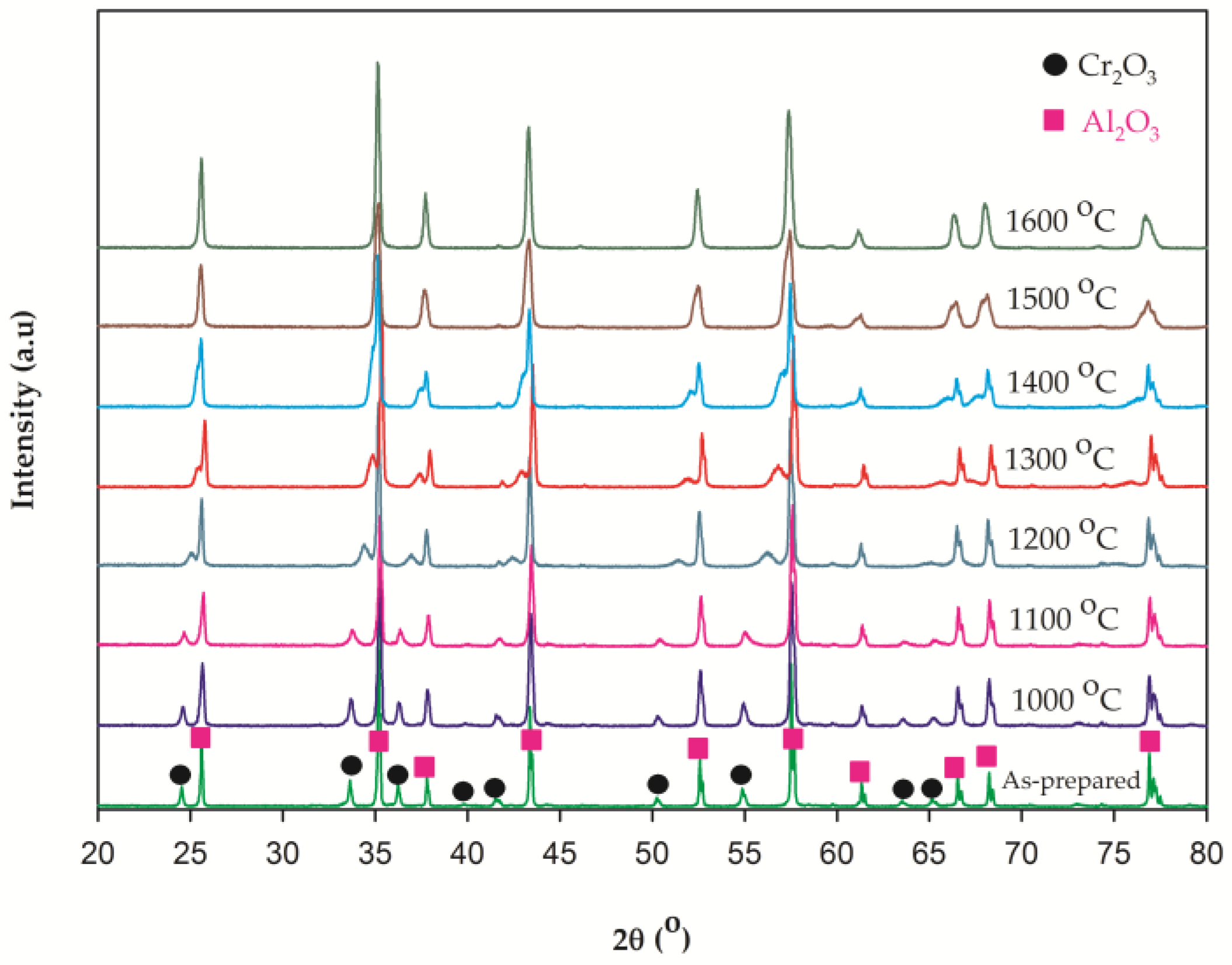
2.2.1. X-ray Diffraction (XRD) Spectra
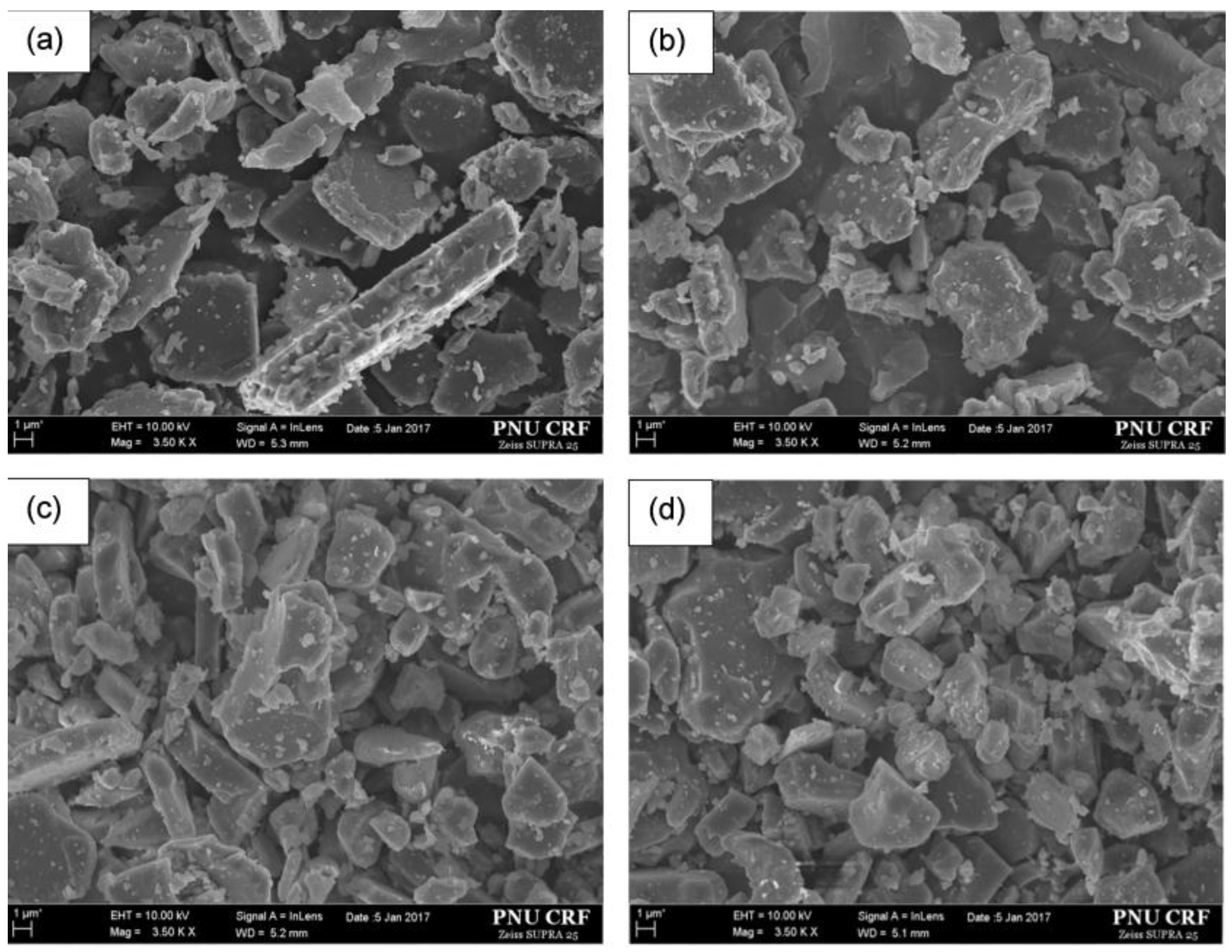
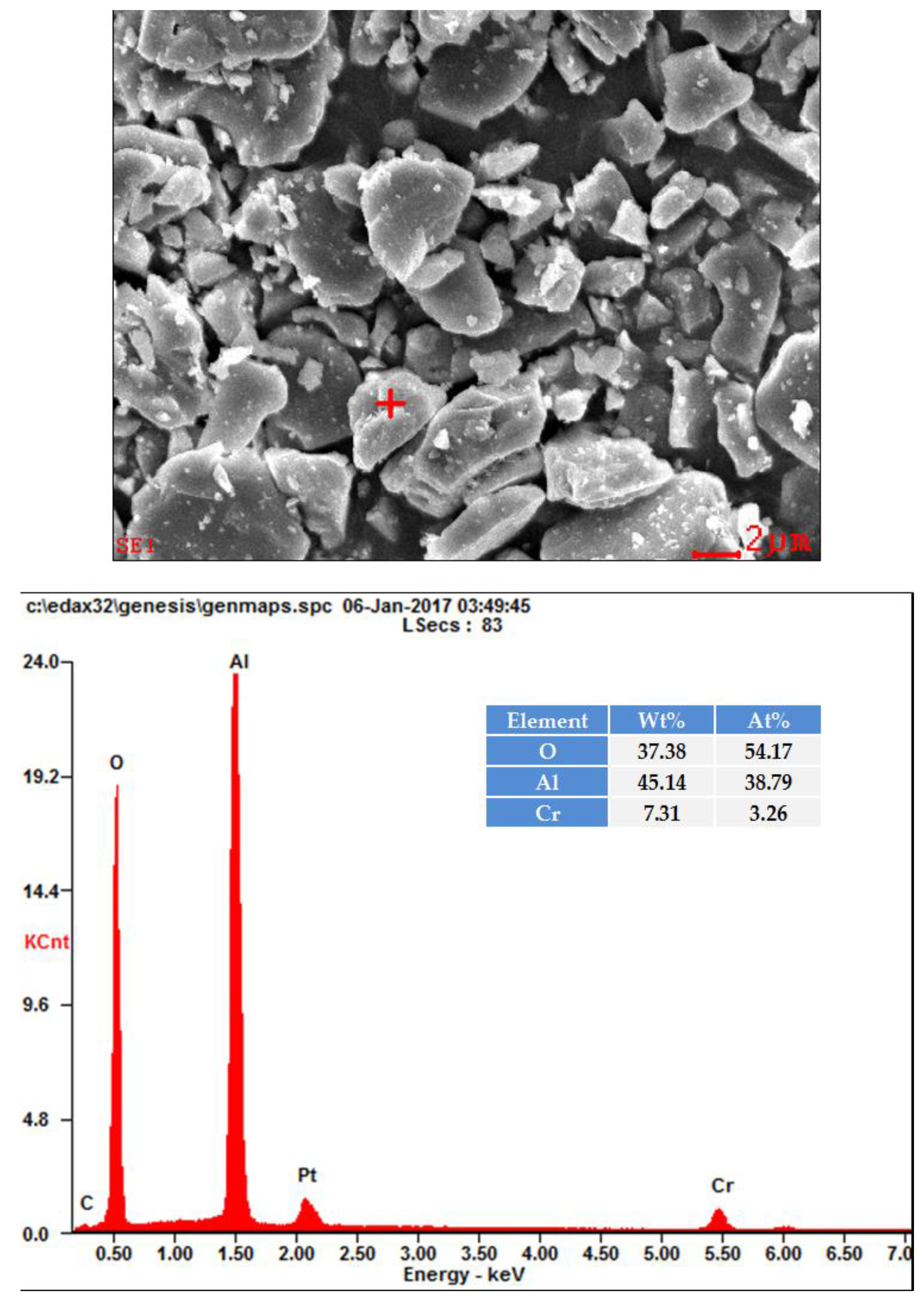
2.2.2. SEM Observations and Spot-Chemical Analysis.
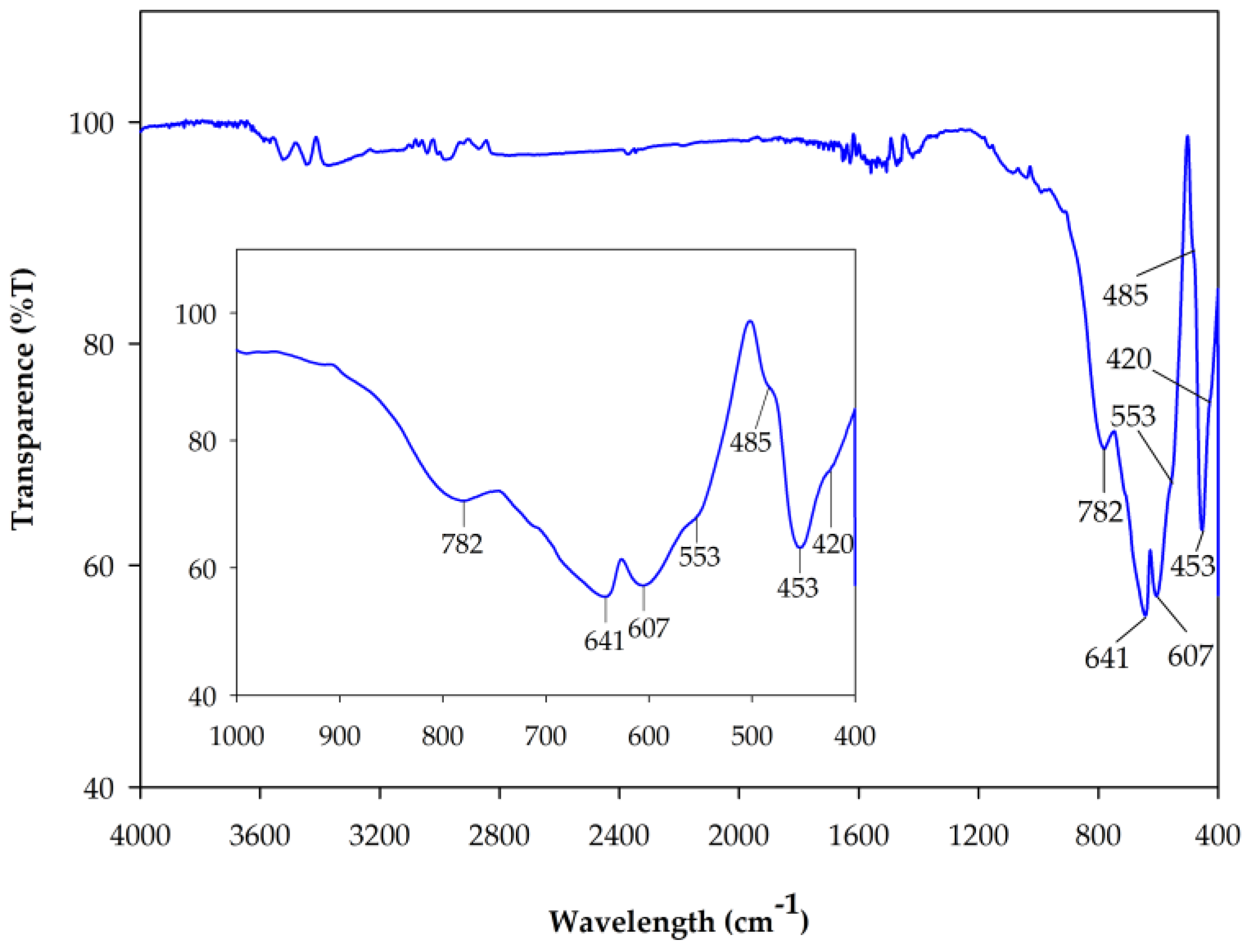
2.2.3. Fourier Transform Infrared Spectra (FT-IR)
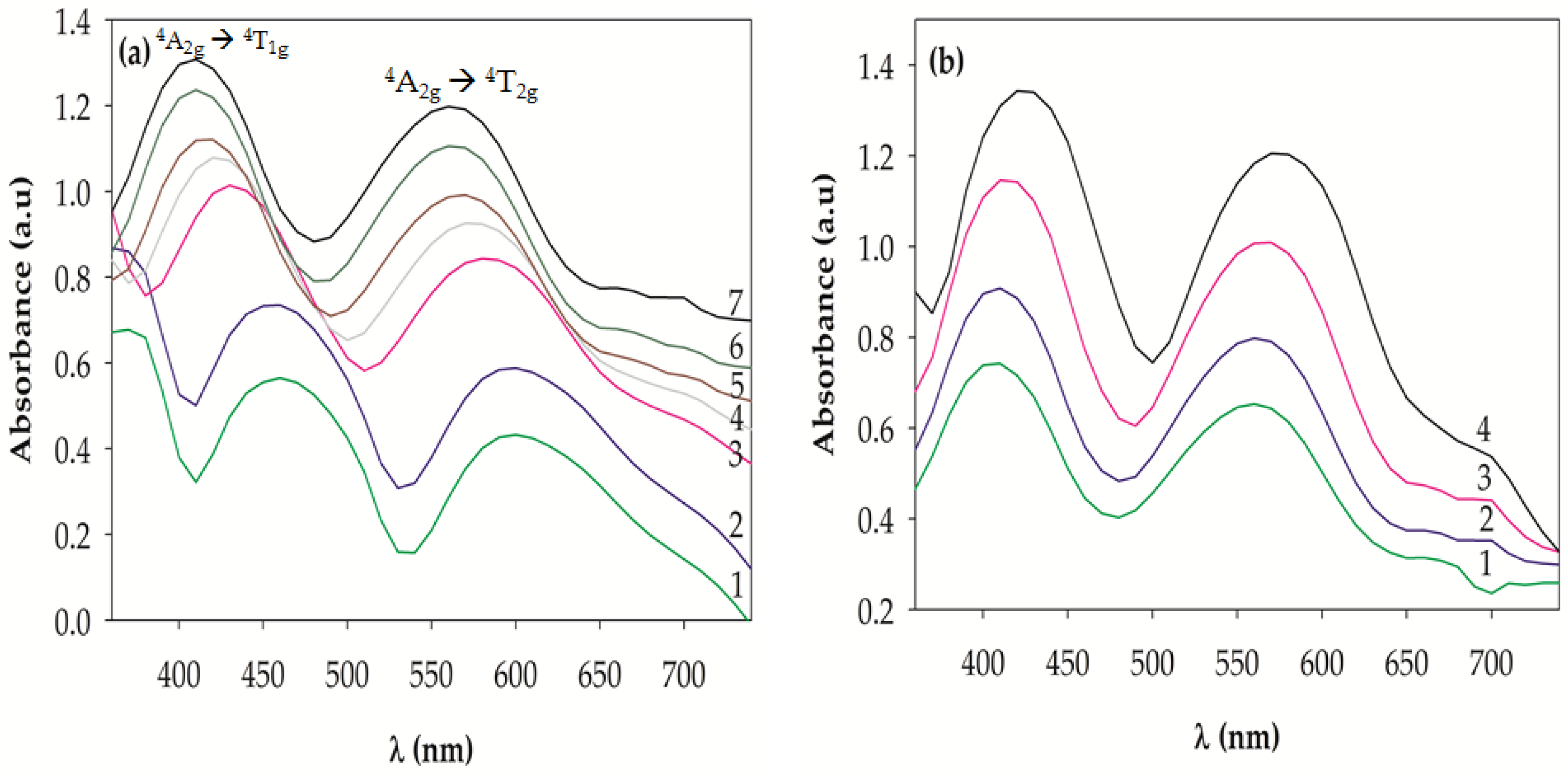
2.3. UV-VIS Spectra
2.4. Color Property
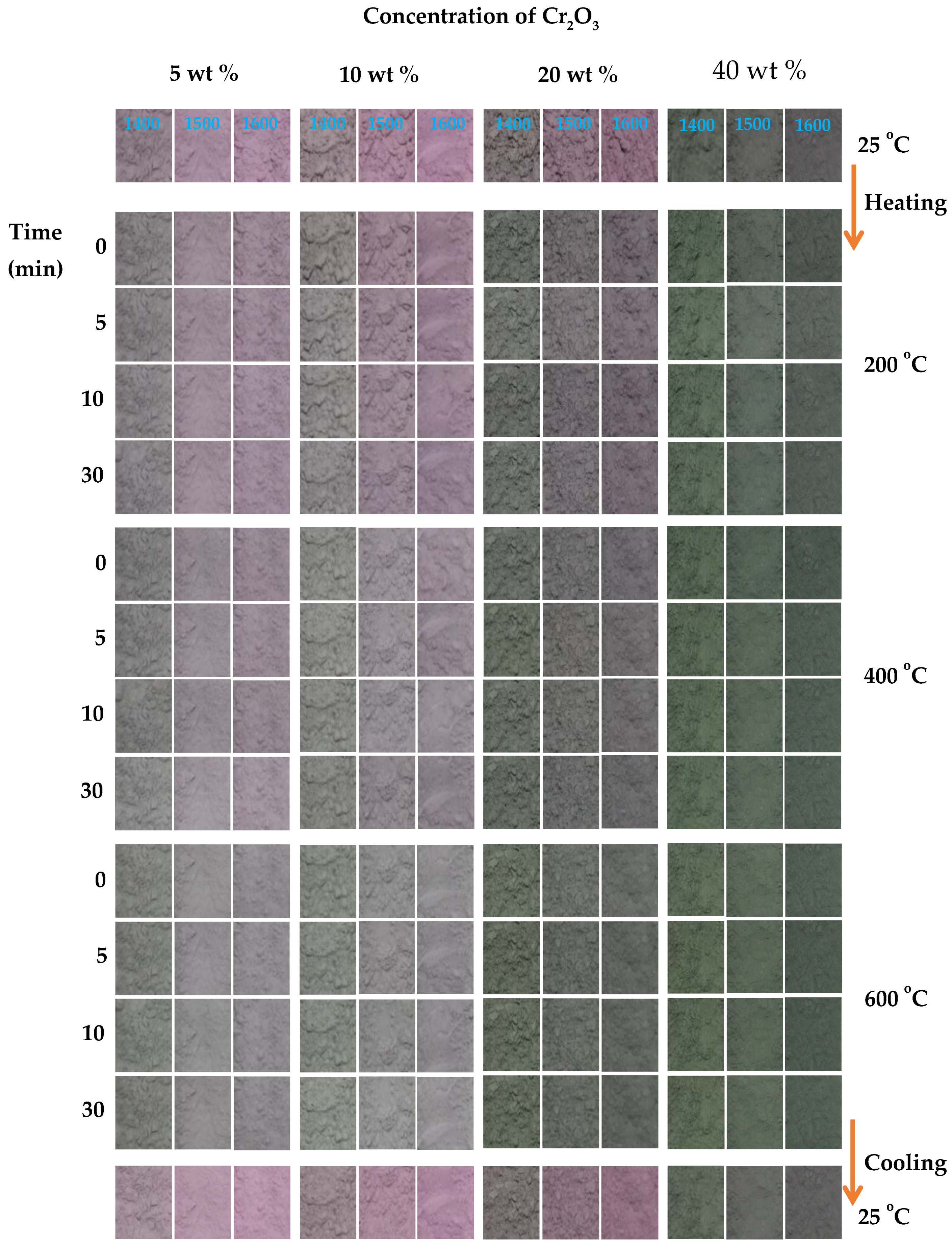
2.5. Thermochromism of Synthesized Pigment Powders
3. Materials and Methods
3.1. Synthesis
3.2. Characterization Techniques
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Popescu, M.; Serban, L.; Popescu, M. Thermo-indicating paint for damage warning. J. Therm. Anal. 1996, 46, 317–321. [Google Scholar] [CrossRef]
- Lempereur, C.; Andral, R.; Prudhomme, J.Y. Surface temperature measurement on engine components by means of irreversible thermal coating. Meas. Sci. Technol. 2008, 19, 105501–105512. [Google Scholar] [CrossRef]
- Pelvich, C.W.; Foulk, D.L.; Polec, T.W. Method of Sensing High Surface Temperature in an Aircraft. Patent EP1959246 A2, 19 June 2008. [Google Scholar]
- Belykh, A.V.; Efremov, A.M.; Mikhailov, M.D. Thermochromic Material. Patent EP1405890 B1, 17 October 2012. [Google Scholar]
- Lataste, E.; Demourgues, A.; Salmi, J.; Naporea, C.; Gaudon, M. Thermochromic behaviour (400 < T °C < 1200 °C) of barium carbonate/binary metal oxide mixtures. Dyes Pigm. 2011, 91, 396–403. [Google Scholar]
- Salek, G.; Demourgues, A.; Jubera, V.; Garcia, A.; Gaudon, M. Mn2+ doped Zn3(PO4)2 phosphors: Irreversible thermochromic materials useful as thermal sensors. Opt. Mater. 2015, 47, 323–327. [Google Scholar] [CrossRef]
- Salek, G.; Devoti, A.; Lataste, E.; Demourgues, A.; Garcia, A.; Jubera, V.; Gaudon, M. Optical properties versus temperature of Cr-doped γ- and α-Al2O3: Irreversible thermal sensors application. J. Lumin. 2016, 179, 189–196. [Google Scholar] [CrossRef]
- Wang, S.; Liu, M.; Kong, L.; Long, Y.; Jiang, X.; Yu, A. Recent progress in VO2 smart coating: Strategies to improve the thermochromic properties. Prog. Mater. Sci. 2016, 81, 1–54. [Google Scholar] [CrossRef]
- Ke, Y.; Balin, I.; Wang, N.; Lu, Q.; Tok, A.I.Y.; White, T.J.; Magdassi, S.; Abdulhalim, I.; Long, Y. Two-dimensional SiO2/VO2 photonic crystals with statically visible and dynamically infrared modulated for smart window deployment. ACS Appl. Mater. Interfaces 2016, 8, 33112–33120. [Google Scholar] [CrossRef] [PubMed]
- Rašković-Lovre, Ž.; Mongstad, T.; Karazhanov, S.; Lindberg, S.; You, C.C.; Deledda, S. Thermochromic and photochromic colour change in Mg-Ni-H thin films. Mater. Lett. 2017, 188, 403–405. [Google Scholar] [CrossRef]
- Zuo, C.; Jagodzinski, P.W. R-line Luminescence from Trace Amounts of Cr3+ in Aluminum Oxide and Its Dependence on Sample Hydration. Appl. Spectrosc. 2002, 56, 1055–1058. [Google Scholar] [CrossRef]
- Nassau, K. The Physics and Chemistry of Color: The Fifteen Causes of Color, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1983. [Google Scholar]
- Dang, K.Q.; Takei, S.; Kawahara, M.; Nanko, M. Pulsed electric current sintering of transparent Cr-doped Al2O3. Ceram. Int. 2011, 37, 957–963. [Google Scholar] [CrossRef]
- Sone, K.; Fukuda, Y. Thermochromism of transition metal complexes in the solid state. In Inorganic Thermochromism, 1st ed.; Springer: Berlin/Heidelbreg, Germany; New York, NY, USA, 1987; pp. 104–128. [Google Scholar]
- Fahlman, B.D.; Barron, A.R. CVD of Chromium-Doped Alumina “Ruby” Thin Films. Chem. Vap. Depos. 2001, 7, 62–65. [Google Scholar] [CrossRef]
- Wang, S.; Shao, M.W.; Shao, G.; Wang, H.; Cheng, L. Room temperature and long-lasting blue phosphorescence of Cr-doped α-Al2O3 nanowires. Chem. Phys. Lett. 2008, 460, 200–204. [Google Scholar] [CrossRef]
- Ianos, R.; Muntean, E.; Băbută, R.; Lazău, R.; Păcurariu, C.; Bandas, C. Combustion synthesis of pink chromium-doped alumina with excellent near-infrared reflective properties. Ceram. Int. 2017, 43, 2568–2572. [Google Scholar] [CrossRef]
- Salah, N.; Khan, Z.H.; Habid, S.S. Nanoparticles of Al2O3:Cr as a sensitive thermoluminescent materials for high exposures of gamma rays irradiations. Nucl. Instrum. Method Phys. Res. Sect. B 2011, 269, 401–404. [Google Scholar] [CrossRef]
- Kakooei, S.; Rouhi, J.; Mohamadpour, E.; Alimanesh, M.; Dehzangi, A. Synthesis and characterization of Cr-doped Al2O3 nanoparticles prepared via aqueous combustion method. Casp. J. Appl. Sci. Res. 2012, 1, 16–22. [Google Scholar]
- Mi, X.; Zhang, X.; Ba, X.; Bai, Z.; Lu, L.; Wang, X.; Liu, Q. Preparation and luminescent properties of Cr3+:Al2O3 nano-powders by low-temperature combustion synthesis. Adv. Powder Technol. 2009, 20, 164–168. [Google Scholar] [CrossRef]
- Badar, N.; Kamarulzaman, N.; Rusdi, R.; Aziz, N.D.A.; Fun, H.K. Increased conductivities of Cr doped Al2-xCrxO3 powders due to band gap narrowing. Phys. B Condens. Matter 2014, 437, 32–35. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Q.H.; Zhao, G.G.; Lu, S.Z.; Zhang, H.J. The thermoluminescence and optically stimulated luminescence properties of Cr-doped alpha alumina transparent ceramics. J. Alloys Compd. 2013, 579, 259–262. [Google Scholar] [CrossRef]
- Munoz, R.; Masó, N.; Julián, B.; Márquez, F.; Beltrán, H.; Escribano, P.; Cordoncillo, E. Environmental study of Cr2O3-Al2O3 green ceramic pigment synthesis. J. Eur. Ceram. Soc. 2004, 24, 2087–2094. [Google Scholar] [CrossRef]
- Martos, M.; Martínez, M.; Cordoncillo, E.; Escribano, P. Towards more ecological ceramic pigments: Study of the influence of glass composition on the colour stability of a pink chromium-doped ceramic pigment. J. Eur. Ceram. Soc. 2007, 27, 4561–4567. [Google Scholar] [CrossRef]
- Prim, S.R.; Garcia, A.; Galindo, R.; Cerro, S.; Llusar, M.; Folgueras, M.V.; Monros, G. Pink ceramic pigments based on chromium doped M(Al2−xCrx)O4, M = Mg, Zn, normal spinel. Ceram. Int. 2013, 39, 6981–6989. [Google Scholar] [CrossRef]
- Patra, A.; Tallman, R.E.; Weinstein, B.A. Effect of crystal structure and dopant concentration on the luminescence of Cr3+ in Al2O3 nanocrystals. Opt. Mater. 2005, 27, 1396–1401. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Galassi, C. Chromium doped γ-Al2O3 powders. Features of the electrical double layer and state of the surface species. J. Organomet. Chem. 2000, 490, 48–53. [Google Scholar] [CrossRef]
- Fujita, K.; Tokudome, Y.; Nakanishi, K.; Miura, K.; Hirao, K. Cr3+-doped macroporous Al2O3 monoliths prepared by the metal-salt-derived sol-gel method. J. Non-Cryst. Solids 2008, 354, 659–664. [Google Scholar] [CrossRef]
- Xuanmeng, H.; Zhenfeng, Z.; Hui, L.; Lu, F. In-situ Cr-doped alumina nanorods powder prepared by hydrothermal method. Rare Met. Mater. Eng. 2016, 45, 1659–1663. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, Z.; Liu, H.; Zhang, Z.; Zhang, Y.; Li, G. Al2O3:Cr3+ microfibers by hydrothermal route: Luminescence properties. Mater. Res. Bull. 2012, 47, 2332–2335. [Google Scholar] [CrossRef]
- Dang, K.Q.; Nanko, M. Transparent Cr-doped Al2O3 made by pulsed electric current sintering process. AZo J. Mater. Online 2010, 6. [Google Scholar] [CrossRef]
- Dang, K.Q.; Nanko, M. Effects of pressure and temperature on sintering of Cr-doped Al2O3 by pulsed electric current sintering process. In International Symposium Multifunctional Ceramic Materials Based on Nanotechnology, IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2011; Volume 20, p. 012004. [Google Scholar]
- Zhu, Z.; Liu, D.; Liu, H.; Du, J.; Yu, H.; Deng, J. Fabrication and luminescent properties of Al2O3:Cr3+ microspheres via a microwave solvothermal route followed by heat treatment. Opt. Commun. 2012, 285, 3140–3142. [Google Scholar] [CrossRef]
- Liu, D. Effects of Cr content and morphology on the luminescence properties of the Cr-doped alpha-Al2O3 powders. Ceram. Int. 2013, 39, 4765–4769. [Google Scholar] [CrossRef]
- Ianosev, S.; Lazǎu, R.; Suba, M.; Pǎcurariu, C.; Lazǎu, I. Synthesis and characterization of some thermoresistant pigments based on the Al3+→Cr3+ substitution. Stud. UBB Chem. 2009, 1, 189–201. [Google Scholar]
- Shin, K.H.; Lee, B.H. Synthesis of Cr-doped Y2O3-Al2O3 red pigments and their application. J. Korean Ceram. Soc. 2008, 45, 453–458. [Google Scholar] [CrossRef]
- Shirpour, M.; Faghihi Sani, M.A.; Mirhabibi, A. Synthesis and study of a new class of red pigments based on perovskite YAlO3 structure. Ceram. Int. 2007, 33, 1427–1433. [Google Scholar] [CrossRef]
- Al-Saadi, T.M.; Hameed, N.A. Synthesis and structural characterization of Cr2O3 nanoparticles prepared by using Cr(NO3)3.9H2O and triethanolamine under microwave irradiation. Adv. Phys. Theor. Appl. 2015, 44, 139–148. [Google Scholar]
- Jaswal, V.S.; Arora, A.K.; Kinger, M.; Gupta, V.D.; Singh, J. Synthesis and characterization of chromium oxide nanoparticles. Orient. J. Chem. 2014, 30, 559–566. [Google Scholar] [CrossRef]
- Sato, T. The thermal transformation of alumina monohydrate, boehmite. J. Appl. Chem. 1962, 12, 9–12. [Google Scholar] [CrossRef]
- Marfunin, A.S. Physics of Minerals and Inorganic Materials; Springer: Berlin/Heidelbreg, Germany; New York, NY, USA, 1979. [Google Scholar]
- Orgel, L.E. Ion compression and the colour of ruby. Nature 1957, 179, 1348. [Google Scholar] [CrossRef]
- Cheng, B.; Qu, S.; Zhou, H.; Wang, Z. Al2O3:Cr3+ nanotubes synthesized via homogenization precipitation followed by heat treatment. J. Phys. Chem. B 2006, 110, 15749–15754. [Google Scholar] [CrossRef] [PubMed]
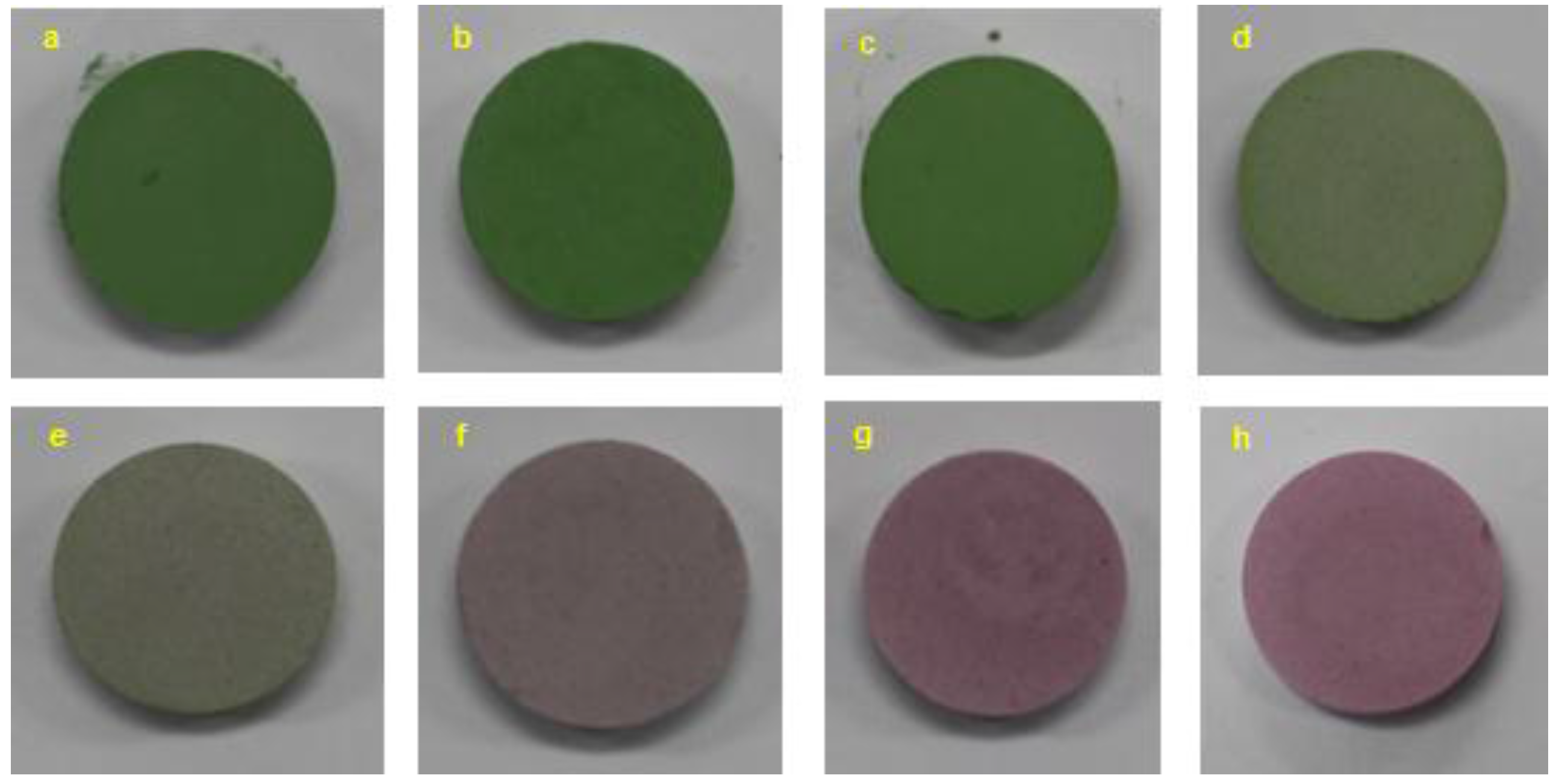
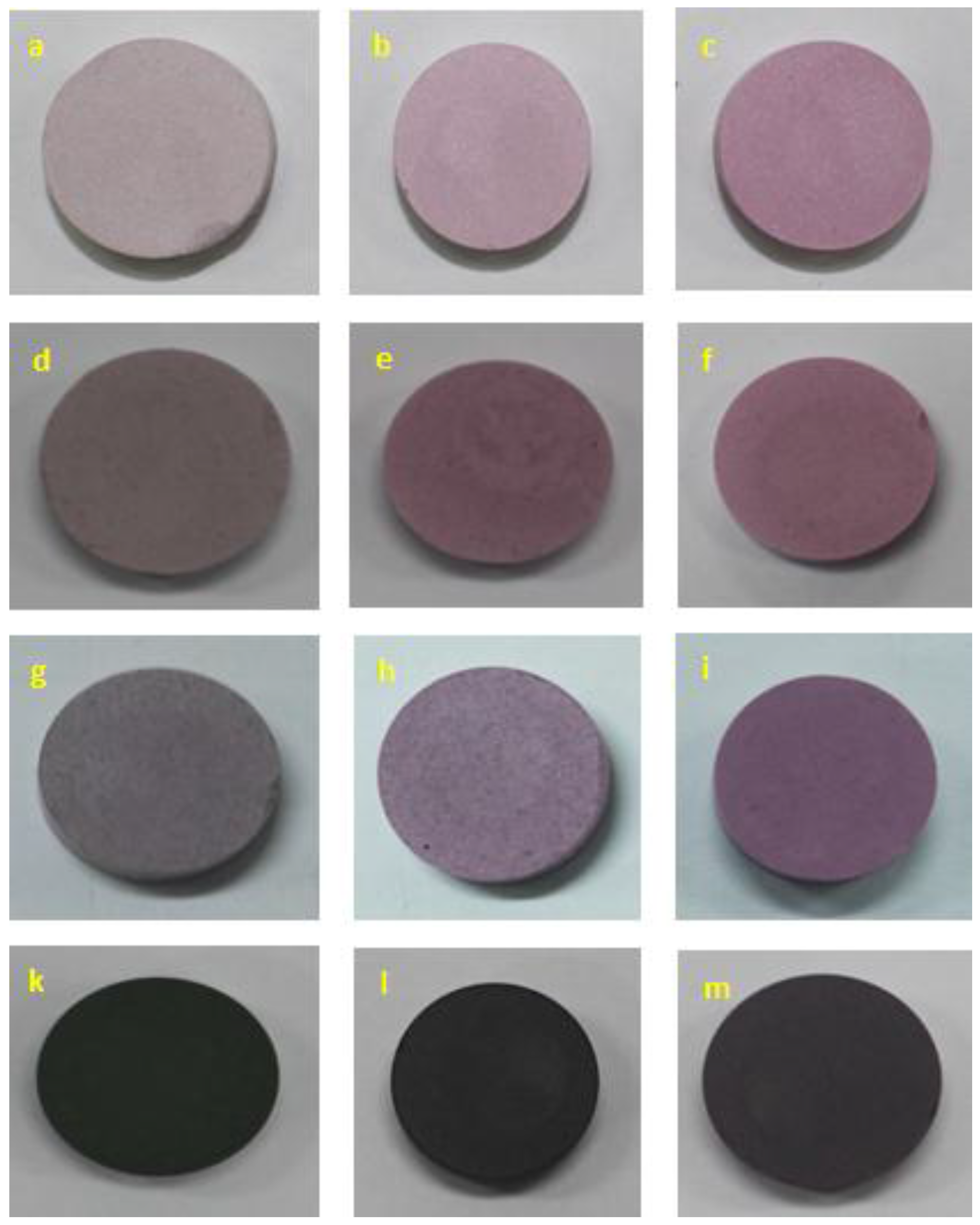


| Cr2O3 Content (wt %) | Annealing Temperature T (°C) | L* | a* | b* |
|---|---|---|---|---|
| 5% | 1400 | 71.45 | 0.72 | 3.02 |
| 1500 | 72.51 | 6.34 | −1.05 | |
| 1600 | 73.48 | 8.1 | −2.4 | |
| 10% | 1000 | 56.38 | −13.56 | 16.97 |
| 1100 | 56.12 | −14.54 | 18.44 | |
| 1200 | 61.24 | −14.26 | 15.78 | |
| 1300 | 62.57 | −9.23 | 10.45 | |
| 1400 | 65.08 | −1.89 | 4.65 | |
| 1500 | 65.43 | 4.68 | 0.47 | |
| 1600 | 65.94 | 7.03 | −1.43 | |
| 20% | 1400 | 57.18 | −6.24 | 7.43 |
| 1500 | 55.85 | −0.45 | 3.9 | |
| 1600 | 55.77 | 2.2 | 1.96 | |
| 40% | 1400 | 45.7 | −13.04 | 11.86 |
| 1500 | 43.7 | −10.68 | 9.72 | |
| 1600 | 42.02 | −9.09 | 8.48 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.K.; Lee, H.; Kim, I.-T. Synthesis and Thermochromic Properties of Cr-Doped Al2O3 for a Reversible Thermochromic Sensor. Materials 2017, 10, 476. https://doi.org/10.3390/ma10050476
Nguyen DK, Lee H, Kim I-T. Synthesis and Thermochromic Properties of Cr-Doped Al2O3 for a Reversible Thermochromic Sensor. Materials. 2017; 10(5):476. https://doi.org/10.3390/ma10050476
Chicago/Turabian StyleNguyen, Duy Khiem, Heesoo Lee, and In-Tae Kim. 2017. "Synthesis and Thermochromic Properties of Cr-Doped Al2O3 for a Reversible Thermochromic Sensor" Materials 10, no. 5: 476. https://doi.org/10.3390/ma10050476






