Osteoblast Cell Response on the Ti6Al4V Alloy Heat-Treated
Abstract
:1. Introduction
2. Results and Discussion
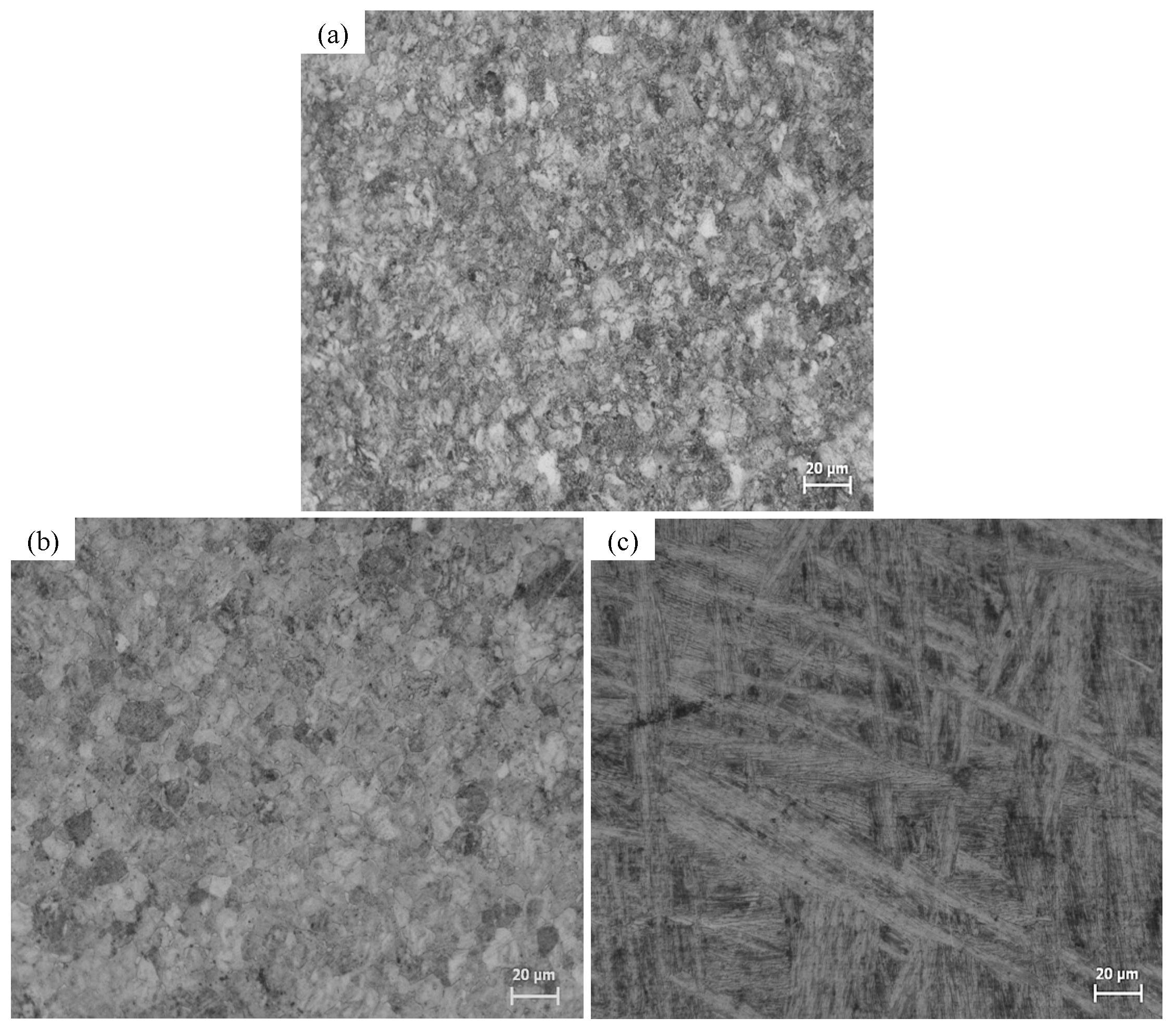
2.1. Microstructural Characterization
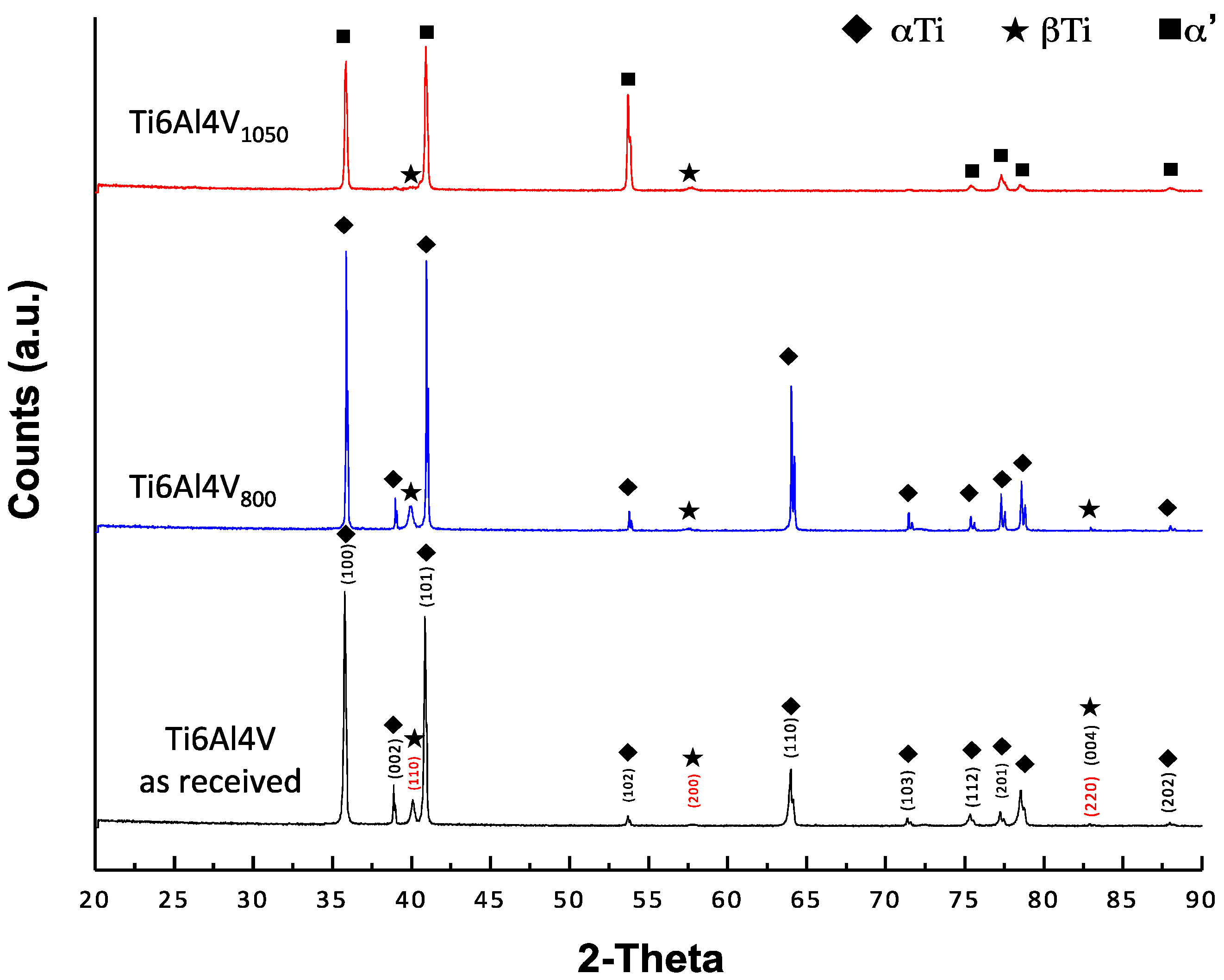
2.2. X-ray Diffraction Analysis (XRD)
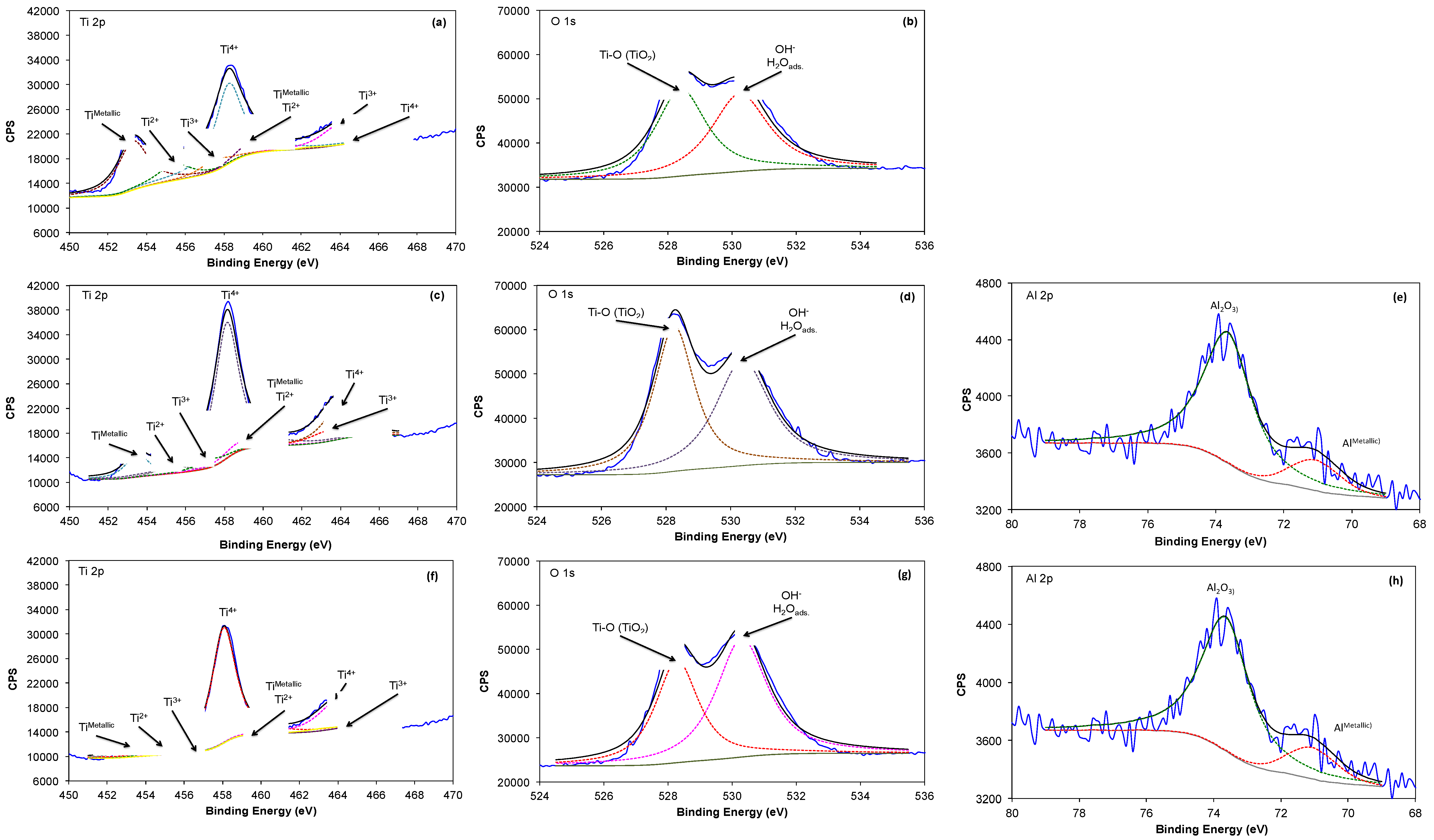
2.3. X-ray Photoelectron Spectroscopy Analysis (XPS)
2.4. Electrochemical Characterization
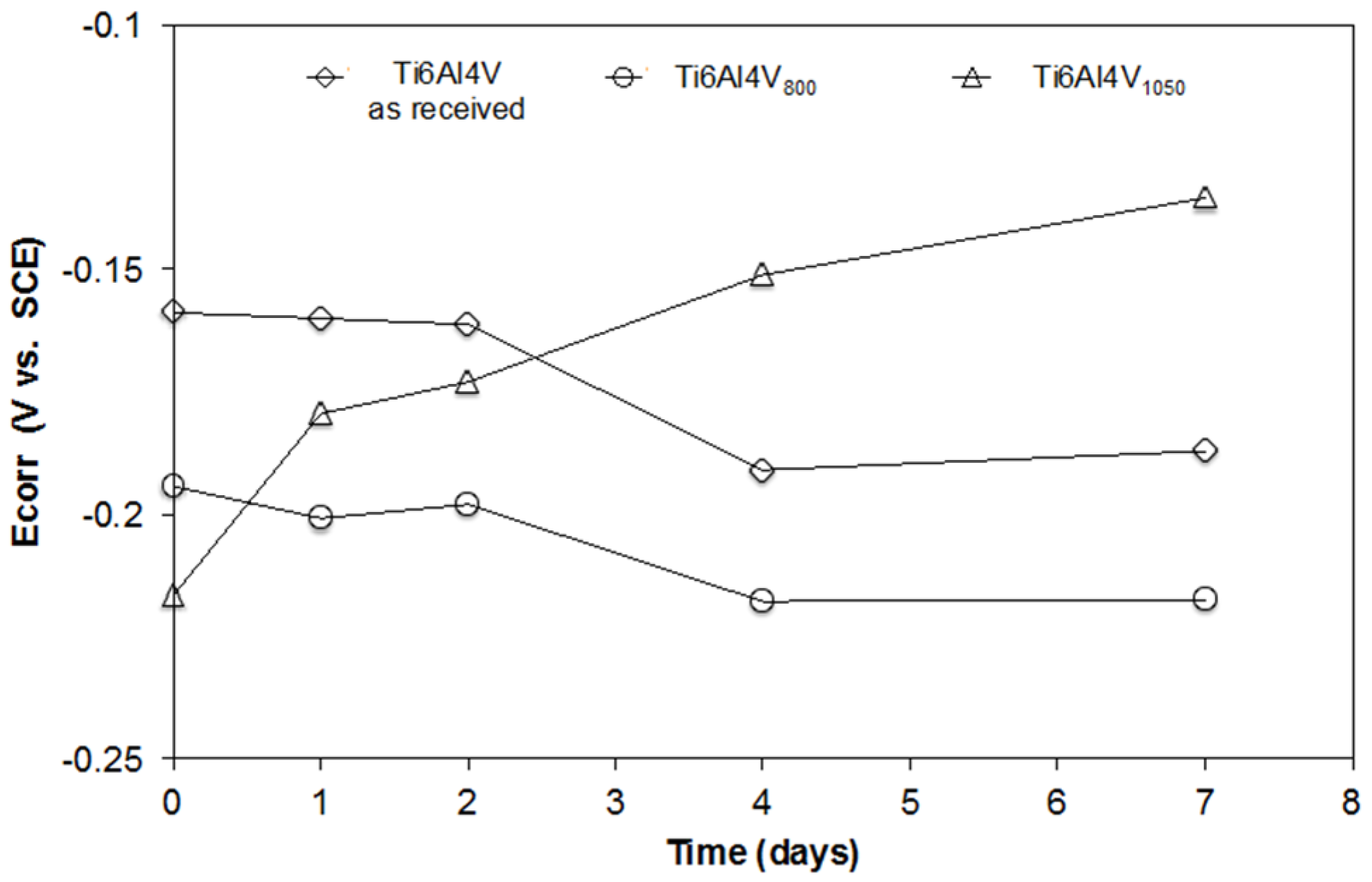
2.4.1. Evolution of the Open Circuit Potential (OCP)
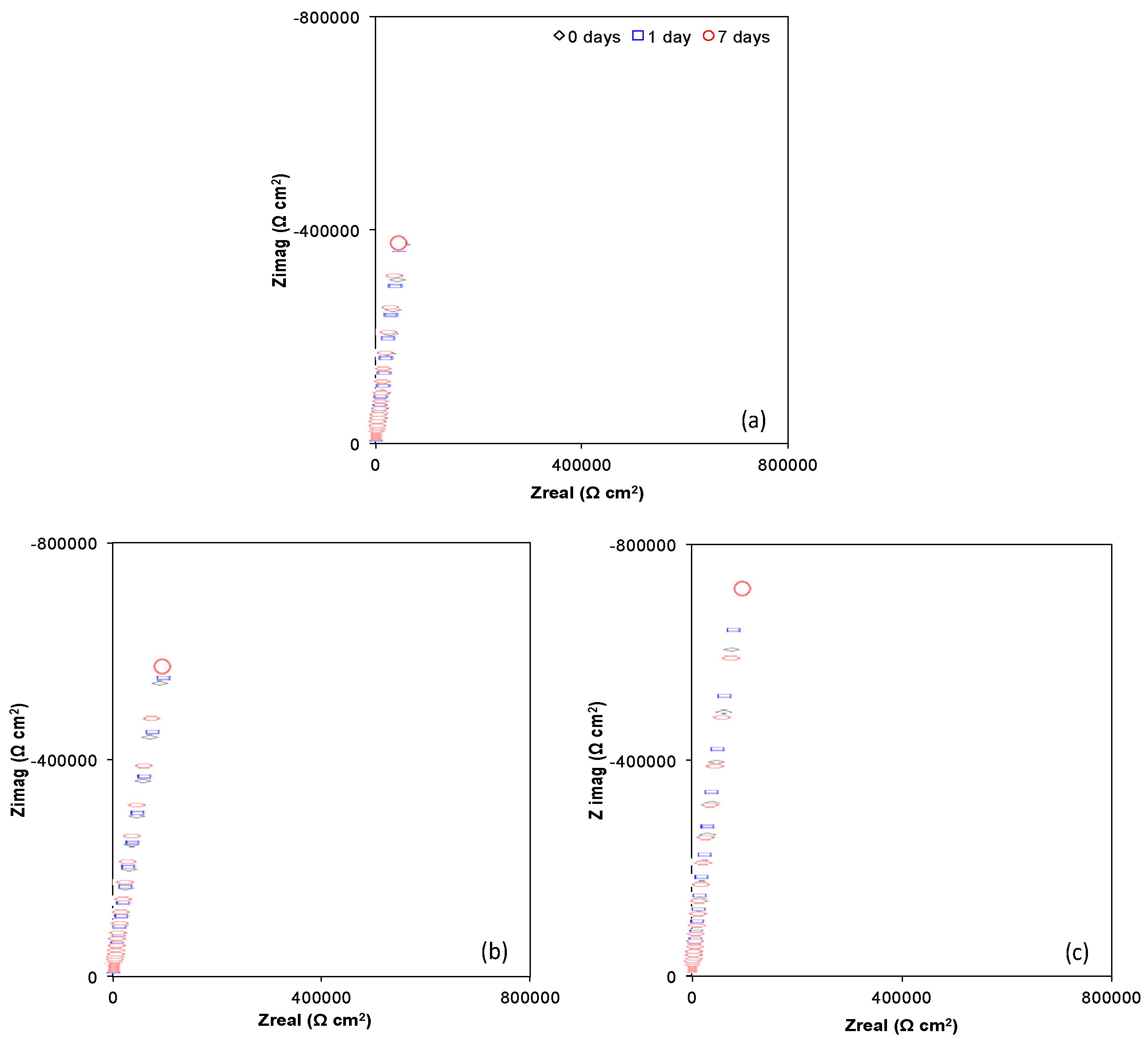
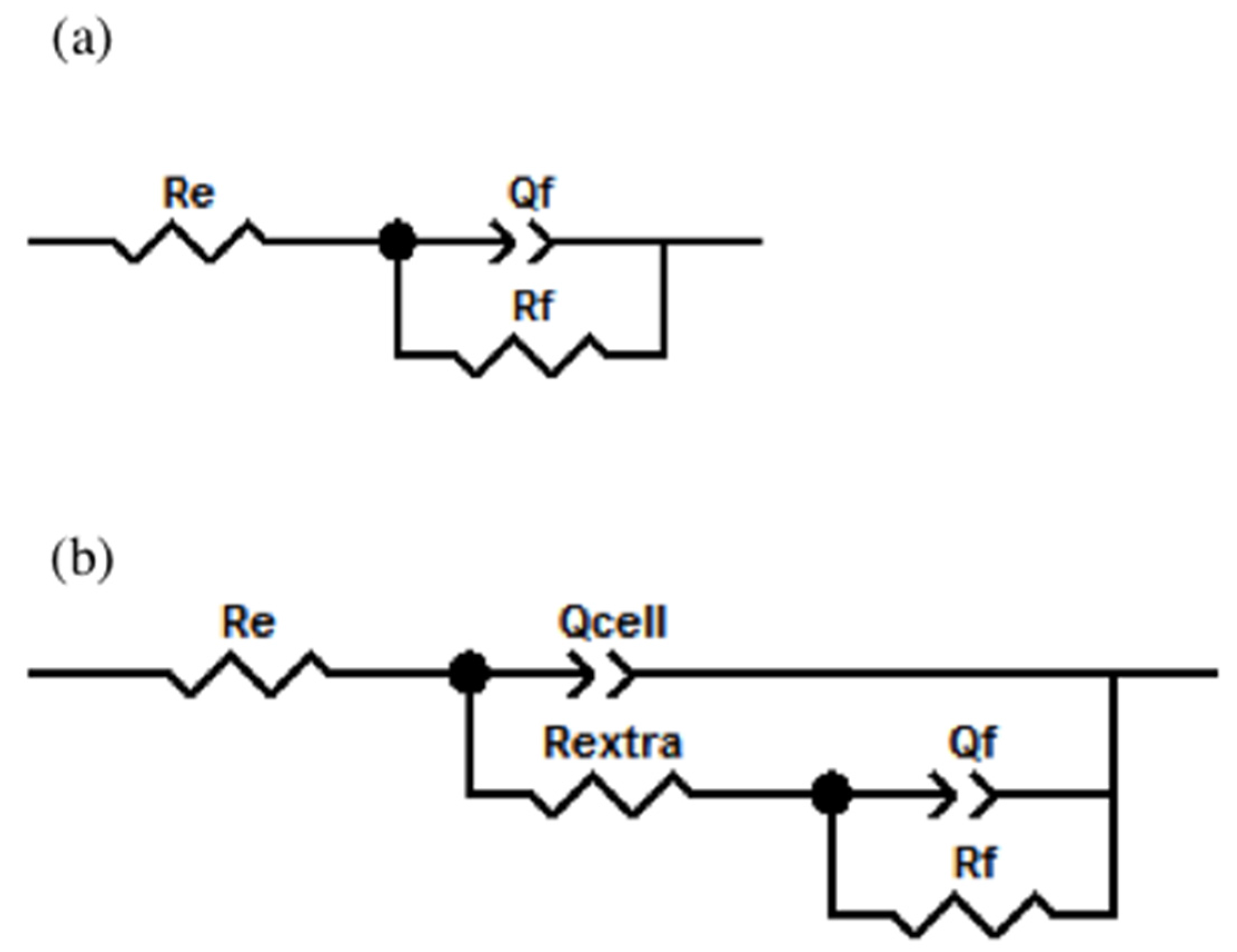
2.4.2. Electrochemical Impedance Spectroscopy Characterization (EIS)
2.4.3. Morphological Observation
3. Materials and Methods
3.1. Heat Treatments
3.2. Microstructure Revealing
3.3. Electrochemical Cell
3.4. Cell Culture Medium
3.5. In Vitro Assays
3.6. Cell Fixation
3.7. Surface Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier Academic Press: New York, NY, USA, 2004. [Google Scholar]
- Scholz, M.S.; Blanchfield, J.P.; Bloom, L.D.; Coburn, B.H.; Elkington, M.; Fuller, J.D.; Gilbert, M.E.; Muflahi, S.A.; Pernice, M.F.; Rae, S.I.; et al. The use of composite materials in modern orthopedic medicine and prosthetic device. Comps. Sci. Technol. 2011, 71, 1791–1803. [Google Scholar] [CrossRef]
- Waite, D.E. Overview and historical perspective of oral reconstructive surgery. Oral Surg. Oral Med. Oral Pathol. 1989, 68, 495–498. [Google Scholar] [CrossRef]
- Lizuca, T.; Hallermann, W.; Seto, I.; Smolka, W. A titanium arch bar for maxillomandibular fixation in oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 2006, 64, 989–992. [Google Scholar]
- Castner, D.G.; Ratner, B.D. Biomedical surface science: Foundations to frontiers. Surf. Sci. 2002, 500, 28–60. [Google Scholar] [CrossRef]
- Sumita, M. Present status and future trend of metallic materials used in orthopedics. Orthop. Surg. 1997, 48, 927–934. [Google Scholar]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys and related materials for biomedical applications. Mater. Sci. Eng. R. Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Bhure, R.; Mahapatro, A. Surface pattering using self-assembled monolayers (SAMs). Biomaterials 2010, 10, 65–107. [Google Scholar]
- Williams, D.F. (Ed.) Titanium and titanium alloys. In Biocompatibility of Clinical Implant Materials; CRC Press Inc.: Boca Raton, FL, USA, 1981; Volume I. [Google Scholar]
- Goldberg, J.R.; Gilbert, J.L. The electrochemical and mechanical behavior of passivated and TiN/AlN coated CoCrMo and Ti6Al4V alloys. Biomaterials 2004, 25, 851–864. [Google Scholar] [CrossRef]
- Ramires, J.; Guastaldi, A.C. Study of Ti-6Al-4V biomaterial using electrochemistry and XPS techniques. Qui. Nova. 2002, 25, 10–14. [Google Scholar] [CrossRef]
- Browne, M.; Gregson, P.J. Effect of mechanical surface pretreatment on metal ion release. Biomaterials 2000, 21, 385–392. [Google Scholar] [CrossRef]
- Popa, M.V.; Demetrescu, I.; Vasilescu, E.; Drob, P.; Santana López, A.; Mirza-Rosca, J.; Vasilescu, C.; Ionita, D. Corrosion susceptibility of implant materials Ti-5Al-4V and Ti-6Al-4Fe in artificial extra-cellular fluids. Electrochim. Acta. 2004, 49, 2121–2123. [Google Scholar] [CrossRef]
- Contu, F.; Elsener, B.; Bohni, H. Characterization of implant materials in fetal bovine serum and sodium-sulfate by electrochemical impedance spectroscopy-I-Mechanically polished samples. J. Biomed. Mater. Res. 2002, 62, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Lee, T.M. Effect of surface chemistry and characteristics of Ti6Al4V on the Ca and P adsorption and ion dissolution in Hanks ethylene diamine tetraacetic acid solution. Biomaterials 2002, 23, 2917–2995. [Google Scholar] [CrossRef]
- Frauchiger, L.; Taborelli, M.; Aronsson, B.O.; Descouts, P. Ion adsorption on titanium surface exposed to a physiological solution. Appl. Surf. Sci. 1999, 143, 67–77. [Google Scholar] [CrossRef]
- Jandt, K.D. Atomic force microscopy of biomaterials surfaces and interfaces. Surf. Sci. 2001, 49, 303–332. [Google Scholar] [CrossRef]
- Mathieu, H.J. Bioengineered material-surfaces for medical applications. Surf. Interface Anal. 2001, 32, 303–332. [Google Scholar] [CrossRef]
- Karimzadeh, F.; Heidarbeigy, M.; Saatchi, A. Effect of heat treatment on corrosion behavior of Ti-6Al-4V alloy weldments. J. Mat. Process. Tech. 2008, 206, 388–394. [Google Scholar] [CrossRef]
- Gil, F.J.; Fernández, E.; Arcas, R.; Planell, J.A. Endurecimiento superficial mediante tratamiento térmicos y anodizado de la aleación Ti-6A1–4U para implantes quirúrgicos. Biomecánica 1994, 2, 51–53. [Google Scholar]
- Geetha, M.; Kamachi Mudali, U.; Gogia, A.K.; Asokamani, R.; Raj, B. Influence of microstructure and alloying elements on corrosion behavior of Ti-13Nb-13Zr alloy. Corros. Sci. 2004, 46, 877–892. [Google Scholar] [CrossRef]
- Vinothkumar, T.S.; Miglani, R.; Lakshminarayananan, L. Influence of deep dry cryogenic treatment on cutting efficiency and wear resistance of nickel–titanium rotary endodontic instruments. J. Endodontics 2007, 33, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Lin, J.; Hodgson, P.D.; Wen, C.E. Effect of heat-treatment atmosphere on the bond strength of apatite layer on Ti substrate. Dent. Mater. 2008, 24, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.C.; Wu, E.Y.; Pan, Y.N.; Ou, K.L. Effects of chemical and heat treatments on surface characteristics and biocompatibility of titanium-niobium alloys. Mat. Trans. 2007, 48, 2978–2985. [Google Scholar] [CrossRef]
- Farhang, P.; Pupak, A.; Sirous, A. Influence of mechanical and chemical surface treatments on the formation of bone-like structure in cpTi for endosseous dental implants. Appl. Surf. Sci. 2012, 259, 283–287. [Google Scholar]
- Lee, B.H.; Lee, C.; Kim, D.G.; Choi, K.; Lee, K.H.; Kim, Y.H. Effect of surface structure on biomechanical properties and osseointegration. Mat. Sci. and Eng. C. 2008, 28, 1448–1461. [Google Scholar] [CrossRef]
- Geetha, M.; Durgalaksshmi, D.; Asokamani, R. Biomedical Implants: Corrosion and its Prevention-A Review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants–A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Alipour, R.; Khani, A.; Mohammadi, R.; Rostami, S. The effect of formation of titanium nitride thin film on surface characteristics of titanium by nitrogen ion implantation. J. Chem. Res. 2016, 40, 1–62. [Google Scholar] [CrossRef]
- Cooper, L.F.; Masuda, T.; Whitson, S.W.; Yliheikkila, P.; Felton, D.A. Formation of mineralizing osteoblast cultures on machined, titanium oxide grit–blasted, and plasma-sprayed titanium surfaces. Int. J. Oral Maxillofac. Implants 1999, 14, 37–47. [Google Scholar] [PubMed]
- Mróz, W.; Bunder, B.; Syroka, R.; Niedzielski, K.; Golanski, G.; Slósarczyk, A.; Schwarz, D.; Douglas, T.E. In vivo implantation of porous titanium alloy implants coated with magnesium-doped octacalcium phosphate and hydroxyapatite thin films using pulsed laser deposition. J. Biomed. Mater. Res. B 2015, 103, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Sing, S.L.; Yeong, W.Y.; Wiria, F.E.; Tay, B.Y. Characterization of titanium lattice structures fabricated by selective laser melting using an adapted compressive test method. Exp. Mech. 2016, 56, 735–748. [Google Scholar] [CrossRef]
- Vydehi Arun, J. (Ed.) Titanium Alloys: An Atlas of Structures and Fracture Features, Physical Metallurgy of Titanium Alloys; CRC Tylor & Francis: Boca Ratón, FL, USA, 2006. [Google Scholar]
- Donachie, M.J. (Ed.) Titanium a Technical Guide. Understanding the Metallurgy of Titanium; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mat. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Hanawa, T.; Asami, K.; Asaoka, K. Repassivation of titanium and surface oxide film regenerated in simulated bioliquid. J. Biomed. Mater. Res. A 1998, 40, 530–538. [Google Scholar] [CrossRef]
- Da Fonseca, C.; Boudin, S.; da Cunha-Belo, M.J. Characterization of titanium passivation films by in situ ac impedance measurements and XPS analysis. J. Electroanal. Chem. 1994, 379, 173–180. [Google Scholar] [CrossRef]
- Vrancken, B.; Thijs, L.; Kruth, J.P.; van Humbeeck, J. Heat treatment of Ti6Al4V produced by Selective Laser Melting: Microstructure and mechanical properties. J. Alloys Comp. 2012, 541, 177–185. [Google Scholar] [CrossRef]
- Sallica-Leva, E.; Jardini, A.L.; Fogagnolo, J.B. Microstructure and mechanical behavior of porous Ti–6Al–4V parts obtained by selective laser melting. J. Mech. Behav. Biomed. 2013, 26, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Yang, R.; Niinomi, M.; Hao, Y.L.; Cui, Y.Y. Formation and growth of calcium phosphate on the surface of oxided Ti-29Nb-13Ta-4.6Zr alloy. Biomaterials 2004, 25, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Malinov, S.; Guo, Z.; Sha, W.; Wilson, A. Differential scanning calorimetry study and computer modelling of β ⇒ α phase transformation in Ti-6Al-4VAlloy. Met. Mat. Trans. A 2001, 32, 879–887. [Google Scholar] [CrossRef]
- Malinov, S.; Sha, W.; Guo, Z.; Tang, C.C.; Long, A.E. Synchrotron X-ray diffraction study of the phase transformations in titanium alloys. Mater. Charact. 2002, 48, 279–295. [Google Scholar] [CrossRef]
- Elmer, J.W.; Palmer, T.A.; Babu, S.S.; Specht, E.D. In situ observations of lattice expansion and transformation rates of α and β phases in Ti–6Al–4V. Mat. Sci. Eng. A 2005, 391, 104–113. [Google Scholar] [CrossRef]
- Feliu, S., Jr.; Barranco, V. XPS study of the surface chemistry of conventional hot-dip galvanised pure Zn, galvanneal and Zn–Al alloy coatings on steel. Acta Mater. 2003, 51, 5413–5424. [Google Scholar] [CrossRef]
- Variola, F.; Yi, J.H.; Richert, L.; Wuest, J.D.; Rosei, F.; Nanci, A. Tailoring the surface properties of Ti6Al4V by controlled chemical oxidation. Biomaterials 2008, 29, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nakai, M.; Akahori, T.; Niinomi, M.; Tsutsumi, Y.; Doi, H.; Hanawa, T. Characterization of air-formed surface oxide film on Ti–29Nb–13Ta–4.6Zr alloy surface using XPS and AES. Corros. Sci. 2008, 50, 2111–2116. [Google Scholar] [CrossRef]
- Bunker, B.C.; Nelson, G.C.; Zavadil, K.R.; Barbour, J.C.; Wall, F.D.; Sullivan, J.P. Hydration of passive oxide films on aluminum. J. Phys. Chem. B 2002, 106, 4705–4713. [Google Scholar] [CrossRef]
- Armstrong, N.R. Auger and X-Ray Photoelectron spectroscopic and electrochemical characterization of titanium thin film electrodes. Surf. Sci. 1977, 67, 415–468. [Google Scholar] [CrossRef]
- Strohmeier, B.R. An ESCA method for determining the oxide thickness on aluminium-alloys. Surf. Interface Anal. 1990, 15, 51–56. [Google Scholar] [CrossRef]
- Milosev, I.; Metikos-Hukovic, M.; Strehblow, H.H. Passive film on orthopaedic TiAlV alloy formed in physiological solution investigated by X-ray photoelectron spectroscopy. Biomaterials 2000, 21, 2103–2113. [Google Scholar] [CrossRef]
- Hiromoto, S.; Hanawa, T. pH near cells on stainless steel and titanium. Electrochem. Solid State Lett. 2004, 7, B9–B11. [Google Scholar] [CrossRef]
- Hiromoto, S. Corrosion of metallic biomaterials in cell culture environments. Electrochem. Soc. Interface 2008, 17, 41–44. [Google Scholar]
- De Assis, S.L.; Wolynec, S.; Costa, I. Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim. Acta. 2006, 51, 1815–1819. [Google Scholar] [CrossRef]
- Souto, M.R.; Laz, M.M.; Reis, R.L. Degradation characteristics of hydroxyapatite coatings on orthopaedic TiAlV in simulated physiological media investigated by electrochemical impedance spectroscopy. Biomaterials 2003, 24, 4213–4221. [Google Scholar] [CrossRef]
- Lavos-Valereto, I.C.; Wolynec, S.; Ramires, I.; Guastaldi, A.C.; Costa, I. Electrochemical impedance spectroscopy characterization of passive film formed on implant Ti6Al7Nb alloy in Hank’s solution. J. Mater. Sci.: Mater. Med. 2004, 15, 55–59. [Google Scholar]
- Chikarakara, E.; Fitzpatrick, P.; Moore, E.; Levingstone, T.; Grehan, L.; Higginbotham, C.; Vázquez, M.; Bagga, K.; Naher, S.; Brabazon, D. In vitro fibroblast and pre-osteoblastic cellular responses on laser surface modified Ti–6Al–4V. Appl. Surf. Sci. 2016, 366, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Kubies, D.; Himmlová, L.; Riedel, T.; Chánová, E.; Balík, K.; Douděrová, M.; Bártová, J.; Pešáková, V. The Interaction of Osteoblasts with Bone-Implant Materials: 1. The effects of physicochemical surface properties of implant materials. Physiol. Res. 2011, 60, 95–111. [Google Scholar] [PubMed]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Castellani, C.; Lindtner, R.A.; Hausbrandt, P.; Tschegg, E.; Stanzl-Tschegg, S.E.; Zanoni, G.; Beck, S.; Weinberg, A.M. Bone–implant interface strength and osseointegration: Biodegradable magnesium alloy versus standard titanium control. Acta Biomater. 2011, 1, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Pan, J.; Wroblewsli, J.; Leygraf, C.; Arvidson, K. Electrochemical impedance spectroscopy and X-ray photoelectron spectroscopy analysis of titanium surfaces cultured with osteoblast-like cells derived from human mandibular bone. J. Biomed. Mater. Res. 2002, 59, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef]
- Keller, J.C.; Stanford, C.M.; Wightman, J.P.; Draughn, R.A.; Zaharias, R. Characterizations of titanium implant surface III. J. Biomed. Mater. Res. 1994, 28, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Chesmel, K.D.; Clark, C.C.; Brighton, C.T.; Black, J. Cellular responses to chemical and morphologic aspects of biomaterial surfaces. II. The biosynthetic and migratory response of bone cell populations. J. Biomed. Mater. Res. 1995, 29, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Lausma, J.; Kasemo, B. Surface spectroscopic characterization on titanium implant materials. Appl. Surf. Sci. 1990, 44, 133–146. [Google Scholar] [CrossRef]
- García-Alonso, M.C.; Saldaña, L.; Alonso, C.; Barranco, V.; Muñoz-Morris, M.A.; Escudero, M.L. In situ cell culture monitoring on a Ti–6Al–4V surface by electrochemical techniques. Acta Biomater. 2009, 5, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Göpel, W.; Anderson, J.A.; Frenkel, D.; Jeaning, M.; Philips, K.; Schäfer, J.A.; Rocker, G. Surface defects of TiO2 (1 1 0): a combined XPS, XAES and ELS study. Surf. Sci. 1984, 67, 333–346. [Google Scholar] [CrossRef]
- Acevedo-Peña, P.; Vázquez-Arenas, J.; Cabrera-Sierra, R.; Lartundo-Rojas, L.; González, I. Ti anodization in alkaline electrolyte: The relationship between transport of defects, film hydration and composition. J. Electrochem. Soc. 2013, 160, 277–284. [Google Scholar] [CrossRef]
- Acevedo-Peña, P.; Vázquez-Arenas, J.; Cabrera-Sierra, R.; Lartundo-Rojas, L.; González, I. Hydration and structural transformations during titanium anodization under alkaline conditions. ECS Trans. 2013, 50, 21–32. [Google Scholar] [CrossRef]
- Hanawa, T.; Hiromoto, S.; Asami, K.; Okuno, O.; Asaoka, K. Surface oxide films on titanium alloys regenerated in Hank’s solution. Mater. Trans. 2002, 43, 3000–3004. [Google Scholar] [CrossRef]
- Calderón-Moreno, J.M.; Vasilescu, C.; Drob, S.I.; Ivanescu, S.; Osiceanu, P.; Drob, P.; Popa, M.; Preda, S.; Vasilescu, E. Microstructural and mechanical properties, surface and electrochemical characterization of a new Ti-Zr-Nb alloy for implant applications. J. Alloys Compd. 2014, 612, 398–410. [Google Scholar] [CrossRef]
- Niinomi, M. Fatigue performance and cytotoxicity of low rigidity titanium alloy Ti-29Nb-13Ta-4.6Zr. Biomaterials 2003, 24, 2673–2683. [Google Scholar] [CrossRef]


| Sample | dTiO2 (nm) |
|---|---|
| Ti6Al4V as received | 2.1 |
| Ti6Al4V800 | 4.8 |
| Ti6Al4V1050 | 5.0 |
| Sample | Time Days | Re (Ω cm2) | Rextra (Ω cm2) | Qcell (Siemens sn) (cm−2) | n | Rf (Ω cm2) | Qf (Siemens sn) (cm−2) | n | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| Ti6Al4V as received | 0 | 57.74 | --- | --- | --- | 1.91 × 107 | 3.19 × 10−5 | 0.862 | 9.9 × 10−3 |
| 1 | 46.13 | 381.2 | 2.82 × 10−5 | 0.887 | 1.78 × 107 | 6.14 × 10−6 | 0.940 | 2.4 × 10−3 | |
| 7 | 50.78 | 146.7 | 2.69 × 10−5 | 0.900 | 1.76 × 107 | 6.77 × 10−6 | 0.958 | 2.3 × 10−3 | |
| Ti6Al4V800 | 0 | 61.11 | --- | --- | --- | 1.75 × 107 | 2.25 × 10−5 | 0.882 | 5.6 × 10−3 |
| 1 | 62.66 | 292.9 | 2.19 × 10−5 | 0.896 | 1.79 × 107 | 2.06 × 10−7 | 0.907 | 2.4 × 10−3 | |
| 7 | 58.29 | 105 | 2.14 × 10−5 | 0.909 | 1.89 × 107 | 2.83 × 10−7 | 0.919 | 2.2 × 10−3 | |
| Ti6Al4V1050 | 0 | 51.20 | --- | --- | --- | 2.10 × 107 | 2.20 × 10−5 | 0.913 | 6.6 × 10−3 |
| 1 | 53.31 | 166.3 | 2.05 × 10−5 | 0.927 | 1.99 × 107 | 2.89 × 10−7 | 0.939 | 2.7 × 10−3 | |
| 7 | 57.82 | 109.6 | 1.82 × 10−5 | 0.936 | 2.19 × 107 | 2.81 × 10−7 | 0.957 | 1.9 × 10−3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Díaz, M.P.; Escudero-Rincón, M.L.; Arce-Estrada, E.M.; Cabrera-Sierra, R. Osteoblast Cell Response on the Ti6Al4V Alloy Heat-Treated. Materials 2017, 10, 445. https://doi.org/10.3390/ma10040445
Chávez-Díaz MP, Escudero-Rincón ML, Arce-Estrada EM, Cabrera-Sierra R. Osteoblast Cell Response on the Ti6Al4V Alloy Heat-Treated. Materials. 2017; 10(4):445. https://doi.org/10.3390/ma10040445
Chicago/Turabian StyleChávez-Díaz, Mercedes Paulina, María Lorenza Escudero-Rincón, Elsa Miriam Arce-Estrada, and Román Cabrera-Sierra. 2017. "Osteoblast Cell Response on the Ti6Al4V Alloy Heat-Treated" Materials 10, no. 4: 445. https://doi.org/10.3390/ma10040445





