7.1. First Researches
As has been noted in the introduction to this review, it is rather surprising that despite the great practical importance of this issue it has only recently that it has started to attract the interest of corrosion scientists.
It is well known that the presence of atmospheric pollutants (natural or anthropogenic) notably accelerates the AC process of CS. The two most common pollutants, which have drawn the majority of research efforts, are SO
2 and marine chlorides. In principle most of the attention has been focused on SO
2, and considerable progress has been made in this respect [
4]. However, as Nishimura et al. [
10,
39] point out, it was not until the final decade of the 20th century that major research started to be carried out on the fundamental mechanisms of rust formation in Cl
−-rich marine atmospheres. Until then very little research was undertaken in this field, the most notable being the work of Keller [
103], Feitknecht [
24], Henriksen [
168] and Misawa [
169,
170,
171].
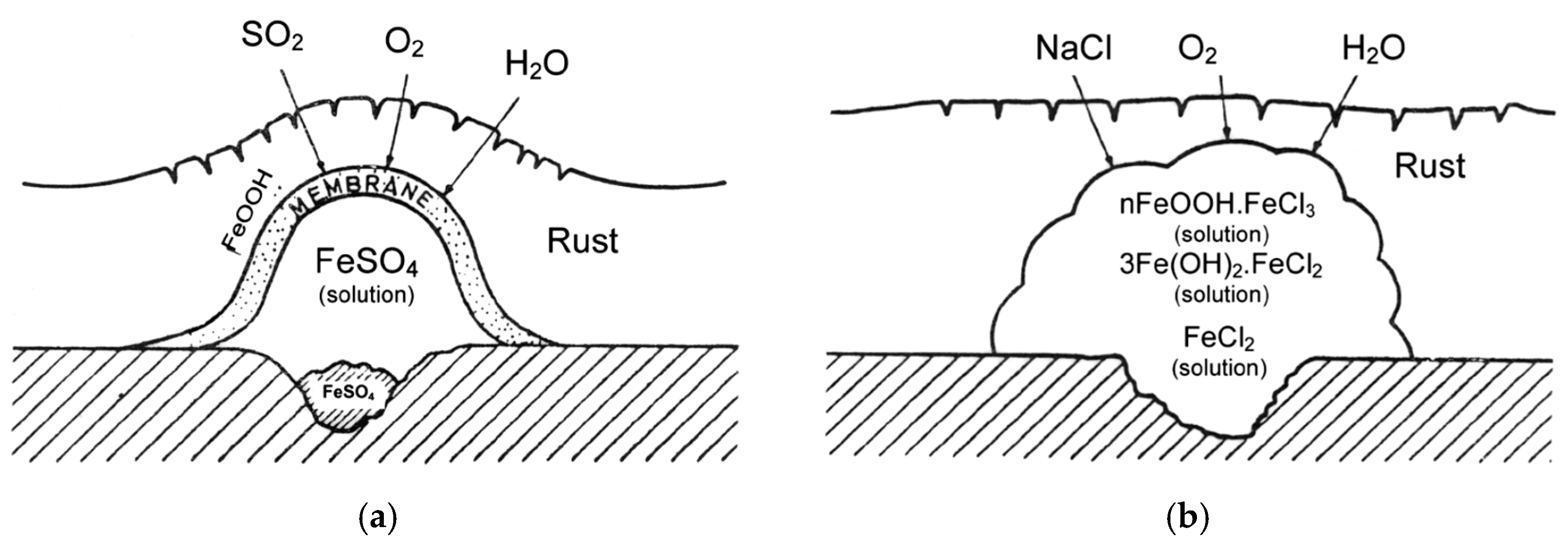
Keller in 1948 [
103] reported three basic chlorides obtained by partial precipitation from FeCl
2 solution in various concentrations, noting that these three basic chlorides were presumably the precursors of GR1. Feitknecht [
24] reported the existence of chloride accumulations (nests) containing FeCl
2 in the rust layers formed on steel exposed in coastal areas and their role in stimulating AC. Henriksen [
168], using autoradiography, noted that the AC of CS in marine atmospheres starts at weak spots in the oxide film. Na
+ and Cl
− in the aqueous adlayer migrate to these weak spots and once Cl
− has been adsorbed and corrosion has begun, Na
+ migrates to the cathodic areas. Misawa et al. [
169,
170,
171], who had carried out important basic research on the formation mechanisms of the different AC products of iron, noted in their work the formation of an intermediate compound, GR1, in marine atmospheres, also in accordance with Keller [
103].
At around the same time, Barton [
4], in his important book on AC, pointed out three causes which explained the high corrosion rates of steel exposed to marine atmospheres: (a) the increase in the ionic conductivity of the aqueous adlayer due to the presence of ionising substances (chlorides); (b) the hygroscopic nature of the Cl
−-containing corrosion products formed; and (c) the solubility of the latter, unlike the stable basic chlorides that form in the case of other metals (Cu, Zn, etc.), indicating that the mechanism which governs the effects of Cl
− ions in AC had not been completely explained.
In addition to the above, two other causes of enormous importance should be mentioned: (a) the strong cathodic depolarising role of Cl
− ions, accelerating the cathodic process by tens and hundreds of times, as formulated in 1961 by Rozenfeld [
18]; and (b) the catalytic role of chlorides [
23,
172]. With regard to the latter effect, it is noted that the anodic reaction generates cations by dissolution and H
+ by hydrolysis of the dissolved cations. Both Fe
2+ and H
+ require neutralisation, which is accomplished by the ingress of chlorides. The locally higher Cl
− concentration enhances local metal dissolution, then draws more Cl
− and enhances dissolution even further [
23]. Migration of Cl
− to the corroding substrate is facilitated by its high permeability in the rust layer. As noted in [
23], this is a feedback mechanism, sometimes referred to as autocatalytic. The high concentrations of Cl
− in the inner rust layer will facilitate the formation of akaganeite, as was also previously noted by Keller [
103] and Misawa [
169].
According to Misawa et al. [
169], when the aqueous adlayer on the metal surface is neutral or slightly acidic, Fe(OH)
2 cannot be formed, but various Fe(II) hydroxo-complexes may be formed, depending on the existing anion in the aqueous solution. Fe(II) hydroxo-complexes thus formed are oxidised by dissolved oxygen, resulting in lepidocrocite through an intermediate GR.
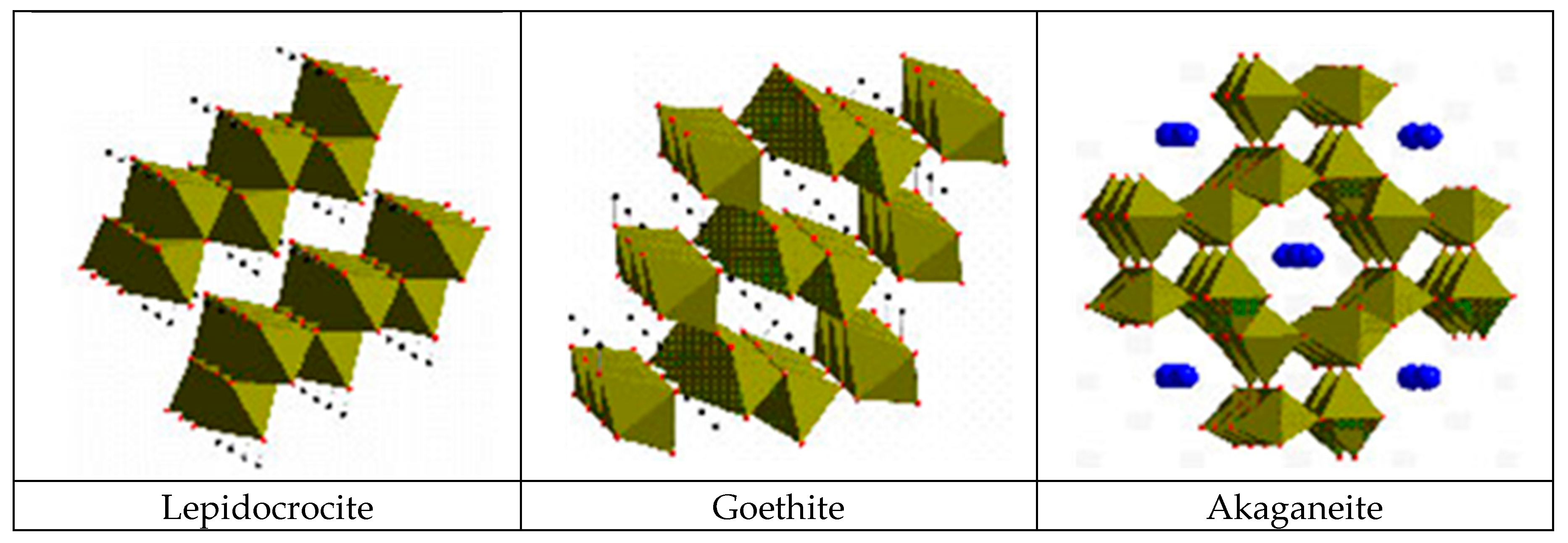
In marine atmospheres, the intermediate generally has a GR1 structure that forms in the presence of Cl
− ion [
96,
107,
108]. GR1 is converted to black magnetite by slow oxidation in solution and this reaction is considered to correspond to the formation of magnetite in the underlying rust layer where the oxygen supply is limited. Also, akaganeite can be obtained by the dry oxidation of solid β-Fe
2(OH)
3Cl precipitated from the slightly acidic solution in the presence of Cl
− ions.
Worch et al. reported in 1983 [
173] that GR1 is often seen on iron and steel exposed to marine environments and that chloride may also be involved catalytically in the formation of akaganeite. Akaganeite is produced only in the presence of sufficient concentrations of Cl
− [
107,
108].
Askey et al. [
25] suggest a cyclical rust formation process, similar to the acid regeneration cycle proposed by Schikorr [
26,
174] for the action of SO
2 in iron, by which the accumulation of Cl
− ions in the underlying steel gives rise to the formation of FeCl
2, which hydrolyses water according to
Releasing HCl. It should be noted that this represents a reaction cycle in which rereleased HCl will react with iron to form fresh FeCl
2. Once started, therefore, the cycle will be independent of incoming HCl. Corrosion will continue until the Cl
− ions are removed (possibly by the washing away of FeCl
2).
7.2. The Fundamental Role of Akaganeite in the Atmospheric Corrosion Process of Steel in Marine Atmospheres
A fundamental advance in relation with the role played by akaganeite in the AC process of steel in marine atmospheres was made by Nishimura et al. in studies carried out in the last decade of the 20th century [
10,
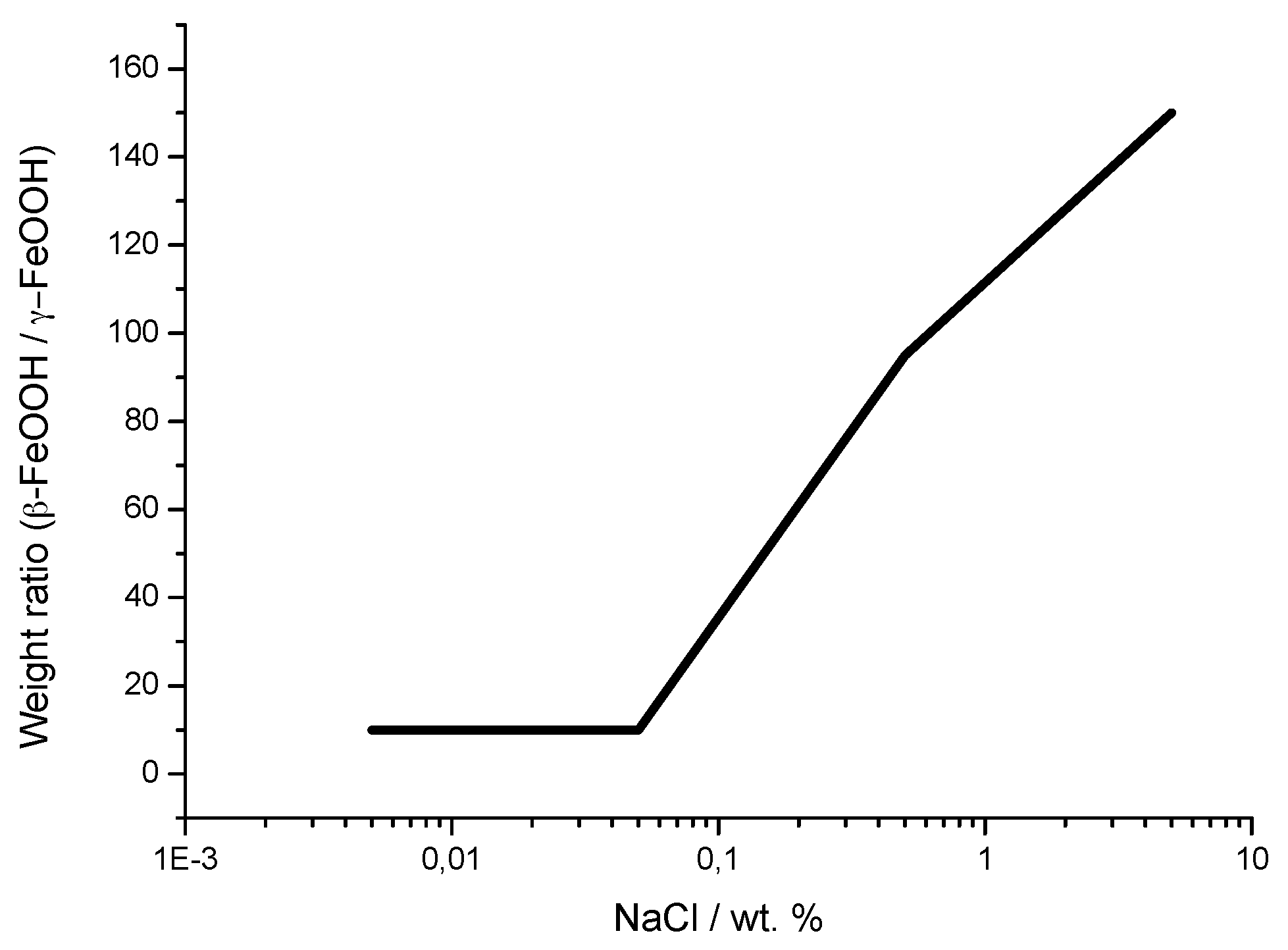
39]. Nishimura et al. in 1995 used a wet and dry corrosion test to study the relationship between steel corrosion resistance and NaCl concentration, analysing the corrosion products by in-situ XRD [
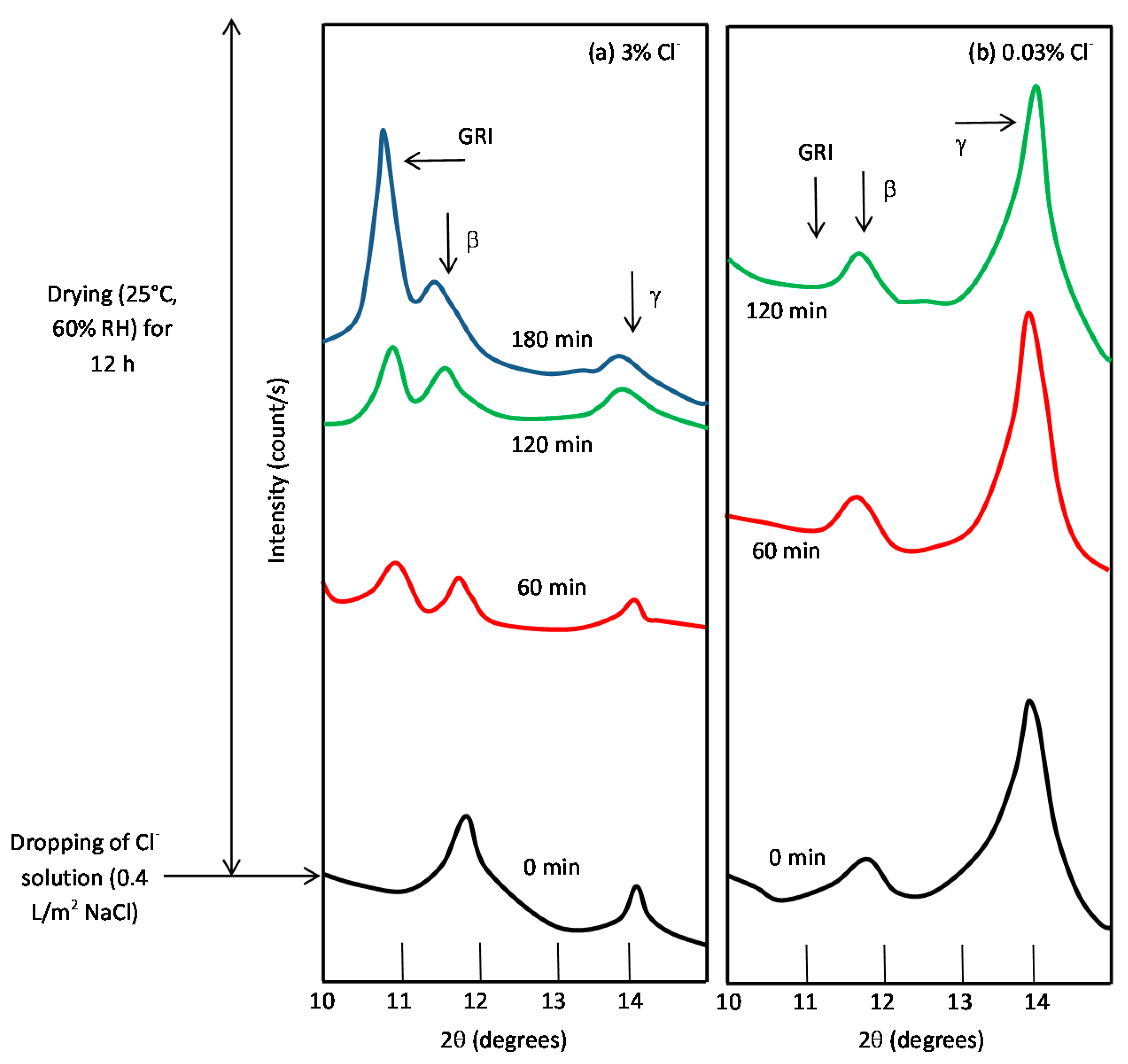
39]. The steel corrosion rate increased as the NaCl concentration rose, and a very strong increase in the akaganeite/lepidocrocite weight ratio was observed from a NaCl concentration of 0.05 wt % (
Figure 30).
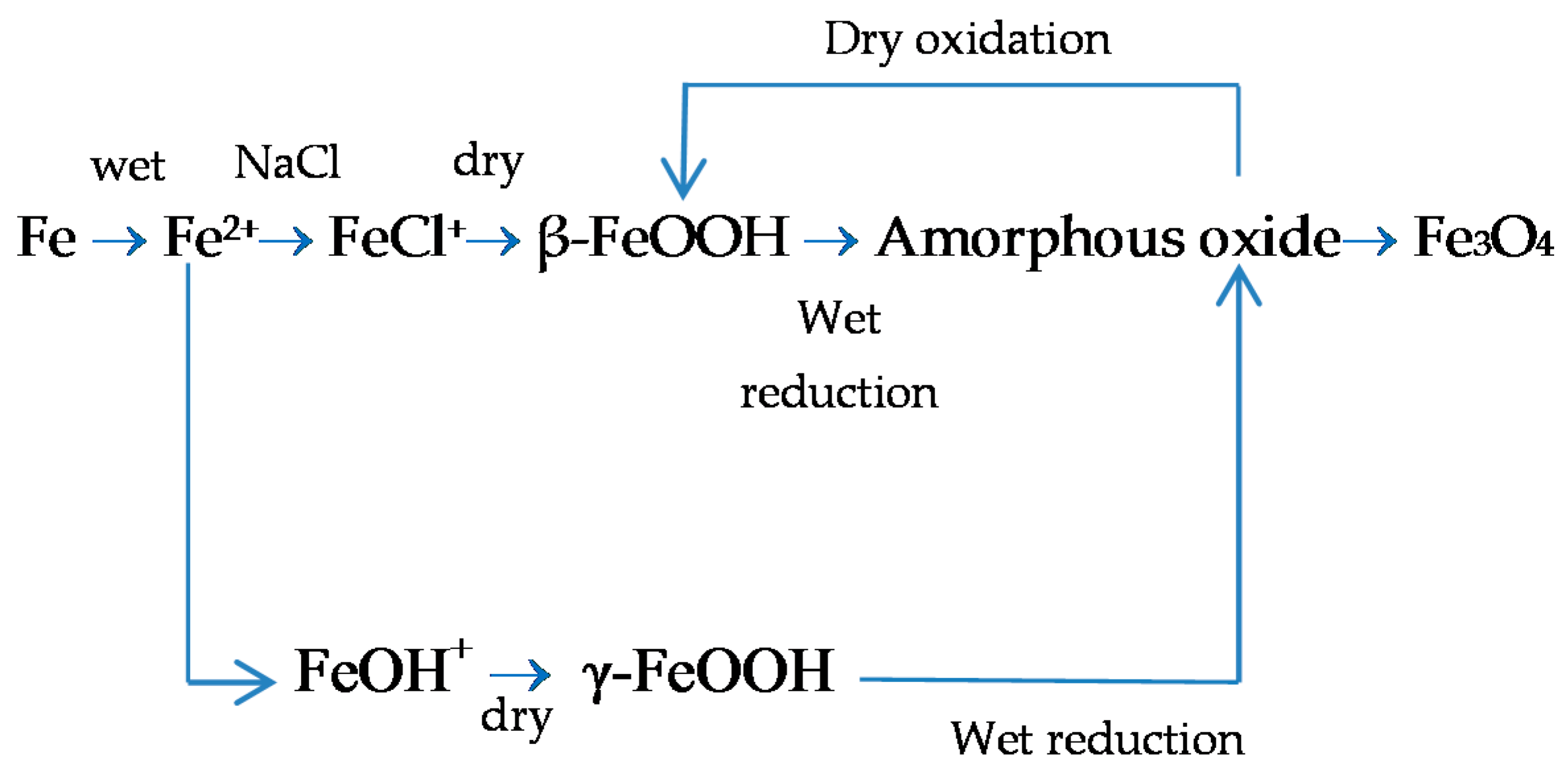
Akaganeite was reduced to an amorphous intermediate oxide during the wet stage of the cycle and reproduced in the dry stage, giving rise to the proposal of the following rusting model of iron in wet and dry corrosion in the presence of NaCl (
Figure 31).
Later, in the year 2000, continuing with the in-situ XRD technique but here in combination with alternating current impedance, Nishimura et al. observed the transition of akaganeite from GR1 in the dry process; the amount of GR1 also depended on the Cl
− ion concentration [
10]. After dripping a Cl
− solution (3% Cl
−) on the steel surface, the dry process progressed with the formation of akaganeite at a high corrosion rate. When a low Cl
− concentration was used (0%–0.3% Cl
−), lepidocrocite was formed from Fe(OH)
2 instead of akaganeite and the corrosion rate was low.
The integral intensity of akaganeite decreased after dripping a Cl
− solution, which implied that akaganeite was consumed in the wet process. After 60 min in the dry process of the cycle the presence of GR1 was detected. GR1 could still be detected after 180 min (
Figure 32), but disappeared after 12 h of testing, indicating that the transformation to akaganeite was complete.
Quantitative analysis of the identified phases was carried out using an XRD standard method. Akaganeite was the most abundant crystalline phase in the iron rust, and its proportion grew considerably as the Cl
− concentration increased, as was previously seen [
39]. The other large phase was goethite. In contrast, the amounts of lepidocrocite and magnetite were low. These two phases were not affected by the Cl
− concentration. Nishimura et al. concluded that in a Cl
−-rich environment the corrosion process was dominated by the formation of akaganeite, rather than lepidocrocite, which acts as the oxidation agent that accelerates corrosion in Cl
−-free environments [
38].
The authors carried out XPS and TEM observations on those portions of iron rust that could not be detected by XRD. It was determined that they contained large amounts of spinel oxide (magnetite structure) with bivalent/trivalent iron. This spinel oxide may have been formed by reduction of akaganeite during the wet process of the cycle.
These findings mark a turning point in the knowledge of the MAC mechanisms of steel, determining that steel corrosion progresses by the formation of akaganeite from GR1 (dry stage or “drying-out stage” following the terminology of Stratmann [
38]) and its reduction (wet stage).
7.3. Initial Stages of MAC
In marine atmospheres, corrosion is generally driven by the deposition of hygroscopic sea salt aerosols that absorb moisture from the environment and form salt droplets. These aerosols range from a few angstroms to several hundred microns in diameter. Lan et al. [
175] point out that coarse sea salt particles are those that contribute most to CS corrosion in marine atmospheres. In coastal marine locations (<2 km from the shoreline) the most common aerosols deposited are in the “coarse mode” size range: 1–100 μm in diameter [
57].
Li and Hihara [
55] studied salt particle deposition and the initial stage of MAC at severe marine test sites. They found both: (a) small sea-salt particles (D < 5 μm) and sea-salt clusters (D < 10 μm) formed by dehydration on the steel substrate that did not corrode under relatively small seawater droplets (D < 30 μm); and (b) sea-salt clusters integrated with iron corrosion products formed on the steel substrate that did corrode from larger seawater droplets (D > 30 μm). The corrosion that occurred under larger seawater droplets (D > 30 μm) showed the typical characteristics of droplet corrosion, with Cl
− and Na
+ ions migrated to the central anode and peripheral cathode, respectively. The corrosion products were identified as lepidocrocite.
These same researchers [
176,
177], in a laboratory study in which they manually deposited NaCl droplets of different diameters on CS steel, used RS to analyse the very initial stage of NaCl particle-induced corrosion. They found that corrosion did not initiate under NaCl droplets with diameters of less than 45 μm after 6 h (at 80% RH). At larger NaCl droplet diameters, corrosion initiated quickly under the droplets in the form of pitting. In-situ and ex-situ Raman spectra show the formation of GR in regions close to the anodic sites and the precipitation of lepidocrocite clusters over cathodic sites surrounding the GR region. Magnetite was detected mostly in the rust clusters formed in the transitional region from GR to lepidocrocite. Upon exposure to ambient air, GR transformed to the more stable lepidocrocite due to oxidation. Li and Hihara underline the need for more research effort in droplet electrochemistry [
178].
Risteen et al. [
179] recently used a new methodology to study corrosion under NaCl solution droplets ranging in diameter from 20 to 1000 μm. They also observed the dependence of the occurrence of corrosion on drop size, noting that this behaviour appears to be strongly dependent on the microstructure and surface finish: corrosion initiation on 1010 steel was dominated by manganese sulfide inclusions when a mirror surface finish was maintained. In contrast, for high purity iron, initiation was dominated by surface roughness.
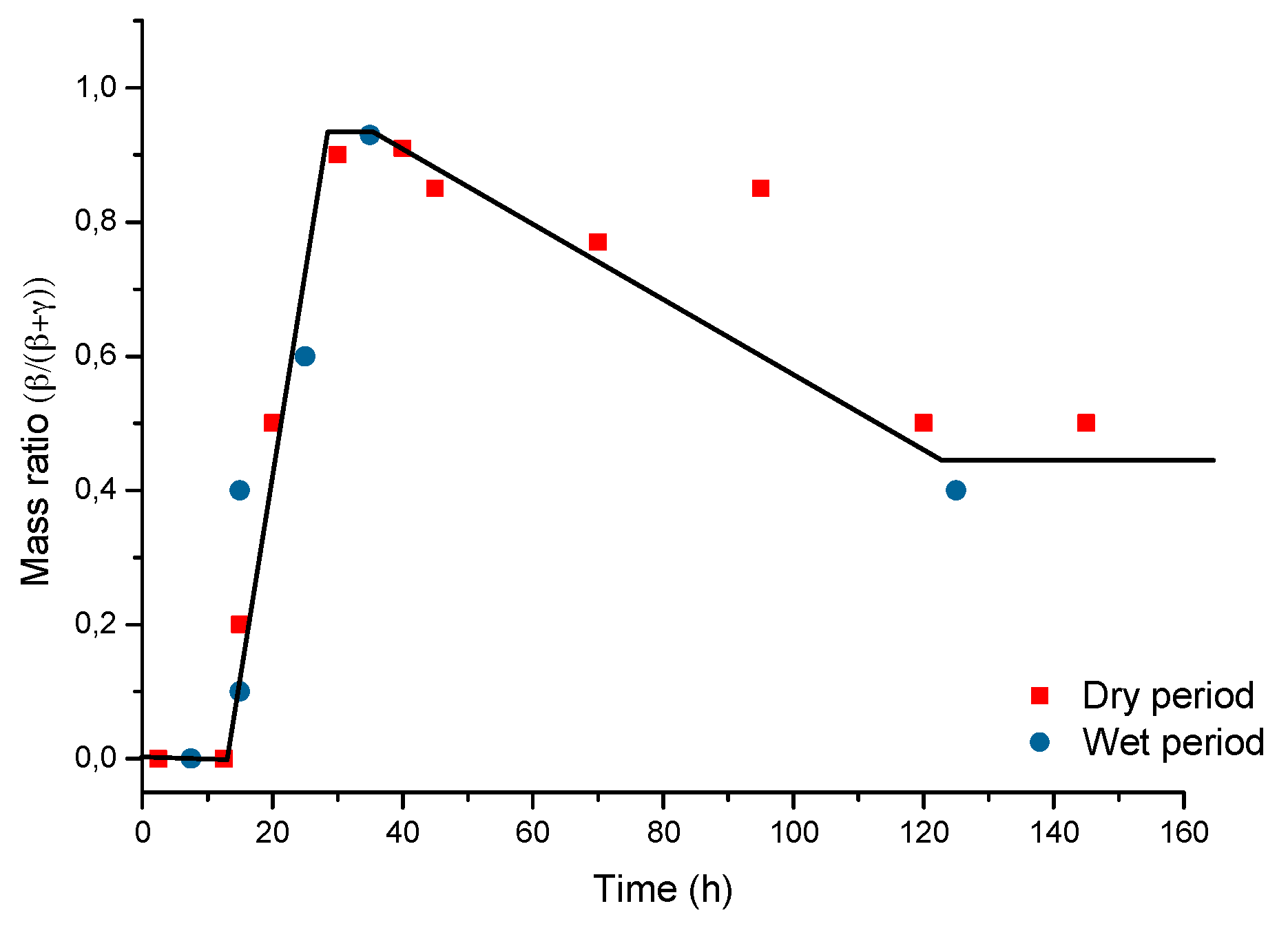
Ohtsuka and Tanaka have recently used RS to carry out a study of changes in rust composition on CS over six days of cyclic exposure: 4 h wet (90% RH)/4 h dry (10% RH) in the presence of NaCl droplets (0.93 and 0.11 mg/cm
2) [
180]. The NaCl solution was first dripped and immediately dried in a vacuum desiccator. A Raman spectrum was recorded every 15 min.
The Raman spectra of the rust surface in the presence of 0.93 mg/cm
2 NaCl deposits corresponded to lepidocrocite and magnetite in the initial 12 h of exposure. After 12 h of exposure, akaganeite started to form, and its molar ratio on the rust surface increased to 90% at 30 h of exposure (
Figure 33). They assume that in order for akaganeite to form, a lepidocrocite + magnetite rust layer of some thickness is required. The Raman spectra further changed after 30 h of exposure, when lepidocrocite again emerged. The reappearance of lepidocrocite is assumed to be caused by the capture of Cl
− ions in the akaganeite, resulting in a decrease in the free Cl
− ions in the aqueous adlayer.
When the amount of NaCl deposit is decreased to 0.11 mg/cm2, the steel surface does not reach high enough concentrations to form akaganeite. Only after repeated wet/dry cycles may a spot with a high concentration of NaCl emerge on the surface and akaganeite form on that spot.
7.4. Formation and Growth of the Corrosion Layer
Once the corrosion process has started on the steel surface it will be necessary to consider the possible mechanisms that take place in the formation and growth of the rust layer, where, as is known, the corrosion products transform from one compound to a more stable form and may involve any of a number of processes including hydrolysis, nucleation, crystallisation, precipitation, dehydration, thermal transformation, dehydroxylation, etc. [
91]. Temperature, time and pH are the main factors governing such transformations [
97].
In 1965, Evans [
181] formulated the first electrochemical method for atmospheric rusting, in which the oxidation of iron (wet periods)
Is balanced by the reduction of ferric rust to magnetite
Later, after partial drying of the pore structure of the rust (dry period), magnetite is reoxidised by oxygen that now has free access through the pores due to gas diffusion
The autocatalytic cycle responsible for the fact that rust promotes further rusting involves alternate reduction and reoxidation of the preexisting rust.
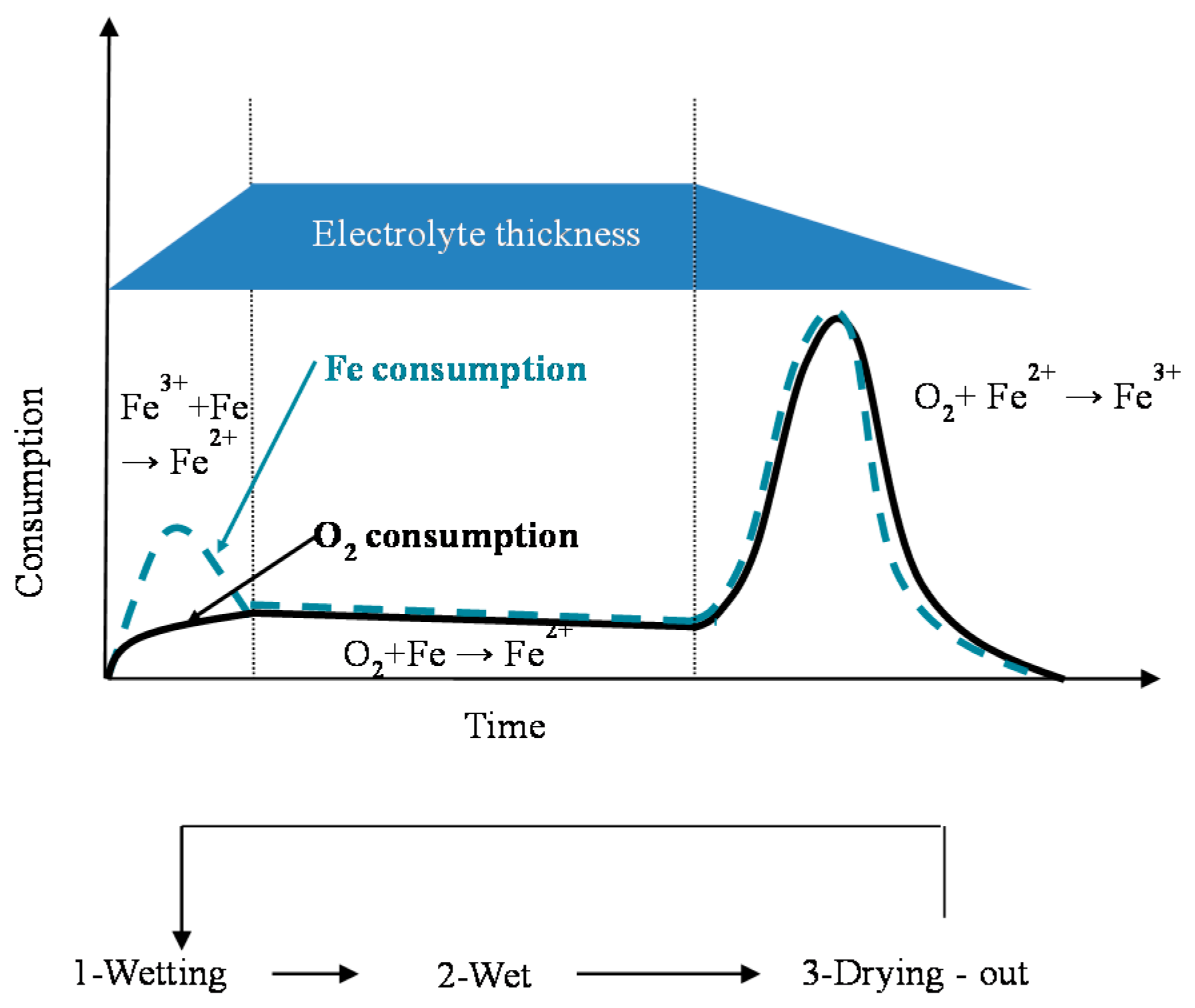
Subsequently, Stratmann et al. [
182], in an electrochemical study of phase transitions in rust layers, experimentally showed that the oxidation of magnetite to lepidocrocite, as proposed by Evans, was not possible. Stratmann et al. used a combination of magnetic and volumetric measurements to show that when a prerusted iron sample is wetted, iron dissolution is not immediately balanced by a reaction with oxygen, but rather by reduction of the preexisting rust
With later reoxidation of the reduced species
Thus, Stratmann [
38] proposed dividing the AC mechanism of pure iron into the following three stages: wetting of the dry surface, wet surface, and drying-out of the surface (see
Figure 2).
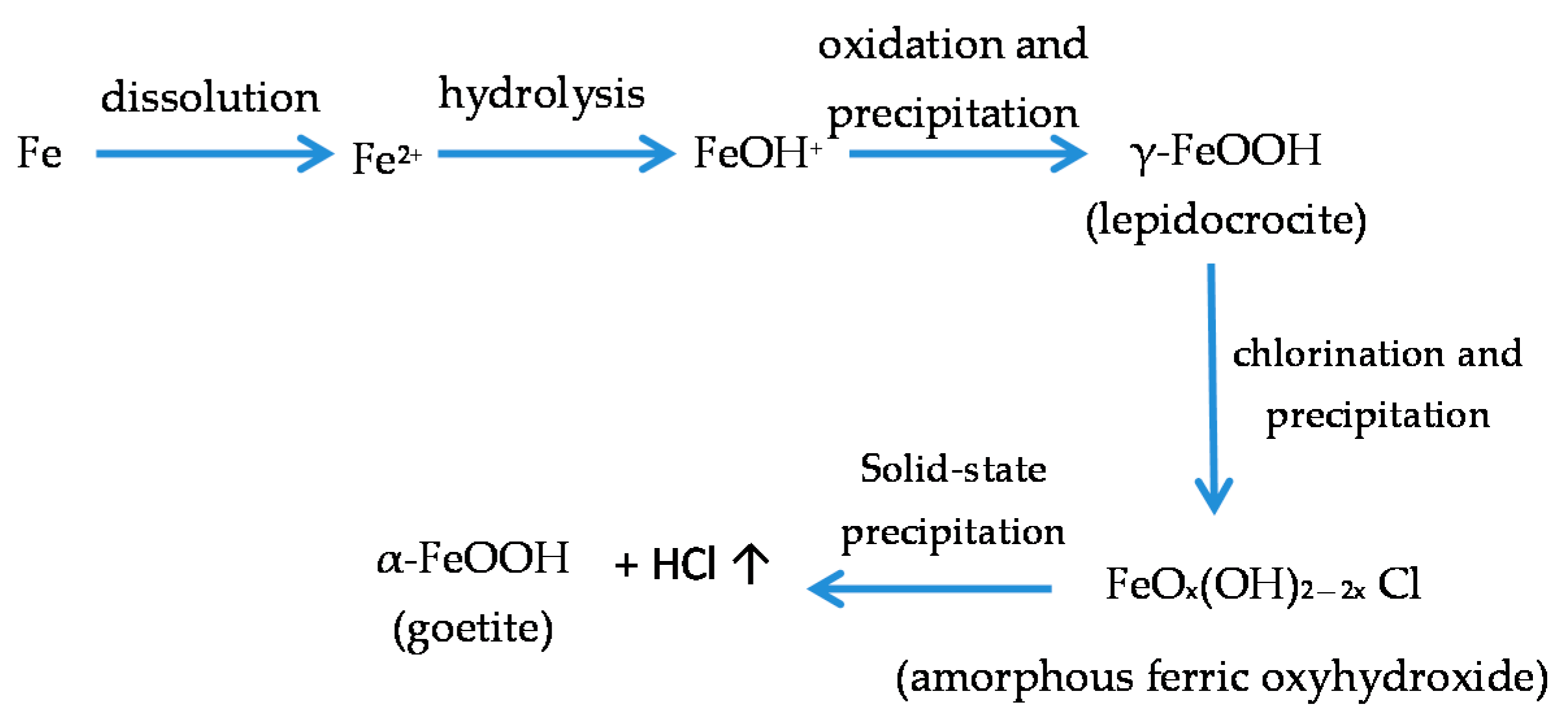
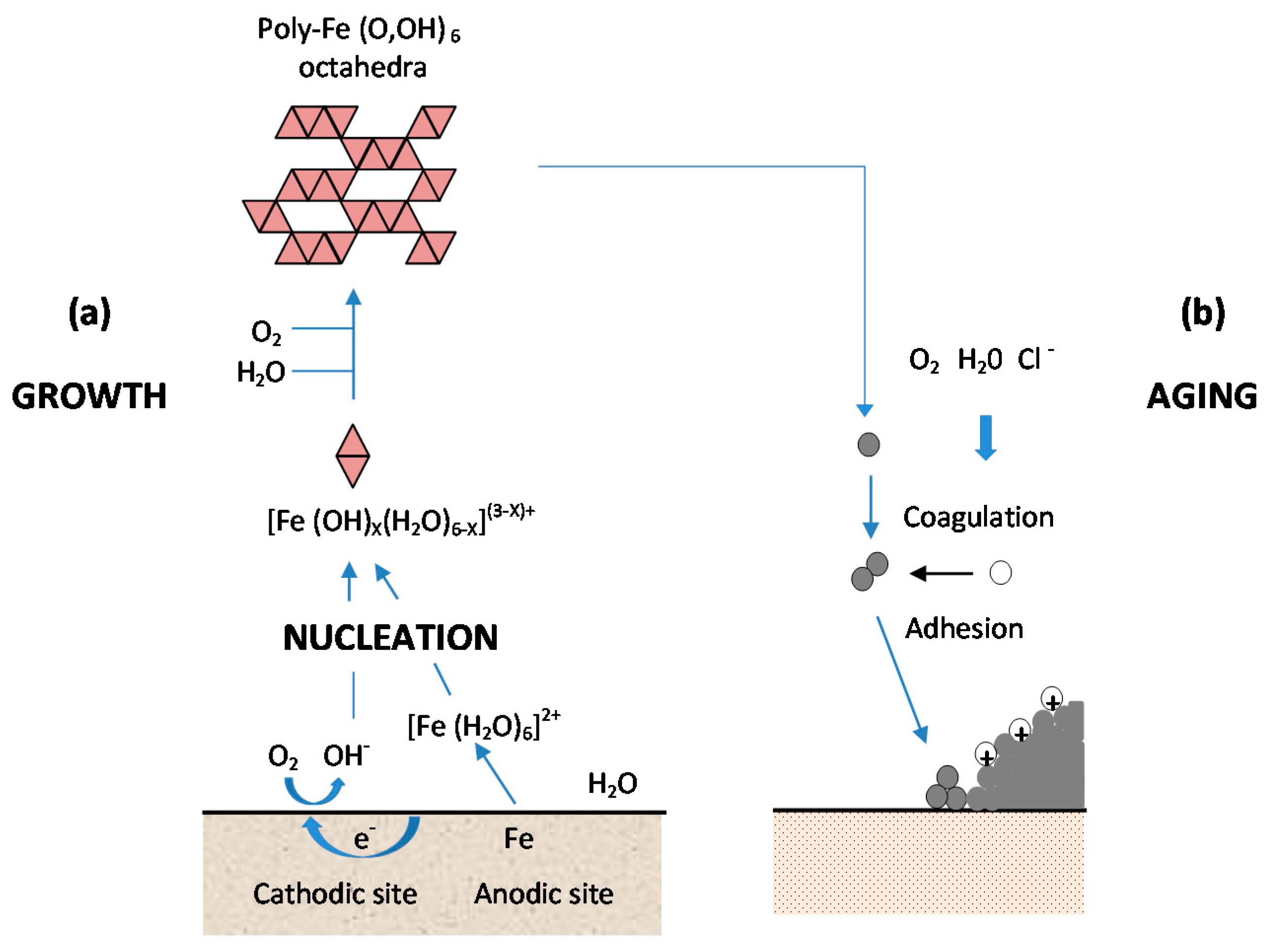
Misawa [
170] notes the following mechanism for the rusting process (
Figure 34):
- (a)
In the first stage of rusting the aerial oxidation of ferrous ions, dissolved from the steel into a slightly acidic thin water layer formed by rain on the steel surface, leads to the precipitation of lepidocrocite. Fine weather accelerates the precipitation and crystallisation of lepidocrocite by drying.
- (b)
The lepidocrocite is formed on the steel surface and transformed to amorphous ferric oxyhydroxide and goethite during the atmospheric rusting process. The amorphous ferric oxyhydroxide transforms to goethite by deprotonation using hydroxyl ions provided by the rainwater.
Another important aspect to consider is how the rust layer grows. Horton [
183] in 1964 observed that rust layers grow by several mechanisms: (i) by iron ions diffusing outward through the rust to form fresh rust at the air-rust interface; (ii) at the steel-rust surface; and (iii) within the rust layer to fill pores and cracks. It was the first time that this observation was reported in scientific literature. Years later, Burger et al. [
184] using an ingenious technique known as the “gold marker method”, addressed the following two aspects:
- (a)
the location at which precipitation of corrosion products occurs within the corrosion system (steel/rust/atmosphere), and
- (b)
the structural evolution of the corrosion product layer during wet/dry cycles.
With regard to (a), they observed a significant contribution of inward diffusion of oxidant through the corrosion product layer. With regard to (b) they note that the continuous decrease in the reactivity of the corrosion product layer seems to be related with a two-step process in the corrosion mechanisms: the preliminary formation of ferrihydrite (a highly reactive hydrated iron oxide) close to the metal/rust interface, followed by its progressive transformation into goethite, a more stable oxyhydroxide. This progressive transformation may be the consequence of incremented cyclic reduction/reoxidation reactions which are not completely reversible. As these cyclic electrochemical reactions require electrical contact between the reactive phase and the metallic substrate, and given the complex morphology of the corrosion patterns, an important outlook is to take into account the connectivity and conductivity of the different phases constituting the corrosion product layer and their influence on its structural evolution. Due to the expansive nature of the corrosion products, mechanical stresses may develop in these materials, thus inducing two opposing effects: pore blocking and formation of cracks/spalling in the rust layer.
In the last decade great advances have been made in the understanding of AC mechanisms. As has been mentioned above, many of these advances have been due to the French research groups of Professors Legrand and Dillmann [
40,
41,
44,
47,
93,
131,
185,
186]. Both groups have made important advances in: (a) the electrochemical reactivity of the ferric phases that constitute rust; (b) the localisation of oxygen reduction sites; (c) the decoupling of anodic and cathodic reactions; (d) in-situ characterisation of reduction and reoxidation processes, etc.
With a view to the development of a model of the AC process that can predict the long term AC behaviour of iron, they note the need to consider several important parameters in order to describe rust layer: average lepidocrocite fraction, thickness, average porosity, tortuosity and specific area, connectivity of the phases inside the rust layer, etc. [
41,
47]. As these researchers note, there is still a long way to go before long-term AC mechanisms are fully clarified.
As has been mentioned several times in this paper, considerable advances have been made in the knowledge of AC mechanisms in atmospheres polluted with SO
2 (e.g., urban and industrial atmospheres) while less progress has been made on corrosion mechanisms in marine atmospheres. In addition to the proposals of Nishimura et al. [
10,
39] referred to in 7.2., other authors have made contributions relating to the subject of MAC which will be enumerated below, and more are sure to appear in the forthcoming years, considering the growing interest of researchers in this field of knowledge.
In 1988 Nomura et al. [
187] applied conversion electron MS to study the formation of akaganeite on iron in a NaCl (3 wt %) solution. On the basis of the study on early stages of Fe(OH)
2 formation, the reaction that takes place on the iron surface in a Cl
− solution can be expressed as follows:
In conditions of high dissolved oxygen (initial stages)
Poorly crystalline FeOOH is considered to deposit on the iron surface by the initial corrosion reaction listed above.
However, in conditions of low dissolved oxygen, when a first rust layer is formed, the supply of OH
− (Equation (28)) is suppressed, and Cl
− ions begin to have a relatively strong affinity to the iron(III) ion, thus iron(III) oxyhydroxide complexes containing Cl
− may be formed.
Before the start of the polymerisation process to form rust according to the following equation:
In short, when unstable oxyhydroxide is first formed on iron, lepidocrocite is formed on the surface where dissolved oxygen has easy access, and then akaganeite and magnetite start to be produced by the transformation of the Fe(OH)2 complex containing Cl− at the intermediate surface between the lepidocrocite layer and the iron substrate and by the slow oxidation of iron, respectively, because the supply of dissolved oxygen to the intermediate layers is restricted by the top lepidocrocite layer.
It is relevant to note at this point the important laboratory studies carried out by Refait and Genin [
113,
115] and subsequently by Remazeilles and Refait [
112,
114] on the formation conditions of Fe(II) hydroxychlorides, GR1 and akaganeite previously mentioned in
Section 5.
More recently, Ma et al. [
188,
189], using XRD and IRS, detected the formation of akaganeite in the inner rust layer accompanied by an acceleration of the corrosion rate on steel exposed to very severe marine atmospheres. After six months of exposure the akaganeite content and the corrosion rate decrease and akaganeite is gradually transformed into maghemite until it completely disappears. The authors speculate on the need to exceed a critical chloride threshold in order for akaganeite to form.
In atmospheres with less Cl
− pollution akaganeite was not formed, though the Cl
− content facilitated the transformation of lepidocrocite into goethite (
Figure 35). The wet/dry cycle accelerates these transformation processes, and especially in the dry cycle HCl is released into the environment.
7.5. Proposal of an Overall Mechanism for the MAC Process of Steel
The composition of the rust layer depends on the conditions in the aqueous adlayer and thus varies according to the type of atmosphere. It is unanimously accepted that lepidocrocite is the primary crystalline corrosion product formed in the atmosphere. As the exposure time increases and the rust layer becomes thicker, the active lepidocrocite is partially transformed into goethite and magnetite.
In mildly acidic solutions lepidocrocite is transformed into goethite. Schwertmann and Taylor established that the transformation occurs in solution through different steps: dissolution of lepidocrocite, formation of goethite nuclei, and nuclei growth [
190].
Magnetite may be formed by oxidation of Fe(OH)
2 or intermediate ferrous-ferric species such as GR [
119], but also by lepidocrocite reduction in the presence of a limited oxygen supply [
119,
120]:
Thus it is not surprising that magnetite is usually detected in the inner part of rust adhering to the steel surface, where oxygen depletion may occur.
With a broader view, Ishikawa et al. [
121] and Tanaka et al. [
122] found that the formation of magnetite particles was caused by the reaction of dissolved ferric species of oxyhydroxides with ferrous species in the solution. The formation of magnetite rust can be represented by the following cathodic reaction:
In marine atmospheres, where the surface electrolyte contains chlorides, akaganeite is also formed. How does akaganeite form? The high Cl
− concentration in the aqueous adlayer on the steel surface gives rise to the formation of FeCl
2, which hydrolyses the water [
25]:
Notably raising the acidity of the electrolyte. At the steel/corrosion products interface, where Cl
− ions can accumulate, large Cl
− concentrations and acidic conditions give rise to akaganeite formation after the precipitation of ferrous hydroxychloride (β-Fe
2(OH)
3Cl), a very slow process requiring the transformation of metastable precursors [
112,
113].
As Remazeilles and Refait point out, large amounts of dissolved Fe(II) species and high Cl
− concentrations are both necessary for akaganeite formation [
114]. The oxidation process of ferrous hydroxychloride which leads to akaganeite formation passes through different steps via the formation of GR1 intermediate compounds. The whole oxidation process can be summarised as follows [
99,
112,
113,
114,
115]:
Thus requiring a relatively long time. This time will depend on the environmental conditions: temperature, Fe
2+, Cl
− and OH
− conditions, O
2 flow, etc. The acid environment at the steel/rust interface also leads to an acceleration of corrosion of the underlying steel and pitting [
23,
191], where the dominant cathodic reaction is hydrogen evolution.
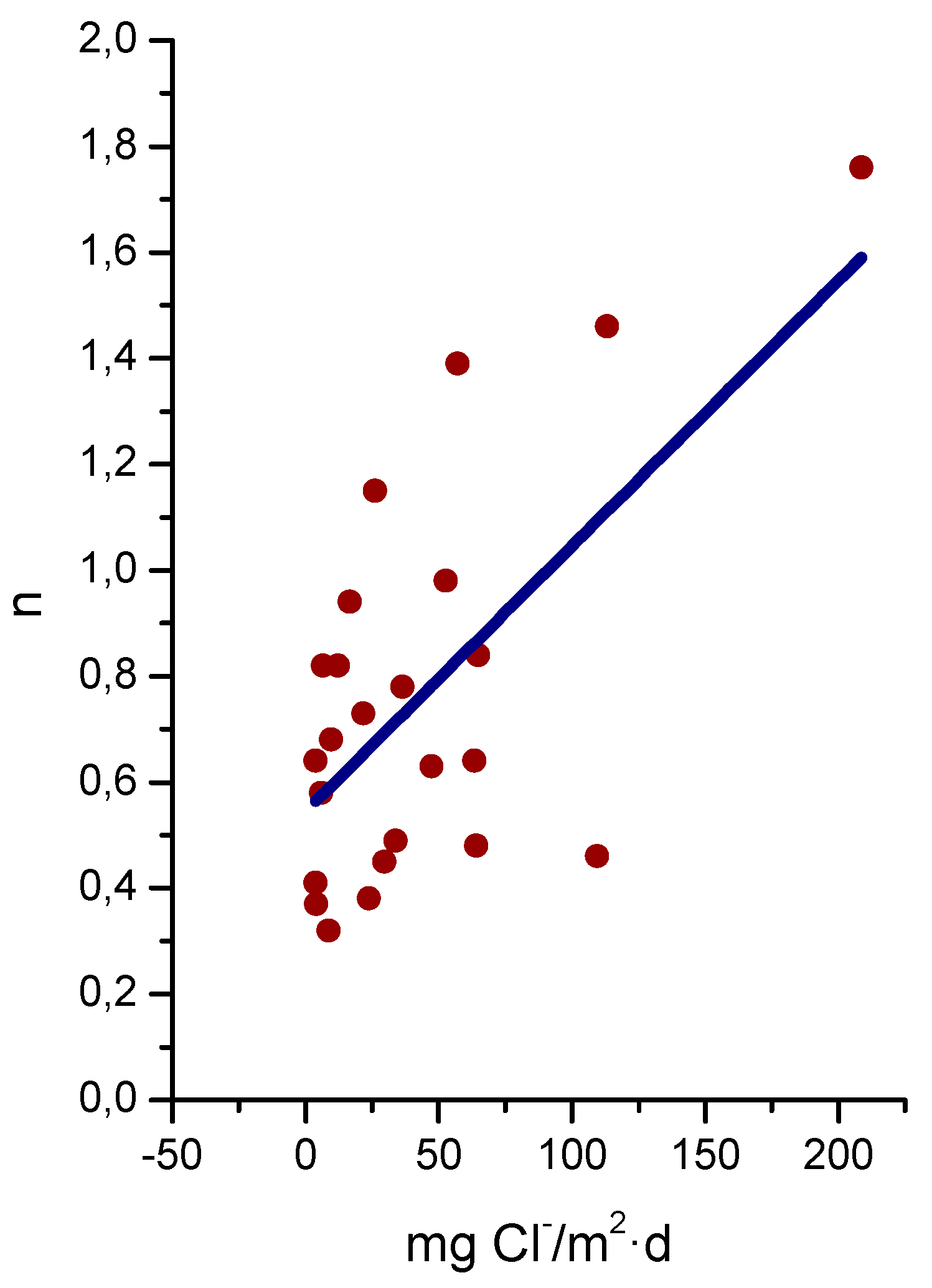
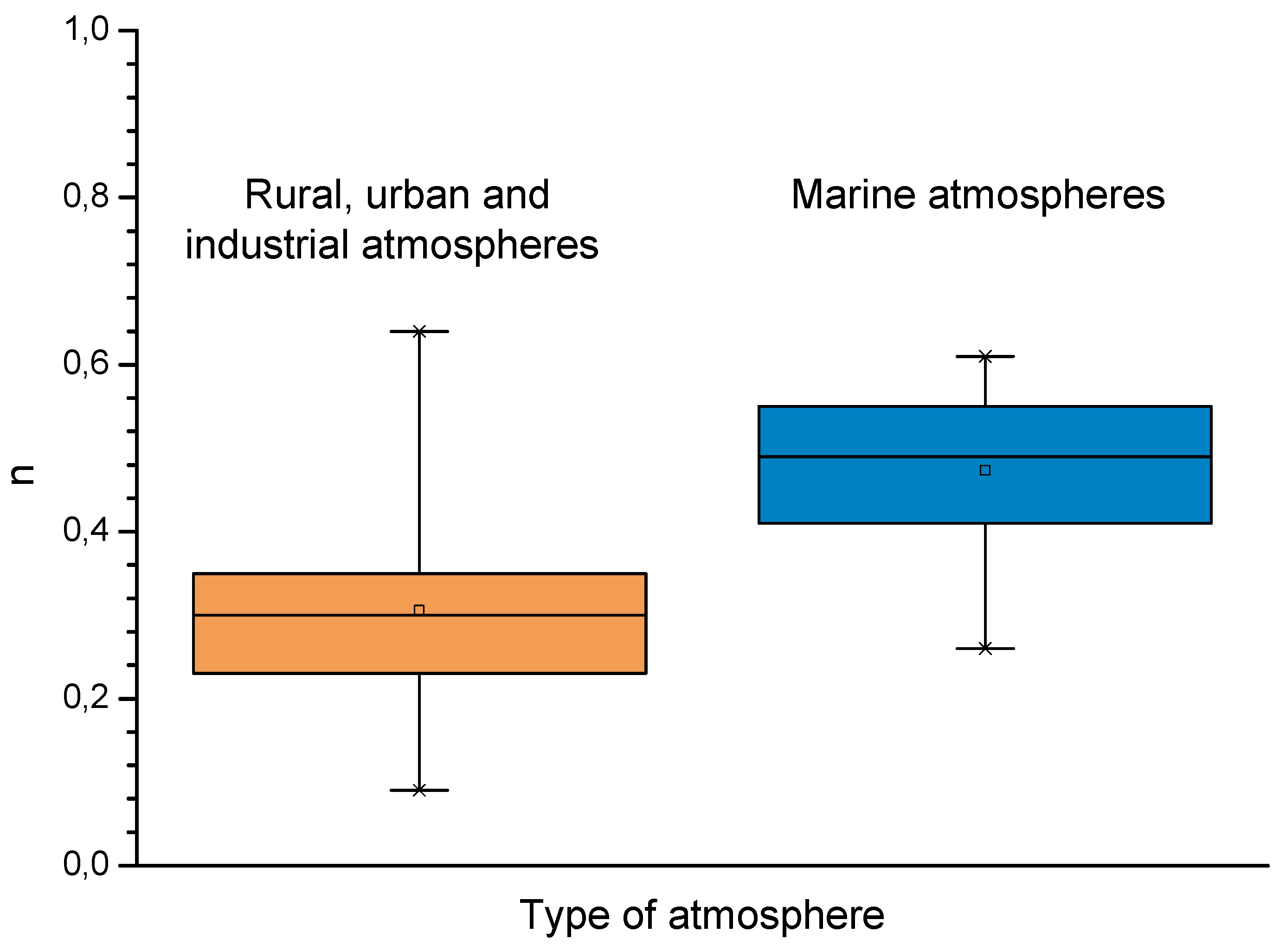
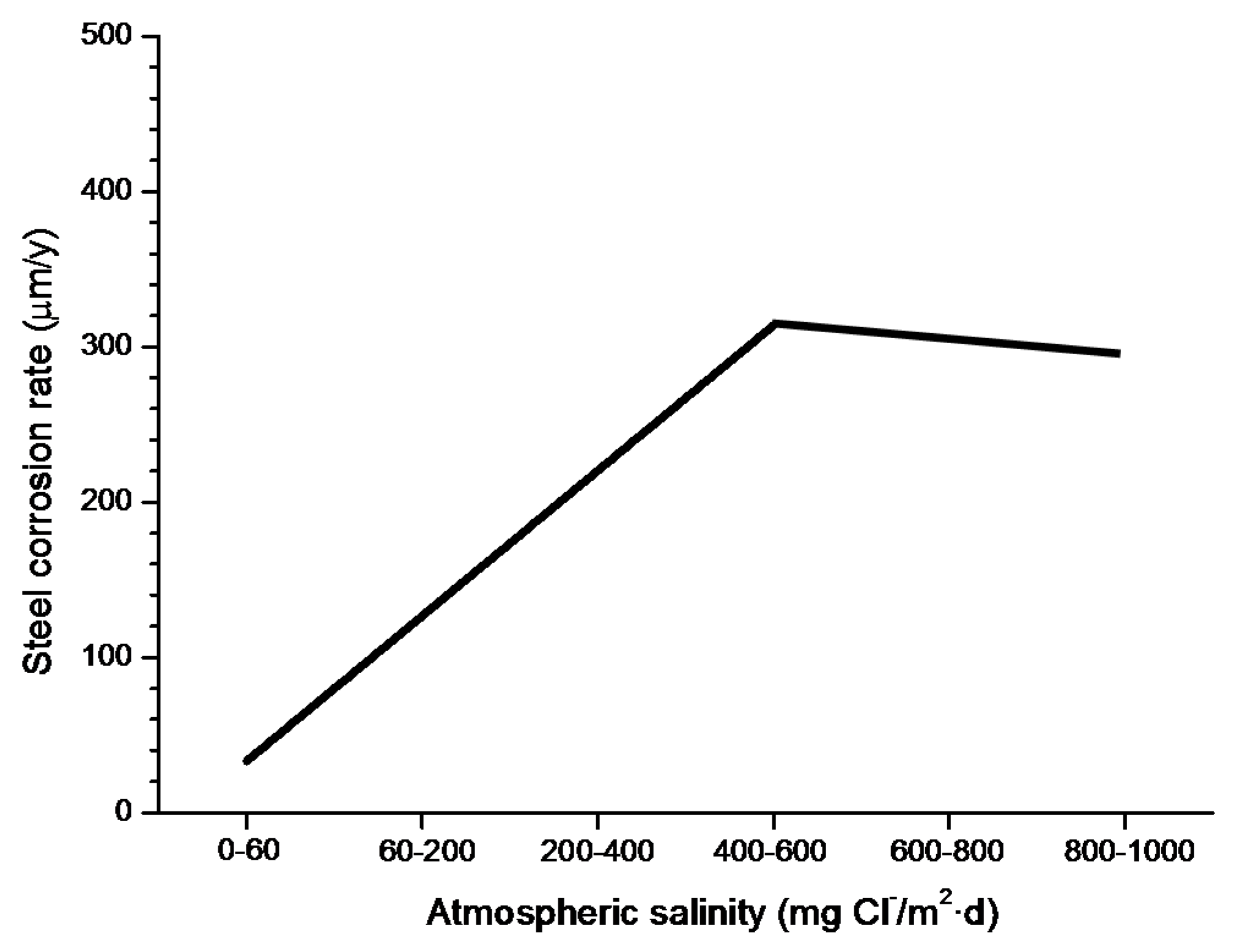
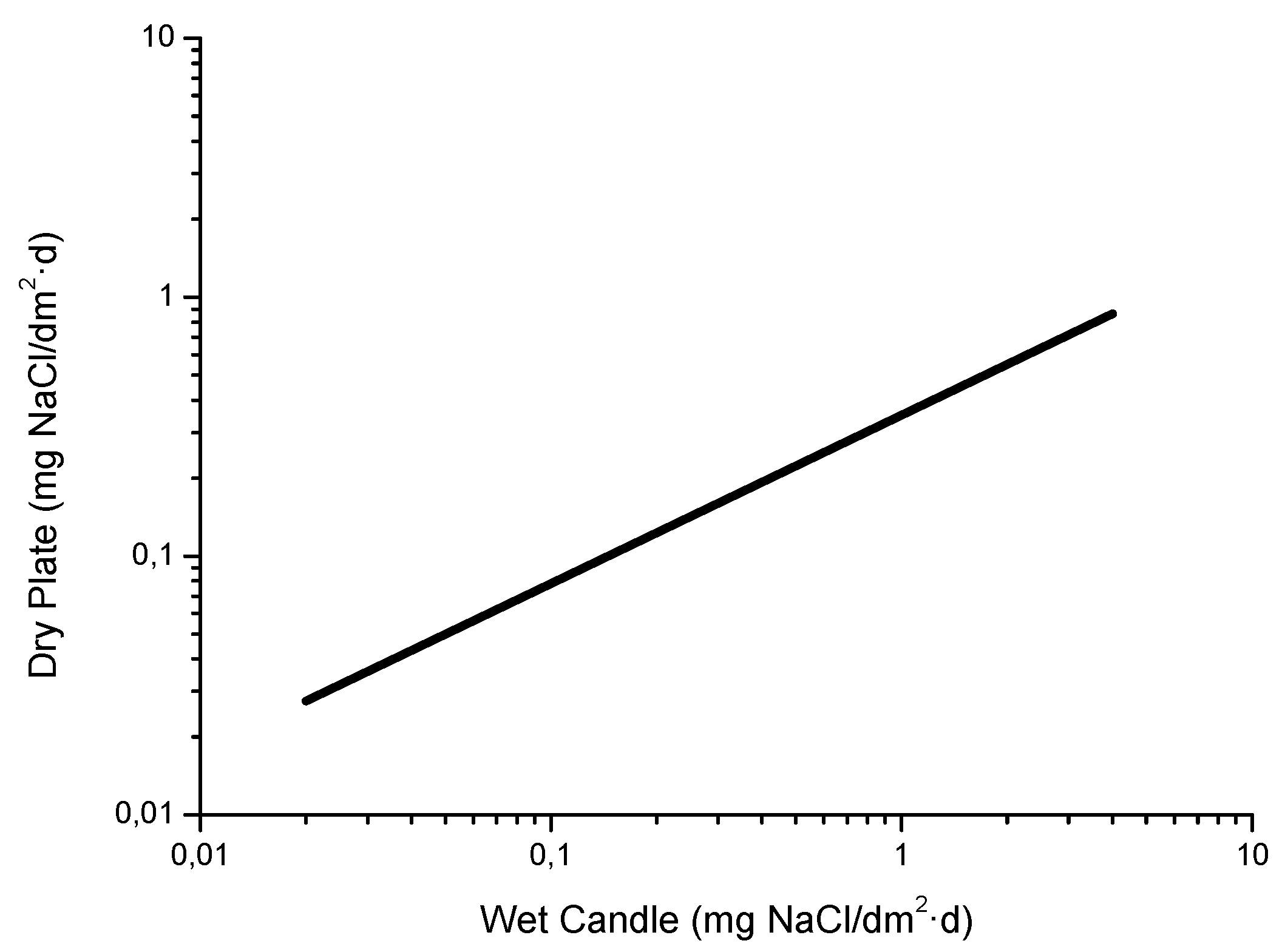
There has been much speculation about the need to exceed a critical atmospheric salinity threshold for akaganeite formation to take place. Morcillo et al. provide a general indication of the environmental conditions in the atmosphere which lead to akaganeite formation: annual average RH around 80% or higher and simultaneously an annual average Cl
− deposition rate of around 60 mg/m
2·d or higher [
192] (
Figure 36). This confirms the laboratory experiments carried out by Remazeilles and Refait [
114], which indicate that a high Cl
− concentration is not the only condition for akaganeite formation. The medium must also be characterised by large dissolved Fe
2+ concentrations, as occur during the high TOW of the metallic surface in high RH atmospheres.
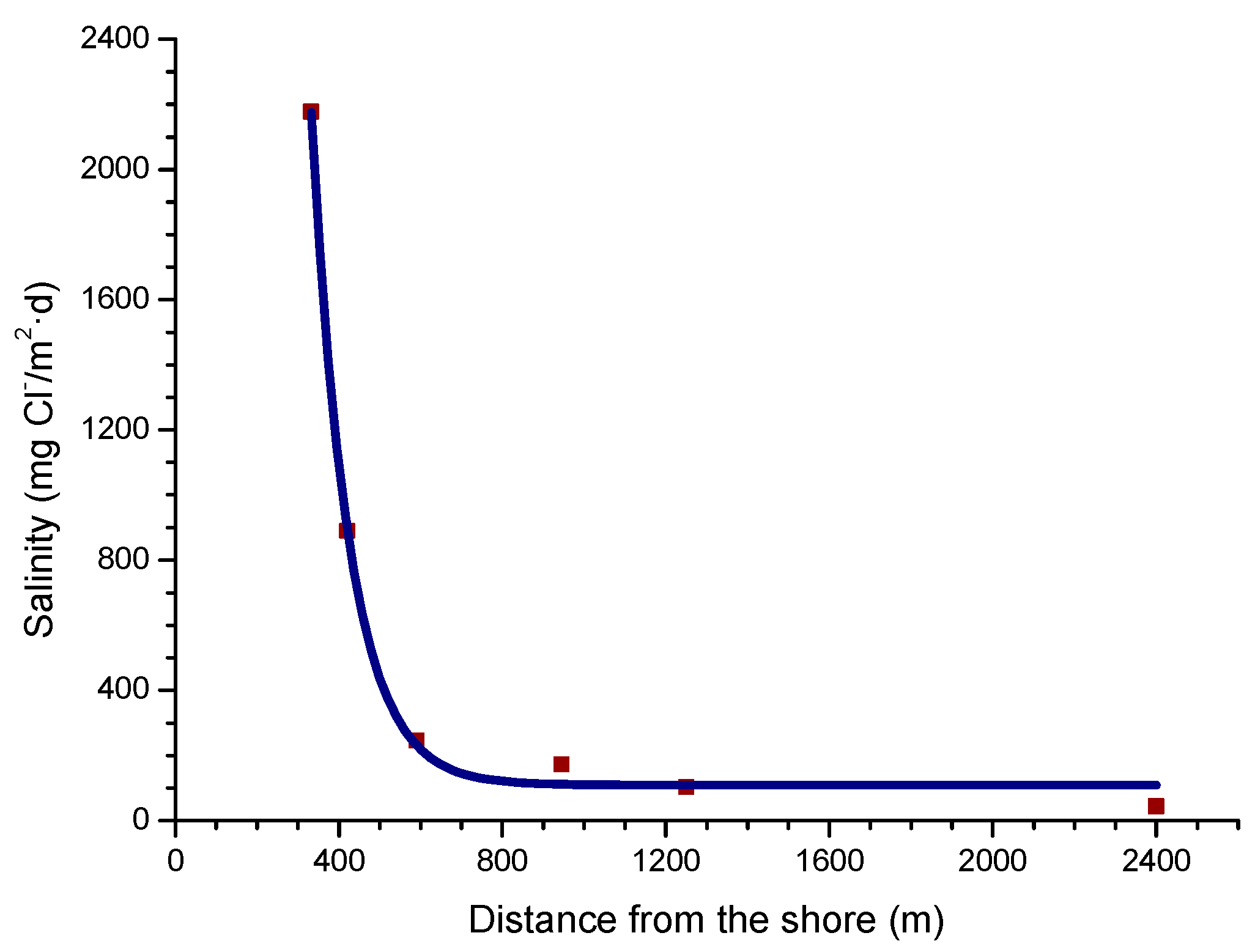
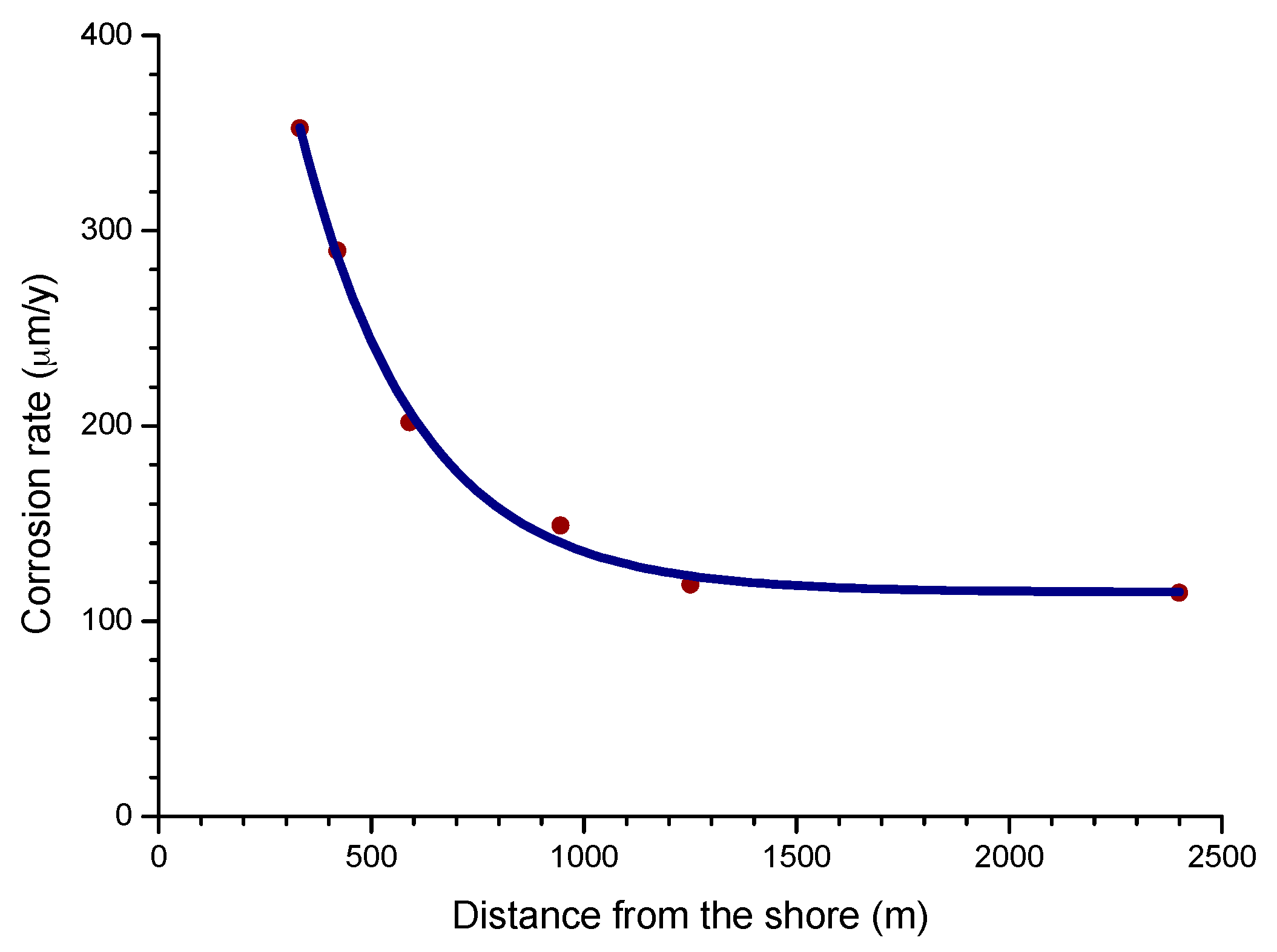
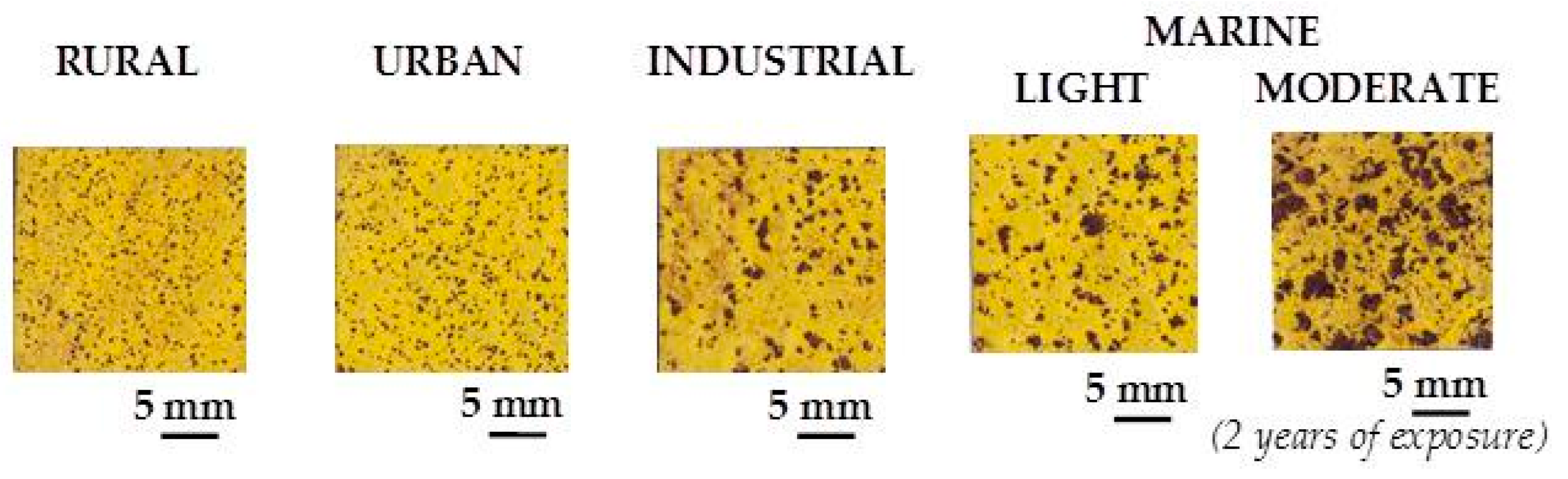
With regard to the corrosion products that form on CS in this type of atmospheres,
Table 4 has been prepared using field data obtained by the authors in different studies carried out at various sites in Spain [
64,
150,
193] and allows the following facts to be deduced: (a) in marine atmospheres with extremely low Cl
− deposition rates (Ponte do Porto) only lepidocrocite and goethite phases form, the latter in a practically insignificant proportion; (b) above a certain atmospheric salinity, akaganeite and spinel (magnetite/maghemite) phases appear; (c) the akaganeite and spinel contents increase notably as the atmospheric salinity rises (Cabo Vilano-2 and Cabo Vilano-3); and (d) it can clearly be seen how an increase in the Cl
− deposition rate is accompanied by a drop in the lepidocrocite phase content of the rust and a rise in the goethite, akaganeite and spinel contents. This fact is well seen in
Figure 37, which shows rusts formed after 3 months exposure of CS in marine atmospheres with different salinities [
95].
Akaganeite could be reduced electrochemically in the corrosion process, being consumed in the wetting of the metallic surface [
10]. Lair et al. [
40] experimentally saw the high reducing capacity of akaganeite in comparison with other oxyhydroxides, reporting the following order: akaganeite > lepidocrocite >> goethite.
This explains the high magnetite contents found in the rust formed on steel exposed to atmospheres with heavy Cl
− ion pollution. As Hiller noted some time ago, the rust formed in atmospheres with high Cl
− deposition rates contains more magnetite than that formed in Cl
− free atmospheres [
123].
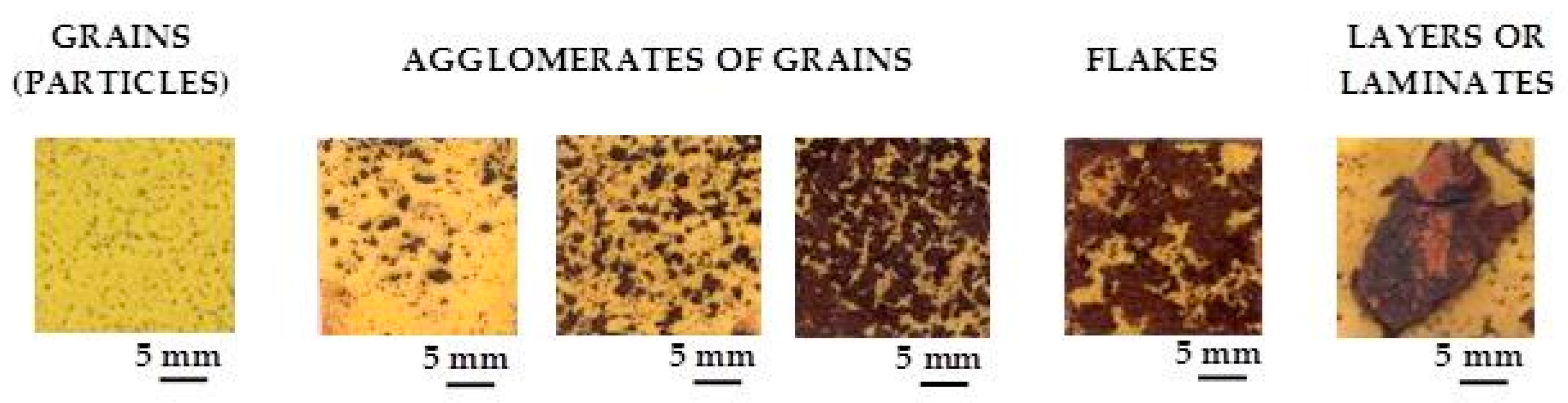
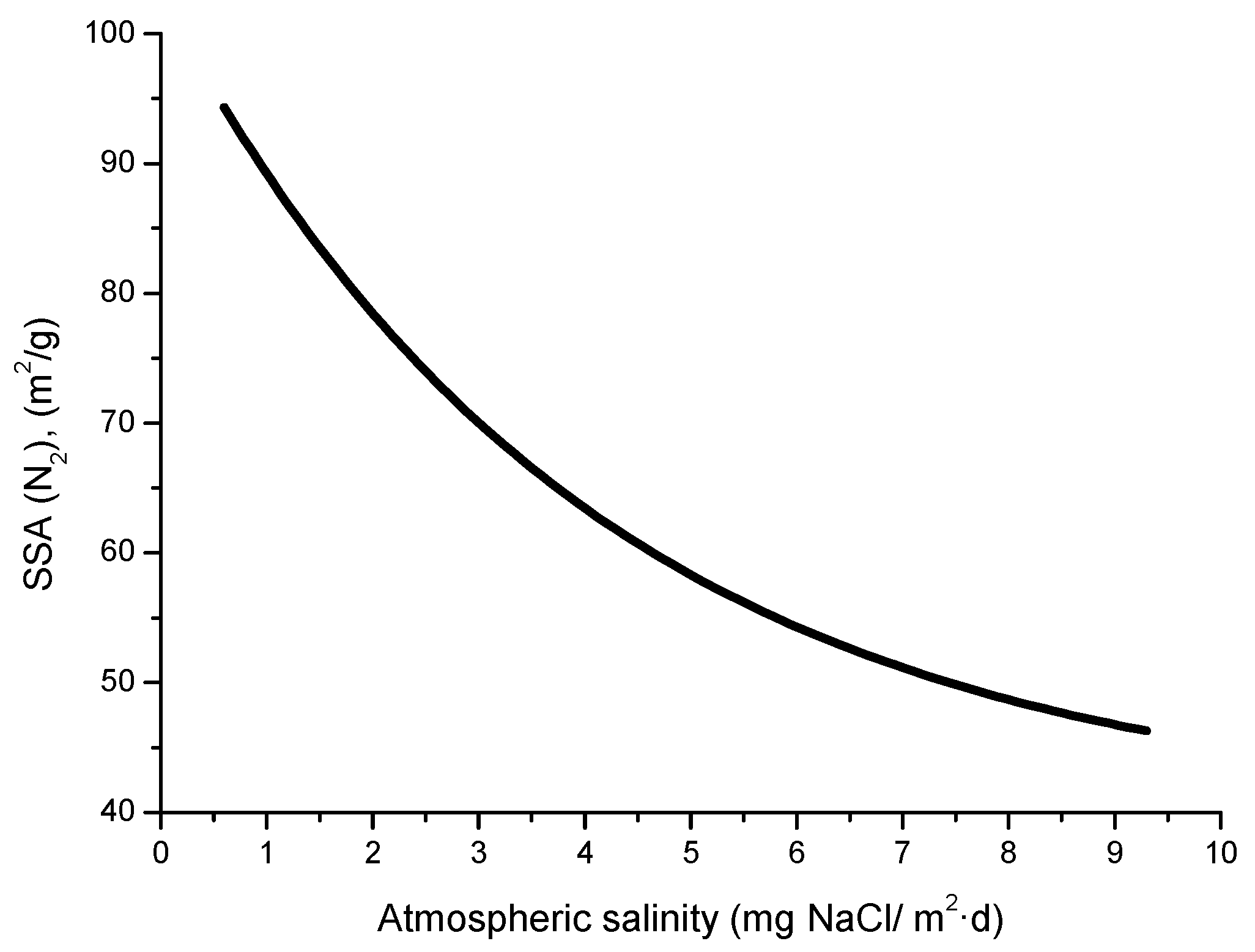
Rust layers present considerable porosity and cracking. Ishikawa et al., examining the textures of rusts, note that the rusts formed at coastal sites are agglomerates of large particles and have larger pores than rusts formed at rural and urban sites. NaCl promotes rust particle growth, resulting in the formation of larger pores and voids between larger particles in the rust layer and facilitating further corrosion [
145,
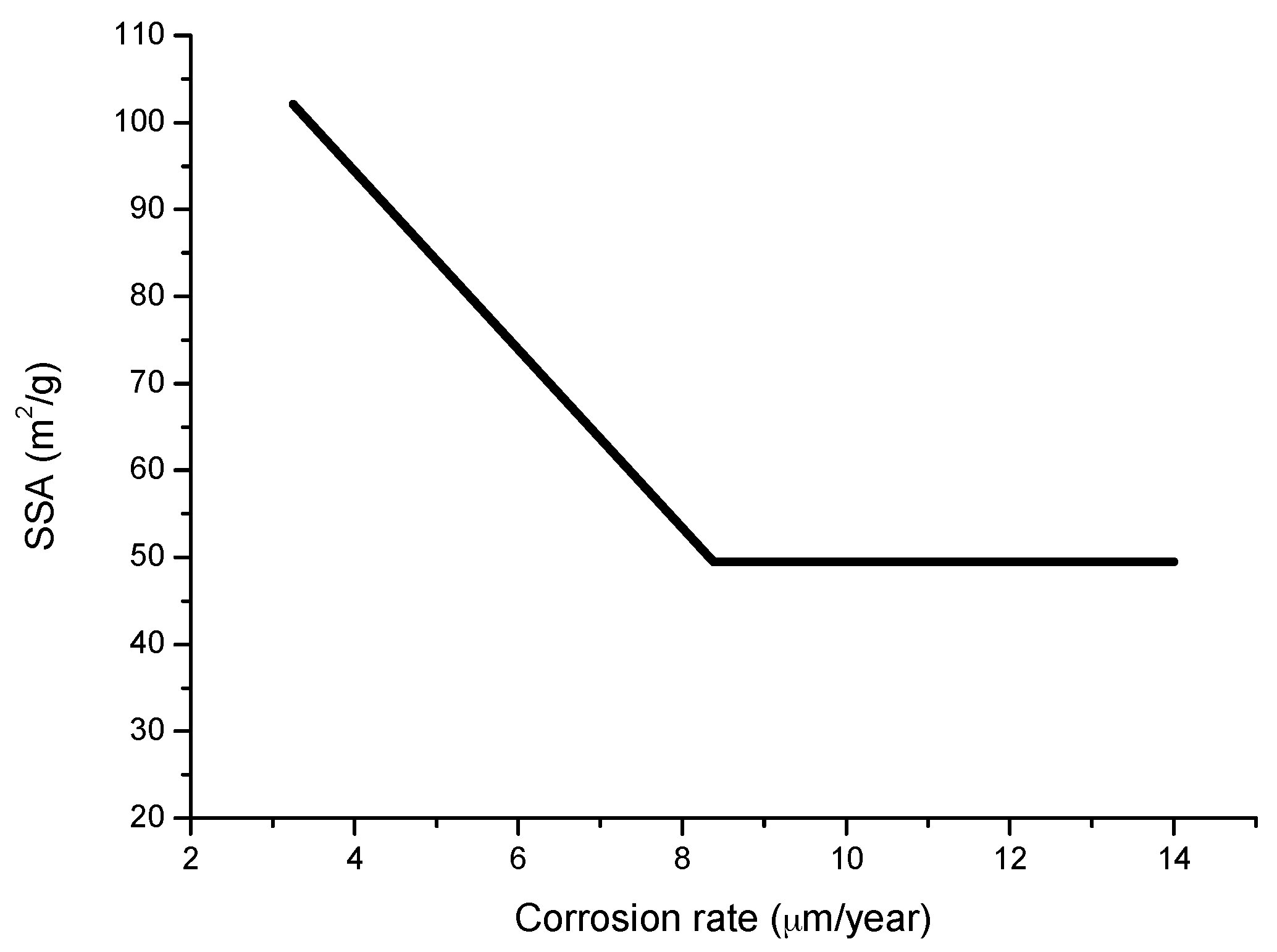
163]. The SSA of rusts decreases as salinity increases, enlarging the diameter of the pores and forming less and less compact rust layers with low protective properties.
Thus the compactness of the corrosion product layers formed is dependent on the salinity of the atmosphere at the exposure site. At low Cl
− deposition rates, even when the time of wetness (TOW) of the metallic surface is high, relatively consistent (less porous) layers, whose thickness does not usually exceed 100 μm, are formed. However, high Cl
− deposition rates lead to the formation of very porous rust layers showing cracks and even flaking and exfoliation [
129].
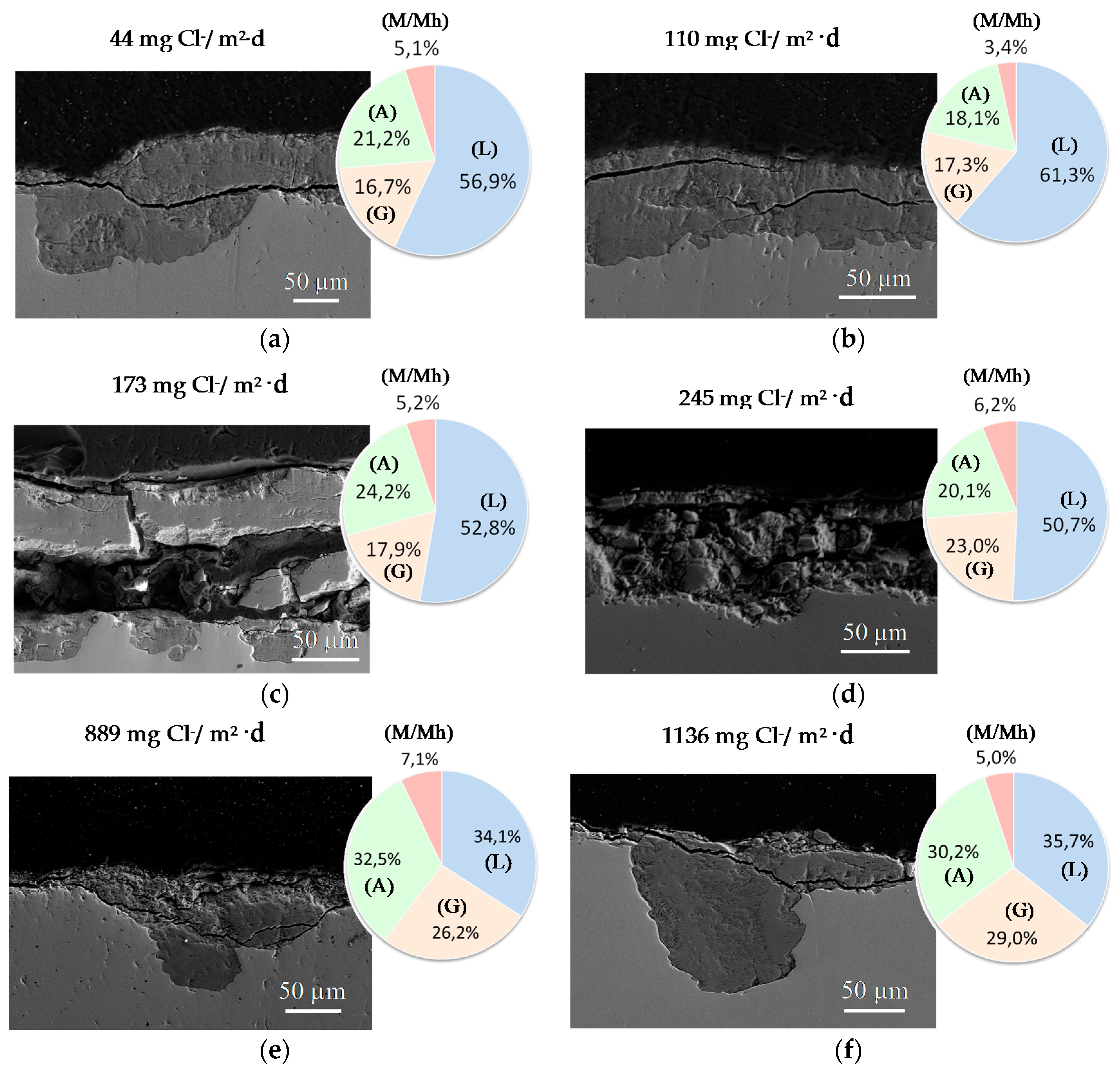
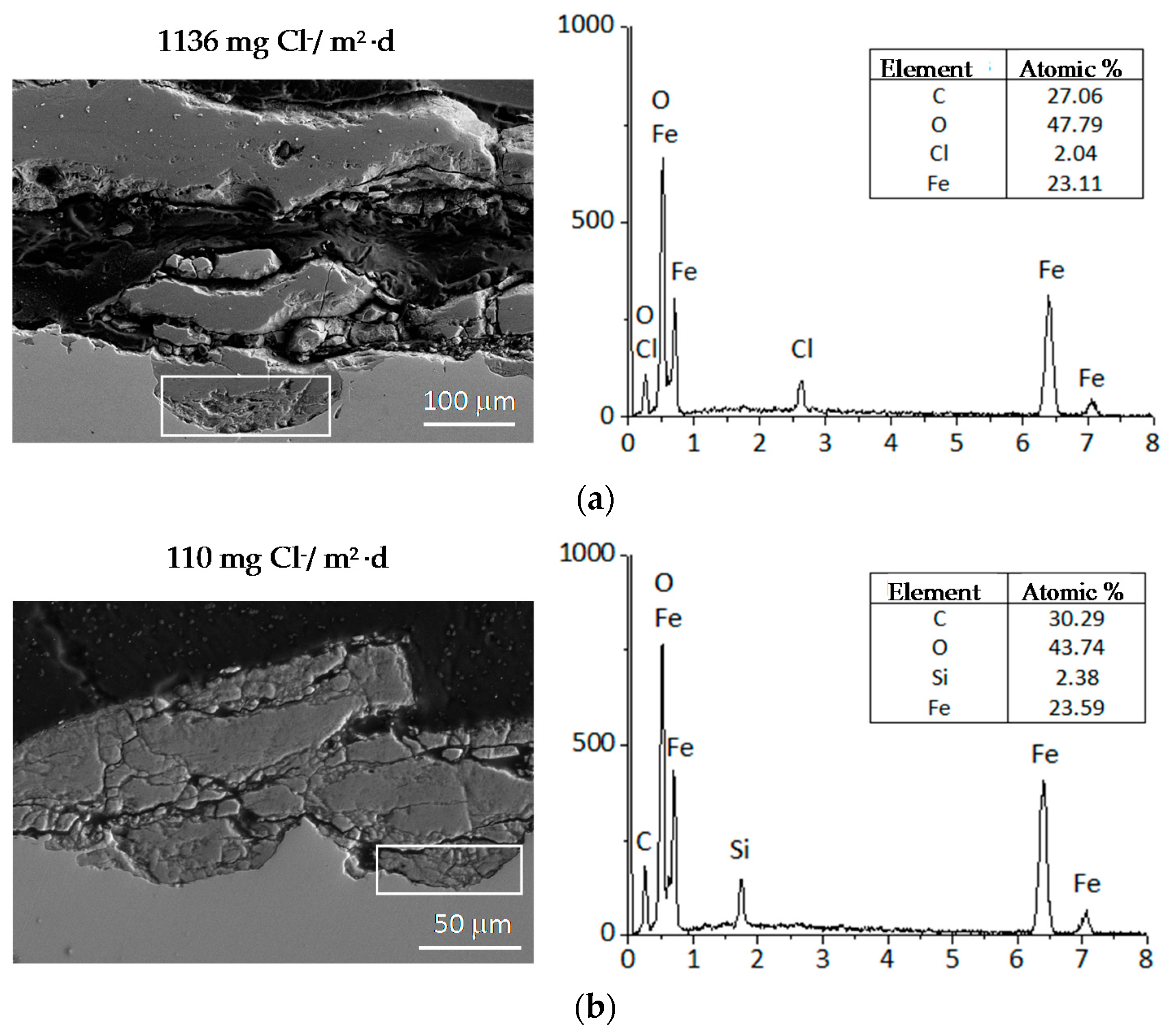
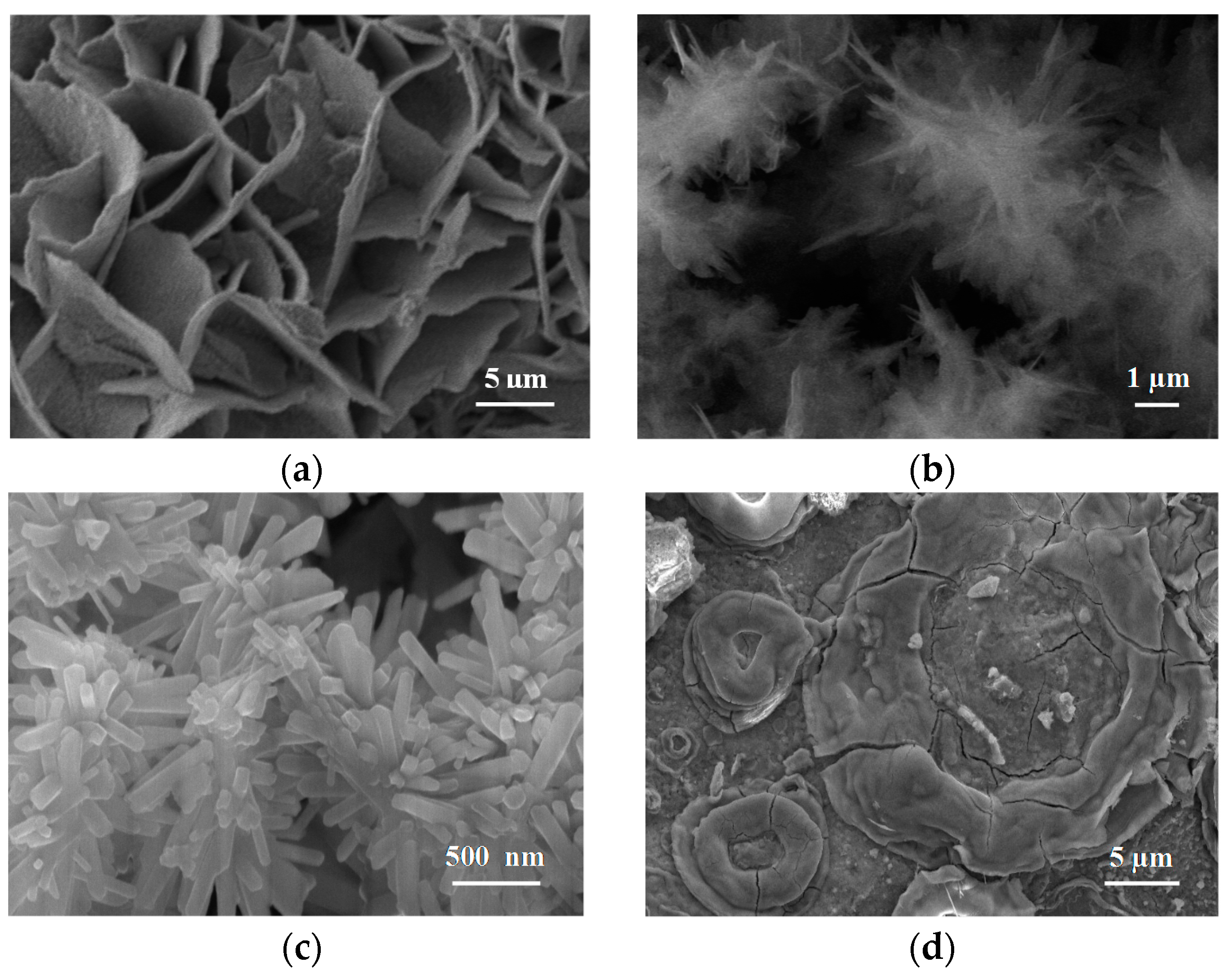
In
Figure 38 it is possible to see the variation in the structure of the rust layer as the atmospheric salinity rises. While at relatively low atmospheric salinities (44 and 110 mg Cl
−/m
2·d) the rust layers are fairly compact (though they can show the presence of longitudinal cracks), at higher atmospheric salinities (173 and 245 mg Cl
−/m
2·d) the rust layers present abundant cracking which facilitates their subsequent detachment (exfoliation), as can clearly be seen at the highest atmospheric salinities (889 and 1136 mg Cl
−/m
2·d) [
64].
Figure 38 also indicates how the content of the different phases in the rust layers varies as the atmospheric salinity rises. The lepidocrocite phase decreases while the goethite and akaganeite phase contents increase [
64].
The base steel shows the formation of pits when exposed to marine atmospheres. As the atmospheric salinity rises, pitting becomes more significant and the Cl signal obtained by EDS inside the pits also rises (
Figure 39) [
64]. As has been seen in
Section 6 (
Figure 22), there is a strong presence of akaganeite in the interior of the pits formed on the base steel in severe marine environments.
Thus there seem to be two notably different situations with regard to the mechanisms involved in the MAC of CS: (a) establishment of a consistent (consolidated), adherent and continuous rust layer (at low Cl
− deposition rates); and (b) formation of a thick rust layer that is easily detached (exfoliated) from the base steel, leaving large areas uncovered (at high Cl
− deposition rates) [
193].
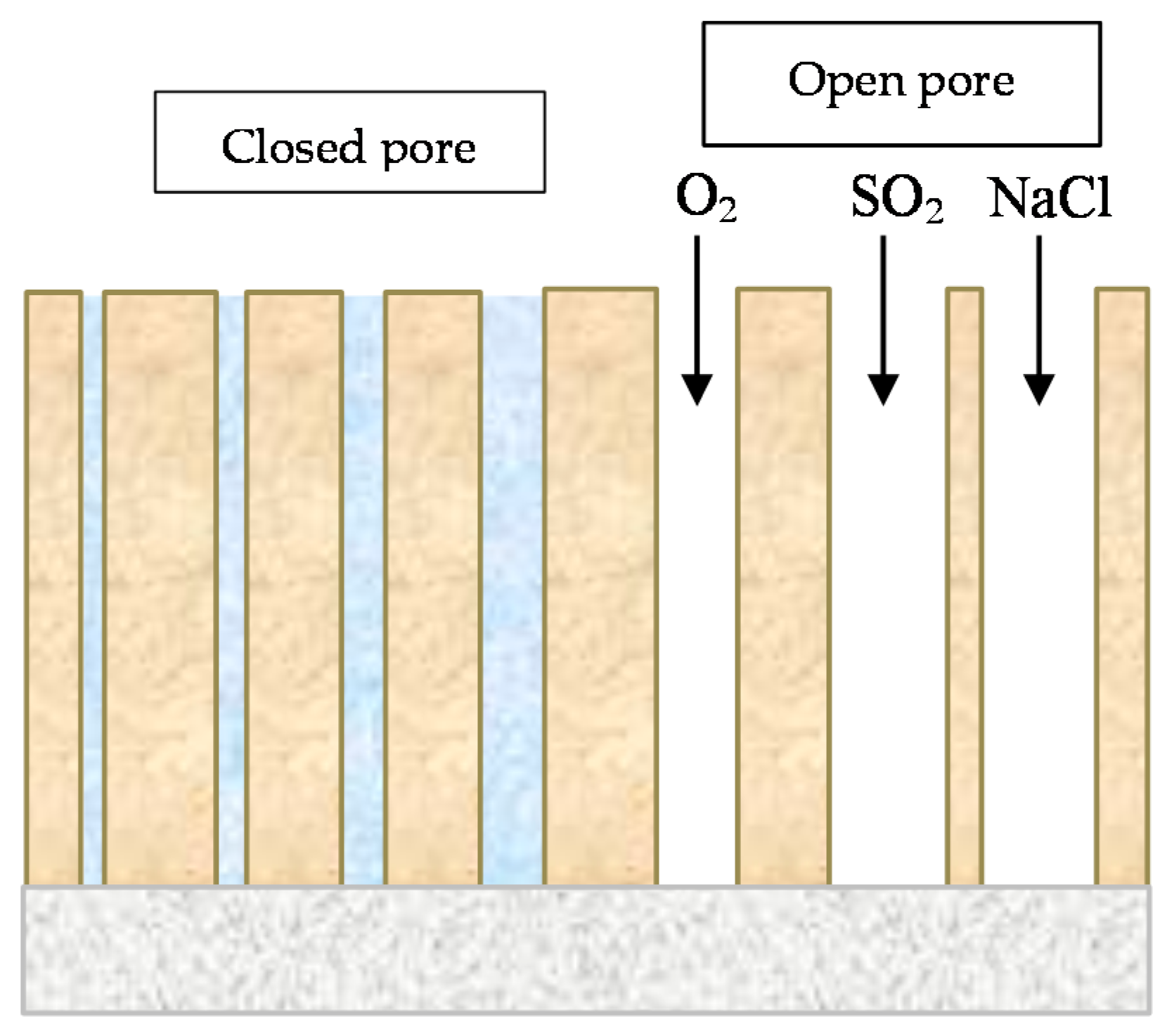
When a consolidated layer of corrosion products remains on the steel surface, the conditions are right for a diffusion-controlled corrosion mechanism to act, in which the aggressive species from the atmosphere (O
2, H
2O and Cl
−) pass through the rust layer to interact with the underlying steel (
Figure 38a). This situation seems to occur in relatively low Cl
−-containing atmospheres. The steel corrosion process consists of the following reactions:
Steel starts to corrode according to the anodic reaction:
where the cathodic process consists of reduction of oxygen dissolved in the aqueous adlayer:
The OH
− ions formed migrate towards the anodic zones forming Fe(OH)
2 as the initial rust product:
Under this basic mechanism, the steel corrosion rate will be highly influenced by the concentration of ionisable substances in the aqueous adlayer, as in the case of chlorides present in marine atmospheres. This explains the notable increase in the steel corrosion rate at station Cabo Vilano-2 compared to the Ponte Do Porto background station (
Table 4), as the Cl
− deposition rate rises from 3.6 mg Cl
−/m
2·d at Ponte Do Porto to 70 mg Cl
−/m
2·d at Cabo Vilano-2.
In contrast, the exposure of CS to severe marine atmospheres can lead in certain circumstances to the formation of thick rust layers. High times of wetness of the metallic surface and an atmosphere with a high Cl
− deposition rate lead to the formation of this type of rust. These thick rust layers tend to become detached from the steel substrate, leaving it uncovered and without protection and thus accelerating the metallic corrosion process. The formation of anomalous thick rust layers and the accompanying exfoliation phenomenon has also been observed in studies carried out by the authors and other researchers [
42,
129,
153,
161].
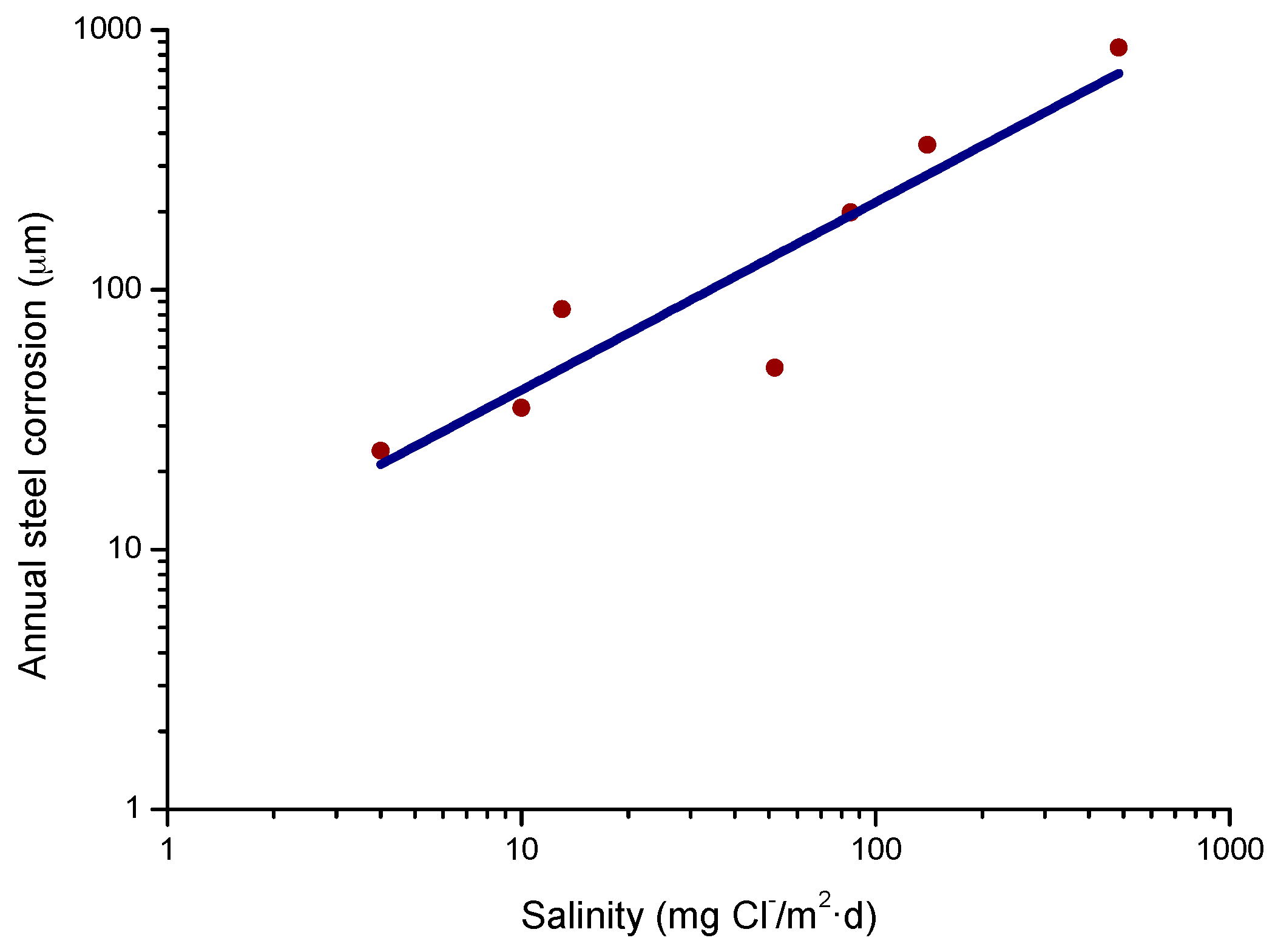
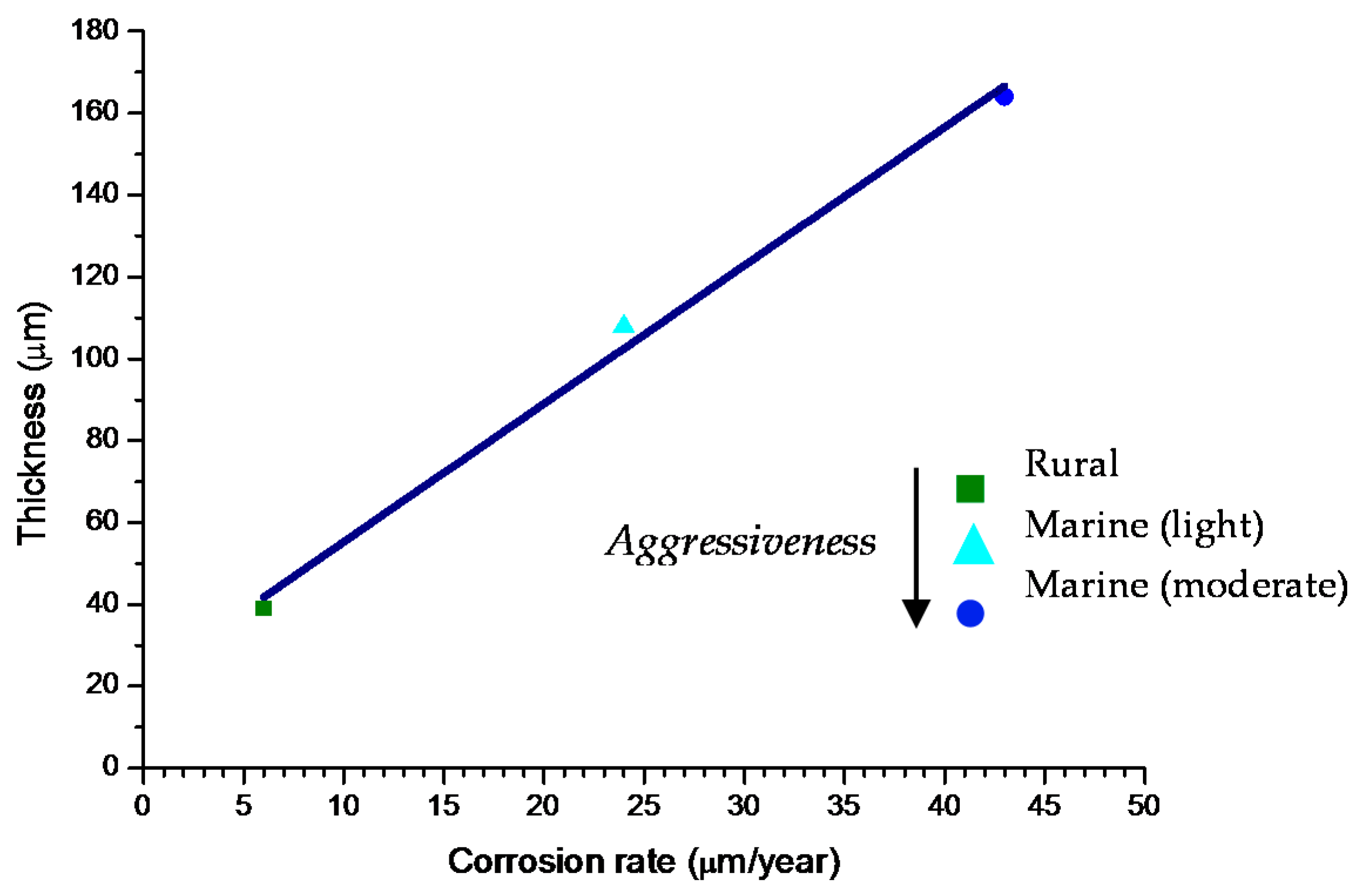
In studies by Chico et al. on CS in Cl
−-rich atmospheres, the average Cl
− deposition rate needed to exceed a critical threshold of close to 300 mg Cl
−/m
2·d for exfoliation to take place. The annual steel corrosion at that atmospheric salinity was higher than 100 μm [
129].
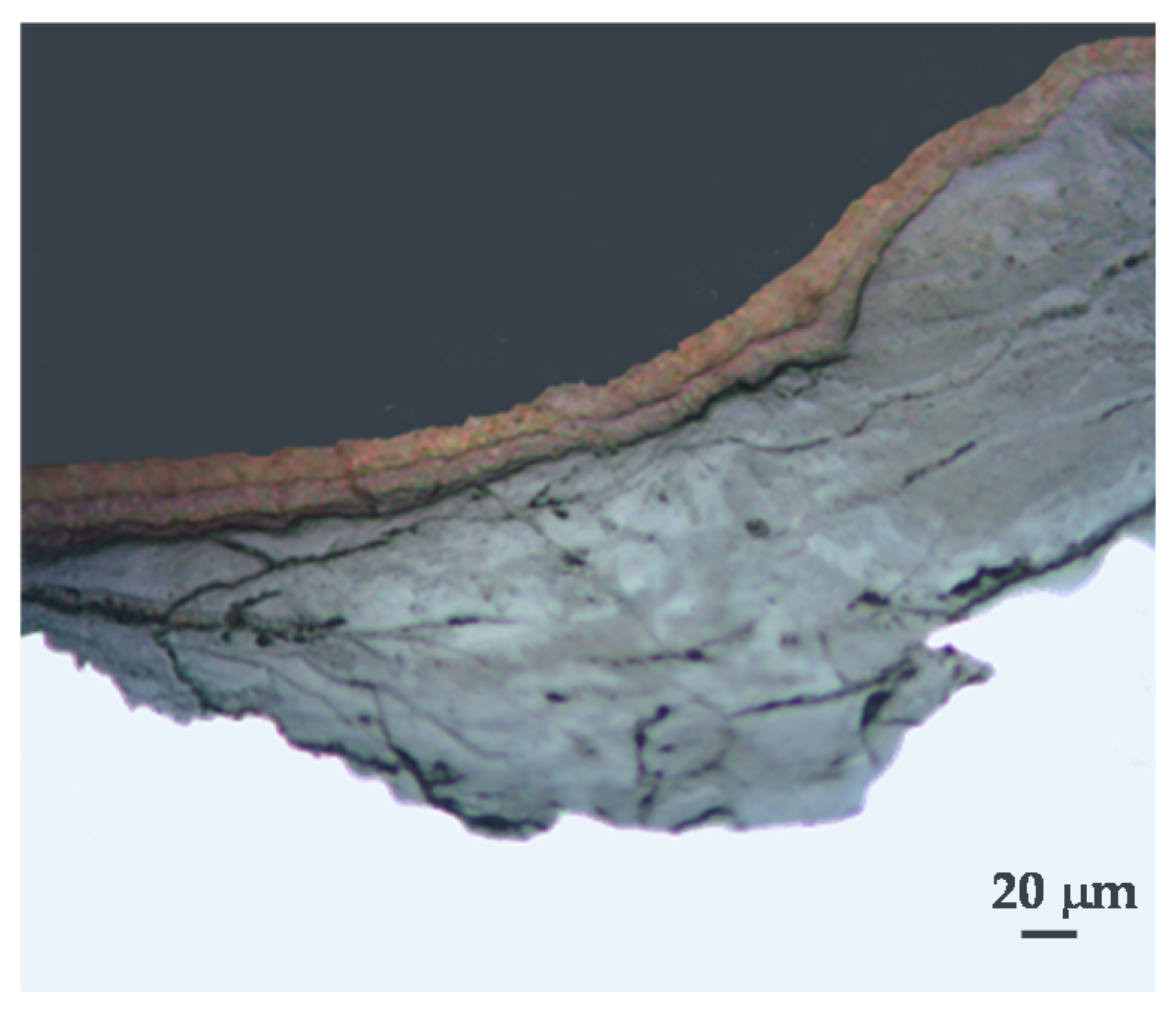
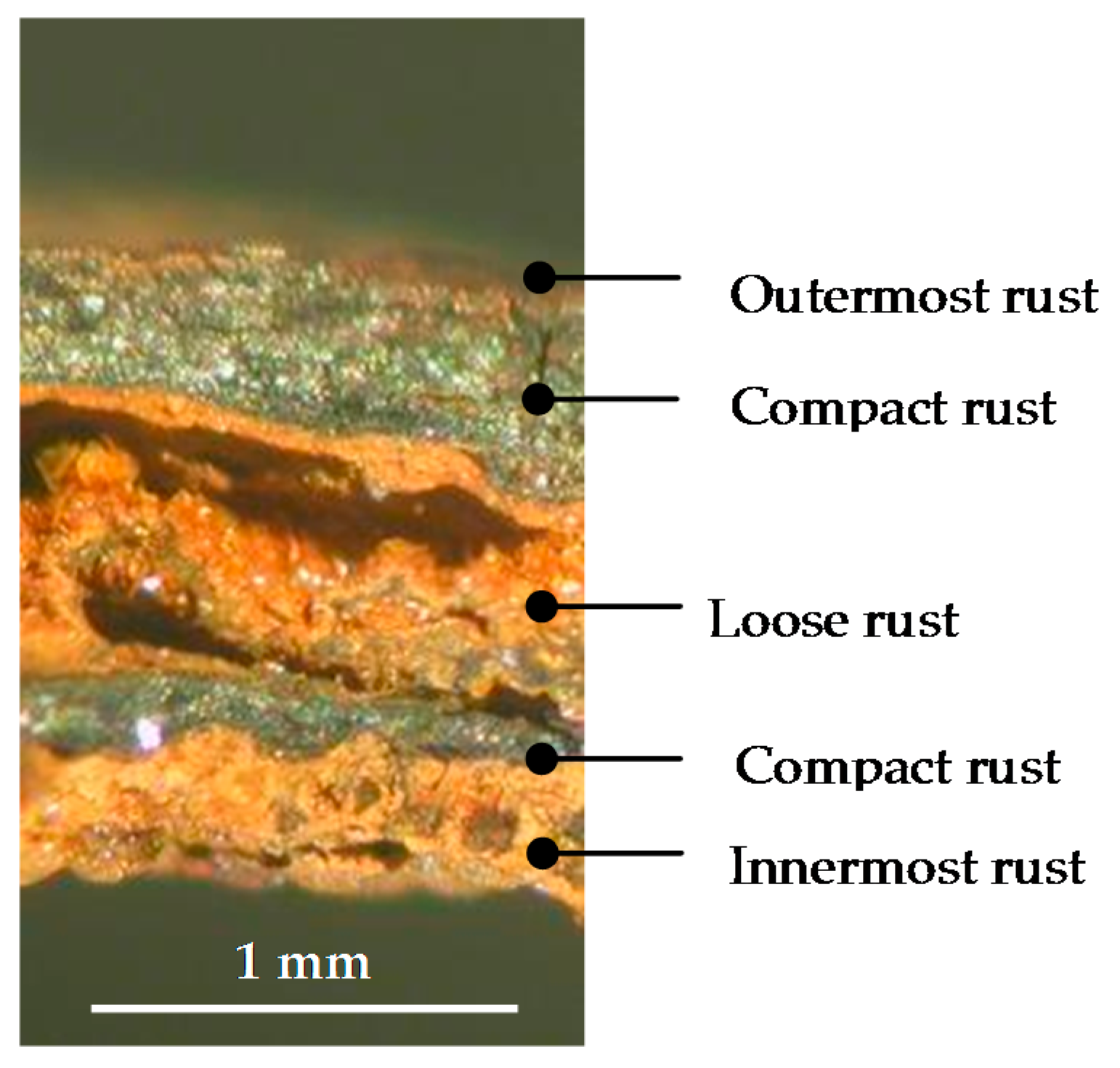
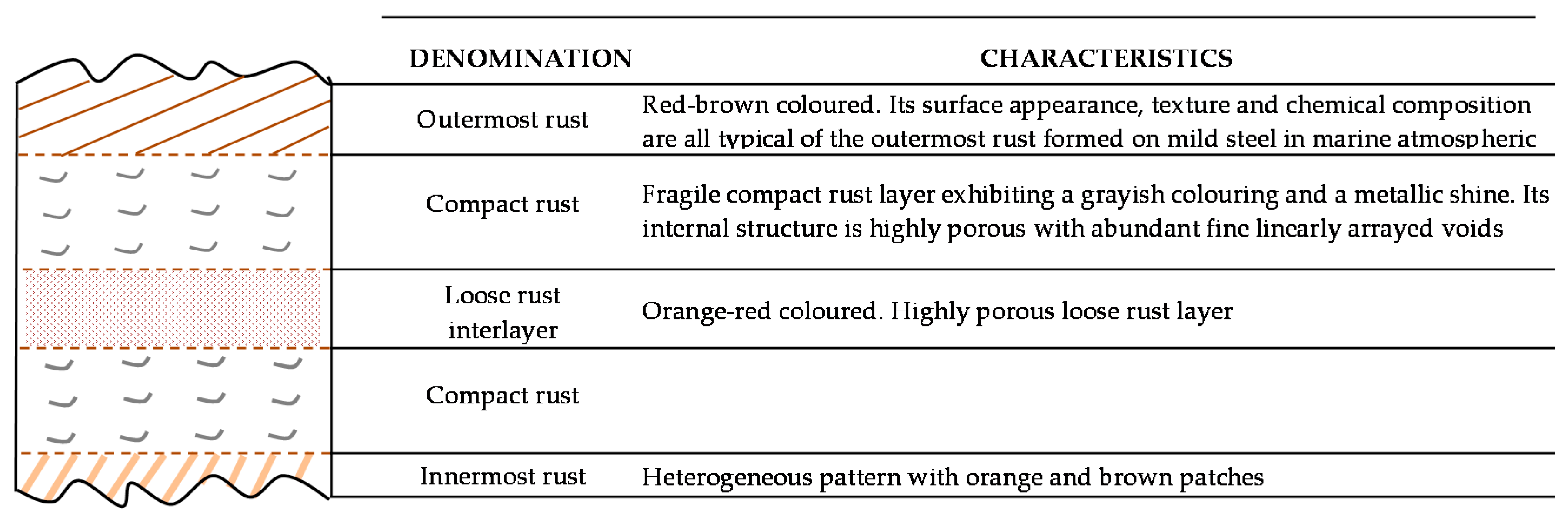
Exfoliated rust layers are composed of multiple rust strata, as can clearly be seen in the cross-section of
Figure 23. The characteristics of the different rust sublayers within the rust multilayer are described in
Figure 24: the outermost rust layer (OR) (rich in lepidocrocite and goethite), and a succession of alternating strata of fragile compact rust (CR) and loose interlayer rust (LIR) layers [
12,
129,
153,
162].
The CRs present high goethite and maghemite contents, low lepidocrocite contents and the practical absence of akaganeite. The mechanism that is proposed for the formation of CRs consists of two stages: (i) the formation of magnetite by electrochemical reduction of lepidocrocite and akaganeite phases (wet stage); and (ii) the solid-state transformation of magnetite into maghemite (dry stage). The LIR presents high goethite and akaganeite contents along with low lepidocrocite and spinel contents. It is proposed that the akaganeite and lepidocrocite phases will be electrochemically reduced to magnetite (maghemite at a later stage) and the formation of the CR layer takes place by consumption of the akaganeite and lepidocrocite phases leading to the complete disappearance of the interlayer rust stratum. An extremely dry period may cause the corrosion process to end without fully exhausting the interlayer rust stratum.
Subsequently, once the extremely dry period has come to an end and a new wet period starts, the formation of a second CR layer would begin, and so on, giving rise to the formation of a sandwich-type structure constituted by alternate CR and LIR layers. A scheme of a feasible multilayered rust formation and rust exfoliation mechanism for CS exposed to severe marine atmospheres is shown in
Figure 40 [
129,
153].
The detachment (exfoliation) of multilayered rust from the steel substrate takes place after complete drying of the whole rust layer, creating expansion stresses that exceed the adhesion forces which keep the multilayered rust joined to the steel substrate.
The difference in density between the sublayers involved (CR and LIR) suggests that compactness combined with mechanical properties may play an important role in the triggering of rust exfoliation. A closer look at the molar volume of the rust phases, i.e., the ratio between the molar mass and the density of each phase expressed in cm
3/mol, indeed shows great variations [
162].
Table 5 displays a factor of 5 when going from the most compact rust phases involved (goethite, lepidocrocite; molar volume around 20 cm
3/mol), over medium compact phases (magnetite, maghemite; around 40 cm
3/mol), to the least compact phase (akaganeite; around 100 cm
3/mol) [
194]. The much lower compactness of akaganeite is due to the presence of tunnels of the akaganeite lattice into which the Cl
− ions can enter and become integrated, resulting in a much less dense structure than the other rust phases [
162].
Taking this difference in molar volume into consideration, it is possible to anticipate a great volume contraction and consequent void formation when the least compact phase akaganeite is structurally transformed into the much more compact spinel phase during primarily wet periods. Similarly, great volume expansion and stress introduction is induced when lepidocrocite is transformed into spinel. Hence, considering the molar volume data, it is not surprising that the compact rust sublayer contains the rust phases with the lowest molar volume (goethite and spinel) while the loose rust interlayer is dominated by akaganeite with a higher molar volume than the main phases in the solid rust sublayer [
162].
Thus it is suggested that rust exfoliation is the result of frequent phase transformations, together with great variations in compactness between the rust phases involved. At some critical point the changes in compactness, compressive stresses and void formation become too large and the whole rust sublayer collapses mechanically and results in a fracture along the innermost rust layer [
162].






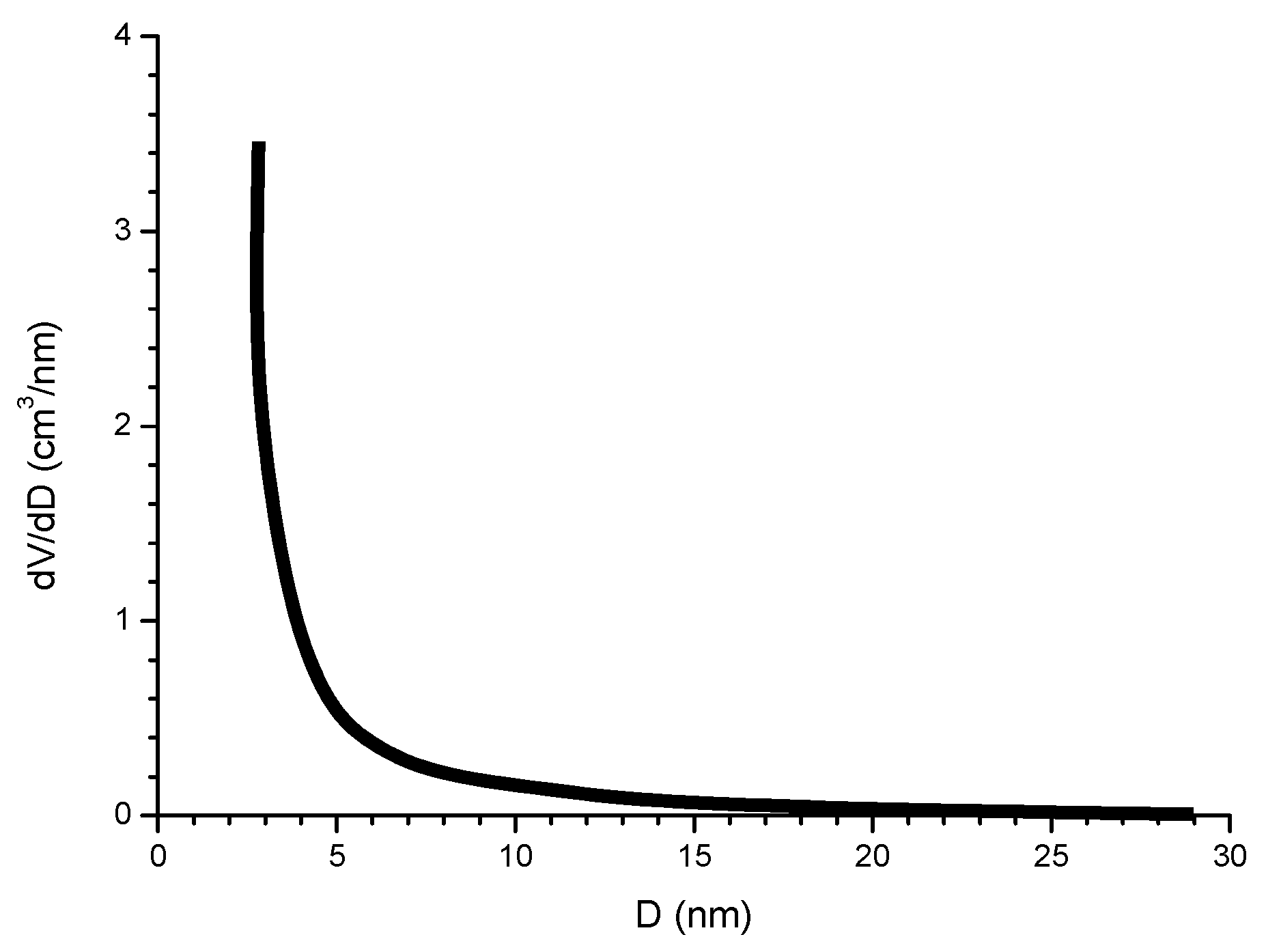
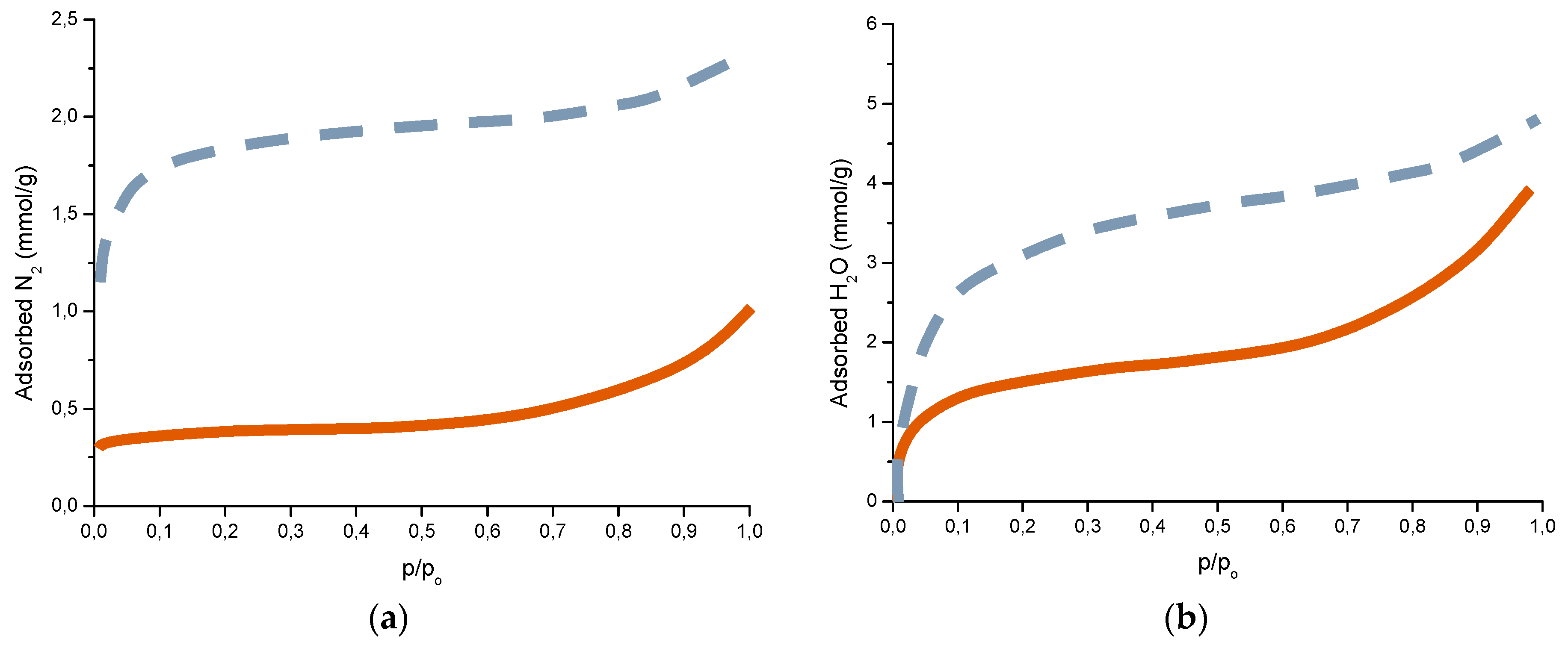
 5.6 mg NaCl/m2; wet period: 56%;
5.6 mg NaCl/m2; wet period: 56%;  10.7 mg NaCl /m2; wet period: 82%; p/po is the relative pressure of water or relative humidity (RH).
10.7 mg NaCl /m2; wet period: 82%; p/po is the relative pressure of water or relative humidity (RH).
 5.6 mg NaCl/m2; wet period: 56%;
5.6 mg NaCl/m2; wet period: 56%;  10.7 mg NaCl /m2; wet period: 82%; p/po is the relative pressure of water or relative humidity (RH).
10.7 mg NaCl /m2; wet period: 82%; p/po is the relative pressure of water or relative humidity (RH).